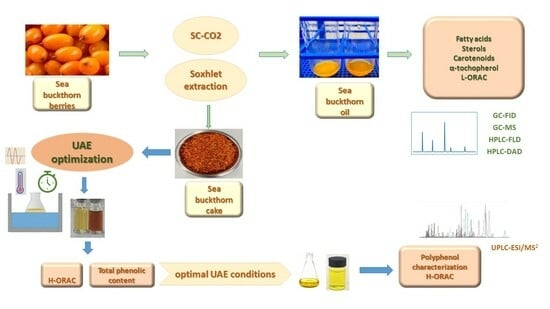
Efficiency of Supercritical CO2 and Ultrasound-Assisted Extraction Techniques for Isolation of Bioactive Molecules from Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) Berry Oils and Cakes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Preparation
2.3. Chemicals and Reagents
2.4. Supercritical CO2 Extraction
2.5. Soxhlet Extraction
2.6. Ultrasound-Assisted Extraction of by-Products of Oil Extraction
2.7. Characterization of Fatty Acids and Sterols in SB Oils
2.8. Characterization of Carotenoids in Oils by HPLC Analysis
2.9. Determination of α-Tocopherol
2.10. Determination of Total Phenolic Content
2.11. Characterization of Phenolic Compounds by UPLC/ESI-MS2 Analysis
2.12. Determination of Antioxidant Activity by the ORAC Method
2.13. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition of SB Berry Oil
3.2. Sterol Composition of SB Berry Oil
3.3. Carotenoid Content of Leikora and Ascola SBO Obtained by SC-CO2 and SE
3.4. α-Tocopherol Content in SB Berry Oil
3.5. Antioxidant Activity of SB Berry Oil
3.6. Polyphenolic Compounds in SB Cake Obtained after Oil Extraction
3.6.1. Optimization of the UAE of Polyphenols from SB Cake
3.6.2. Polyphenolic Characterization of Different SB Cake Extracts
3.7. Antioxidant Activity of SB Cake
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, T.S.C.; Beveridge, T.H.J. Sea Buckthorn: Hippophae rhamnoides L.: Production and Utilization; NRC Research Press: Ottawa, ON, Canada, 2003. [Google Scholar]
- Guo, R.X.; Guo, X.B.; Li, T.; Fu, X.; Liu, R.H. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of Sea buckthorn (Hippophae rhamnoides L.) berries. Food Chem. 2017, 221, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.K.; Sharma, K.; Sharma, N.; Sharma, A.; Singh, H.P.; Sinha, A.K. Microwave-assisted efficient extraction of different parts of for the comparative evaluation of antioxidant activity and quantification of its phenolic constituents by reverse-phase high-performance liquid chromatography (RP-HPLC). J. Agric. Food Chem. 2008, 56, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Jasniewska, A.; Diowksz, A. Wide Spectrum of Active Compounds in Sea Buckthorn (Hippophae rhamnoides L.) for Disease Prevention and Food Production. Antioxidants 2021, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Dwivedi, P.; Borkar, P.; Shinde, L. Pharmacological and nutritional importance of sea buckthorn (Hippophae). Pharma Innov. 2018, 7, 258. [Google Scholar]
- Zielinska, A.; Nowak, I. Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis. 2017, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, Ľ.; Panovská, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, G.; Zhang, J.; Zhang, Y.; Li, J.J.; Xiong, C.; Zhang, Q.; Li, X.D.; Lai, X.R. Metabolic discrimination of sea buckthorn from different species by H NMR based metabolomics. Sci. Rep. 2017, 7, 1585. [Google Scholar] [CrossRef] [PubMed]
- Kallio, H.; Yang, B.R.; Peippo, P. Effects of different origins and harvesting time on vitamin C, tocopherols, and tocotrienols in sea buckthorn (Hippophae rhamnoides) berries. J. Agric. Food Chem. 2002, 50, 6136–6142. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.C.; Rumpunen, K.; Johansson, E.; Olsson, M.E. Tocopherols and tocotrienols in sea buckthorn (Hippophae rhamnoides L.) berries during ripening. J. Agric. Food. Chem. 2008, 56, 6701–6706. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.R.; Kallio, H. Composition and physiological effects of sea buckthorn (Hippophae rhamnoides L.) lipids. Trends Food Sci. Technol. 2002, 13, 160–167. [Google Scholar] [CrossRef]
- Li, T.S.C.; Beveridge, T.H.J.; Drover, J.C.G. Phytosterol content of sea buckthorn (Hippophae rhamnoides L.) seed oil: Extraction and identification. Food Chem. 2007, 101, 1633–1639. [Google Scholar] [CrossRef]
- Baoru, Y.; Kallio, H.P.; Koponen, J.; Tahvonen, R.L.; Pfannhauser, W.; Fenwick, G.R.; Khokhar, S. Free and esterified sterols in seed oil and pulp/peel oil of sea buckthorn (Hippophaë rhamnoides L.). In Proceedings of the EUROFOODCHEM XI Meeting, Norwich, UK, 26–28 September 2001. [Google Scholar]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Fatty acid composition, bioactive phytochemicals, antioxidant properties and oxidative stability of edible fruit seed oil: Effect of preharvest and processing factors. Heliyon 2020, 6, e04962. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidovic, S.; Redovnikovic, I.R.; Jokic, S. Green solvents for green technologies. J. Chem. Technol. Biot. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Brunton, N.; Brennan, C.; Cullen, P.J.; O’Donnell, C.P. Application of Supercritical Carbon Dioxide to Fruit and Vegetables: Extraction, Processing, and Preservation. Food Rev. Int. 2012, 28, 253–276. [Google Scholar] [CrossRef]
- Uquiche, E.; Romero, V.; Ortíz, J.; del Valle, J.M. Extraction of Oil and Minor Lipids from Cold-Press Rapeseed Cake with Supercritical Co. Braz. J. Chem. Eng. 2012, 29, 585–597. [Google Scholar] [CrossRef]
- Miekus, N.; Iqbal, A.; Marszalek, K.; Puchalski, C.; Swiergiel, A. Green Chemistry Extractions of Carotenoids from Hippophae rhamnoides L.-Supercritical Carbon Dioxide and Enzyme-Assisted Methods. Molecules 2019, 24, 4339. [Google Scholar] [CrossRef] [PubMed]
- Dienaitė, L.; Pukalskas, A.; Pukalskienė, M.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Phytochemical Composition, Antioxidant and Antiproliferative Activities of Defatted Sea Buckthorn (Hippophae rhamnoides L.) Berry Pomace Fractions Consecutively Recovered by Pressurized Ethanol and Water. Antioxidants 2020, 9, 274. [Google Scholar] [CrossRef]
- Teh, S.S.; Birch, E.J. Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrason. Sonochem 2014, 21, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.P.; Pang, S.X.; Zhong, M.M.; Sun, Y.F.; Qayum, A.; Liu, Y.X.; Rashid, A.; Xu, B.G.; Liang, Q.F.; Ma, H.L.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem 2023, 101, 106646. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.Q.; Zhang, H.H.; Wen, C.T.; Zhang, J.X.; Chen, G.Y.; Ma, H.L. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT-Food Sci. Technol. 2018, 89, 284–290. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid- and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MS identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef]
- Petrarca, M.H.; Vicente, E.; Tfouni, S.A.V. Single-run gas chromatography-mass spectrometry method for the analysis of phthalates, polycyclic aromatic hydrocarbons, and pesticide residues in infant formula based on dispersive microextraction techniques. Microchem. J. 2024, 197, 109824. [Google Scholar] [CrossRef]
- Pavlović, N.; Valek Lendić, K.; Miškulin, M.; Moslavac, T.; Jokić, S. Supercritical CO2 Extraction of Sea Buckthorn. Food Health Dis. 2016, 5, 55–61. [Google Scholar]
- Harkat, H.; Bousba, R.; Benincasa, C.; Atrouz, K.; Gueltekin-oezgueven, M.; Altuntas, U.; Demircan, E.; Zahran, H.A.; Oezcelik, B. Assessment of Biochemical Composition and Antioxidant Properties of Algerian Date Palm (Hippophae rhamnoides L.) Seed Oil. Plants 2022, 11, 381. [Google Scholar] [CrossRef]
- ISO 12966-22017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids [WWW Document]. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/72142.html (accessed on 1 October 2022).
- Determination of Individual and Total Sterols Contents—Gas Chromatographic Method—Part 1: Animal and Vegetable Fats and Oils [WWW Document]. ISO: Geneva, Switzerland, 2014. Available online: https://www.iso.org/standard/60248.html (accessed on 20 October 2022).
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdés, A.; Ibrahim, O.H.M.; Suarez-Alvarez, S.; Ferragut, J.A.; Micol, V.; Cifuentes, A.; Ibáñez, E.; García-Cañas, V. Pressurized liquid extraction of Neochloris oleoabundans for the recovery of bioactive carotenoids with anti-proliferative activity against human colon cancer cells. Food Res. Int. 2017, 99, 1048–1055. [Google Scholar] [CrossRef]
- Pop, R.M.; Weesepoel, Y.; Socaciu, C.; Pintea, A.; Vincken, J.-P.; Gruppen, H. Carotenoid composition of berries and leaves from six Romanian sea buckthorn (Hippophae rhamnoides L.) varieties. Food Chem. 2014, 147, 1–9. [Google Scholar] [CrossRef] [PubMed]
- ISO 9936-1:2016; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Contents by Highperformance Liquid Chromatography [WWW Document]. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/69595.html (accessed on 20 October 2022).
- Culina, P.; Repajic, M.; Garofulic, I.E.; Dragovic-Uzelac, V.; Pedisic, S. Evaluation of Polyphenolic Profile and Antioxidant Activity of Sea Buckthorn (Eleagnus rhamnoides (L.) A Nelson) Extraction. Processes 2024, 12, 126. [Google Scholar] [CrossRef]
- Culina, P.; Cvitkovic, D.; Pfeifer, D.; Zoric, Z.; Repajic, M.; Garofulic, I.E.; Balbino, S.; Pedisic, S. Phenolic Profile and Antioxidant Capacity of Selected Medicinal and Aromatic Plants: Diversity upon Plant Species and Extraction Technique. Processes 2021, 9, 2207. [Google Scholar] [CrossRef]
- Garofulic, I.E.; Kruk, V.; Martic, A.; Martic, I.; Zoric, Z.; Pedisic, S.; Dragovic, S.; Dragovic-Uzelac, V. Evaluation of Polyphenolic Profile and Antioxidant Activity of L. Leaves and Fruit Extract Obtained by Optimized Microwave-Assisted Extraction. Foods 2020, 9, 1556. [Google Scholar] [CrossRef]
- Naguib, Y.M.A.; Hari, S.P.; Passwater, R.; Huang, D.J. Antioxidant activities of natural vitamin E formulations. J. Nutr. Sci. Vitaminol. 2003, 49, 217–220. [Google Scholar] [CrossRef]
- Dulf, F.V. Fatty acids in berry lipids of six sea buckthorn (Hippophae rhamnoides L., subspecies carpatica) cultivars grown in Romania. Chem. Cent. J. 2012, 6, 106. [Google Scholar] [CrossRef]
- Barkhuu, B.; Lodonjav, M.; Ganzorig, O.; Tumurtogoo, N. The Physicochemical Composition of Sea Buckthorn (Hippophae rhamnoides L.) Oil and Its Treatment Characteristics; Atlantis Press: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Teleszko, M.; Wojdylo, A.; Rudzinska, M.; Oszmianski, J.; Golis, T. Analysis of Lipophilic and Hydrophilic Bioactive Compounds Content in Sea Buckthorn (Hippophae rhamnoides L.) Berries. J. Agric. Food Chem. 2015, 63, 4120–4129. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Y.X.; Liu, G.M.; Wang, Q.; Zhao, H. Optimization of supercritical carbon dioxide extraction of sea buckthorn (Hippophae rhamnoides L.) oil using response surface methodology. LWT-Food Sci. Technol. 2008, 41, 1223–1231. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Y.X.; Liu, G.M.; Zheng, Y.Y.; Zhao, J. Supercritical CO extraction of whole berry oil from sea buckthorn fruit. Food Sci. Biotechnol. 2008, 17, 470–474. [Google Scholar]
- Vaitkeviciene, N.; Jariene, E.; Danilcenko, H.; Kulaitiene, J.; Mazeika, R.; Hallmann, E.; Blinstrubiene, A. Comparison of Mineral and Fatty Acid Composition of Wild and Cultivated Sea Buckthorn Berries from Lithuania. J. Elem. 2019, 24, 1101–1113. [Google Scholar] [CrossRef]
- Yang, B.R.; Kallio, H.P. Fatty acid composition of lipids in sea buckthorn (Hippophae rhamnoides L.) berries of different origins. J. Agric. Food Chem. 2001, 49, 1939–1947. [Google Scholar] [CrossRef]
- Cakir, A. Essential oil and fatty acid composition of the fruits of Hippophae rhamnoides L. (Sea Buckthorn) and Myrtus communis L. from Turkey. Biochem. Syst. Ecol. 2004, 32, 809–816. [Google Scholar] [CrossRef]
- Máté, M.; Selimaj, G.; Simon, G.; Szalóki-Dorkó, L.; Gitta, F. Assessment of Berries of Some Sea Buckthorn Genotypes by Physicochemical Properties and Fatty Acid Content of the Seed. Plants 2022, 11, 3412. [Google Scholar] [CrossRef]
- Kuhkheil, A.; Badi, H.N.; Mehrafarin, A.; Abdossi, V. Chemical constituents of sea buckthorn (Hippophae rhamnoides L.) fruit in populations of central Alborz Mountains in Iran. Res. J. Pharmacogn. 2017, 4, 1–12. [Google Scholar]
- Zheng, L.; Shi, L.-K.; Zhao, C.-W.; Jin, Q.-Z.; Wang, X.-G. Fatty acid, phytochemical, oxidative stability and in vitro antioxidant property of sea buckthorn (Hippophaë rhamnoides L.) oils extracted by supercritical and subcritical technologies. LWT 2017, 86, 507–513. [Google Scholar] [CrossRef]
- St George, S.; Cenkowski, S. Influence of Harvest Time on the Quality of Oil-Based Compounds in Sea Buckthorn (Hippophae rhamnoides L. ssp. sinensis) Seed and Fruit. J. Agric. Food Chem. 2007, 55, 8054–8061. [Google Scholar] [CrossRef]
- Arimboor, R.; Venugopalan, V.; Sarinkumar, K.; Arumughan, C.; Sawhney, R.C. Integrated processing of fresh Indian sea buckthorn (Hippophae rhamnoides) berries and chemical evaluation of products. J. Sci. Food Agric. 2006, 86, 2345–2353. [Google Scholar] [CrossRef]
- Madawala, S.R.P.; Brunius, C.; Adholeya, A.; Tripathi, S.B.; Hanhineva, K.; Hajazimi, E.; Shi, L.; Dimberg, L.; Landberg, R. Impact of location on composition of selected phytochemicals in wild sea buckthorn (Hippophae rhamnoides). J. Food Compos. Anal. 2018, 72, 115–121. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, L.; Zhao, C.; Jin, Q.; Wang, X. Chemical composition and antioxidant capacity of extracts from the whole berry, pulp and seed of Hippophaë rhamnoides ssp. yunnanensis. Nat. Prod. Res. 2019, 33, 3596–3600. [Google Scholar] [CrossRef]
- Seglina, D.; Krasnova, I.; Grygier, A.; Radziejewska-Kubzdela, E.; Rudzińska, M.; Górnaś, P. Unique bioactive molecule composition of sea buckthorn (Hippophae rhamnoides L.) oils obtained from the peel, pulp, and seeds via physical “solvent-free” approaches. J. Am. Oil Chem. Soc. 2021, 98, 1009–1020. [Google Scholar] [CrossRef]
- Yang, B.; Karlsson, R.M.; Oksman, P.H.; Kallio, H.P. Phytosterols in sea buckthorn (Hippophaë rhamnoides L.) berries: Identification and effects of different origins and harvesting times. J. Agric. Food Chem. 2001, 49, 5620–5629. [Google Scholar] [CrossRef]
- Sajfrtová, M.; Licková, I.; Wimmerová, M.; Sovová, H.; Wimmer, Z. β-Sitosterol: Supercritical carbon dioxide extraction from sea buckthorn (Hippophae rhamnoides L.) seeds. Int. J. Mol. Sci. 2010, 11, 1842–1850. [Google Scholar] [CrossRef]
- Yang, B.; Ahotupa, M.; Määttä, P.; Kallio, H. Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Res. Int. 2011, 44, 2009–2017. [Google Scholar] [CrossRef]
- Sovová, H.; Galushko, A.A.; Stateva, R.P.; Rochová, K.; Sajfrtová, M.; Bártlová, M. Supercritical fluid extraction of minor components of vegetable oils: β-Sitosterol. J. Food Eng. 2010, 101, 201–209. [Google Scholar] [CrossRef]
- Tudor, C.; Bohn, T.; Iddir, M.; Dulf, F.V.; Focsan, M.; Rugina, D.O.; Pintea, A. Sea Buckthorn Oil as a Valuable Source of Bioaccessible Xanthophylls. Nutrients 2020, 12, 76. [Google Scholar] [CrossRef]
- Mihalcea, L.; Turturica, M.; Ghinea, I.O.; Barbu, V.; Ionita, E.; Cotârlet, M.; Stanciuc, N. Encapsulation of carotenoids from sea buckthorn extracted by CO supercritical fluids method within whey proteins isolates matrices. Innov. Food Sci. Emerg. 2017, 42, 120–129. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Quantitative analysis, assessment of bioavailability and antioxidant activity of food carotenoids-A review. J. Food Compos. Anal. 2010, 23, 726–740. [Google Scholar] [CrossRef]
- Ranjith, A.; Kumar, K.S.; Venugopalan, V.V.; Arumughan, C.; Sawhney, R.C.; Singh, V. Fatty acids, tocols, and carotenoids in pulp oil of three sea buckthorn species grown in the Indian Himalayas. J. Am. Oil Chem. Soc. 2006, 83, 359–364. [Google Scholar] [CrossRef]
- Gao, X.Q.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Korekar, G.; Dolkar, P.; Singh, H.; Srivastava, R.B.; Stobdan, T. Variability and the genotypic effect on antioxidant activity, total phenolics, carotenoids and ascorbic acid content in seventeen natural population of Seabuckthorn (Hippophae rhamnoides L.) from trans-Himalaya. LWT-Food Sci. Technol. 2014, 55, 157–162. [Google Scholar] [CrossRef]
- Zadernowski, R.; Naczk, M.; Amarowicz, R. Tocopherols in sea buckthorn (Hippophaë rhamnoides L.) berry oil. J. Oil Fat. Ind. 2003, 80, 55–58. [Google Scholar] [CrossRef]
- Bal, L.M.; Meda, V.; Naik, S.N.; Satya, S. Sea buckthorn berries: A potential source of valuable nutrients for nutraceuticals and cosmoceuticals. Food Res. Int. 2011, 44, 1718–1727. [Google Scholar] [CrossRef]
- Otgonbayar, C.; Matthaus, B.; Odonmajig, P. Fatty acid, Tocopherol and Sterol Composition in Sea buckthorn (Hippophae rhamnoides L.) of Mongolia. Mong. J. Chem. 2014, 12, 126–130. [Google Scholar] [CrossRef]
- Eisenmenger, M.; Dunford, N.T. Bioactive Components of Commercial and Supercritical Carbon Dioxide Processed Wheat Germ Oil. J. Am. Oil Chem. Soc. 2008, 85, 55–61. [Google Scholar] [CrossRef]
- Gracia, I.; Rodríguez, J.F.; de Lucas, A.; Fernandez-Ronco, M.P.; García, M.T. Optimization of supercritical CO2 process for the concentration of tocopherol, carotenoids and chlorophylls from residual olive husk. J. Supercrit. Fluids 2011, 59, 72–77. [Google Scholar] [CrossRef]
- Cuco, R.P.; Cardozo-Filho, L.; Silva, C.D. Simultaneous extraction of seed oil and active compounds from peel of pumpkin (Cucurbita maxima) using pressurized carbon dioxide as solvent. J. Supercrit. Fluids 2019, 143, 8–15. [Google Scholar] [CrossRef]
- Sun, Q.; Shi, J.; Scanlon, M.; Xue, S.J.; Lu, J. Optimization of supercritical-CO2 process for extraction of tocopherol-rich oil from canola seeds. LWT 2021, 145, 111435. [Google Scholar] [CrossRef]
- Verleyen, T.; Verhe, R.; Huyghebaert, A.; Dewettinck, K.; De Greyt, W. Identification of α-Tocopherol Oxidation Products in Triolein at Elevated Temperatures. J. Agric. Food Chem. 2001, 49, 1508–1511. [Google Scholar] [CrossRef]
- Sabliov, C.; Fronczek, C.; Astete, C.; Khachaturyan, M.; Khachatryan, L.; Leonardi, C. Effects of Temperature and UV Light on Degradation of α-Tocopherol in Free and Dissolved Form. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 895–902. [Google Scholar] [CrossRef]
- Andrei, S.; Bunea, A.; Pop, E.A.; Dulf, F.; Pintea, A. The Protective Effect of Hippophae rhamnoides Carotenoid Extract Against Lipid Peroxidation in Crude Vegetable Oils. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca 2014, 71, 101–106. [Google Scholar] [CrossRef]
- Zardo, I.; de Espíndola Sobczyk, A.; Marczak, L.D.F.; Sarkis, J. Optimization of Ultrasound Assisted Extraction of Phenolic Compounds from Sunflower Seed Cake Using Response Surface Methodology. Waste Biomass Valorization 2019, 10, 33–44. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Rodríguez Barragan, Ó.; Bona, S.; Rodríguez-Turienzo, L.; Staebler, A. Ultrasound-Assisted Extraction of Polyphenols from Olive Pomace: Scale up from Laboratory to Pilot Scenario. Processes 2022, 10, 2481. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Rodríguez, M.; González Pereira, A.; Rivo, F.N.; Soria López, A.; García-Pérez, P.; Otero, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; et al. Critical Variables Influencing the Ultrasound-Assisted Extraction of Bioactive Compounds—A Review. Chem. Proc. 2021, 5, 50. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate peel. LWT-Food Sci. Technol. 2019, 101, 342–350. [Google Scholar] [CrossRef]
- Gomes, W.F.; Tiwari, B.K.; Rodriguez, O.; de Brito, E.S.; Fernandes, F.A.N.; Rodrigues, S. Effect of ultrasound followed by high pressure processing on prebiotic cranberry juice. Food Chem. 2017, 218, 261–268. [Google Scholar] [CrossRef]
- Borrás-Enríquez, A.; Reyes-Ventura, E.; Villanueva, S.; Moreno-Vilet, L. Effect of Ultrasound-Assisted Extraction Parameters on Total Polyphenols and Its Antioxidant Activity from Mango Residues (Mangifera indica L. var. Manililla). Separations 2021, 8, 94. [Google Scholar] [CrossRef]
- Pedisic, S.; Culina, P.; Pavlesic, T.; Vahcic, N.; Garofulic, I.E.; Zoric, Z.; Dragovic-Uzelac, V.; Repajic, M. Efficiency of Microwave and Ultrasound-Assisted Extraction as a Green Tool for Polyphenolic Isolation from Monofloral Honeys. Processes 2023, 11, 3141. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Martini, S. Sonocrystallization of Fats: Considerations for Industrial Applications. J. Am. Oil Chem. Soc. 2021, 98, 5. [Google Scholar]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Stability of 40 Phenolic Compounds during Ultrasound-Assisted Extractions (UAE). AIP Conf. Proc. 2016, 1755, 080009. [Google Scholar] [CrossRef]
- Shehata, M.G.; Aziz, N.M.A.; Youssef, M.M.; El-Sohaimy, S.A. Optimization conditions of ultrasound-assisted extraction of phenolic compounds from orange peels using response surface methodology. J. Food Process Preserv. 2021, 45, e15870. [Google Scholar] [CrossRef]
- Aguilar-Hernández, G.; Vivar-Vera, M.D.; García-Magaña, M.D.; González-Silva, N.; Pérez-Larios, A.; Montalvo-González, E. Ultrasound-Assisted Extraction of Total Acetogenins from the Soursop Fruit by Response Surface Methodology. Molecules 2020, 25, 1139. [Google Scholar] [CrossRef] [PubMed]
- Dobrincic, A.; Repajic, M.; Garofulic, I.E.; Tuden, L.; Dragovic-Uzelac, V.; Levaj, B. Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols. Processes 2020, 8, 1008. [Google Scholar] [CrossRef]
- Galviz-Quezada, A.; Ochoa, A.; Zabala, M.; Ochoa, S.; Osorio-Tobón, J. Obtaining phenolic compounds from iraca waste (Carludovica palmata Ruiz & Pav) through ultrasound-assisted extraction. Biomass Convers. Biorefinery 2021, 13, 4965–4976. [Google Scholar] [CrossRef]
- Rösch, D.; Krumbein, A.; Mügge, C.; Kroh, L.W. Structural investigations of flavonol glycosides from sea buckthorn (Hippophaë rhamnoides) pomace by NMR spectroscopy and HPLC-ESI-MS(n). J. Agric. Food Chem. 2004, 52, 4039–4046. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Halttunen, T.; Raimo, O.; Price, K.; Kallio, H. Flavonol glycosides in wild and cultivated berries of three major subspecies of and changes during harvesting period. Food Chem. 2009, 115, 657–664. [Google Scholar] [CrossRef]
- Kallio, H.; Yang, B.; Halttunen, T. Flavonol glycosides of berries of three major sea buckthorn subspecies, Hippophaë rhamnoides ssp. Rhamnoides, ssp. Sinensis and ssp. Mongolica. In Proceedings of the 2nd International Seabuckthorn Association Conference, ISA2005, Beijing, China, 26–29 August 2005; pp. 29–35. [Google Scholar]
- Pop, R.M.; Socaciu, C.; Pintea, A.; Buzoianu, A.D.; Sanders, M.G.; Gruppen, H.; Vincken, J.P. UHPLC/PDA-ESI/MS Analysis of the Main Berry and Leaf Flavonol Glycosides from Different Carpathian Hippophae rhamnoides L. Varieties. Phytochem. Anal. 2013, 24, 484–492. [Google Scholar] [CrossRef]
- Fatima, T.; Kesari, V.; Watt, I.; Wishart, D.; Todd, J.F.; Schroeder, W.R.; Paliyath, G.; Krishna, P. Metabolite profiling and expression analysis of flavonoid, vitamin C and tocopherol biosynthesis genes in the antioxidant-rich sea buckthorn (Hippophae rhamnoides L.). Phytochemistry 2015, 118, 181–191. [Google Scholar] [CrossRef]
- Guo, X.; Guo, R.; Chang, X.; Brennan, C.; Li, T.; Fu, X.; Liu, R. Phenolic compounds, antioxidant activity, antiproliferative activity and bioaccessibility of Sea buckthorn (Hippophaë rhamnoides L.) berries as affected by in vitro digestion. Food Funct. 2017, 8, 4229–4240. [Google Scholar] [CrossRef]
- Bittová, M.; Krejzová, E.; Roblova, V.; Kubáň, P.; Kubáň, V. Monitoring of HPLC profiles of selected polyphenolic compounds in sea buckthorn (Hippophae rhamnoides L.) plant parts during annual growth cycle and estimation of their antioxidant potential. Cent. Eur. J. Chem. 2014, 12, 1152–1161. [Google Scholar] [CrossRef]
- Zadernowski, R.; Naczk, M.; Czaplicki, S.; Rubinskiene, M.; Szlakiewicz, M. Composition of phenolic acids in sea buckthorn (Hippophae rhamnoides L.) berries. J. Am. Oil Chem. Soc. 2005, 82, 175–179. [Google Scholar] [CrossRef]
- Arimboor, R.; Kumar, K.S.; Arumughan, C. Simultaneous estimation of phenolic acids in sea buckthorn (Hippophaë rhamnoides) using RP-HPLC with DAD. J. Pharm. Biomed. Anal. 2008, 47, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, F.; Wei, P.; Chai, X.; Hou, G.; Meng, Q. Phytochemistry, health benefits, and food applications of sea buckthorn (Hippophae rhamnoides L.): A comprehensive review. Front. Nutr. 2022, 9, 1036295. [Google Scholar] [CrossRef]
- Urcan, A.; Criste, A.; Dezmirean, D.; Mărgăoan, R.; Caeiro, A.; Campos, M. Similarity of Data from Bee Bread with the Same Taxa Collected in India and Romania. Molecules 2018, 23, 2491. [Google Scholar] [CrossRef] [PubMed]
- Tańska, M.; Roszkowska, B.; Czaplicki, S.; Borowska, E.J.; Bojarska, J.; Dąbrowska, A. Effect of Fruit Pomace Addition on Shortbread Cookies to Improve Their Physical and Nutritional Values. Plant Foods Hum. Nutr. 2016, 71, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Ficzek, G.; Mátravölgyi, G.; Furulyas, D.; Рэнцэндава, Ч.; Jócsák, I.; Papp, D.; Simon, G.; Végvári, G.; Stéger-Máté, M. Analysis of bioactive compounds of three sea buckthorn cultivars (Hippophaë rhamnoides L. ‘Askola’, ‘Leikora’, and ‘Orangeveja’) with HPLC and spectrophotometric methods. Eur. J. Hortic. Sci. 2018, 84, 31–38. [Google Scholar] [CrossRef]
- Orhan, E.; Çakir, Ö.; Şengül, M. The genotypic effects on the chemical composition and antioxidant activity of sea buckthorn (Hippophae rhamnoides L.) berries grown in Turkey. Sci. Hortic. 2007, 115, 27–33. [Google Scholar] [CrossRef]
- Rop, O.; Ercisli, S.; Mlcek, J.; Jurikova, T.; Hoza, I. Antioxidant and radical scavenging activities in fruits of 6 sea buckthorn (Hippophae rhamnoides L.) cultivars. Turk. J. Agric. 2014, 38, 224–232. [Google Scholar] [CrossRef]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A 2003, 1000, 657–691. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Env. Sci. Pollut. Res. Int. 2022, 29, 81112–81129. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acids | Leikora | Ascola | ||
|---|---|---|---|---|
| SC-CO2 | SE | SC-CO2 | SE | |
| SATURATED | ||||
| Myristic | 0.26 ± 0.01 b | 0.25 ± 0.01 b | 0.15 ± 0.01 a | 0.15 ± 0.01 a |
| Pentadecanoic acid | 0.06 ± 0.01 a | 0.05 ± 0.01 a | 0.07 ± 0.01 a | 0.08 ± 0.01 a |
| Palmitic | 42.86 ± 1.01 b | 42.85 ± 1.14 b | 25.44 ± 1.05 a | 25.43 ± 0.05 a |
| Heptadecanoic acid | 1.04 ± 0.01 b | 1.03 ± 0.01 b | 0.11 ± 0.01 a | 0.12 ± 0.01 a |
| Stearic | 0.77 ± 0.01 a | 0.77 ± 0.01 a | 1.59 ± 0.02 b | 1.59 ± 0.02 b |
| Arachidic acid | 0.18 ± 0.01 a | 0.18 ± 0.01 a | 0.28 ± 0.02 b | 0.29 ± 0.01 b |
| Docosanoic acid | 0.09 ± 0.01 a | 0.09 ± 0.01 a | 0.12 ± 0.01 a | 0.12 ± 0.01 a |
| UNSATURATED | ||||
| MONOUNSATURATED | ||||
| Palmitoleic acid | 37.47 ± 1.11 b | 37.46 ± 2.01 b | 31.85 ± 1.15 a | 31.83 ± 2.08 a |
| Elaidic acid | 0.05 ± 0.01 a,b | 0.07 ± 0.01 b | 0.01 ± 0.01 a | 0.01 ± 0.00 a |
| Gondoic acid | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.13 ± 0.01 b | 0.14 ± 0.00 b |
| Oleic acid | 5.35 ± 0.02 a | 5.36 ± 0.01 a | 16.38 ± 1.04 b | 16.37 ± 1.24 b |
| Vaccenic acid | 0.03 ± 0.00 a | 0.04 ± 0.00 a | 7.43 ± 1.03 b | 7.43 ± 1.07 b |
| POLYUNSATURATED | ||||
| Linoleic acid | 11.16 ± 0.05 a | 11.16 ± 0.05 a | 11.20 ± 0.45 a | 11.19 ± 0.63 a |
| α-Linolenic acid | 0.66 ± 0.01 a | 0.66 ± 0.01 a | 5.24 ± 0.14 b | 5.25 ± 0.01 b |
| Source of Variation: | SUM SFAs: | SUM UFAs: | SUM MUFAs: | SUM PUFAs: | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SC-CO2 | SE | SC-CO2 | SE | SC-CO2 | SE | SC-CO2 | SE | |||||
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | |||||
| Leikora | p = 0.90 | 45.25 ± 0.02 b,A | 45.22 ± 0.21 b,A | p = 0.92 | 54.75 ± 0.03 a,A | 54.78 ± 0.26 a,A | p = 0.95 | 42.93 ± 0.37 a,A | 42.96 ± 0.24 a,A | p = 1.00 | 11.82 ± 0.17 a,A | 11.82 ± 0.30 a,A |
| Ascola | p = 0.93 | 27.75 ± 0.20 a,A | 27.78 ± 0.26 a,A | p = 0.85 | 72.25 ± 0.10 b,A | 72.22 ± 0.09 b,A | p = 0.99 | 55.80 ± 0.82 b,A | 55.78 ± 0.66 b,A | p = 1.00 | 16.44 ± 0.27 b,A | 16.44 ± 0.47 b,A |
| Compound | Leikora | Ascola | ||
|---|---|---|---|---|
| SC-CO2 | SE | SC-CO2 | SE | |
| Desmosterol | 0.53 ± 0.01 a | 0.31 ± 0.01 a | 2.68 ± 0.01 b | 2.20 ± 0.01 b |
| Campesterol | 32.30 ± 0.15 d | 22.31 ± 0.14 c | 16.91 ± 0.12 a,b | 11.89 ± 0.11 a |
| Stigmasterol | 3.08 ± 0.01 d | 1.11 ± 0.05 a,b | 1.43 ± 0.01 c | 0.45 ± 0.01 a |
| 5-α-kolestanol | 0.76 ± 0.01 b | 0.22 ± 0.00 a | 1.36 ± 0.01 c | 0.85 ± 0.01 b |
| β-Sitosterol | 589.96 ± 2.45 c | 473.85 ± 2.14 b | 433.82 ± 2.11 b | 352.56 ± 2.41 a |
| Stigmast-8-en-3β-ol | 6.51 ± 0.08 c | 3.45 ± 0.02 a | 7.28 ± 0.07 c | 5.26 ± 0.02 b |
| 24(Z)-stigmasta-5,24(241)-dien-3β-ol | 8.83 ± 0.05 a | 7.81 ± 0.05 a | 30.26 ± 0.45 b | 23.23 ± 0.52 b |
| Obtusifoliol | 13.31 ± 0.12 a | 12.30 ± 0.05 a | 24.27 ± 0.25 c | 18.51 ± 0.12 b |
| Δ5-avenasterol | 5.09 ± 0.01 a | 4.10 ± 0.01 a | 17.67 ± 0.23 b | 15.21 ± 0.11 b |
| Stigmasta-7,24(28)-dien-3-ol | 7.50 ± 0.02 b | 3.10 ± 0.01 a | 9.85 ± 0.05 c | 6.86 ± 0.05 b |
| Lanosta-7,9(11)-dien-3-ol | 1.92 ± 0.01 c | 0.58 ± 0.00 a | 1.43 ± 0.00 a,b | 1.12 ± 0.01 a,b |
| Uvaol | 22.02 ± 0.05 a | 19.01 ± 0.04 a | 26.23 ± 0.11 b | 20.25 ± 0.06 a |
| Eritrodiol | 5.12 ± 0.03 b | 3.02 ± 0.01 a | 6.84 ± 0.01 c | 5.85 ± 0.00 b |
| Citrostadienol | 7.05 ± 0.02 b | 6.01 ± 0.01 a,b | 5.91 ± 0.01 a,b | 4.78 ± 0.01 a |
| Δ7-avenasterol | 3.35 ± 0.01 a,b | 3.14 ± 0.01 a | 4.36 ± 0.01 c | 3.95 ± 0.00 b,c |
| Ursolic aldehyde | 1.99 ± 0.04 a,b | 1.01 ± 0.01 a | 3.04 ± 0.01 b | 2.52 ± 0.00 b |
| Total Sterol Content (mg/100 g) | |||
|---|---|---|---|
| Source of Variation: | SC-CO2 | SE | |
| p < 0.05 | p < 0.05 | ||
| Leikora | p < 0.05 | 709.32 ± 4.20 b,B | 561.33 ± 3.10 b,A |
| Ascola | p < 0.05 | 593.34 ± 4.18 a,B | 475.49 ± 4.20 a,A |
| Carotenoids: | Mass Concentration (mg/100 g dm) | |||
|---|---|---|---|---|
| Leikora | Ascola | |||
| SC-CO2 | SE | SC-CO2 | SE | |
| Zeaxanthin | 8.50 ± 0.15 a | 5.42 ± 0.16 a | 8.77 ± 0.01 a | 8.45 ± 0.05 a |
| Lutein | 3.38 ± 0.05 a | 4.33 ± 0.01 b | nd | nd |
| β-cryptoxanthin | 5.21 ± 0.06 b | 3.76 ± 0.01 a | 6.85 ± 0.02 c | 5.75 ± 0.01 b |
| γ-carotene | 0.07 ± 0.00 a | 0.05 ± 0.00 a | 0.18 ± 0.00 b | 0.09 ± 0.00 a |
| cis γ-carotene | 0.44 ± 0.01 b | 0.19 ± 0.00 a | 1.18 ± 0.01 d | 0.99 ± 0.01 c |
| β-carotene | 2.09 ± 0.01 b | 1.73 ± 0.00 a | 2.09 ± 0.01 b | 1.95 ± 0.15 a,b |
| β-cryptoxanthin palmitate | 0.73 ± 0.02 b | 0.78 ± 0.01 b | 0.14 ± 0.00 a | nd |
| Zeaxanthin-myristate | 26.66 ± 0.15 a | 24.76 ± 0.25 a | 28.65 ± 0.75 a | 27.52 ± 0.25 a |
| Lutein-palmitate | 5.85 ± 0.01 b | 3.07 ± 0.01 a | 3.30 ± 0.00 a | 2.95 ± 0.00 a |
| Zeaxanthin-pamitate | 10.86 ± 0.14 a | 8.18 ± 0.15 a | 31.01 ± 0.20 b | 30.82 ± 0.45 b |
| Lutein di-myristate | nd | nd | 10.39 ± 0.15 b | 9.12 ± 0.12 a |
| Zeaxanthin-palmitate-myristate | 19.97 ± 0.25 b | 12.87 ± 0.16 a | 36.22 ± 1.05 c | 35.98 ± 1.21 c |
| Lutein di-palmitate | 6.44 ± 0.05 a | 5.40 ± 0.05 a | 24.17 ± 0.95 b | 23.25 ± 0.85 b |
| Zeaxanthin-di-palmitate | 56.73 ± 1.12 b | 43.38 ± 1.25 a | 58.08 ± 1.75 b | 57.05 ± 1.44 b |
| Lutein-palmitate-stearate | 35.05 ± 1.15 c | 26.94 ± 0.45 b | 4.37 ± 0.00 a | 3.75 ± 0.00 a |
| Total Carotenoid Content (mg/100 g) | |||
| Source of Variation: | SC-CO2 | SE | |
| p < 0.05 | p < 0.001 | ||
| Leikora | p < 0.05 | 181.98 ± 1.73 a,B | 140.86 ± 1.50 a,A |
| Ascola | p = 0.09 | 215.4 ± 2.18 b,A | 207.67 ± 1.11 b,A |
| α-Tocopherol (mg/100 g dm) | |||
|---|---|---|---|
| Source of Variation: | SC-CO2 | SE | |
| p = 0.33 | p = 0.14 | ||
| Leikora | p < 0.05 | 106.86 ± 2.61 a,B | 84.23 ± 1.00 a,A |
| Ascola | p < 0.05 | 102.45 ± 2.33 a,B | 81.06 ± 0.85 a,A |
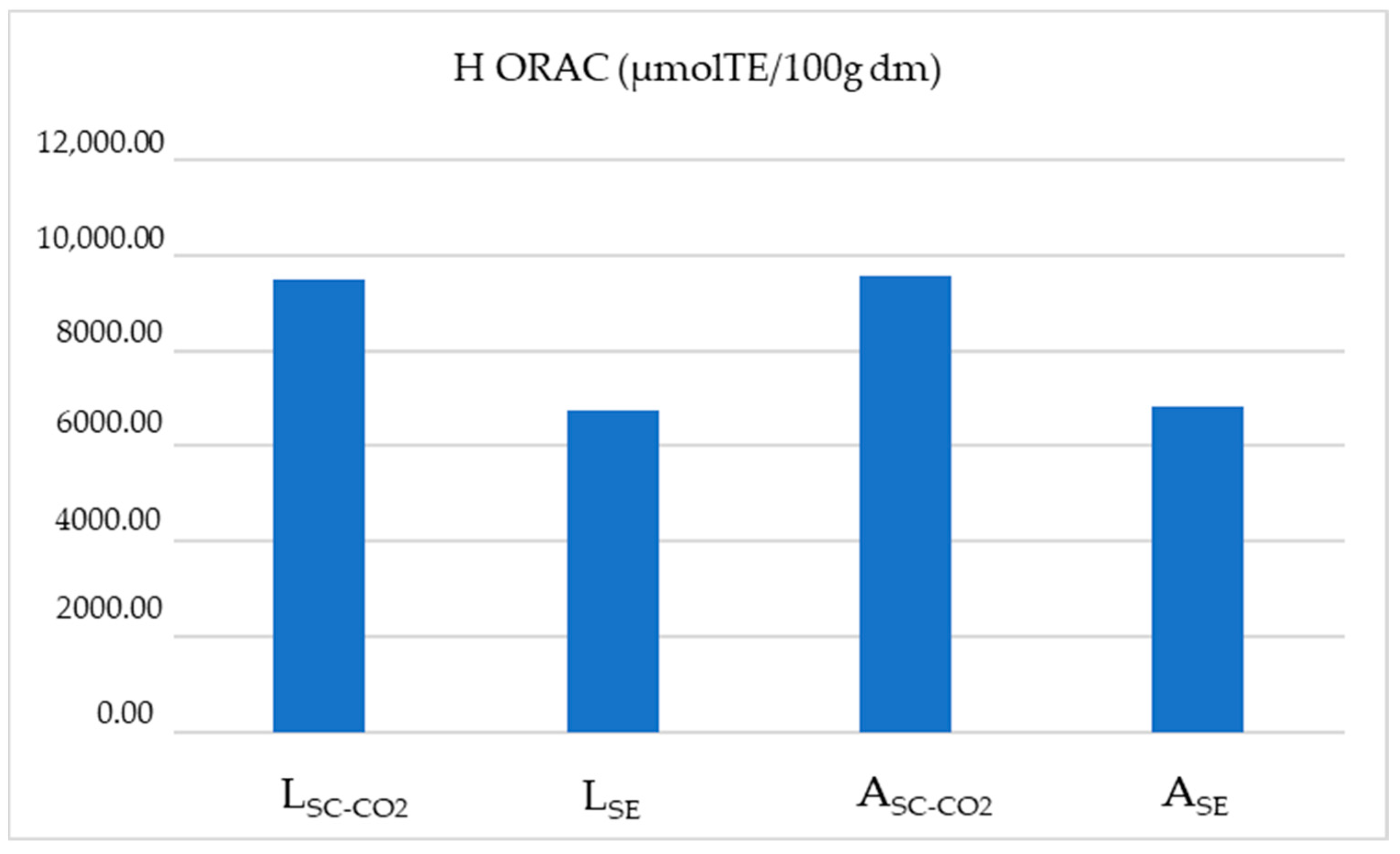
| Source of Variation: | L-ORAC (μmolTE/100 g dm) | ||
|---|---|---|---|
| SC-CO2 | SE | ||
| p = 0.20 | p = 0.55 | ||
| Leikora | p < 0.05 | 1537.10 ± 4.19 a,B | 1356.58 ± 19.62 a,A |
| Ascola | p < 0.05 | 1558.50 ± 10.34 a,B | 1372.63 ± 10.47 a,A |
| Source of Variation | Ultrasound Power (%) | ||||||
|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 30 | 60 | 90 | ||
| Temperature | Time | TPC | AA | ||||
| (°C) | (min) | mg/100 g dm | μmol TE/100 g dm | ||||
| 35 | 10 | 1123.25 ± 25.02 | 1254.77 ± 22.15 | 1242.25 ± 24.81 | 6871.34 ± 121.45 | 7524.12 ± 65.74 | 7192.18 ± 132.14 |
| 20 | 1256.36 ± 31.45 | 1596.75 ± 18.45 | 1591.90 ± 35.62 | 7322.90 ± 147.23 | 8194.90 ± 111.41 | 8280.29 ± 101.81 | |
| 30 | 1499.83 ± 33.15 | 1366.05 ± 21.75 | 1437.08 ± 85.41 | 8759.13 ± 150.74 | 8113.61 ± 121.71 | 8189.16 ± 108.45 | |
| 50 | 10 | 1572.21 ± 45.18 | 1851.72 ± 75.41 | 1749.01 ± 58.41 | 8983.91 ± 101.41 | 8756.20 ± 125.41 | 8751.92 ± 102.71 |
| 20 | 1795.40 ± 52.41 | 1998.98 ± 33.45 | 1792.11 ± 62.35 | 8700.93 ± 121.74 | 9504.85 ± 125.36 | 8795.53 ± 125.85 | |
| 30 | 1735.74 ± 65.85 | 1965.86 ± 25.41 | 1846.55 ± 55.24 | 8440.58 ± 122.52 | 9448.77 ± 145.87 | 8931.60 ± 123.52 | |
| 65 | 10 | 1303.05 ± 25.42 | 1381.00 ± 25.74 | 1281.03 ± 55.24 | 8887.88 ± 95.41 | 8631.72 ± 155.71 | 8427.25 ± 185.41 |
| 20 | 1753.91 ± 75.41 | 1853.88 ± 65.41 | 1829.31 ± 52.36 | 8994.28 ± 127.34 | 9332.67 ± 191.74 | 9485.58 ± 155.24 | |
| 30 | 1808.65 ± 48.26 | 1793.14 ± 75.61 | 1761.33 ± 75.14 | 8931.72 ± 154.25 | 8912.14 ± 185.74 | 9104.90 ± 151.74 | |
| Source of Variation | TPC | AA |
|---|---|---|
| mg/100 g dm | μmol TE/100 g dm | |
| Ultrasound power (%) | p = 0.29 | p = 0.39 |
| 30 | 1538.71 ± 59.40 a | 8432.52 ± 179.75 a |
| 60 | 1673.57 ± 64.51 a | 8735.44 ± 161.66 a |
| 90 | 1614.51 ± 54.90 a | 8706,49 ± 167.81 a |
| Temperature (°C) | p ≤ 0.001 | p ≤ 0.001 |
| 35 | 1374.25 ± 38.75 a | 7827.51 ± 142.20 a |
| 50 | 1811.95 ± 30.04 c | 8946.03 ± 90.92 b |
| 65 | 1640.59 ± 55.80 b | 9100.90 ± 67.87 b |
| Time (min) | p ≤ 0.001 | p = 0.11 |
| 10 | 1417.59 ± 57.62 a | 8336.28 ± 205.74 a |
| 20 | 1718.73 ± 49.37 b | 8756.88 ± 170.40 a |
| 30 | 1690.47 ± 47.21 b | 8781.29 ± 105.64 a |
| Phenolic Compounds | Precursor Ion (m/z) | Fragment Ions (m/z) | Ionization Mode | Mass Concentration (mg/100 g dm) | |||
|---|---|---|---|---|---|---|---|
| LSC-CO2 | LSE | ASC-CO2 | ASE | ||||
| FLAVONOLS | |||||||
| Isorhamnetin | 317 | 201 | positive | 0.45 ± 0.01 a | 0.93 ± 0.02 b | 1.05 ± 0.01 b | 0.98 ± 0.01 b |
| Isorhamnetin-3-sinapoyglucose-glucoside-7-rhamnoside | 993 | 463, 317 | positive | 1.71 ± 0.05 b | 0.62 ± 0.01 a | 1.80 ± 0.02 b | 0.53 ± 0.00 a |
| Ishorhamnetin-3-sophoroside-7-rhamnoside | 787 | 463, 317 | positive | 11.67 ± 0.12 c | 6.79 ± 0.01 b | 8.17 ± 0.02 b | 3.20 ± 0.01 a |
| Isorhamnetin-3-rutinoside-7-glucoside | 787 | 479, 317 | positive | 1.98 ± 0.05 b,c | 1.46 ± 0.01 b | 2.25 ± 0.01 c | 0.33 ± 0.00 a |
| Isorhamnetin-3-hexoside | 479 | 317 | positive | 65.37 ± 2.14 c | 35.90 ± 1.45 a | 79.44 ± 2.74 d | 56.54 ± 1.85 b |
| Isorhamnetin-3-rhamnoside | 463 | 317 | positive | 2.20 ± 0.00 a | 4.01 ± 0.01 b | 4.67 ± 0.01 b | 2.68 ± 0.00 a |
| Isorhamnetin-3.7-dihexoside | 641 | 479, 317 | positive | 0.55 ± 0.00 a,b | 1.25 ± 0.01 b | 0.51 ± 0.00 a | 2.91 ± 0.01 c |
| Isorhamnetin-3-rutinoside | 625 | 479, 317 | positive | 16.01 ± 0.54 b | 9.64 ± 0.65 a | 30.88 ± 0.17 c | 15.87 ± 0.28 b |
| Kaempferol | 287 | 145 | positive | 7.56 ± 0.01 b | 3.53 ± 0.00 a | 8.53 ± 0.01 b | 2.97 ± 0.00 a |
| Kaempferol-3-O-sophorose-7-O-rhamnoside | 757 | 287 | positive | 1.76 ± 0.01 a | 1.71 ± 0.01 a | 1.36 ± 0.01 a | 3.98 ± 0.01 b |
| Kaemferol-3-O-glucoside-7-O-rhamnoside | 595 | 433, 287 | positive | 15.71 ± 0.54 b | 12.38 ± 0.25 a | 18.72 ± 0.23 c | 13.89 ± 0.08 a,b |
| Kaempferol-3-rutinoside | 595 | 287 | positive | 1.97 ± 0.01 a | 0.42 ± 0.02 a | 2.03 ± 0.02 a | 1.22 ± 0.02 a |
| Kaempferol-rhamnoside | 433 | 287 | positive | 3.32 ± 0.01 b | 3.12 ± 0.01 b | 9.39 ± 0.05 c | 1.27 ± 0.05 a |
| Quercetin-3-sophoroside-7-rhamnoside | 773 | 611, 303 | positive | 2.32 ± 0.00 b | 0.20 ± 0.00 a | 2.11 ± 0.01 b | 0.33 ± 0.00 a |
| Quercetin-3-rhamnosylglucoside-7-rhamnoside | 757 | 303 | positive | 1.97 ± 0.02 b | 1.65 ± 0.01 b | 0.75 ± 0.02 a | 0.55 ± 0.01 a |
| Quercetin-3-rutinoside (rutin) | 611 | 303 | positive | 47.14 ± 2.45 b | 32.49 ± 1.24 a | 41.74 ± 0.98 b | 33.27 ± 0.98 a |
| Quercetin-3-glucoside | 465 | 303 | positive | 20.93 ± 0.04 b | 13.46 ± 0.02 a | 48.16 ± 0.51 c | 16.43 ± 0.09 a |
| Quercetin-3-rhamnoside (quercitrin) | 449 | 303 | positive | 1.70 ± 0.01 b | 1.60 ± 0.01 b | 0.95 ± 0.00 a | 2.95 ± 0.01 c |
| Quercetin-3-pentoside | 435 | 303 | positive | 0.81 ± 0.00 b | 0.50 ± 0.00 a | 0.54 ± 0.00 a | 0.38 ± 0.00 a |
| SUM: | 205.13 ± 6.01 c | 131.66 ± 3.52 a | 263.05 ± 4.81 d | 160.28 ± 4.92 b | |||
| FLAVAN-3-OLS | |||||||
| Catechin | 291 | 139 | positive | 2.15 ± 0.00 b | 0.64 ± 0.00 a | 3.16 ± 0.00 c | 2.10 ± 0.00 b |
| Epicatechin | 291 | 165 | positive | 0.99 ± 0.00 a | 0.51 ± 0.00 a | 3.82 ± 0.00 c | 2.50 ± 0.00 b |
| SUM: | 3.14 ± 0.01 b | 1.15 ± 0.00 a | 6.98 ± 0.01 c | 4.6 ± 0.01 b | |||
| PHENOLIC ACIDS | |||||||
| Caffeic acid | 179 | 135 | negative | 23.14 ± 1.41 a | 21.01 ± 0.95 a | 23.03 ± 1.01 a | 22.05 ± 1.11 a |
| Chorogenic acid | 353 | 191 | negative | 2.33 ± 0.01 a | 14.35 ± 0.85 b | 3.46 ± 0.01 a | 17.63 ± 1.14 c |
| Gallic acid | 169 | 125 | negative | 23.92 ± 1.05 a | 21.82 ± 0.98 a | 21.30 ± 1.75 a | 20.71 ± 1.14 a |
| p-hydroxybenzoic acid | 137 | 93 | negative | 31.49 ± 2.14 b | 18.75 ± 0.85 a | 38.13 ± 1.74 c | 29.72 ± 1.12 b |
| p-coumaric acid | 163 | 119 | negative | 14.67 ± 0.85 b | 11.51 ± 0.08 b | 13.13 ± 0.75 b | 7.55 ± 0.05 a |
| Protocatechuic acid | 153 | 109 | negative | 38.08 ± 1.74 c | 9.95 ± 0.83 a | 29.53 ± 1.41 b | 9.55 ± 0.81 a |
| Vanillic acid | 169 | 125 | positive | 46.80 ± 1.28 a | 42.63 ± 1.95 a | 60.46 ± 2.53 b | 48.32 ± 1.75 a |
| SUM: | 180.43 ± 8.48 b | 140.02 ± 6.49 a | 189.04 ± 8.94 b | 155.53 ± 6.21 a, b | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čulina, P.; Balbino, S.; Jokić, S.; Dragović-Uzelac, V.; Pedisić, S. Efficiency of Supercritical CO2 and Ultrasound-Assisted Extraction Techniques for Isolation of Bioactive Molecules from Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) Berry Oils and Cakes. Processes 2024, 12, 698. https://doi.org/10.3390/pr12040698
Čulina P, Balbino S, Jokić S, Dragović-Uzelac V, Pedisić S. Efficiency of Supercritical CO2 and Ultrasound-Assisted Extraction Techniques for Isolation of Bioactive Molecules from Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) Berry Oils and Cakes. Processes. 2024; 12(4):698. https://doi.org/10.3390/pr12040698
Chicago/Turabian StyleČulina, Patricija, Sandra Balbino, Stela Jokić, Verica Dragović-Uzelac, and Sandra Pedisić. 2024. "Efficiency of Supercritical CO2 and Ultrasound-Assisted Extraction Techniques for Isolation of Bioactive Molecules from Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) Berry Oils and Cakes" Processes 12, no. 4: 698. https://doi.org/10.3390/pr12040698
APA StyleČulina, P., Balbino, S., Jokić, S., Dragović-Uzelac, V., & Pedisić, S. (2024). Efficiency of Supercritical CO2 and Ultrasound-Assisted Extraction Techniques for Isolation of Bioactive Molecules from Sea Buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) Berry Oils and Cakes. Processes, 12(4), 698. https://doi.org/10.3390/pr12040698