Abstract
This study aimed to investigate the antioxidant activity and antibacterial effect of total flavonoids from Persicaria hydropiper (L.) Spach (TFs-Ph) and to provide a theoretical basis for the development of drugs for the treatment of pathogenic Escherichia coli and Salmonella spp. of broiler origin. Firstly, the response surface optimization heating reflux method was used to extract TFs-Ph, and the effects of ethanol concentration, solid–liquid ratio, heating reflux time, heating reflux temperature, and number of extraction times on the extraction yield of TFs-Ph were analyzed to determine the optimal extraction conditions. The antioxidant activity of TFs-Ph was determined by measuring the scavenging ability against hydroxyl radicals (•OH), 1,1-diphenyl-2-picrylhydrazyl (DPPH), superoxide anion (•O2−), and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). The antibacterial effect of TFs-Ph was determined by the disk diffusion method. The results showed that the optimal extraction parameters of TFs-Ph were as follows: ethanol concentration of 51%, solid-liquid ratio of 1:24 g/mL, heating reflux time of 74 min, heating reflux temperature of 70 °C, and three extraction times; in this case, the extraction yield of TFs-Ph was 6.37%. TFs-Ph had a strong scavenging ability against the free radicals of •OH, DPPH, •O2−, and ABTS, and the antioxidant activity was better than that of vitamin C (Vc). In addition, it showed a better antibacterial effect against pathogenic Escherichia coli and Salmonella of broiler origin compared with ampicillin (AMP). Therefore, TFs-Ph have a certain potential to replace antibiotics.
1. Introduction
Persicaria hydropiper (L.) Spach, a traditional Chinese herbal medicine, is an annual herb of the genus Polygonum in the family Polygonaceae [1]. It has the effects of detumescence, clearing away heat and toxic materials, activating blood and dissolving stasis, and treating rheumatic pain [2], and flavonoids are the main active components [3,4]. In traditional medicine, P. hydropiper is widely used to treat gastrointestinal disorders, dysentery, pain, excessive menstrual bleeding, and other diseases [5,6,7], while in veterinary clinics, P. hydropiper is mainly used to treat diarrhea and dysentery in livestock or poultry and can also be used to treat uterine bleeding and intestinal parasites in animals, with remarkable curative effects [8,9,10].
China’s broiler farming market is huge and tends to be large-scale with intensive development. Large-scale and intensive farming is characterized by advanced equipment, efficient management, and high feed-to-meat ratio; however, there are also problems, such as high stocking density and frequent occurrence of bacterial diseases [11,12,13], where E. coli and Salmonella spp. are the main bacterial infections [14,15]. With the implementation of the “anti-antibiotics order”, the addition of antibiotics to feed is prohibited, so the development of natural antimicrobials that can replace antibiotics is currently the only way [16,17,18,19]. Therefore, it is of great significance to extract TFs-Ph and explore their antioxidant properties and inhibitory effects on pathogenic E. coli and Salmonella from broilers, as they can be applied as veterinary medicine in the animal husbandry industry [20].
There have been many previous studies on the extraction of total flavonoids from plants. Zhao et al. [21] extracted flavonoids from Nitrariasibirica leaves by the ultrasound-assisted response surface methodology, and the extracts showed good antioxidant activity, with 86.99% DPPH scavenging. Miao et al. [22] extracted flavonoids from Xanthoceras sorbifolium Bunge leaves by the ultrasound-assisted method and determined that the flavonoid extraction yield under optimal process conditions was 5.52% and the extracts had strong antioxidant ability against DPPH and ABTS. To determine the medicinal value of flavonoids, extensive research has been carried out by previous researchers and has shown significant differences in the antioxidant and bacteriostatic activities of flavonoids from different species or different sources. Ożarowski et al. [23] reviewed the antioxidant properties of flavonoids from Passiflora species and concluded that Passiflora flavonoids have strong antioxidant activity and can be a valuable anti-oxidative medication for the prevention or treatment of many diseases associated with inflammation. Hu et al. [24] studied the antioxidant capacity of flavonoids from Folium Artemisia Argyi and the molecular mechanism in Caenorhabditis elegans. The results demonstrated that the flavonoids had a good scavenging effect in vitro and exerted antioxidant effects by improving the activity of antioxidant enzymes under acute stress to reduce reactive oxygen species (ROS) accumulation in vivo in C. elegans. Lu et al. [25] extracted flavonoids from Fengdan Peony and found that the flavonoids had a certain antibacterial effect on Gram-positive bacteria such as S. aureus and B. subtilis but had no antibacterial effect on Gram-negative bacteria such as E. coli and Salmonella. The research study by El-Zahar et al. [26] suggested that the synergistic effect of extracts from Berberis vulgaris with both antibacterial and antioxidant compounds may enhance their pathogenic antimicrobial capabilities.
So far, there have been few studies on the extraction of TFs-Ph, and the antioxidant and antibacterial activities of these extracts are unknown. Therefore, in this study, TFs-Ph were extracted by the heating reflux method, and the antioxidant activities, as well as the antibacterial effects against pathogenic E. coli and Salmonella from broilers, were determined to explore the possibility of their use as antibiotic substitutes, with a view to providing a theoretical basis for the further utilization of TFs-Ph as veterinary medicine resources.
2. Materials and Methods
2.1. Materials and Equipment
2.1.1. Materials and Reagents
The plant we studied is a common wild herb that grows in agricultural fields or watersides. The herb P. hydropiper was collected whole in October 2022 in Wuyishan City (27°32′36″ N, 117°24′12″ E), Fujian Province, China, and identified by Professor Yanyan Li, horticulture major at Wuyi University. It was dried naturally and then dried in an oven (DHG-9123A; Shanghai Jinghong Experimental Equipment Co., Ltd., Shanghai, China) at 50 °C, ground into powder with a grinder (WJX-500A; Yongkang Red Sun Electromechanical Co., Ltd., Zhongshan, China), and then passed through a 100-mesh sieve.
Rutin standard, anhydrous ethanol, Vc, salicylic acid, H2O2, FeSO4, NaOH, Al(NO3)3, NaNO2, and pyrogallol were supplied by Sinopharm Chemical Reagent Co., Ltd., Shanghai, China; DPPH and ABTS were supplied by Shanghai Macklin Biochemical Co., Ltd., Shanghai, China; Mueller–Hinton Agar (MHA), blank disk, and ampicillin disk (AMP), were purchased from Oxoid company, Basingstoke, UK.
2.1.2. Bacterial Strains
Bacterial strains used in this study and the related information are shown in Table 1. E. coli ATCC25922 and Salmonella ATCC14028 were purchased from China Microbial Resource Center. All the other strains were isolated from diseased or dead white-feathered broilers which were from farms in northern Fujian Provience, China. The 16S rDNA sequence of the strains were identified as E. coli or Salmonella by 16Sr DNA.

Table 1.
Bacterial Strains used in this study.
2.2. Plotting of Rutin Standard Curve
The standard curve was plotted using NaNO2-Al(NO3)3-NaOH colorimetric method [27]: 0.2 mg/mL rutin standard solution was prepared with 60% ethanol, completely dissolved by ultrasound. Different volumes of rutin standard solution (0.0, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0 mL) were added into 8 volumetric flasks. Then 1.0 mL 0.5% NaNO2 was added, shaken, and stood for 6 min. 1.0 mL 10% Al(NO3)3 was added, shaken, and stood for 6 min. 10.0 mL 0.1% NaOH was added, the volume was fixed to 25 mL with 60% ethanol, shaken, and stood for 15 min. Finally, the absorbance (OD) value was measured by ultraviolet spectrophotometer (V-1100D, Jiangsu Tianrui Instrument Co., Ltd., Kunshan, China) at 510 nm.
2.3. Calculation of the Extraction Yield of TFs-Ph
The method of Zhao et al. [28] was used to extract TFs-Ph with h a few adjustments. P. hydropiper powder (10.0 g) was added into 500 mL of extraction bottle, mixed well, and soaked for 30 min. The heated reflux was carried out in a water bath (HH-S4, Changzhou Zhongjie Testing Instrument Co., Ltd., Changzhou, China) at different temperatures. After filtration and collection of the filtrate, it was evaporated to 100 mL in a rotary evaporator (RE-2000A, Shanghai Yarong Biochemical Instrument Factory, Shanghai, China). Then the concentrate was transferred to a centrifuge tube and centrifuged (TGL-16G, Shanghai Anting Scientific Instrument Factory, Shanghai, China) at 3500 r/min for 10 min, and the supernatant was collected as the TFs-Ph.
TFs-Ph extract was calibrated according to the method of the rutin standard curve, and the OD value was measured. Then the TFs-Ph extraction yield was calculated.
where:
- C: the concentration of TFs-Ph (mg/mL);
- V: the volume of extraction solution after calibration (mL);
- X: the dilution times;
- M: the mass of P. hydropiper powder (mg).
2.4. Single-Factor Experiment
The single-factor tests were carried out based on the fixed solid-liquid ratio of 1:20, heating reflux time of 60 min, heating reflux temperature of 60 °C, ethanol concentration of 50%, and extraction times of 3 to investigate the solid-liquid ratio (g/mL) (1:10, 1:15, 1:20, 1:25, 1:30), heating reflux time (min) (30, 60, 90, 120, 150), heating reflux temperature (°C) (40, 50, 60, 70, 80), ethanol concentration (%) (30, 40, 50, 60, 70), and extraction times (times) (1, 2, 3, 4) on the extraction yield of TFs-Ph.
2.5. Plackett-Burman (PB) Design
According to the single factor tests, the PB design was used to evaluate every single factor and screen the main influencing effectors. The levels of each factor are shown in Table 2.

Table 2.
Factors and levels of PB design.
2.6. Box-Behnken (BB) Optimization Design
According to the screening results of the PB design, solid-liquid ratio, heating reflux time, and ethanol concentration were selected as the main influencing factors, and the BB optimization design was carried out with the extraction yield of TFs-Ph as the response value. The levels of each factor are shown in Table 3.

Table 3.
Factors and levels of BB design.
2.7. Antioxidant Activity Evaluation of TFs-Ph
The P. hydropiper extract was concentrated to paste, and the sample solution of TFs-Ph was prepared into 1.0 mg/mL with optimal ethanol extraction concentration solution (51%), then was diluted into the appropriate concentration of TFs-Ph to be measured. The positive control vitamin C (Vc) solution was prepared as above.
The antioxidant activity of TFs-Ph was evaluated by referring to some literature with minor modifications, including the scavenging activity of •OH, DPPH, •O2−, and ABTS [29,30,31].
2.7.1. Determination of •OH Scavenging Activity
1.0 mL the sample solution was mixed with 0.3 mL 9 mmol/L salicylic acid solution and 0.3 mL 9 mmol/L FeSO4 solution, replenished to 6 mL with ultrapure water, and shaken well. Then 0.3 mL of 8.8 mmol/L H2O2 solution was added and bathed in water for 10 min at 37 °C. The absorbance (Asample) was measured at 510 nm; Ultrapure water was used instead of salicylic acid solution to determine the background absorbance (Abackground); Ultrapure water was substituted for the sample solution as a negative control and the absorbance (Acontrol) was measured. The •OH scavenging rate was calculated as Formula (2).
2.7.2. Determination of DPPH Scavenging Activity
0.1 mL the sample solution was mixed with 4.0 mL 0.06 mg/mL DPPH ethanol solution, shaken well, and reacted for 30 min away from light. The absorbance (Asample) was measured at 517 nm; Anhydrous ethanol was used instead of DPPH solution to determine the background value absorbance (Abackground); anhydrous ethanol was used instead of the sample solution as a negative control and the absorbance (Acontrol) was measured. The DPPH scavenging rate was calculated as Formula (3).
2.7.3. Determination of •O2− Scavenging Activity
3 mL the sample solution was mixed with 0.5 mL 25 mmol/L pyrogallol and 4.5 mL Tris-HCl (pH 8.0) in a water bath at 25 °C for 5 min; Then the reaction was stopped with 0.1 mL 8 mmol/L HCl, and the absorbance (Asample) was measured at 325 nm; ultrapure water was used instead of pyrogallol solution to determine the background absorbance (Abackground); Ultrapure water was substituted for the sample solution as a negative control and the absorbance (Acontrol) was measured. The •O2− scavenging rate was calculated as Formula (4).
2.7.4. Determination of ABTS Scavenging Activity
100 µL sample solution was mixed with 6.0 mL ABTS solution, and the reaction was protected from light for 6 min; The absorbance (Asample) was measured at 734 nm, ultrapure water was used instead of ABTS solution to determine the background absorbance (Abackground); Ultrapure water was substituted for the sample solution as a negative control and the absorbance (Acontrol) was measured. The ABTS scavenging rate was calculated as Formula (5).
2.8. Antibacterial Effect Evaluation of TFs-Ph
The TFs-Ph was concentrated to paste and then was prepared into high (80 mg/mL), medium (40 mg/mL), and low (20 mg/mL) dosage groups with sterile normal saline. Stored at 4 °C for later use. The strains to be tested (Table 1) were removed from −80 °C, and activated to an OD600 value of 0.6.
The antibacterial effect of TFs-Ph was examined according to the method of disk diffusion issued by the Clinical and Laboratory Standards Institute (CLSI) [32]. Briefly, 100 μL bacterial suspension was uniformly spread on the MH agar plate, then blank disks (6 mm) were pasted on the agar surface and 10 μL TFs-Ph concentrate was added onto each blank disk. Ampicillin (AMP) was used as a positive control while sterile normal saline as a negative control. The plates were incubated (SPJ-250, Tianhong Experimental Instrument Factory, Wuhan, China) at 37 °C for 24 h. The antibacterial effect was evaluated by measuring the diameters of antibacterial circles.
The following zone diameter criteria were used to assign susceptibility or resistance to TFs-Ph: Susceptible (S) ≥ 15 mm, Intermediate (I) = 11–14 mm, and Resistant (R) ≤ 10 mm. While criteria for assigning susceptibility or resistance to AMP were as follows: (S) ≥ 17 mm, (I) = 14–16 mm, and (R) ≤ 13 mm [33].
2.9. Data Analysis
All the tests in this study were conducted in three parallel trials and the results were expressed as mean ± standard deviation (SD). Data analyses were done by the software Origin Pro 2022, SPSS Statistics 22, and Design Expert 12.
3. Results and Discussion
3.1. Standard Curve Result
The rutin standard curve is shown in Figure 1. The regression equation: Y = 12.732X − 0.0094 (R2 = 0.9995), which showed good linearity and can be used for the determination of flavonoid content.
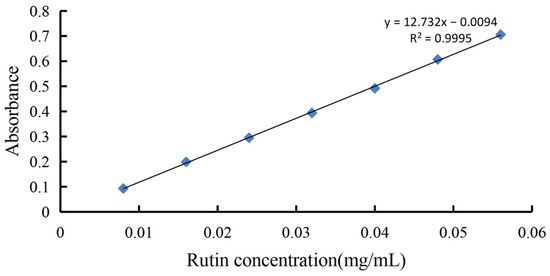
Figure 1.
Rutin standard curve.
3.2. The Influence of Single-Factor Experiment on TFs-Ph Extraction Yield
The influence of solid-liquid ratio: As illustrated in Figure 2A, the extraction yield of TFs-Ph was increased from 5.96% to 6.11% with the increase of solid-liquid ratio from 1:10 to 1:25, which may be attributed to the fact that the increase in the solid-liquid ratio can promote the solvent to penetrate the plant cellular tissues more sufficiently to allow the TFs-Ph to maximize the degree of dissolution. Whereas, the extraction yield decreased after the solid-liquid ratio was greater than 1:25, which may be that during flavonoid extraction, there are non-flavonoids dissolved, competing with TFs-Ph for solvent [25]. Thus, the solid-liquid ratio should be about 1:25.
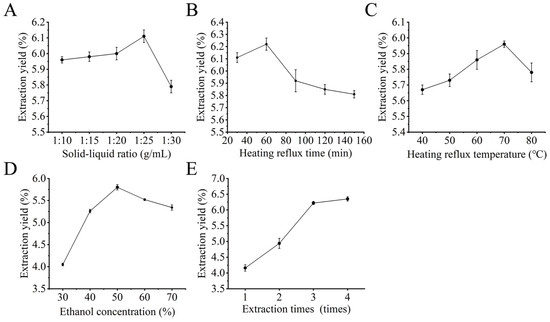
Figure 2.
Effect of single factors on the extraction yield of TFs: (A) solid-liquid ratio; (B) heating reflux time; (C) heating reflux temperature; (D) ethanol concentration; (E) extraction times.
The influence of heating reflux time: As illustrated in Figure 2B, the extraction yield of TFs-Ph increased until reached to a maximum value of 6.22% between the heating reflux time of 30–60 min. However, the extraction yield decreased with extended heating reflux time. It may be that the heating reflux time was too long, resulting in hydrolysis of TFs-Ph or volatilization of solvent [34]. Thus, the optimal heat reflux time was about 60 min.
The influence of heating reflux temperature: As illustrated in Figure 2C, the extraction yield of TFs-Ph increased with the increase of heating reflux temperature, and reached a maximum value of 5.96% at 70 °C. While the extraction yield decreased when the temperature continued to increase. This is because an appropriate increase in temperature can accelerate the diffusion rate of molecules, and thus increase the release of bioactive substances in plant cells. But when the temperature is higher than the optimal level it would lead to the decomposition of heat-sensitive compounds such as flavonoids [29]. Thus, the best heat reflux temperature was 70 °C.
The effect of ethanol concentration on the extraction yield of TFs-Ph was determined and the result was illustrated in Figure 2D: the TFs-Ph increased gradually between 30–50% ethanol concentration and reached a maximum of 5.80% at 50% ethanol concentration, then the extraction yield decreased, and the extraction yield was greatly affected by the concentration of ethanol. This reason may be related to the polarity of ethanol and the solubility of TFs-Ph [35]. Thus, the optimal ethanol concentration may be about 50%.
The influence of extraction times: As illustrated in Figure 2E, the extraction yield increased with increasing extraction times. The second extraction yield increased by 0.78% compared to the first one, the third one increased by 1.28% compared to the second one, and the fourth one increased by 0.13% compared to the third one. Briefly, although the extraction yield increased with the increase number of extraction times, there was not much growth in the extraction yield. Thus, taking into account factors such as extraction efficiency and economic cost, the appropriate extraction times was 3.
3.3. Screening of Main Influencing Factors by PB Design
A two-level PB design of 12 runs was performed in this study to unbiasedly screen the main influencing factors that significantly affect the extraction of TFs-Ph. The PB design and results are shown in Table 4. And Table 5 shows the result of significance analysis.

Table 4.
Design and results of PB test.

Table 5.
Significance analysis of PB design.
The regression model between each significant factor and the Extraction yield (Y) was established by PB design as follows:
Y = 5.44 + 0.3458 X1 + 0.2025 X2 − 0.1975 X3 − 0.1008 X4 + 0.1892 X5
As shown in Table 5, according to the effect value, the key factors influencing the extraction yield of TFs-Ph were as follows: X1 (ethanol concentration) > X2 (solid-liquid ratio) > X3 (heating reflux time) > X5 (extraction times) > X4 (heating reflux temperature). The effect of ethanol concentration on the extraction yield of TFs-Ph attained a highly significant level (p ≤ 0.01), solid-liquid ratio, heating reflux time, and extraction times reached a significant level (p ≤ 0.05), while heating reflux temperature was not significant. So, ethanol concentration, solid-liquid ratio, and heating reflux time were selected to optimize the response surface. Meanwhile, in order to save time and economic cost, combined with single factor result, the extraction times was fixed at 3 times, and the heating reflux temperature was 70 °C.
3.4. Optimization of Significant Factors by BB Design
A two-level PB design of 12 runs was performed in this study to unbiasedly screen the main influencing factors.
According to the principle of BB design, response surface analysis of the TFs-Ph extraction yield was carried out by Design Expert 12 software. The results were illustrated in Table 6 and the regression model between each significant factor and the response value (Y) was established as follows:
Y = 6.43 + 0.05A − 0.01B + 0.0575C − 0.0675AB + 0.4275AC − 0.0675BC −
0.8943A2 − 0.1993B2 − 0.1392C2
0.8943A2 − 0.1993B2 − 0.1392C2

Table 6.
BB design and results.
The regression model was analyzed for significance and the results are shown in Table 7. In this regression model, the probability value (p < 0.0001) indicated that the established regression model reached an unusually significant level. The lack of fit value (p = 0.2303 > 0.05) was not significant, the coefficient of variation (CV% = 0.8631 < 10) indicated that the unknown factors had little effect on the results and the model had good experimental stability. The coefficient of determination (R2 = 0.9961) and adjusted coefficient of determination (R2adj = 0.9912), indicated that 99.61% of the experimental data could be accounted for by this regression model with high reliability. Therefore, the regression model can be used for the prediction of TFs-Ph extraction yield.

Table 7.
BB regression analysis.
As shown in Figure 3, 3-D response surface and 2-D contour plots were drawn to reveal the interactions between two variables. The response surface diagrams were all open downward, which indicated that there was a maximum value. The highest points of Figure 3A,C,E were the optimal conditions for the interaction factors of AB, AC, and BC. The 2-D contour slopes AB (Figure 3B) and AC (Figure 3D) were steeper, while BC (Figure 3F) were relatively flat, indicating that the interaction of AB and AC had a greater influence on the extraction yield of TFs-Ph than BC. Which was consistent with the results of single-factor experiments and PB design.
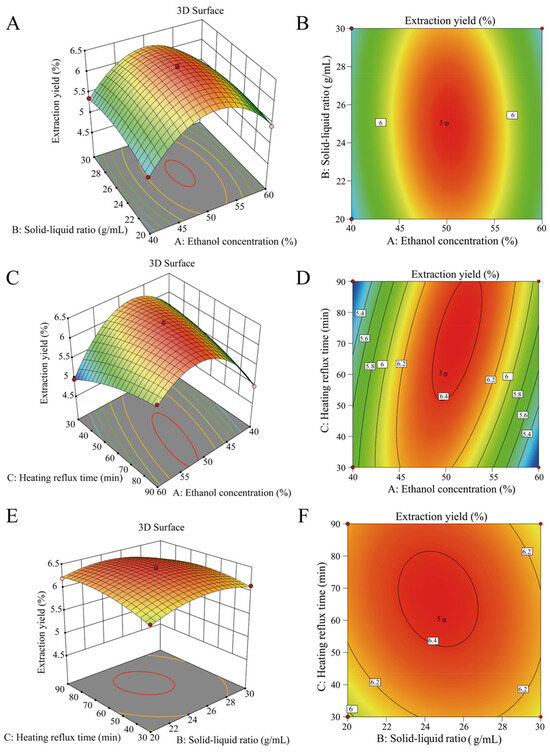
Figure 3.
3-D response surface for AB (A), AC (C) and BC (E) to extraction yield of TFs-Ph. 2-D contour plots for AB (B), AC (D) and BC (F) to extraction yield of TFs-Ph.
After analyzing the regression model by Design Expert 12.0 software, the optimal extraction condition of TFs-Ph was predicted as follows: the concentration of ethanol was 51.42%, the solid-liquid ratio was 1:24.37 g/mL, and the heating reflux time was 73.62 min. In this case, the extraction yield of TFs-Ph was 6.44%. Considering the practical operation, the theoretical parameters were adjusted as follows: the ethanol concentration was 51%, the solid-liquid ratio was 1:24 g/mL, and the heating reflux time was 74 min. Combined with the optimal reflux temperature of 70 °C, the extraction times of 3, the actual extraction yield of TFs-Ph was 6.37 ± 0.04%. The relative error with the predicted value is 1.09% (<5%), indicating that the extraction conditions are highly reliable and can be used to guide the actual operation.
3.5. Study on the Antioxidant Activities of TFs-Ph
•OH is highly active and is the most cytotoxic ROS, which can quickly react with any biomolecules and then cause severe damage to the adjacent organs or tissues [36]. Therefore, it is important to study the scavenging ability of flavonoids on •OH. According to Figure 4A, the scavenging ability of TFs-Ph and Vc on •OH showed a tendency of increasing and then leveling off: in the concentration range of 0.10–0.40 mg/mL, it showed a significant upward trend, and at a concentration of 0.50 mg/mL, the •OH scavenging rates of TFs-Ph and Vc were 98.1% and 89.9% respectively. The IC50 value of TFs-Ph for •OH was 0.14 mg/mL, while that of Vc was 0.27 mg/mL. At the same concentration, the scavenging ability of TFs-Ph on •OH was always higher than that of Vc.
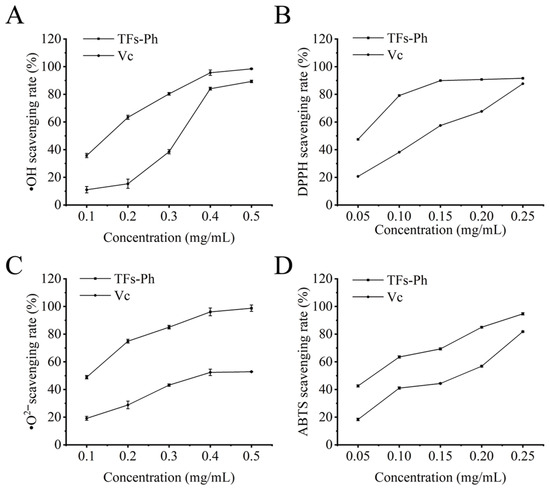
Figure 4.
Antioxidant activities of TFs-Ph. (A) •OH scavenging rate; (B) DPPH scavenging rate; (C) •O2− scavenging rate; (D) ABTS scavenging rate.
According to Figure 4B, with the increase of concentration, the scavenging ability of TFs-Ph on DPPH gradually increased and eventually stabilized, whereas the scavenging effect of Vc on DPPH showed a certain quantitative effect relationship. At the concentration of 0.25 mg/mL for both, their DPPH scavenging rates were basically the same, 91.9% and 87.7% respectively. The IC50 value of TFs-Ph for DPPH was 0.06 mg/mL, while that of Vc was 0.12 mg/mL. Thus scavenging effect of TFs-Ph on DPPH was significant and was higher than that of Vc at lower concentrations. This is agreeable with the findings of Zhang et al. and Cui et al. [37,38].
Compared with other ROS, •O2− has a longer life span, then it can move a longer distance within the cell and is involved in the formation of other toxic oxygen species such as peroxyl, alkoxyl, hydroxyl, and nitric oxide [39,40]. Therefore, it is great important to detect the scavenging ability of •O2−. As shown in Figure 4C, the •O2− scavenging rate of TFs-Ph was apparently higher than that of Vc, with TFs-Ph (98.7%) about twice as that of Vc (52.8%) at the concentration of 0.50 mg/mL. The IC50 value of TFs-Ph for •O2− was 0.10 mg/mL, while that of Vc was 0.42 mg/mL. Thus, the TFs-Ph has a strong •O2− scavenging ability and can be developed as a natural antioxidant.
According to Figure 4D, TFs-Ph and Vc showed a similar trend in scavenging ABTS and the scavenging rate showed a linear relationship with the TFs-Ph or Vc concentration. When the concentration was 0.50 mg/mL, the ABTS scavenging rate reached 94.7% or 81.8%, respectively. The IC50 value of TFs-Ph for ABTS was 0.07 mg/mL, while that of Vc was 0.14 mg/mL. This is inconsistent with the study results of Wang et al., which could be that flavonoids from different plant sources have different antioxidant activities and different ABTS scavenging abilities [41].
There has been many research on the antioxidant mechanisms of genus Polygonum plant extract. Zhao et al. [42] identified the main compound from Polygonum cuspidatum was polydatin and used SD rats model to study the antioxidant mechanism. They found that polydatin can reduced ROS level, inhibit Keap1 and activate Nrf2 pathway. Ren et al. [43] determined that the Polygonum hydropiper extract were flavonoids (including rutin, quercitrin, and quercetin) which exerted protective effects against gastric injury through antioxidant pathway used human gastric epithelial cells and SD rats models. However, there have no research on the antioxidant mechanism of TFs-Ph in broilers. We speculate that its antioxidant mechanism may be similar to that in rats and plan to confirm it in subsequent study.
3.6. Study on the Antibacterial Effects of TFs-Ph
The antibacterial effects of TFs-Ph against pathogenic bacteria of broiler origin are shown in Table 8. The antibacterial effect of AMP on broiler pathogenic E. coli only 20% (2 strains/10 strains) was intermediate, while at high dosage concentration, that of TFs-Ph 50% (5 strains/10 strains) was susceptible, 10% (1 strain/10 strains) was intermediate; and the antibacterial effect was enhanced with the increase in the concentration of TFs-Ph; Compared with standard E. coli ATCC25922, the antibacterial effect was similar. The antibacterial effect of AMP on broiler pathogenic Salmonella 30% (3 strains/10 strains) was susceptible, 20% (2 strains/10 strains) was intermediate, while at high dosage concentration, that of TFs-Ph 50% (5 strains/10 strains) was susceptible, and the antibacterial effect was enhanced with the increase in the concentration of TFs-Ph; compared with standard Salmonella ATCC14028, the antibacterial effect was similar. Hence, compared with AMP, TFs-Ph had better antibacterial effects against pathogenic E. coli and Salmonella of broiler origin. Ayaz et al. [44] studied the antibacterial effects of Polygonum hydropiper extracts and found that compared with gram-positive bacteria, Polygonum hydropiper extracts had a stronger inhibitory effect on gram-negative bacteria, which further proved the positive certainty of this study. Kovacs et al. [45] investigated the antibacterial effect of two flavonoids extracted from grape seeds against pathogenic E. coli and Salmonella of swine origin and determined that the antibacterial effect was obvious, which was comparable to the results of this study. Thus, there was some potential for TFs-Ph to replace antibiotics.

Table 8.
Inhibitory zone diameter of TFs-Ph against pathogenic bacteria of broiler origin (mm).
4. Conclusions
In this study, extraction yield was used as the index, and the TFs-Ph were extracted by ethanol heating reflux method. The ethanol concentration, solid-liquid ratio, and heating reflux time were determined as the main influencing factors through single factor tests, Plackett-Burman design, and Box-Behnken response surface optimization. The optimal extraction parameters were as follows: the concentration of ethanol of 51%, the solid-liquid ratio of 1:24 g/mL, the heating reflux time of 74 min, the reflux temperature of 70 °C, the extraction times of 3, in this case, the extraction yield of TFs-Ph was 6.37%.
With Vc as the positive control, the antioxidant activities of TFs-Ph were studied. It was determined that the scavenging abilities of free radicals such as •OH, DPPH, •O2− and ABTS were gradually enhanced with increasing concentration. All the free radicals scavenging rate was always higher than that of Vc. Therefore, TFs-Ph had excellent antioxidant activity and could effectively scavenge a variety of free radicals.
With AMP as the positive control and sterile normal saline as the negative control, the antibacterial effect of TFs-Ph was studied. It was determined that compared with AMP, the TFs-Ph had better antibacterial effects against pathogenic E. coli and Salmonella of broiler origin, and had the certain potential to replace antibiotics. Further validation work is underway in our laboratory to develop the TFs-Ph into a drug for the treatment of pathogenic E. coli and Salmonella of broiler origin.
Author Contributions
T.Z.: designed the study, wrote the paper, and made critical revisions; Y.H. and H.X.: performed the experiments; Y.Z. and W.T.: analysis the data; Z.L.: and B.H.: supplied the bacterial Strains; L.C. and L.F. critically read and revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Department guided project of Fujian Province, grant number: 2021N0034; Resource chemical industry science and technology innovation joint funding Co-financed of Nanping city, grant number: N2022Z002.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We gratefully acknowledge Yanyan Li of Wuyi University for the help in identifying Persicaria hydropiper (L.) Spach.
Conflicts of Interest
Author Zhongbao Luo and Baoqin Huang were employed by the company Fujian Sunner Development Company Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bairagi, J.; Saikia, P.J.; Boro, F.; Hazarika, A. A review on the ethnopharmacology, phytochemistry and pharmacology of Polygonum hydropiper Linn. J. Pharm. Pharmacol. 2022, 74, 619–645. [Google Scholar] [CrossRef]
- Kong, Y.D.; Qi, Y.; Cui, N.; Zhang, Z.H.; Wei, N.; Wang, C.F.; Zeng, Y.N.; Sun, Y.P.; Kuang, H.X.; Wang, Q.H. The traditional herb Polygonum hydropiper from China: A comprehensive review on phytochemistry, pharmacological activities and applications. Pharm. Biol. 2023, 61, 799–814. [Google Scholar] [CrossRef]
- Ren, C.Z.; Hu, W.Y.; Li, J.C.; Xie, Y.H.; Jia, N.N.; Shi, J.; Wei, Y.Y.; Hu, T.J. Ethyl acetate fraction of flavonoids from Polygonum hydropiper L. modulates pseudorabies virus-induced inflammation in RAW264.7 cells via the nuclear factor-kappa B and mitogen-activated protein kinase pathways. J. Vet. Med. Sci. 2020, 82, 1781–1792. [Google Scholar] [CrossRef]
- Peng, Z.F.; Strack, D.; Baumert, A.; Subramaniam, R.; Goh, N.K.; Chia, T.F.; Tan, S.N.; Chia, L.S. Antioxidant flavonoids from leaves of Polygonum hydropiper L. Phytochemistry 2003, 62, 219–228. [Google Scholar] [CrossRef]
- Nasir, A.; Khan, M.; Rehman, Z.; Khalil, A.A.K.; Farman, S.; Begum, N.; Irfan, M.; Sajjad, W.; Parveen, Z. Evaluation of Alpha-Amylase Inhibitory, Antioxidant, and Antimicrobial Potential and Phytochemical Contents of Polygonum hydropiper L. Plants 2020, 9, 852. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ahmed, J.; Ullah, F.; Sadiq, A.; Ahmad, S.; Imran, M. Phenolic contents, antioxidant and anticholinesterase potentials of crude extract, subsequent fractions and crude saponins from Polygonum hydropiper L. BMC Complement. Altern. Med. 2014, 14, 145. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, Y.; Sun, G.; Yu, S.; Xu, Y.; He, C.; Li, Z.; Jin, S.; Qin, X. Polygonum perfoliatum L., an Excellent Herbal Medicine Widely Used in China: A Review. Front. Pharmacol. 2020, 11, 581266. [Google Scholar] [CrossRef]
- Ren, C.Z.; Hu, W.Y.; Song, M.L.; Wei, Y.Y.; Hu, T.J. An ethyl acetate fraction of flavonoids from Polygonum hydropiper L. exhibits an anti-inflammatory activity in PCV2-infected porcine alveolar macrophages via PI3K/Akt and NF-κB pathways. Vet. Res. Forum 2022, 13, 339–347. [Google Scholar]
- Shen, S.; Qian, J.; Ren, J. Ethnoveterinary plant remedies used by Nu people in NW Yunnan of China. J. Ethnobiol. Ethnomed. 2010, 6, 24. [Google Scholar] [CrossRef]
- Nath, A.; Joshi, S.R. Endophytic fungi from tropical ethnoveterinary plants and their antibacterial efficacy against Pasteurella multocida Capsular Type A strain. Rev. Biol. Trop. 2016, 64, 733–745. [Google Scholar] [CrossRef]
- Hao, L.; Xiaolin, Z.; Quanli, X.; Xizhong, Z.; Changfen, Y. Pros and cons analysis of company-type, professional household-type and farmer-type poultry farms. China Poult. 2005, 17, 22–24. [Google Scholar]
- Ailian, G.; Baoming, L.; Furong, Z.; Gang, C. Investigation and study of broiler breeders’ health and welfare under intensive production systems. China Poult. 2009, 31, 10–15. [Google Scholar]
- Jeżak, K.; Kozajda, A. Occurrence and spread of antibiotic-resistant bacteria on animal farms and in their vicinity in Poland and Ukraine-review. Environ. Sci. Pollut. Res. Int. 2022, 29, 9533–9559. [Google Scholar] [CrossRef]
- Lutful Kabir, S.M. Avian colibacillosis and salmonellosis: A closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health 2010, 7, 89–114. [Google Scholar] [CrossRef]
- Tan, M.F.; Li, H.Q.; Yang, Q.; Zhang, F.F.; Tan, J.; Zeng, Y.B.; Wei, Q.P.; Huang, J.N.; Wu, C.C.; Li, N.; et al. Prevalence and antimicrobial resistance profile of bacterial pathogens isolated from poultry in Jiangxi Province, China from 2020 to 2022. Poult. Sci. 2023, 102, 102830. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Shaheen, H.; Samak, D.; Othman, S.I.; Allam, A.A.; Taha, A.E.; Khafaga, A.F.; Arif, M.; Osman, A.; et al. Ginger and its derivatives as promising alternatives to antibiotics in poultry feed. Animals 2020, 10, 452. [Google Scholar] [CrossRef]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef]
- Song, Z.T.; Zhu, M.J. Feed additive production by fermentation of herb Polygonum hydropiper L. and cassava pulp with simultaneous flavonoid dissolution. Biotechnol. Appl. Biochem. 2017, 64, 290–300. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, P.; Zhang, B.; Kong, L.; Xiao, C.; Song, Z. Progress on gut health maintenance and antibiotic alternatives in broiler chicken production. Front. Nutr. 2021, 8, 692839. [Google Scholar] [CrossRef]
- Cherian, S.; Hacisayidli, K.M.; Kurian, R.; Mathews, A. Therapeutically important bioactive compounds of the genus Polygonum L. and their possible interventions in clinical medicine. J. Pharm. Pharmacol. 2023, 75, 301–327. [Google Scholar] [CrossRef]
- Zhao, T.; Ding, Y.; Sun, W.; Turghun, C.; Han, B. Ultrasonic-assisted extraction of flavonoids from Nitrariasibirica leaf using response surface methodology and their anti-proliferative activity on 3T3-L1 preadipocytes and antioxidant activities. J. Food Sci. 2023, 88, 2325–2338. [Google Scholar] [CrossRef]
- Miao, M.; Chen, X.; Wu, Z.; Liu, J.; Xu, C.; Zhang, Z.; Wang, J. Extraction, composition, and antioxidant activity of flavonoids from Xanthoceras sorbifolium bunge leaves. J. AOAC Int. 2023, 106, 769–777. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M. Extracts and flavonoids of Passiflora species as promising anti-inflammatory and antioxidant substances. Curr. Pharm. Des. 2021, 27, 2582–2604. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, Z.; Guo, Y.; Lu, S.; Du, H.; Cao, Y. Antioxidant capacity of flavonoids from Folium Artemisiae Argyi and the molecular mechanism in Caenorhabditis elegans. J. Ethnopharmacol. 2021, 279, 114398. [Google Scholar] [CrossRef]
- Lu, J.; Huang, Z.; Liu, Y.; Wang, H.; Qiu, M.; Qu, Y.; Yuan, W. The optimization of extraction process, antioxidant, whitening and antibacterial effects of Fengdan Peony flavonoids. Molecules 2022, 27, 506. [Google Scholar] [CrossRef]
- El-Zahar, K.M.; Al-Jamaan, M.E.; Al-Mutairi, F.R.; Al-Hudiab, A.M.; Al-Einzi, M.S.; Mohamed, A.A. Antioxidant, antibacterial, and antifungal activities of the ethanolic extract obtained from Berberis vulgaris roots and leaves. Molecules 2022, 27, 6114. [Google Scholar] [CrossRef]
- Rafińska, K.; Pomastowski, P.; Rudnicka, J.; Krakowska, A.; Maruśka, A.; Narkute, M.; Buszewski, B. Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem. 2019, 289, 16–25. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Sheng, L.; Wang, N.; Ren, S.Z. Optimization of Extraction Process of Total Flavonoids from Polygonum hydropiper by Response Surface Methodology and Its Antioxidant Activity. Chin. J. Vet. Drug 2019, 53, 49–56. [Google Scholar]
- Dong, J.; Zhou, K.; Ge, X.; Xu, N.; Wang, X.; He, Q.; Zhang, C.; Chu, J.; Li, Q. Effects of extraction technique on the content and antioxidant activity of flavonoids from Gossypium Hirsutum linn. flowers. Molecules 2022, 27, 5627. [Google Scholar] [CrossRef]
- Xie, J.H.; Dong, C.J.; Nie, S.P.; Li, F.; Wang, Z.J.; Shen, M.Y.; Xie, M.Y. Extraction, chemical composition and antioxidant activity of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves. Food Chem. 2015, 186, 97–105. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, X.; Duan, X.; Zhou, C.; Zhao, Z.; Chen, H.; Tang, Z.; Wan, Y.; Xiao, Y.; Chen, H. Optimal extraction, purification and antioxidant activity of total flavonoids from endophytic fungi of Conyza blini H. Lév. PeerJ 2021, 9, e11223. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Jalili, S. Phytochemical content, antioxidant properties, and antibacterial activities of Centella asiatica L. Nat. Prod. Res. 2023, 1–6. [Google Scholar] [CrossRef]
- Awolola, G.V.; Koorbanally, N.A.; Chenia, H.; Shode, F.O.; Baijnath, H. Antibacterial and anti-biofilm activity of flavonoids and triterpenes isolated from the extracts of Ficus sansibarica Warb. subsp. sansibarica (Moraceae) extracts. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 124–131. [Google Scholar] [CrossRef]
- Zhang, P.; Song, Y.; Wang, H.; Fu, Y.; Zhang, Y.; Pavlovna, K.I. Optimization of flavonoid extraction from Salix babylonica L. buds, and the antioxidant and antibacterial activities of the extract. Molecules 2022, 27, 5695. [Google Scholar] [CrossRef]
- Huang, H.S.; Liaw, E.T. Extraction optimization of flavonoids from Hypericum formosanum and matrix metalloproteinase-1 inhibitory activity. Molecules 2017, 22, 2172. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef]
- Zhang, K.; Han, M.; Zhao, X.; Chen, X.; Wang, H.; Ni, J.; Zhang, Y. Hypoglycemic and antioxidant properties of extracts and fractions from Polygoni Avicularis Herba. Molecules 2022, 27, 3381. [Google Scholar] [CrossRef]
- Cui, L.; Ma, Z.; Wang, D.; Niu, Y. Ultrasound-assisted extraction, optimization, isolation, and antioxidant activity analysis of flavonoids from Astragalus membranaceus stems and leaves. Ultrason. Sonochem. 2022, 90, 106190. [Google Scholar] [CrossRef]
- Niu, Q.; Gao, Y.; Liu, P. Optimization of microwave-assisted extraction, antioxidant capacity, and characterization of total flavonoids from the leaves of Alpinia oxyphylla Miq. Prep. Biochem. Biotechnol. 2020, 50, 82–90. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.H.; Zhang, J.; Yu, B.Y. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J. Agric. Food Chem. 2008, 56, 3876–3883. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Liu, Q.; Lin, Y.; Zhang, Z.; Li, S. Study on extraction and antioxidant activity of flavonoids from Hemerocallis fulva (Daylily) leaves. Molecules 2022, 27, 2916. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Chen, B.; Ma, Z.; Hu, H.; Xie, Y. Polygonum hydropiper extract attenuates ethanol-induced gastric damage through antioxidant and anti-inflammatory pathways. Braz. J. Med. Biol. Res. 2021, 54, e10841. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Junaid, M.; Ullah, F.; Sadiq, A.; Ovais, M.; Ahmad, W.; Ahmad, S.; Zeb, A. Chemical profiling, antimicrobial and insecticidal evaluations of Polygonum hydropiper L. BMC Complement. Altern. Med. 2016, 16, 502. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Karancsi, Z.; Farkas, O.; Jerzsele, Á. Antioxidant activity of flavonoids in LPS-treated IPEC-J2 porcine intestinal epithelial cells and their antibacterial effect against bacteria of swine origin. Antioxidants 2020, 9, 1267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).