Finite Element Modeling of Crystallization with Temperature Jump to Improve Cryopreservation of Fish Germ Cells
Abstract
1. Introduction
- -
- Slow freezing (less than 10 K/min) [23];
- -
- Vitrification (fast freezing, more than 10 K/min).
- -
- Construction of a continuous mathematical model of the crystallization process [37] taking into account the phase transition, but without identification of the phase boundary;
- -
- It is subsequent discretization based on the finite element method and carrying out the numerical experiments.
2. Materials and Methods
2.1. Materials (Cryoprotector Substance)
2.2. Semen Scoring and Freezing Procedure
2.3. Mathematical Model for Crystallization
- -
- Linear theory of elasticity;
- -
- Linear theory of electroelasticity;
- -
- Equations of motion of liquid and gaseous media (in the acoustic approximation).
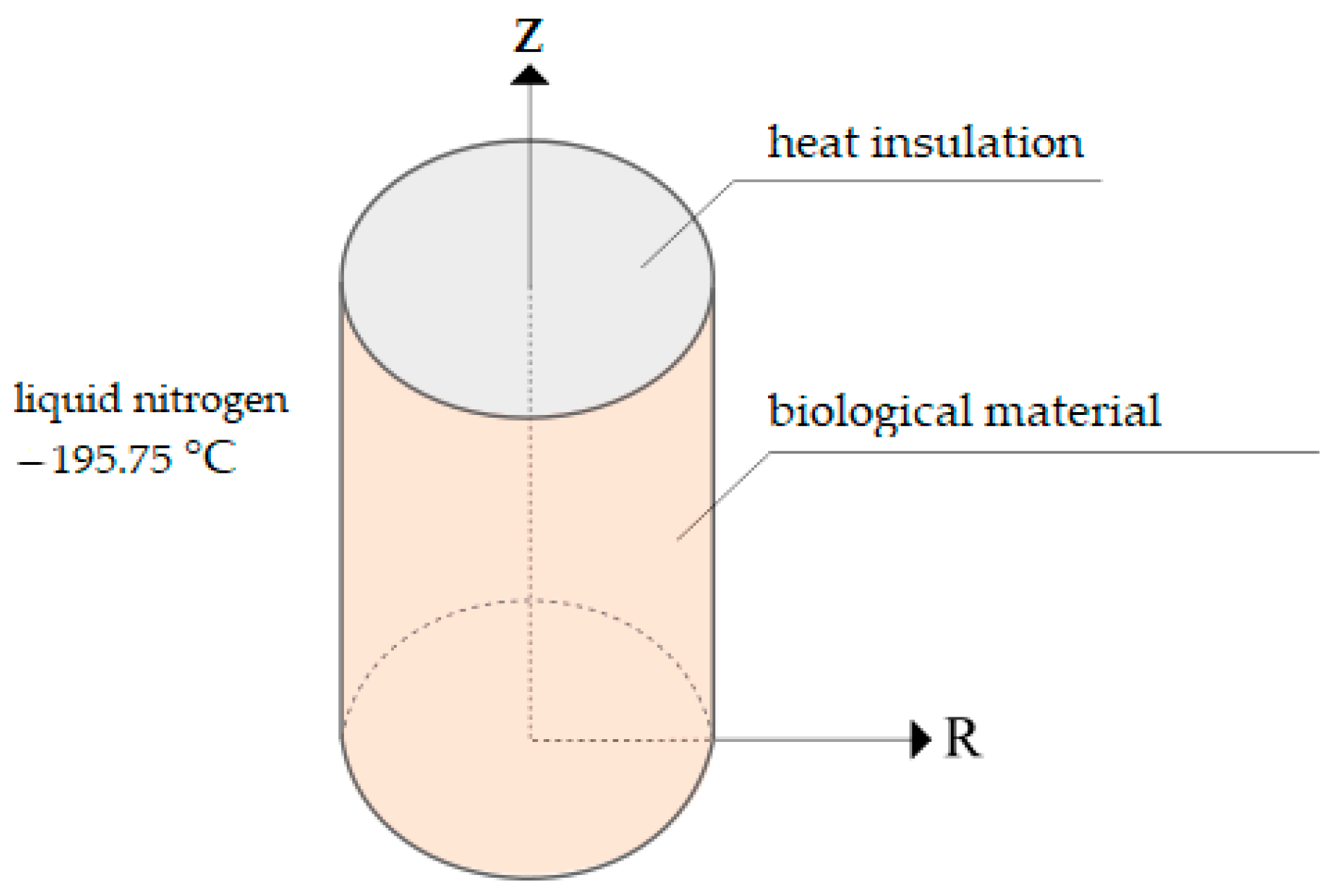
2.4. Finite Element Modeling
- -
- Condition (5) is set at the lower end; that is, a constant temperature of minus 195.75 °C is set;
- -
- Condition (5) is also set on the lateral cylindrical boundary; that is, a constant temperature of minus 195.75 °C is set;
- -
- The upper end of the container is thermally insulated (Figure 1), which is described by Condition (6).
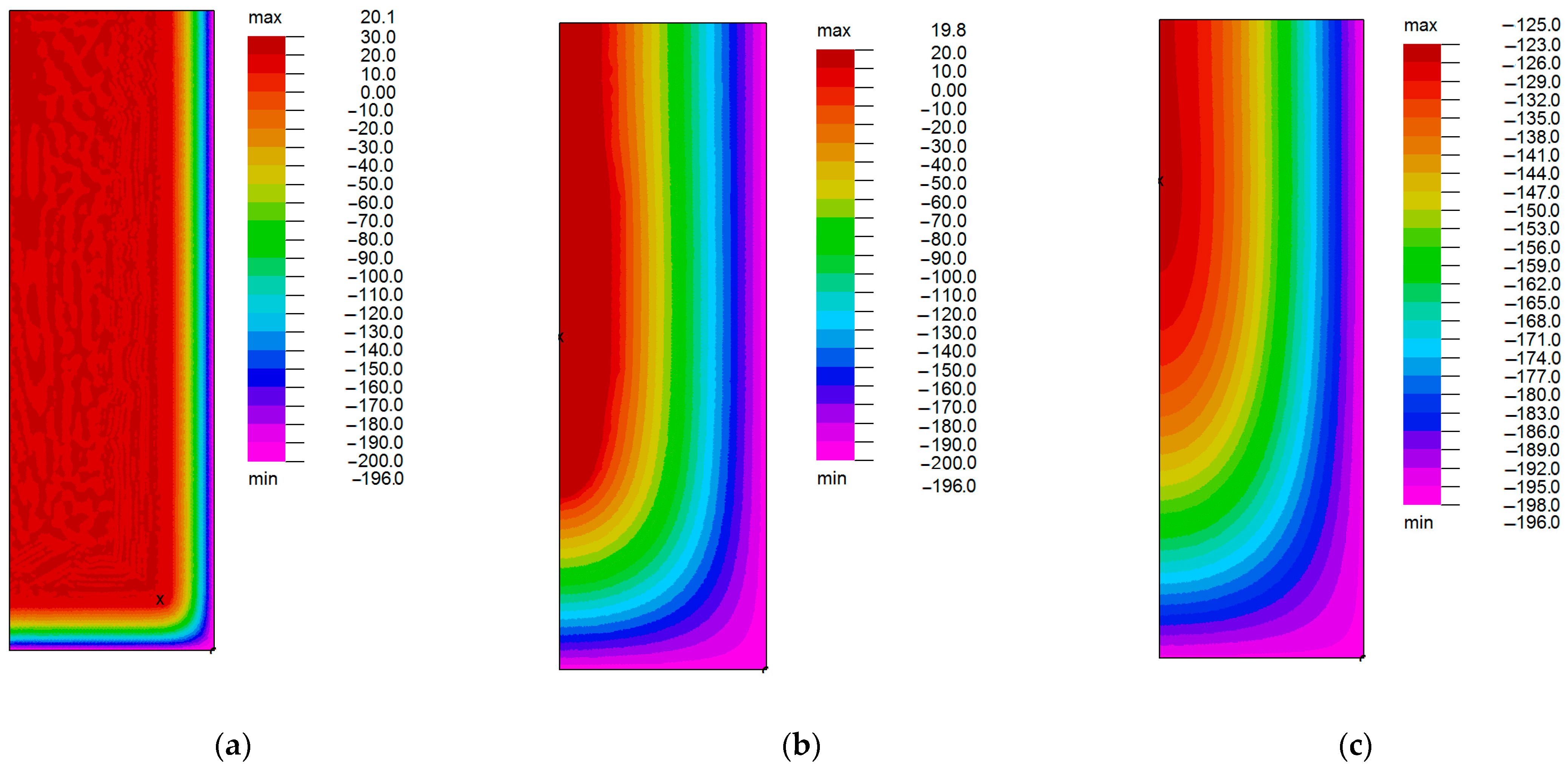
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online. Available online: https://www.wfp.org/ (accessed on 10 November 2023).
- Global Report on Food Crises. Joint Analysis for Better Decisions, 2023. WFP, 2023. Available online: https://www.fsinplat-form.org/sites/default/files/resources/files/GRFC2023-hi-res.pdf/ (accessed on 10 November 2023).
- Brander, K.M. Global fish production and climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 19709–19714. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Koehn, J.Z.; Holsman, K.K.; Halpern, B.S. Emerging trends in science and news of climate change threats to and adaptation of aquaculture. Aquaculture 2021, 549, 737812. [Google Scholar] [CrossRef]
- Di Iorio, M.; Rusco, G.; Esposito, S.; D’andrea, M.; Roncarati, A.; Iaffaldano, N. The role of semen cryobanks for protecting endangered native salmonids. Front. Mar. Sci. 2022, 9, 1075498. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Horváth; Labbé, C.; Zhang, T.; Robles, V.; Herráez, P.; Suquet, M.; Adams, S.; Viveiros, A.; Tiersch, T.R.; et al. Cryobanking of aquatic species. Aquaculture 2016, 472, 156–177. [Google Scholar] [CrossRef] [PubMed]
- Comizzoli, P.; Holt, W.V. Recent advances and prospects in germplasm preservation of rare and endangered species. Adv. Exp. Med. Biol. 2014, 753, 331–356. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I. The Role of Reproductive Sciences in the Preservation and Breeding of Commercial and Threatened Teleost Fishes. In Reproductive Sciences in Animal Conservation. Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Judycka, S.; Nynca, J.; Ciereszko, A. Opportunities and challenges related to the implementation of sperm cryopreservation into breeding of salmonid fishes. Theriogenology 2019, 132, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Asturiano, J.F.; Cabrita, E.; Horváth, A. Progress, challenges and perspectives on fish gamete cryopreservation: A mini-review. Gen. Comp. Endocrinol. 2017, 245, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T. Importance of cryobanking in aquatic species conservation and aquaculture. Cryobiology 2018, 80, 169. [Google Scholar] [CrossRef]
- Diwan, A.D.; Harke, S.N.; Gopalkrishna; Panche, A.N. Cryobanking of Fish and Shellfish Egg, Embryos and Larvae: An Overview. Front. Mar. Sci. 2020, 7, 00251. [Google Scholar] [CrossRef]
- Hrytsyniak, I.; Shvets, T. Cryopreservation of fish germ cells. Thematic bibliography. Ribogospodarsʹka nauka Ukraïni. 2018, 4, 79–101. [Google Scholar] [CrossRef]
- Kilbride, P.; Meneghel, J.; Manilal Chawda, M.; Ross, S.; Crompton, T. Scaling up Cryopreservation from Cell Suspensions to Tissues: Challenges and Successes. Biomed. Eng. IntechOpen 2023, 84457. [Google Scholar] [CrossRef]
- Jaiswal, A.N.; Vagga, A. Cryopreservation: A Review Article. Cureus 2022, 14, e31564. [Google Scholar] [CrossRef]
- Assylbekova, A.; Barinova, G.; Aubakirova, G.; Makhanbetova, A.; Kuanchaleyev, Z.; Mussin, S.; Mussina, A. Cryopreservation of Reproductive Cells of Male Russian Sturgeon. Exp. Biol. 2022, 92, 132–138. [Google Scholar] [CrossRef]
- de Siqueira-Silva, D.H.; Saito, T.; dos Santos-Silva, A.P.; Costa, R.d.S.; Psenicka, M.; Yasui, G.S. Biotechnology applied to fish reproduction: Tools for conservation. Fish Physiol. Biochem. 2018, 44, 1469–1485. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.J. Cryopreservation: Vitrification and Controlled Rate Cooling. Methods Mol Biol. 2017, 1590, 41–77. [Google Scholar] [CrossRef]
- Lujić, J.; Marinović, Z.; Bajec, S.S.; Djurdjevič, I.; Kása, E.; Urbányi, B.; Horváth. First successful vitrification of salmonid ovarian tissue. Cryobiology 2017, 76, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Pšenička, M.; Saito, T.; Rodina, M.; Dzyuba, B. Cryopreservation of early stage Siberian sturgeon Acipenser baerii germ cells, comparison of whole tissue and dissociated cells. Cryobiology 2016, 72, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Igna, V.; Telea, A.; Florea, T.; Popp, R.; Grozea, A. Evaluation of Russian sturgeon (Acipenser gueldenstaedtii) Semen Quality and Semen Cryopreservation. Animals 2022, 12, 2153. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, T.; Zhao, F.; Feng, G.; Liu, J.; Yang, G.; Zhang, L.; Zhuang, P. Effects of cryopreservation on acrosin activity and DNA damage of Russian sturgeon (Acipenser guelden-staedtii) semen. Cryoletters 2021, 42, 129–136. [Google Scholar] [PubMed]
- Marques, L.S.; Fossati, A.A.N.; Rodrigues, R.B.; Da Rosa, H.T.; Izaguirry, A.P.; Ramalho, J.B.; Moreira, J.C.F.; Santos, F.W.; Zhang, T.; Streit, D.P. Slow freezing versus vitrification for the cryopreservation of zebrafish (Danio rerio) ovarian tissue. Sci. Rep. 2019, 9, 15353. [Google Scholar] [CrossRef] [PubMed]
- Magnotti, C.; Cerqueira, V.; Lee-Estevez, M.; Farias, J.G.; Valdebenito, I.; Figueroa, E. Cryopreservation and vitrification of fish semen: A review with special emphasis on marine species. Rev. Aquac. 2016, 10, 15–25. [Google Scholar] [CrossRef]
- Rajan, R.; Matsumura, K. Development and application of cryoprotectants. In Survival Strategies in Extreme Cold and Desiccation: Adaptation Mechanisms and Their Applications; Springer Nature Singapore Pte Ltd. Springer: Singapore, 2018; pp. 339–354. [Google Scholar] [CrossRef]
- Bhattacharya, M.S. A review on cryoprotectant and its modern implication in cryonics. Asian J. Pharm. 2016, 10, 154–159. [Google Scholar] [CrossRef]
- Niu, J.; Wang, X.; Liu, P.; Liu, H.; Li, R.; Li, Z.; He, Y.; Qi, J. Effects of Cryopreservation on Sperm with Cryodiluent in Viviparous Black Rockfish (Sebastes schlegelii). Int. J. Mol. Sci. 2022, 23, 3392. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Siddique, M.A.M.; Dzyuba, B.; Cuevas-Uribe, R.; Shaliutina-Kolešová, A.; Linhart, O. Progress and challenges of fish sperm vitrification: A mini review. Theriogenology 2017, 98, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Kása, E.; Bernáth, G.; Kollár, T.; Żarski, D.; Lujić, J.; Marinović, Z.; Bokor, Z.; Hegyi; Urbányi, B.; Vílchez, M.C.; et al. Development of sperm vitrification protocols for freshwater fish (Eurasian perch, Perca fluviatilis) and marine fish (European eel, Anguilla anguilla). Gen. Comp. Endocrinol. 2016, 245, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Rusco, G.; Di Iorio, M.; Gibertoni, P.P.; Esposito, S.; Penserini, M.; Roncarati, A.; Cerolini, S.; Iaffaldano, N. Optimization of Sperm Cryopreservation Protocol for Mediterranean Brown Trout: A Comparative Study of Non-Permeating Cryoprotectants and Thawing Rates In Vitro and In Vivo. Animals 2019, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Park, J.Y.; Lee, I.Y.; Zidni, I.; Lim, H.K. Effects of cryoprotective agents and treatment methods on sperm cryopreservation of stone flounder, Kareius bicoloratus. Aquaculture 2020, 531, 735969. [Google Scholar] [CrossRef]
- Hu, E.; Daniels, H.; Gill, A.O.; Tiersch, T.R.; Cuevas-Uribe, R. Vitrification as an Alternative Approach for Sperm Cryopreservation in Marine Fishes. North Am. J. Aquac. 2017, 79, 187–196. [Google Scholar] [CrossRef]
- Ren, S.; Shu, Z.; Pan, J.; Peng, J.; Wang, J.; Zhao, C.; Gao, D. Development of a Novel Electromagnetic Rewarming Technology for the Cryopreservation of Stem Cells with Large Volume. In Novel Perspectives of Stem Cell Manufacturing and Therapies; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Rahman, S.M.; Strüssmann, C.A.; Suzuki, T.; Majhi, S.K.; Hattori, R.S.; Alam, A. Effects of ultrasound on permeation of cryoprotectants into Japanese whiting Sillago japonica embryos. Cryobiology 2017, 77, 19–24. [Google Scholar] [CrossRef]
- Ponomareva, E.; Firsova, A.; Krasilnikova, A.; Kovalenko, M.; Rudoy, D. Acoustic-mechanical effect on the sperm of sturgeon fish using piezoactuators. E3S Web Conf. 2022, 363, 03020. [Google Scholar] [CrossRef]
- Alcalá, E.; Encabo, L.; Barroso, F.; Puentes, A.; Risco, I.; Risco, R. Sound waves for solving the problem of recrystallization in cryopreservation. Sci. Rep. 2023, 13, 7603. [Google Scholar] [CrossRef] [PubMed]
- Blagin, A.V.; Blagina, L.V.; Knyazev, S.Y.; Shcherbakova, E.E.; Nefyodova, N.A. Crystallization Processes in Gradient Fields; DSTU: Rostov-on-Don, Russia, 2021; 196p. (In Russian) [Google Scholar]
- Agarwal, N.K. Cryopreservation of Fish Semen. In Himalayan Aquatic Biodiversity Conservation & New Tools in Biotechnology Edition, 1st ed.; Chapter 8; Transmedia Publication: Srinagar (Garhawal) Uttarakhand, India, 2011. [Google Scholar] [CrossRef]
- Union for Conservation of Nature and Natural Resources. Available online: https://iucn.org/ (accessed on 10 November 2023).
- Convention on International Trade in Endangered Species of Wild Fauna and Flora. Available online: https://www.fisheries.noaa.gov/national/international-affairs/convention-international-trade-endangered-species-wild-fauna-and (accessed on 10 November 2023).
- Red Book of the Russian Federation. Available online: https://www.mnr.gov.ru/activity/red_book/krasnaya-kniga-rossiyskoy-federatsii/?ysclid=ls4q0e9eui253200674 (accessed on 10 November 2023).
- Ponomareva, E.N.; Soloviev, A.N.; Matrosov, A.A.; Chebanenko, V.A.; Nizhnik, D.A.; Egorova, A.A.; Krasilnikova, A.A. Mathematical Simulation of the Acoustic Effect on a Cryoprotectant with Fish Sperm at Equilibration. Biophysics 2022, 67, 549–558. [Google Scholar] [CrossRef]
- Matrosov, A.A.; Nizhnik, D.A.; Ponomareva, E.N.; Soloviev, A.N. Modeling the Impact of Piezoactuator on Fish Reproductive Cells in the Cryoprotective Environment; DSTU: Rostov-on-Don, Russia, 2022; pp. 266–269. (In Russian) [Google Scholar]
- Matrosov, A.A.; Nizhnik, D.A.; Pakhomov, V.I.; Soloviev, A.N. Diffusion of Cryoprotectant Through the Membrane of Re-productive Cells During Equilibration. In Physics and Mechanics of New Materials and Their Applications (PHENMA); Springer: Berlin/Heidelberg, Germany, 2023; pp. 508–514. [Google Scholar] [CrossRef]
- Krishna, R. Modelling of Cell Suspention During Cryopreservation. National Institute of Technology, Rourkela, 2013. 53p. Available online: http://ethesis.nitrkl.ac.in/5341/1/211BM2008.pdf (accessed on 10 November 2023).
- Cincotti, A.; Fadda, S. Modelling the Cryopreservation Process of a Suspension of Cells: The Effect of a Size-Distributed Cell Population//Computational Modeling in Tissue Engineering. In Studies in Mechanobiology, Tissue Engineering and Biomaterials; Springer: Berlin/Heidelberg, Germany, 2012; pp. 145–182. [Google Scholar] [CrossRef]
- Andreev, A.A.; Sadikova, D.G.; Ponomareva, E.N.; Krasilnikova, A.A.; Belaya, M. Method of Reduction of Low-Temperature Jump of Cryoprotectant. Solutions. Patent RU 2540598 C2, 10 February 2015. [Google Scholar]
- Ponomareva, E.; Matrosov, A.; Kovalenko, M.; Rudoy, D.; Pakhomov, V. Low-temperature preservation of sterlet reproductive cells (Acipenser ruthenus Linnaeus, 1758) by the acoustic-mechanical influence method. E3S Web Conf. 2023, 462, 03058. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matrosov, A.; Soloviev, A.; Ponomareva, E.; Meskhi, B.; Rudoy, D.; Olshevskaya, A.; Serebryanaya, I.; Nizhnik, D.; Pustovalova, O.; Maltseva, T. Finite Element Modeling of Crystallization with Temperature Jump to Improve Cryopreservation of Fish Germ Cells. Processes 2024, 12, 413. https://doi.org/10.3390/pr12020413
Matrosov A, Soloviev A, Ponomareva E, Meskhi B, Rudoy D, Olshevskaya A, Serebryanaya I, Nizhnik D, Pustovalova O, Maltseva T. Finite Element Modeling of Crystallization with Temperature Jump to Improve Cryopreservation of Fish Germ Cells. Processes. 2024; 12(2):413. https://doi.org/10.3390/pr12020413
Chicago/Turabian StyleMatrosov, Andrey, Arkady Soloviev, Elena Ponomareva, Besarion Meskhi, Dmitry Rudoy, Anastasiya Olshevskaya, Irina Serebryanaya, Dariya Nizhnik, Olga Pustovalova, and Tatiana Maltseva. 2024. "Finite Element Modeling of Crystallization with Temperature Jump to Improve Cryopreservation of Fish Germ Cells" Processes 12, no. 2: 413. https://doi.org/10.3390/pr12020413
APA StyleMatrosov, A., Soloviev, A., Ponomareva, E., Meskhi, B., Rudoy, D., Olshevskaya, A., Serebryanaya, I., Nizhnik, D., Pustovalova, O., & Maltseva, T. (2024). Finite Element Modeling of Crystallization with Temperature Jump to Improve Cryopreservation of Fish Germ Cells. Processes, 12(2), 413. https://doi.org/10.3390/pr12020413










