Abstract
The increased concentration of CO2 in the atmosphere has a strong impact on global warming. Therefore, efficient technologies must be used to reduce CO2 emissions. One of the methods is the biofixation of CO2 by microalgae and cyanobacteria. This is now a widely described technology that can improve the economics of biomass production and reduce CO2 emissions. There are no reports on the possibility of using it to clean exhaust gases from biogas combustion. The aim of the research was to determine the possibility of using Arthrospira platensis cultures to remove CO2 from biogas combustion. The efficiency of biomass production and the effectiveness of biological CO2 fixation were evaluated. The use of exhaust gases led to a more efficient increase in cyanobacterial biomass. The growth rate in the exponential phase was 209 ± 17 mgVS/L·day, allowing a biomass concentration of 2040 ± 49 mgVS/L. However, the use of exhaust gases led to a decrease in the pH of the culture medium and a rapid decline in the Arthrospira platensis population. The cyanobacteria effectively fixed CO2, and its concentration was limited from 13 ± 1% to 1.3 ± 0.7%. There was no influence of the exhaust gases on changes in the qualitative composition of the cyanobacterial biomass. In the culture fed with exhaust gas, the A. platensis population quickly entered the death phase, which requires close monitoring. This is an important indication for potential operators of large-scale photobioreactors.
1. Introduction
One of the main environmental threats is the dynamic global warming observed in recent years. This process entails many dynamic and dangerous atmospheric phenomena, leads to an imbalance in ecosystems and has a negative impact on human health, agriculture and the economy [1]. It has been proven that the main cause of the greenhouse effect is the rapidly increasing concentrations of carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) in the air [2,3].
However, CO2 accounts for almost 65% of total global greenhouse gas (GHG) emissions, which is why it is considered the main factor determining the course of currently observed climate changes [4,5]. It is mainly related to human economic activity, which is based on the extraction and utilisation of coal, oil and natural gas [6]. It is therefore necessary to use effective methods to reduce emissions and the concentration of CO2 in the atmosphere. This is accomplished both by primary methods, which are mainly based on renewable and unconventional energy sources, and by secondary methods, which lead to the capture and subsequent long-term storage, conservation or utilisation of CO2 [7,8].
The technology widely described in the literature for capturing and binding CO2 is the mineral carbonisation method [9]. It provides for the contact of contaminated gases with natural or artificial materials and wastes that have the ability to adsorb CO2. The result of the application is the production of stable carbonate compounds [10]. The search is still ongoing for ways to increase the technological efficiency and economic effectiveness of this technology. Research is focussed on the search for and synthesis of new materials, innovative designs of adsorption reactors, the suspension of the contact surface and the residence time in the filtration bed as well as methods for the introduction and distribution of CO2-containing gases [11]. The undeniable advantages of mineral carbonisation include the possibility of long-term binding and safe storage of CO2. The weaknesses include the high investment and operating costs of the reactors, the cost of procuring and storing adsorption materials, the limited availability of effective adsorbents and the technological complications of the process [12].
Other secondary methods to reduce CO2 emissions to the atmosphere include the capture and long-term storage of gases [13]. One of the well-characterised CO2 storage techniques is sequestration in natural geological formations [14]. It appears attractive because it enables the storage of large quantities of gas and a very long retention time. This is important for the possible future utilisation of CO2 in modern technologies and production processes. This concept assumes CO2 storage in deep, natural, permeable formations that are covered and isolated by impermeable layers. Commonly mentioned locations include depleted oil/gas reservoirs, deep aquifers and coal deposits [15]. Other, less technologically based approaches to capture and remove CO2 from gases include membrane separation and absorption processes in water or aqueous solutions [16]. The main disadvantages of these methods are the limited possibility of long-term storage of the captured CO2 in stable forms and the need to use it directly to prevent transfer to the atmosphere [17]. Another group includes biological processes based on photosynthetic organisms, such as forest plantations, planktonic organisms of the seas and oceans and fast-growing aquatic and terrestrial vascular plants [18].
Biological methods of CO2 fixation include those based on the use of intensive cultures of microalgae and cyanobacteria [19,20]. Currently, photosynthetic processes in natural planktonic marine and oceanic biocoenoses play a key role in maintaining the CO2 balance [21]. The use of about 50 gigatonnes of CO2 per year by microalgae and cyanobacteria corresponds to half of the global primary production [22]. Many studies have shown that phytoplankton are characterised by a higher CO2 fixation efficiency and biomass productivity compared to vascular plants. Photosynthetic microbes utilise the carbon concentration mechanism (CCM) to assimilate CO2. This is possible due to the presence of a specialised organelle (pyrenoid) that allows an increase in the CO2 content in the thylakoid membrane environment, which can increase the efficiency of carboxylation/oxidation of ribulose-1,5-bisphosphate (Rubisco), a photosynthetic enzyme that plays the main role in CO2 biofixation [23].
It has been shown that it is also possible to use controlled systems for the production of microalgae and cyanobacteria biomass to capture CO2 from anthropogenic sources [24,25]. The biomass thus produced is a raw material for the production of energy carriers and a source of many economically valuable compounds and chemicals, bioplastics, food supplements, cosmetics, pharmaceuticals, animal feed and fertilisers, which directly enhances the positive economic and environmental aspects of this type of technology [26,27].
It has been proven that microalgae and cyanobacteria can be used for long-term CO2 capture and utilisation. The biomass of these microorganisms is used for the production of cement and bioplastic [28]. The production of biocement involves the precipitation of CaCO3 by some photosynthetic microalgae or cyanobacteria as well as by nonphototrophic bacteria [29]. Bioplastics are environmentally friendly because they do not increase the CO2 pool and biodegrade faster [30]. An alternative direction is the use of biochar to improve the quality of poor-quality soils, marginal soils or degraded lands that require reclamation and biostimulants for crop production [31]. The sequestration of CO2 in soil structures ensures its storage and also serves the development of sustainable and organic crops by reducing the use of synthetic fertilisers [32].
The European Union (EU) has adopted a very ambitious strategy for the development of the bioeconomy, in which photosynthetic microorganisms represent an important biological resource. In particular, microalgae and cyanobacteria are currently being promoted due to their wide use in environmental technologies, bioenergy production and as a source of valuable nutrients for humans and animals [33]. The microalgae sector is growing dynamically, reaching a turnover of EUR 1.5 billion, and indirect activities (research, etc.) generate a further EUR 240 million [34]. Improving the economic viability of these systems is achieved by using waste, including waste water and waste gases, as basic components of the growing medium [35]. A promising solution is therefore the integration of systems for the production of biomass from microalgae and cyanobacteria with plants that can ensure an adequate quantity and quality of nutrients and provide a source of CO2 [36]. The development of systems for CO2 capture by microalgae is also supported by legislative measures that lead to a reduction in greenhouse gas emissions. One example of this is the requirements regarding the proportion of BIO components in fuels, which require a wider use of biofuel technologies. Microalgae are at the top of the list of potential raw materials for the production of biofuels [37].
The dynamic development of bioenergy systems based on the use of methane fermentation processes in biogas and biomethane CHP plants leads to the formation of leachate after fermentation and CO2 emissions [38,39]. Many previous studies have shown the possibility of utilising post-digestion leachate for the production of microalgae and cyanobacteria biomass [40,41]. It is a source of biogenic compounds, microelements and CO2 in the culture medium [42,43]. The high CO2 concentration in the leachate has a positive effect on the growth rate and determines the achievement of higher technological effects of the cultivation process [44]. To date, there are few experimental data analysing the possibility of using exhaust gases from biogas combustion in the production processes of microalgae and cyanobacteria [45]. So far, this source of CO2 has been considered promising, but the assumptions described were not based on research [46,47]. Considering the need to fix CO2 and the observed dynamics in the development of biogas production and combustion technologies, it is necessary to reliably assess the possibility of using this type of exhaust gas in the processes of intensive biomass production of microalgae and cyanobacteria.
The aim of the research was to determine the possibility of using cyanobacterial cultures of the species Arthrospira platensis in the process of removing CO2 from biogas combustion exhaust gases. The efficiency and speed of biomass production as well as the effectiveness of biological CO2 fixation were evaluated. The scope of the research included determining the efficiency of Arthrospira platensis by monitoring the concentration of dry matter and chlorophyll a as well as evaluating the final concentration of cyanobacteria obtained in vertical photobioreactors (V-PBR). The effects of the introduction of exhaust gases into the culture medium on the course and duration of the characteristic growth phases of the monitored population of these photosynthetic microorganisms were evaluated. The research determined the effectiveness of CO2 fixation in the biological sequestration process carried out by the Arthrospira platensis population and the effects of the tested technological treatment on the chemical composition of the biomass obtained. The study presents the research methodology, including the organisation of the experiment, the characteristics of the materials and the equipment used, as well as analytical and statistical methods. The results were presented and discussed in detail, and final conclusions were formulated.
2. Materials and Methods
2.1. Organisation of the Research
The research was conducted on a fractional technical scale. The experiments were divided into two variants (V) whose separation criterion was the CO2 source introduced into the vertical column photobioreactors (V-PBR). In variant 1 (V1), the CO2 introduced into the V-PBR came from atmospheric air. In variant 2 (V2), the CO2 source was exhaust gases from biogas combustion. The duration of the experiment was determined by the reaching of the death/lysis phase by the increasing population of the cyanobacteria Arthrospira platensis. In each variant, after cultivation and separation of the A. platensis biomass, the culture medium was returned to the V-PBR and reused in the cultivation process. In order to limit the effects of the process of water absorption of CO2 in the culture medium on the biosequestration results obtained, the results of the first two culture cycles were not included in the data analysis. The organisation chart of the experimental work is shown in Figure 1.
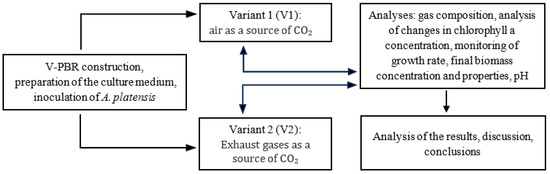
Figure 1.
The organisation chart of the experimental work.
2.2. Materials
2.2.1. Cyanobacteria Biomass and Culture Medium
A. platensis UTEX 3086 (Culture Collection of Algae University of Texas, Austin, TX, USA) was tested in the experiments. A medium based on tap water with the following composition in g/L was formulated: NaHCO2—27, Na2CO3—8, K2HPO4—1, NaNO3—25, K2SO4—2, NaCl—2. At the beginning of the culture, the medium was added to the V-PBR and then the A. platensis inoculum was introduced in an amount that ensures an initial biomass concentration of about 250 mg VS/L. The culture medium was supplemented with tap water in the quantities resulting from the evaporation losses and the separation of the biomass of Arthrospira platensis at the end of the culture cycle.
2.2.2. CO2 Sources
The exhaust gases, cooled to 20 °C, came from the combustion of biogas produced in an agricultural biogas plant on a technical scale. The biogas was produced in a fermentation reactor fed with cattle manure, maize and grass silage and operated under mesophilic conditions. The basic technological parameters for the operation of the biogas plant were as follows: organic loading rate—2.4 kgVS/m3·day, hydraulic retention time—42 days, process temperature—39 °C, substrate dosing 6 times/day with a frequency of every 2 h, complete mixing with vertical axis mixers and plant output—500 kWe. The composition of the purified biogas fed into the CHP module (No. TCG3016V12C, CES Ltd., Sugar Land, TX, USA, 600 kWe) contained 64.3 ± 1.6% CH4; 31.7 ± 1.1% CO2; 80 ± 10ppm H2S; 1.0 ± 0.2% O2 and 3.1 ± 0.7% N2. The average CO2 content in the exhaust gases was 15.7% ± 1.9%. The exhaust gases were collected once a day and stored in tight Tedlar bags with a volume of 70 L. In both series, the CO2 mass flow rate fed to the V-PBR was set to 8.0 mgCO2/min. In V1, atmospheric air with a capacity of 10.8 L/min was supplied to the V-PBR (Mistral 200, Aqua Medic, Brentwood Essex, UK). In V2, the exhaust gases were fed to the V-PBR with a peristaltic pump (FASTLoad Programmable Control Peristaltic Pump, VWR, Darmstadt, Germany) with a capacity of 28 mL/min. In V1, the air was discharged outside the V-PBR after it had flowed through the culture medium. In V2, the exhaust gases were recirculated with a peristaltic pump (VWR Germany) with a capacity of 10.8 L/min. As a result, the volume flow of the gases in both reactors was the same. With the peristaltic pump in V2, the gas was discharged from the reactor with a capacity of 28 mL/min.
2.2.3. Photobioreactors
V-PBRs made of acrylic glass (polymethyl methacrylate) were used, in which the culture medium had a volume of 10.0 L, and the gas phase a volume of 5.0 L. CO2 was introduced into the culture medium through a valve at the bottom of the V-PBR and discharged at the top. The V-PBRs were equipped with pH probes. The pH was measured continuously, once a day, and the results were sorted, averaged and stored in the memory of the pH metre. The reactors were continuously illuminated with fluorescent lamps (T8 Luxine Plus 15 W Sylvania United Kingdom, colour temperature 6500 K), and the illumination intensity on the surface of the reactors from the light side was 2 lux. The temperature of the introduced exhaust gases, the air and the temperature of the culture was 20 °C ± 2 °C. The scheme and armament of the PBRs used are shown in Figure 2.
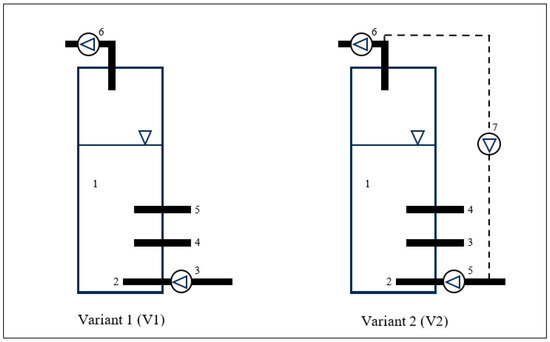
Figure 2.
Scheme of the V-PBRs. Variant 1: (1) V-PBR culture medium; (2) supply of pressurised air to the culture medium; (3) air supply pump; (4) sampling of cyanobacterial biomass; (5) pH measurement; (6) pump for gas removal. Variant 2: (1) V-PBR culture medium; (2) supply of pressurised air to the culture medium; (3) air supply pump; (4) sampling of the cyanobacterial biomass; (4) pH measurement; (6) pump for gas removal; (7) pump for gas recirculation.
2.2.4. Analytical Methods
Volatile solids (VS)—gravimetric method, chlorophyll a—fluorescence method (Algae Online Analyser bbe Moldanke, Schwentinental, Germany) and gas composition—Agilent with TCD detector, Testo 340 Analyser (Testo Ltd., Alton, UK, certificate of conformity EN 50379). The pH—VWR 1000 L pH meter (Germany), total carbon in biomass (TC), total organic carbon (TOC) and total nitrogen (TN)—Flesh 2000 analyser, Thermo. Ptot.—colourimetric method at a wavelength of 390 nm (DR 2800 HACH Lange) after prior mineralisation (HT200S HACH Lange). Total protein—multiplication of the Ntot. value by the conversion factor to protein, which is 6.25. Carbohydrates—colourimetric method with an anthrone reagent at a wavelength of 600 nm with a HACH Lange DR 2800 spectrophotometer. The lipid content—Soxhlet method with a Buchi extraction device.
2.2.5. Statistical Evaluation
The samples for analysis were taken once a day. The tests were carried out in five repetitions for both research variants. The statistical analysis was performed using a one-way analysis of variance with the assumed significance level (p < 0.05). The differences between the mean values of the variables were tested using the Tukey HSD test (Statistica 13.3 PL).
3. Results and Discussion
3.1. CO2 Biofixation and pH Changes
The raw biogas produced in the fermentation reactor and fed to combustion was characterised by the following average qualitative composition: 64.3 ± 1.6% CH4; 31.7 ± 1.1% CO2; 80 ± 10 ppm H2S; 1.0 ± 0.2% O2; 3.1 ± 0.7% N2. The average composition of the exhaust gases and air entering and leaving the V-PBR is shown in Table 1. In V1, the CO2 concentration resulted directly from the average content of the air used and was around 400 ± 20 ppm. The composition contained NOx and SOx in concentrations of 22 ± 2 ppm and 19 ± 2 ppm, respectively. In V2, the CO2 concentration in the exhaust gases from the biogas combustion was significantly higher and averaged 15.7 ± 1.9%. In addition, 112 ± 21 ppm CO, 130 ± 17 ppm NOx and 91 ± 2 ppm SOx were also detected. The oxygen content in V1 was 20.9 ± 0.1%, while in V2, it was 7.1 ± 1.5%.

Table 1.
Characteristics of the composition of the dry gases entering and leaving the V-PRB depending on the experiment variant.
A. platensis is a species with a high tolerance to changing environmental conditions. It is characterised by a relatively simple cultivation, separation and harvesting technique as well as a fast growth rate [48]. Cyanobacteria are among the oldest organisms on earth [49]. The choice of Arthrospira platensis for research was based on the fact that this species is highly adaptable to difficult development conditions [50]. They are eurybionts. Cyanobacteria can colonise the most inhospitable and difficult ecosystems [51]. Those taxa can be found in soil, on rocks, on tree bark, on glaciers and even in hot springs where temperatures can reach 90 °C [52]. They can be found in both salty and inland waters, floating freely in the water column among other phytoplankton groups or forming benthic mats at the bottom of reservoirs [53]. Cyanobacteria can utilise a broad light spectrum, are resistant to low oxygen levels and tolerate high pH values [54]. They are exceptionally proliferating organisms that are resistant to unfavourable environmental conditions. Arthrospira platensis tolerate a lack of oxygen in the water, pH fluctuations and organic contamination as well as long-term periods of drought and high temperatures [55].
It is easy to maintain the purity of the culture as the growing biomass causes a significant increase in the pH of the culture medium. This effectively inhibits the growth of other competitive organisms, including microalgae, fungi and protozoa [56]. The arguments outlined above indicate significant potential for the practical application of technologies for the utilisation of exhaust gases and other pollutants based on the use of A. platensis biomass [57].
It has been proven that too low CO2 concentrations in PBR are one of the main factors limiting the efficiency of biomass production of microalgae and cyanobacteria. Many studies indicate that the use of exhaust gases reduces the costs of supplementing livestock with other sources of CO2 [58]. In addition, this solution can reduce CO2 emissions to the atmosphere and reduce the costs of chemical and physical exhaust gas treatment [59,60]. It has been proven that only a few microalgae species tolerate high levels of SOx and NOx. Therefore, the selection of strains is important for the efficiency of CO2 fixation from exhaust gases [61,62]. The eurybiontic and resistant to harsh environmental conditions genera Chlorella sp. and Scenedesmus sp. are considered very promising. Chlorella sp. achieves CO2 fixation values of 0.73 to 1.79 gCO2/L per culture medium day, depending on the culture conditions [61]. Jiang et al. (2013) showed that the efficiency of CO2 fixation by Scenedesmus dimorphus can reach 75.61% [62]. The authors proved that S. dimorphus can tolerate high concentrations of CO2 and NO. Due to their very high environmental tolerance and resistance to changing growth conditions and pollution, cyanobacteria, including A. platensis, also have a very high potential in the fixation of CO2 from exhaust gases [63].
It was found that the efficiency of biosequestration of CO2 from exhaust gases (V2) was very high, ranging from 87.3 ± 1.1% to 96.2 ± 0.6% depending on the growth phase and progress of the A. platensis culture. The CO2 concentration in the gas flowing out of the V-PBR ranged from 0.5 ± 0.2% to 2.0 ± 0.1% (Figure 3A). The pH increased from 7.17 ± 0.04 at the beginning of the experiment to 9.33 ± 0.09 after 12 days of culture (Figure 3B). In the following days, the death phase of the culture was observed in V2, leading to a decrease in the production of exometabolites by the cyanobacteria and a decrease in pH to 8.70 ± 0.12. Feeding the V-PBR with exhaust gases in V2 also enabled the complete removal of CO, NOx and SOx.
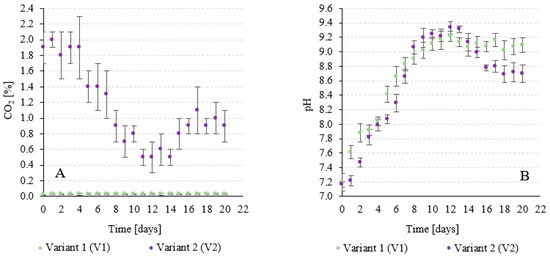
Figure 3.
Changes in the CO2 concentration in the gases flowing out of the V-PBR (A) and the pH value in the culture medium (B) as a function of the experimental variant.
In V1, the CO2 concentration was reduced to an average level of 310 ± 20 ppm (Table 1 and Figure 3A), the amount of oxygen was increased to 21.4 ± 0.2% and the NOx and SOx present in the air were removed. The pH increased significantly from the beginning to the 9th day of culture (Figure 3B). This correlated strongly with the dynamics of A. platensis population development and the increase in microalgae biomass concentration in V-PBR. This phenomenon is characteristic of periodic cultures in which the increasing concentration of exometabolites produced in the photosynthetic process leads to an increase in the pH of the environment [64]. Reaching threshold concentrations and a significant increase in pH limits eventually inhibit the growth of the cyanobacterial population [65]. In the following days of culture, the concentration of A. platensis biomass remained constant, limiting the dynamic changes in pH. On day 20, at the end of the process, the pH in V1 was 9.09 ± 0.1 (Figure 3B).
The literature shows that the final technological effect associated with CO2 biosequestration is generally influenced by two factors. During biosynthesis, CO2 is utilised by the photosynthesising biomass and by its dissolution and absorption in the culture medium [66]. Considering that the culture medium had a hardness of 474 ± 14 mg CaCO3/L, the chemical absorption of CO2 by calcium or magnesium ions could have a significant influence on the observed binding effects. This is confirmed by the studies of Liu et al. (2022), who analysed the efficiency of CO2 fixation in the autotrophic culture of Prymnesium parvum [67]. To limit this phenomenon and saturate the medium with CO2, the culture medium of each variant was returned to the V-PBR after completion of cultivation and separation of the biomass of A. platensis and reused in the research process. Wang et al. (2019) [68] used biofilm technology to cultivate A. platensis biomass. The study was conducted in a pilot plant with an area of 10 m2 under greenhouse conditions. The CO2 fixation efficiency was 75.1% [68]. Sydney et al. (2010) [69] used air enriched with 5% CO2 for the production of A. platensis LEB-52 biomass. A CO2 removal coefficient of 318.61 mg/L/d was achieved. It was found that 80.4% of CO2 was utilised for biomass production [69]. Ramanan et al. (2010) [70] exposed A. platensis to different concentrations of CO2. The efficiency of CO2 biosynthesis was 59%, 51% and 46% for initial concentrations of 1%, 5% and 10%, respectively [70]. Chunzhuk et al. (2023) [71] investigated the efficiency of CO2 capture during the cultivation of A. platensis at high CO2 concentrations. Constant flushing of the medium with a gas–air mixture at initial CO2 concentrations of 1.5 and 9% was used. In the variant with an initial CO2 concentration of 1%, the decrease in CO2 content in the gas–air mixture, which is due to the capture of CO2 during photosynthesis by the microalgae, was 0.06%(CO2)/d. In the variant with 5% CO2, the decrease in concentration was 0.10%(CO2)/d. However, the use of 9% CO2 led to a decrease in concentration of 0.04%(CO2)/d [71]. The results of the first two cultivation cycles were not considered when analysing the experimental data. In V2, when exhaust gases were used, a strong correlation (R2 = 0.9421) was found between the CO2 concentration at the outflow from the V-PBR and the recorded pH, while in V2, no relationship was found between these two monitored factors (R2 = 0.2302) (Figure 4A,B).
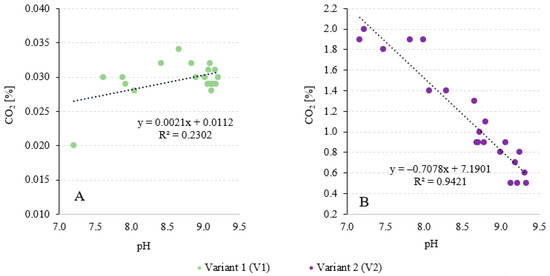
Figure 4.
Relationships between the CO2 content in the gases obtained from the V-PBR and the pH value in variant 1 (A) and variant 2 (B).
3.2. Production and Properties of the Biomass
Significant differences in the rate and amount of cyanobacteria biomass produced were found depending on the variant. Both V1 and V2 were in the lag phase during the first 6 days, and the differences in the achieved efficiency of biomass growth were not significant. In V1, the biomass concentration of A. platensis increased from 250 ± 11 mgVS/L to 621 ± 24 mgVS/L. In V2, an increase from 250 ± 13 mgVS/L to 566 ± 32 mgVS/L was observed (Figure 5A). In the logarithmic growth phase, the biomass growth rate was 148 ± 12 mgVS/L-d in V1 and 209 ± 17 mgVS/L-d in V2. The observed differences were statistically significant (Figure 5B).
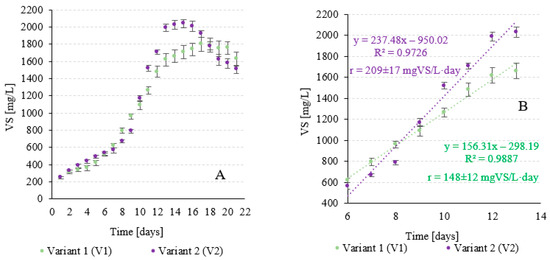
Figure 5.
Changes in the concentration of A. platensis in V-PBR (A) and the recorded growth rates of the biomass in the logarithmic phase (B) depending on experimental variant.
After 13 days of culture, a value of 1660 ± 74 mgVS/L was reached at the end of the exponential growth phase in V1. In V2, the concentration of A. platensis was significantly higher at 2030 ± 51 mgVS/L (Figure 5A). In the following days, the culture entered the stationary growth phase, which was characterised by slight changes in the biomass concentration in V-PBR. In V1, this phase lasted an average of 7 days, and at its end, a cyanobacterial biomass concentration of 1760 ± 72 mgVS/L was observed. In V2, this phase was significantly shorter, lasting 3 days on average, and the biomass concentration was 2010 ± 56 mgVS/L. After this time, the A. platensis culture entered the death phase. After 20 days at the end of the culture, a concentration of 1630 ± 81 mgVS/L was reached in V1 compared to 1510 ± 48 mgVS/L in V2 (Figure 5A).
Experience has shown that the duration of the lag phase and the exponential growth phase was the same for both variants, averaging 5 and 8 days, respectively. The stationary growth phase was significantly longer in V1 and lasted 7 days, while in V2, it was only 3 days until the transition to the death phase (Figure 6A). Wang et al. (2019) [68] determined an average biomass production of A. platensis of 38.3 g/m2/d during CO2 sequestration [68]. Sydney et al. (2010) [69] used air enriched with 5% CO2 to cultivate A. platensis. The maximum cell concentration of 2.18 g/L was observed on day 14. The maximum cell productivity was 0.73 g/L/d [69]. In the study by Ramanan et al. (2010) [70], the growth rate of A. platensis increased with the highest CO2 concentration. The biomass production was 2.91 g/L at a CO2 concentration of 10% [70]. In the study by Chunzhuk et al. (2023) [71], at an initial CO2 concentration of 5%, the highest biomass density value of A. platensis was found to be 1.28 g/L wt% on day 15. The maximum biomass growth rate was 79.4 mg/L/d at an initial CO2 concentration of 1%. In addition, almost complete cell death of A. platensis was observed under the influence of a CO2 concentration of 9% [71]. The relationships related to biomass growth are confirmed by the observations of chlorophyll a concentration in V-PBR. The progression of changes in chlorophyll a content over time is similar, but not identical, to the changes in A. platensis biomass concentration (Figure 6B). From the 6th to the 10th day of culture, significantly higher chlorophyll a concentrations were observed in V1. However, no statistically significant differences were observed from the 11th to the 15th day of culture. Analogue chlorophyll a concentrations were observed in both experimental variants. On day 15, it was 6.47 ± 0.32 mg/L in V1, while it was 6.37 ± 0.18 mg/L in V2 (Figure 6B). In the following days of the culture, a significant and rapid decrease in chlorophyll a concentration was observed in V2, which was 4.99 ± 0.16 mg/L at the end of the culture (Figure 6B). In V1, the concentration of chlorophyll a remained at a steady level until the end of the culture, ranging from 6.67 ± 0.34 mg/L on day 16 to 6.16 ± 0.33 mg/L on day 20 of the culture (Figure 6B).
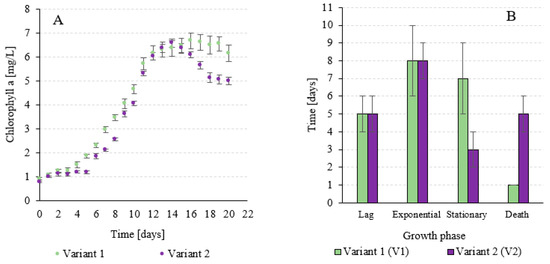
Figure 6.
Duration of the subsequent growth phases of A. platensis (A) and recorded concentrations of chlorophyll a in V-PBR (B) depending on the experimental variant.
Analysing the chlorophyll a content in V-PBR proved that the use of exhaust gases is beneficial in the short term. In the long term, however, it leads to negative changes in the population of A. platensis. This is mainly determined by the rapid transition to the death phase and the dynamic decline in biomass after 15 days of cultivation. The effects of cultivating microalgae with exhaust gases on the change in chlorophyll concentration were also analysed by Yang et al. (2004) [72]. It was found that high concentrations of bisulphites and free radicals above 2 mmol/L destroy chlorophyll in Botryococcus braunii. Chlorophyll bleaching processes and lipid peroxidation of the cell membranes were observed [72].
There was no significant effect of the CO2 source on the composition and characteristics of the biomass of A. platensis. Regardless of the experimental variant, the content of the basic parameters characterising the biomass was similar. The content of volatile solids was about 93%, the amount of protein was close to 37%, lipids 11% and carbohydrates around 26%. The detailed characteristics of the biomass are presented in Table 2. In the study by Sydney et al. (2010) [69], the composition of the biomass of A. platensis LEB-52 after CO2 sequestration was as follows: 42.33 ± 1.9% proteins, 11 ± 2.2% lipids and 11 ± 0.88% carbohydrates [69]. In turn, Chunzhuk et al. (2023) [71] reported 70.0 ± 0.6% proteins and 5.7 ± 0.6% lipids in A. platensis biomass grown at a CO2 concentration of 1%. However, increasing the CO2 concentration to 5% led to a decrease in the concentration of proteins and lipids to 47.7 ± 1.5% and 4.2 ± 0.5%, respectively [71].

Table 2.
Characteristics of the A. platensis biomass composition depending on the experimental variant.
Due to its high nutritional value (Table 2), A. platensis has numerous applications in food, feed, pharmaceuticals, nutraceuticals and cosmetics [73]. It is one of the most commercially produced species and is considered a very good source of proteins, pigments (phycocyanin and chlorophyll), vitamins and antioxidants [74]. A. platensis has great potential as an important ingredient for the development of new functional foods that fulfil consumer demand for nutrient-rich and health-promoting foods [16,17]. It is used in the human diet not only for its high protein content but also for its desirable amino acid (AA) profile in terms of the amount of essential AA and good digestibility, making it a potential alternative protein source. In addition, microalgae proteins also have promising techno-functional properties and are used as foaming agents, gelling agents and emulsifiers [75]. Some studies have shown that microalgae proteins can compete with some commercial proteins used as emulsifiers, such as soya and whey proteins and sodium caseinate [76]. As for the polysaccharides of A. platensis, studies have demonstrated their biological activity and potential for use in the food industry [77]. Microalgae are also an important source of minerals, especially Fe, Zn, Mn and Cu, as well as water-soluble vitamins (B and C complex) and vitamin E [78]. A. platensis is rich in vitamin B12, as it is estimated that it can contain between 1.6 and 3.2 µg of this vitamin per gramme of dry matter, which covers 25 to 133% of the daily requirement [79]. In recent years, microalgae biomass has been successfully used as an additive to foods, offering innovative and healthy alternatives such as biscuits, pasta, mayonnaises, jelly desserts and cold meats [80]. Another important aspect is the use of A. platensis for the production of biofuels [81]. They show a high biochemical tendency to accumulate carbohydrates and proteins through their metabolism [82]. The biomass of A. platensis could easily be converted into biogas [83], bioethanol [84] and biodiesel [85]. However, current processes and technologies are not sufficient to make large-scale production economically viable. It is therefore necessary to introduce improvements and innovations in the biofuel production process, including the development of a biorefinery approach [74].
It should be noted that a complete and reliable assessment of the CO2 fixation efficiency of cyanobacteria can be made by taking into account the energy consumption during cultivation (lighting, nutrient dosing, mixing, gas supply, separation, etc.). This is only possible on the basis of data obtained from plants operated on a pilot or technical scale. This is of course an important aspect that determines the application potential of this technological solution. Studies of this type in an innovative photobioreactor with a total volume of 30 m3 were carried out by Chen et al. (2012) [86]. These researchers determined the CO2 binding potential of the cyanobacteria Spirulina platensis. The total CO2 sequestration in this photoautotrophic culture was 2234 kg CO2/year. However, if the emissions from the operational energy consumption of 1494 kg CO2/year are taken into account, the amount of CO2 bound in the biomass was only 740 kg CO2/year. Ultimately, the estimated amount of bound CO2 would be around 74 tonnes/ha⋅year [86].
4. Conclusions
It was shown that the biomass of A. platensis effectively fixed CO2 from the combustion of biogas. Using this CO2 source, a higher growth rate of the biomass in the logarithmic growth phase and higher concentrations of cyanobacteria in the photobioreactors were also observed. The growth rate in the exponential growth phase was 209 ± 17 mgVS/L·day, which allowed a biomass concentration of 2040 ± 49 mgVS/L. In the control V-PBR, it was a maximum of 1800 mgVS/L. The cyanobacteria effectively captured the CO2 from the biogas combustion, and its concentration was reduced from 13 ± 1% to 1.3 ± 0.7%. Feeding the V-PBR with exhaust gases had no significant effect on the biomass properties in terms of organic matter content, including lipids, proteins and carbohydrates.
The exhaust gas-fed culture quickly transitioned to the A. platensis population die-off phase after only three days of stable growth, requiring close monitoring of cyanobacteria concentrations to enable rapid response and biomass removal. This is an important indication for potential operators of large-scale photobioreactors. Cultivation with air was characterised by much greater stability and a long phase of stationary growth.
Further research should aim to determine the maximum amount of exhaust gas that can be introduced into the cultivation system without significantly limiting the effectiveness of CO2 fixation and inhibiting the production of cyanobacterial biomass. It is important to automate the process and possibly link the amount of exhaust gas added to the pH changes in the culture medium. In the longer term, it is advisable to increase the technical readiness level and conduct research on a larger scale in order to obtain reliable results that are necessary for drawing up a mass, energy and economic balance as well as a complete life cycle assessment.
Author Contributions
Conceptualization, M.D. and M.Z.; methodology, M.D. and M.Z.; validation, M.D.; formal analysis, M.D. and M.Z.; investigation, M.D., M.Z., A.V. and J.K.; resources, M.D., M.Z., A.V. and J.K.; data curation, M.D., M.Z. and A.V.; supervision, M.D.; writing—original draft preparation, M.D. and J.K.; writing—review and editing, M.D., M.Z., A.V. and J.K.; visualization, M.D.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by works no. 29.610.023-110 of the University of Warmia and Mazury in Olsztyn and WZ/WB-IIŚ/3/2022 of the Bialystok University of Technology, funded by the Minister of Education and Science.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kaur, H. Air Pollution and Greenhouse Gases Emissions: Implications in Food Production and Food Security. In Greenhouse Gases: Sources, Sinks and Mitigation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 107–133. [Google Scholar] [CrossRef]
- Arıcı, H.Y.; Ak, H. A Perspective on Sustainable Ecology in the Light of the Qur’an. OPUS J. Soc. Res. 2022, 19, 380–392. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Griffiths, S.; Kim, J.; Bazilian, M. Climate Change and Industrial F-Gases: A Critical and Systematic Review of Developments, Sociotechnical Systems and Policy Options for Reducing Synthetic Greenhouse Gas Emissions. Renew. Sustain. Energy Rev. 2021, 141, 110759. [Google Scholar] [CrossRef]
- Gür, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Timm, W. An Empirical Approach to the Analysis of Local and Global Climate and Weather Data and to the Determination of CO2 Sensitivities. 2021; preprint. [Google Scholar] [CrossRef]
- Dimitriou, K.; Bougiatioti, A.; Ramonet, M.; Pierros, F.; Michalopoulos, P.; Liakakou, E.; Solomos, S.; Quehe, P.Y.; Delmotte, M.; Gerasopoulos, E.; et al. Greenhouse Gases (CO2 and CH4) at an Urban Background Site in Athens, Greece: Levels, Sources and Impact of Atmospheric Circulation. Atmos. Environ. 2021, 253, 118372. [Google Scholar] [CrossRef]
- Borowski, P.F. Management of Energy Enterprises in Zero-Emission Conditions: Bamboo as an Innovative Biomass for the Production of Green Energy by Power Plants. Energies 2022, 15, 1928. [Google Scholar] [CrossRef]
- Sun, X.; Alcalde, J.; Bakhtbidar, M.; Elío, J.; Vilarrasa, V.; Canal, J.; Ballesteros, J.; Heinemann, N.; Haszeldine, S.; Cavanagh, A.; et al. Hubs and Clusters Approach to Unlock the Development of Carbon Capture and Storage—Case Study in Spain. Appl. Energy 2021, 300, 117418. [Google Scholar] [CrossRef]
- Alturki, A. The Global Carbon Footprint and How New Carbon Mineralization Technologies Can Be Used to Reduce CO2 Emissions. ChemEngineering 2022, 6, 44. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Adewuyi, Y.G. State-of-the-Art Review on Capture of CO2 Using Adsorbents Prepared from Waste Materials. Process Saf. Environ. Prot. 2020, 139, 1–25. [Google Scholar] [CrossRef]
- Snæbjörnsdóttir, S.; Sigfússon, B.; Marieni, C.; Goldberg, D.; Gislason, S.R.; Oelkers, E.H. Carbon Dioxide Storage through Mineral Carbonation. Nat. Rev. Earth Environ. 2020, 1, 90–102. [Google Scholar] [CrossRef]
- Sabri, M.A.; Al Jitan, S.; Bahamon, D.; Vega, L.F.; Palmisano, G. Current and Future Perspectives on Catalytic-Based Integrated Carbon Capture and Utilization. Sci. Total Environ. 2021, 790, 148081. [Google Scholar] [CrossRef]
- Leonzio, G.; Fennell, P.S.; Shah, N. Analysis of Technologies for Carbon Dioxide Capture from the Air. Appl. Sci. 2022, 12, 8321. [Google Scholar] [CrossRef]
- Ringrose, P. Why We Need Engineered Geological Storage of CO2. In How to Store CO2 Underground: Insights from Early-Mover CCS Projects. SpringerBriefs in Earth Sciences; Springer: Cham, Switzerland, 2020; pp. 1–12. [Google Scholar] [CrossRef]
- Hameli, A.; Dhuhoori, A.; Gomes, P.; Al Hameli, F.; Belhaj, H.; Al Dhuhoori, M. CO2 Sequestration Overview in Geological Formations: Trapping Mechanisms Matrix Assessment. Energies 2022, 15, 7805. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Yang, R.; Liu, Y.; Jafari, M. CO2 Capture and Storage Monitoring Based on Remote Sensing Techniques: A Review. J. Clean. Prod. 2021, 281, 124409. [Google Scholar] [CrossRef]
- Guduru, R.K.; Gupta, A.A.; Dixit, U. Biological Processes for CO2 Capture. In Emerging Carbon Capture Technologies towards a Sustainable Future; Elsevier: Amsterdam, The Netherlands, 2022; pp. 371–400. [Google Scholar] [CrossRef]
- Molahid, V.L.M.; Kusin, F.M.; Hasan, S.N.M.S.; Ramli, N.A.A.; Abdullah, A.M. CO2 Sequestration through Mineral Carbonation: Effect of Different Parameters on Carbonation of Fe-Rich Mine Waste Materials. Processes 2022, 10, 432. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Yamakawa, C.K.; van der Maas, L.; Dragone, G. New Trends in Bioprocesses for Lignocellulosic Biomass and CO2 Utilization. Renew. Sustain. Energy Rev. 2021, 152, 111620. [Google Scholar] [CrossRef]
- Cabrerizo, M.J.; Medina-Sánchez, J.M.; González-Olalla, J.M.; Sánchez-Gómez, D.; Carrillo, P. Microbial Plankton Responses to Multiple Environmental Drivers in Marine Ecosystems with Different Phosphorus Limitation Degrees. Sci. Total Environ. 2022, 816, 151491. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of Microalgae in Global CO2 Sequestration: Physiological Mechanism, Recent Development, Challenges, and Future Prospective. Sustainability 2021, 13, 13061. [Google Scholar] [CrossRef]
- Sørensen, M.; Andersen-Ranberg, J.; Hankamer, B.; Møller, B.L. Circular Biomanufacturing through Harvesting Solar Energy and CO2. Trends Plant Sci. 2022, 27, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, M.; Krzemieniewski, M.; Zieliński, M.; Kazimierowicz, J. Immobilized Microalgae-Based Photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency Estimation, Technological Parameters, and Prototype Concept. Atmosphere 2021, 12, 1031. [Google Scholar] [CrossRef]
- Farooq, W.; Naqvi, S.R.; Sajid, M.; Shrivastav, A.; Kumar, K. Monitoring Lipids Profile, CO2 Fixation, and Water Recyclability for the Economic Viability of Microalgae Chlorella Vulgaris Cultivation at Different Initial Nitrogen. J. Biotechnol. 2022, 345, 30–39. [Google Scholar] [CrossRef]
- Dębowski, M.; Michalski, R.; Zieliński, M.; Kazimierowicz, J. A Comparative Analysis of Emissions from a Compression–Ignition Engine Powered by Diesel, Rapeseed Biodiesel, and Biodiesel from Chlorella Protothecoides Biomass Cultured under Different Conditions. Atmosphere 2021, 12, 1099. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae Biomass as a Sustainable Source for Biofuel, Biochemical and Biobased Value-Added Products: An Integrated Biorefinery Concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- In-Na, P.; Umar, A.A.; Wallace, A.D.; Flickinger, M.C.; Caldwell, G.S.; Lee, J.G.M. Loofah-Based Microalgae and Cyanobacteria Biocomposites for Intensifying Carbon Dioxide Capture. J. CO2 Util. 2020, 42, 101348. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Vu, M.T.; Vu, H.P.; Johir, M.A.H.; Labeeuw, L.; Ralph, P.J.; Mahlia, T.M.I.; Pandey, A.; Sirohi, R.; Nghiem, L.D. Microalgae-Based Carbon Capture and Utilization: A Critical Review on Current System Developments and Biomass Utilization. Crit. Rev. Environ. Sci. Technol. 2023, 53, 216–238. [Google Scholar] [CrossRef]
- Abe, M.M.; Branciforti, M.C.; Brienzo, M. Biodegradation of Hemicellulose-Cellulose-Starch-Based Bioplastics and Microbial Polyesters. Recycling 2021, 6, 22. [Google Scholar] [CrossRef]
- Poria, V.; Dębiec-Andrzejewska, K.; Fiodor, A.; Lyzohub, M.; Ajijah, N.; Singh, S.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) Integrated Phytotechnology: A Sustainable Approach for Remediation of Marginal Lands. Front. Plant Sci. 2022, 13, 999866. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.S.; Kumar, S.; Yadav, G.S. Soil Carbon Sequestration in Crop Production. In Nutrient Dynamics for Sustainable Crop Production; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–39. [Google Scholar] [CrossRef]
- Morgado, F.; Vieira, L.R. Marine Bioprospecting to Improve Knowledge of the Biological Sciences and Industrial Processes. In Affordable and Clean Energy; Springer: Berlin/Heidelberg, Germany, 2021; pp. 845–858. [Google Scholar] [CrossRef]
- European Commission. The EU Blue Economy Report 2020; European Commission: Luxembourg, 2020. [Google Scholar]
- Chen, J.; Dai, L.; Mataya, D.; Cobb, K.; Chen, P.; Ruan, R. Enhanced Sustainable Integration of CO2 Utilization and Wastewater Treatment Using Microalgae in Circular Economy Concept. Bioresour. Technol. 2022, 366, 128188. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Freitas, J.; Fernandes, I.; Silva, P. Microalgae as Biofertilizers: A Sustainable Way to Improve Soil Fertility and Plant Growth. Sustainability 2023, 15, 12413. [Google Scholar] [CrossRef]
- Leliaert, F.; Verbruggen, H.; Vanormelingen, P.; Steen, F.; López-Bautista, J.M.; Zuccarello, G.C.; De Clerck, O. Key Targets for Improving Algal Biofuel Production. Clean Technol. 2021, 3, 43. [Google Scholar] [CrossRef]
- Kucharska, K.; Makoś-Chełstowska, P.; Słupek, E.; Gębicki, J. Management of Dark Fermentation Broth via Bio Refining and Photo Fermentation. Energies 2021, 14, 6268. [Google Scholar] [CrossRef]
- Morsink-Georgali, P.Z.; Kylili, A.; Fokaides, P.A.; Papadopoulos, A.M. Compost versus Biogas Treatment of Sewage Sludge Dilemma Assessment Using Life Cycle Analysis. J. Clean. Prod. 2022, 350, 131490. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. Outflow from a Biogas Plant as a Medium for Microalgae Biomass Cultivation—Pilot Scale Study and Technical Concept of a Large-Scale Installation. Energies 2022, 15, 2912. [Google Scholar] [CrossRef]
- Zielinski, D.; Fraczyk, J.; Debowski, M.; Zielinski, M.; Kaminski, Z.J.; Kregiel, D.; Jacob, C.; Kolesinska, B. Biological Activity of Hydrophilic Extract of Chlorella Vulgaris Grown on Post-Fermentation Leachate from a Biogas Plant Supplied with Stillage and Maize Silage. Molecules 2020, 25, 1790. [Google Scholar] [CrossRef] [PubMed]
- Bharati Barua, V.; Munir, M.; Vuppala, S.; Ochando-Pulido, J.M. A Review on Synchronous Microalgal Lipid Enhancement and Wastewater Treatment. Energies 2021, 14, 7687. [Google Scholar] [CrossRef]
- Krzemińska, I.; Oleszek, M.; Wiacek, D. Liquid Anaerobic Digestate as a Source of Nutrients for Lipid and Fatty Acid Accumulation by Auxenochlorella protothecoides. Molecules 2019, 24, 3582. [Google Scholar] [CrossRef]
- Chuka-ogwude, D.; Ogbonna, J.; Moheimani, N.R. A Review on Microalgal Culture to Treat Anaerobic Digestate Food Waste Effluent. Algal Res. 2020, 47, 101841. [Google Scholar] [CrossRef]
- Venkiteshwaran, K.; Xie, T.; Seib, M.; Tale, V.P.; Zitomer, D. Anaerobic Digester Biogas Upgrading Using Microalgae. In Integrated Wastewater Management and Valorization Using Algal Cultures; Elsevier: Amsterdam, The Netherlands, 2022; pp. 183–214. [Google Scholar] [CrossRef]
- Estrada-Graf, A.; Hernández, S.; Morales, M. Biomitigation of CO2 from Flue Gas by Scenedesmus Obtusiusculus AT-UAM Using a Hybrid Photobioreactor Coupled to a Biomass Recovery Stage by Electro-Coagulation-Flotation. Environ. Sci. Pollut. Res. 2020, 27, 28561–28574. [Google Scholar] [CrossRef]
- Park, S.; Ahn, Y.; Pandi, K.; Ji, M.K.; Yun, H.S.; Choi, J.Y. Microalgae Cultivation in Pilot Scale for Biomass Production Using Exhaust Gas from Thermal Power Plants. Energies 2019, 12, 3497. [Google Scholar] [CrossRef]
- Bayona-Morcillo, P.J.; Plaza, B.M.; Gómez-Serrano, C.; Rojas, E.; Jiménez-Becker, S. Effect of the Foliar Application of Cyanobacterial Hydrolysate (Arthrospira platensis) on the Growth of Petunia x Hybrida under Salinity Conditions. J. Appl. Phycol. 2020, 32, 4003–4011. [Google Scholar] [CrossRef]
- Waditee-Sirisattha, R.; Kageyama, H. Cyanobacterial Cells. In Cyanobacterial Physiology from Fundamentals to Biotechnology; Academic Press: Cambridge, MA, USA, 2022; pp. 3–16. [Google Scholar] [CrossRef]
- Markou, G.; Kougia, E.; Arapoglou, D.; Chentir, I.; Andreou, V.; Tzovenis, I. Production of Arthrospira Platensis: Effects on Growth and Biochemical Composition of Long-Term Acclimatization at Different Salinities. Bioengineering 2023, 10, 233. [Google Scholar] [CrossRef]
- Wehr, J.; Janse van Vuuren, S. Algae and Cyanobacteria Communities. In Wetzel’s Limnology; Academic Press: Cambridge, MA, USA, 2024; pp. 463–510. [Google Scholar] [CrossRef]
- Saber, A.A.; El-Refaey, A.A.; Saber, H.; Singh, P.; van Vuuren, S.J.; Cantonati, M. Cyanoprokaryotes and Algae: Classification and Habitats. In Handbook of Algal Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–38. [Google Scholar] [CrossRef]
- Sukenik, A.; Kaplan, A. Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems: A Comprehensive Outlook on Current and Emerging Mitigation and Control Approaches. Microorganisms 2021, 9, 1472. [Google Scholar] [CrossRef]
- Yadav, P.; Prasad Singh, R.; Rana, S.; Joshi, D.; Kumar, D.; Bhardwaj, N.; Kumar Gupta, R.; Kumar, A. Mechanisms of Stress Tolerance in Cyanobacteria under Extreme Conditions. Stresses 2022, 2, 36. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; El-Ashram, S.; Yilmaz, S.; Naiel, M.A.E.; Abdul Kari, Z.; Hamid, N.K.A.; Dawood, M.A.O.; Nowosad, J.; Kucharczyk, D. The Effectiveness of Arthrospira platensis and Microalgae in Relieving Stressful Conditions Affecting Finfish and Shellfish Species: An Overview. Aquac. Rep. 2022, 24, 101135. [Google Scholar] [CrossRef]
- Yu, J.; Hu, H.; Wu, X.; Wang, C.; Zhou, T.; Liu, Y.; Ruan, R.; Zheng, H. Continuous Cultivation of Arthrospira platensis for Phycocyanin Production in Large-Scale Outdoor Raceway Ponds Using Microfiltered Culture Medium. Bioresour. Technol. 2019, 287, 121420. [Google Scholar] [CrossRef] [PubMed]
- Vlaskin, M.S.; Kiseleva, S.V.; Chernova, N.I.; Grigorenko, A.V.; Ryndin, K.G.; Popel’, O.S.; Malanii, S.Y.; Slavkina, O.V.; de Farias Naves, F.; Kumar, V. Effectiveness of CO2 Capture by Arthrospira platensis Microalgae from a Mixture Simulating Flue Gases. Therm. Eng. 2023, 70, 370–383. [Google Scholar] [CrossRef]
- Lage, S.; Gojkovic, Z.; Funk, C.; Gentili, F.G. Algal Biomass from Wastewater and Flue Gases as a Source of Bioenergy. Energies 2018, 11, 664. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Chang, J.S.; Ling, T.C.; Juan, J.C. Biosequestration of Atmospheric CO2 and Flue Gas-Containing CO2 by Microalgae. Bioresour. Technol. 2015, 184, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, Y.; Wu, N.; Lan, C.Q. CO2 Bio-Mitigation Using Microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Perspectives on Microalgal CO2-Emission Mitigation Systems—A Review. Biotechnol. Adv. 2011, 29, 189–198. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Wang, J.; Chen, Y.; Shen, S.; Liu, T. Utilization of Simulated Flue Gas for Cultivation of Scenedesmus Dimorphus. Bioresour. Technol. 2013, 128, 359–364. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, J.; Zhang, Z.; Liu, J. Mutation of Arthrospira platensis by Gamma Irradiation to Promote Phenol Tolerance and CO2 Fixation for Coal-Chemical Flue Gas Reduction. J. CO2 Util. 2020, 38, 252–261. [Google Scholar] [CrossRef]
- Zieliński, M.; Kazimierowicz, J.; Dębowski, M. The Possibility of Deploying CO2 from Biogas Combustion to Improve the Productivity of a Periodical Chlorella vulgaris Culture. Front. Biosci. Elit. 2023, 15, 3. [Google Scholar] [CrossRef]
- Fang, F.; Gao, Y.; Gan, L.; He, X.; Yang, L. Effects of Different Initial PH and Irradiance Levels on Cyanobacterial Colonies from Lake Taihu, China. J. Appl. Phycol. 2018, 30, 1777–1793. [Google Scholar] [CrossRef]
- de Morais, M.G.; Vargas, B.P.; da Silva Vaz, B.; Cardias, B.B.; Costa, J.A.V. Advances in the Synthesis and Applications of Nanomaterials to Increase CO2 Biofixation in Microalgal Cultivation. Clean Technol. Environ. Policy 2023, 25, 617–632. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhao, R.Z.; Qiu, X.C.; Guo, Q. Optimization Analysis to Evaluate the Relationships between Different Ion Concentrations and Prymnesium parvum Growth Rate. Water 2022, 14, 928. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, W.; Liu, W.; Wang, H.; Zhang, D.; Qiao, Z.; Jin, G.; Liu, T. Field Study on Attached Cultivation of Arthrospira (Spirulina) with Carbon Dioxide as Carbon Source. Bioresour. Technol. 2019, 283, 270–276. [Google Scholar] [CrossRef]
- Sydney, E.B.; Sturm, W.; de Carvalho, J.C.; Thomaz-Soccol, V.; Larroche, C.; Pandey, A.; Soccol, C.R. Potential Carbon Dioxide Fixation by Industrially Important Microalgae. Bioresour. Technol. 2010, 101, 5892–5896. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.; Kannan, K.; Deshkar, A.; Yadav, R.; Chakrabarti, T. Enhanced Algal CO2 Sequestration through Calcite Deposition by Chlorella Sp. and Spirulina platensis in a Mini-Raceway Pond. Bioresour. Technol. 2010, 101, 2616–2622. [Google Scholar] [CrossRef]
- Chunzhuk, E.A.; Grigorenko, A.V.; Chernova, N.I.; Kiseleva, S.V.; Ryndin, K.G.; Popel, O.S.; Malaniy, S.Y.; Slavkina, O.V.; de Farias Neves, F.; Leng, L.; et al. Direct Study of CO2 Capture Efficiency during Microalgae Arthrospira platensis Cultivation at High CO2 Concentrations. Energies 2023, 16, 822. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Cong, W.; Cai, Z.; Ouyang, F. Effects of Bisulfite and Sulfite on the Microalga Botryococcus braunii. Enzym. Microb. Technol. 2004, 35, 46–50. [Google Scholar] [CrossRef]
- Ramírez-Rodrigues, M.M.; Estrada-Beristain, C.; Metri-Ojeda, J.; Pérez-Alva, A.; Baigts-Allende, D.K. Spirulina platensis Protein as Sustainable Ingredient for Nutritional Food Products Development. Sustainability 2021, 13, 6849. [Google Scholar] [CrossRef]
- Magro, F.G.; Margarites, A.C.; Reinehr, C.O.; Gonçalves, G.C.; Rodigheri, G.; Costa, J.A.V.; Colla, L.M. Spirulina platensis Biomass Composition Is Influenced by the Light Availability and Harvest Phase in Raceway Ponds. Environ. Technol. 2018, 39, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The Potential of Microalgae and Their Biopolymers as Structuring Ingredients in Food: A Review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef]
- Teuling, E.; Schrama, J.W.; Gruppen, H.; Wierenga, P.A. Characterizing Emulsion Properties of Microalgal and Cyanobacterial Protein Isolates. Algal Res. 2019, 39, 101471. [Google Scholar] [CrossRef]
- Ai, X.; Yu, P.; Li, X.; Lai, X.; Yang, M.; Liu, F.; Luan, F.; Meng, X. Polysaccharides from Spirulina platensis: Extraction Methods, Structural Features and Bioactivities Diversity. Int. J. Biol. Macromol. 2023, 231, 123211. [Google Scholar] [CrossRef]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Microalgae as Feed Ingredients for Livestock Production and Meat Quality: A Review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Edelmann, M.; Aalto, S.; Chamlagain, B.; Kariluoto, S.; Piironen, V. Riboflavin, Niacin, Folate and Vitamin B12 in Commercial Microalgae Powders. J. Food Compos. Anal. 2019, 82, 103226. [Google Scholar] [CrossRef]
- Liber, J.A.; Bryson, A.E.; Bonito, G.; Du, Z.Y. Harvesting Microalgae for Food and Energy Products. Small Methods 2020, 4, 2000349. [Google Scholar] [CrossRef]
- Mendoza, Á.; Morales, V.; Sánchez-Bayo, A.; Rodríguez-Escudero, R.; González-Fernández, C.; Bautista, L.F.; Vicente, G. The Effect of the Lipid Extraction Method Used in Biodiesel Production on the Integrated Recovery of Biodiesel and Biogas from Nannochloropsis gaditana, Isochrysis galbana and Arthrospira platensis. Biochem. Eng. J. 2020, 154, 107428. [Google Scholar] [CrossRef]
- Rempel, A.; de Souza Sossella, F.; Margarites, A.C.; Astolfi, A.L.; Steinmetz, R.L.R.; Kunz, A.; Treichel, H.; Colla, L.M. Bioethanol from Spirulina platensis Biomass and the Use of Residuals to Produce Biomethane: An Energy Efficient Approach. Bioresour. Technol. 2019, 288, 121588. [Google Scholar] [CrossRef] [PubMed]
- Varol, A.; Ugurlu, A. Biogas Production from Microalgae (Spirulina platensis) in a Two Stage Anaerobic System. Waste Biomass Valorization 2016, 7, 193–200. [Google Scholar] [CrossRef]
- Bautista, E.G.; Laroche, C. Arthrospira platensis as a Feasible Feedstock for Bioethanol Production. Appl. Sci. 2021, 11, 6756. [Google Scholar] [CrossRef]
- Zaki, M.A.; Ashour, M.; Heneash, A.M.M.; Mabrouk, M.M.; Alprol, A.E.; Khairy, H.M.; Nour, A.M.; Mansour, A.T.; Hassanien, H.A.; Gaber, A.; et al. Potential Applications of Native Cyanobacterium Isolate (Arthrospira platensis NIOF17/003) for Biodiesel Production and Utilization of Its Byproduct in Marine Rotifer (Brachionus plicatilis) Production. Sustainaibility 2021, 13, 1769. [Google Scholar] [CrossRef]
- Chen, H.W.; Yang, T.S.; Chen, M.J.; Chang, Y.C.; Lin, C.Y.; Wang, E.I.C.; Ho, C.L.; Huang, K.M.; Yu, C.C.; Yang, F.L.; et al. Application of Power Plant Flue Gas in a Photobioreactor to Grow Spirulina Algae, and a Bioactivity Analysis of the Algal Water-Soluble Polysaccharides. Bioresour. Technol. 2012, 120, 256–263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).