Experimental and Kinetic Study of Biochar in N-Absorption Reaction of Chemical Looping Ammonia Generation
Abstract
:1. Introduction
2. Experiments
2.1. Feedstock
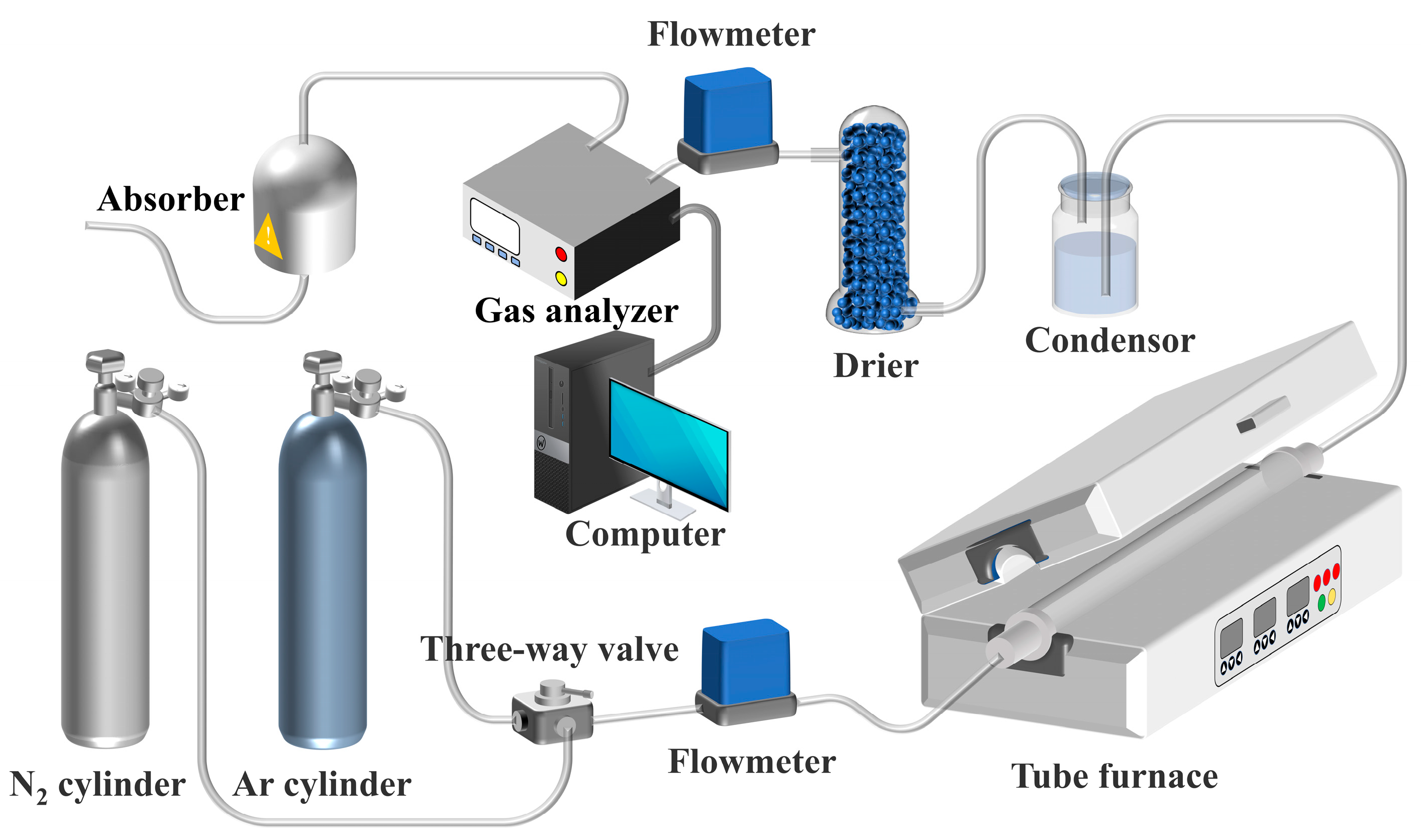
2.2. Experiment Process
2.3. Kinetic Calculation
3. Results and Discussion
3.1. Aspects Influencing Biochar Conversion Efficiency
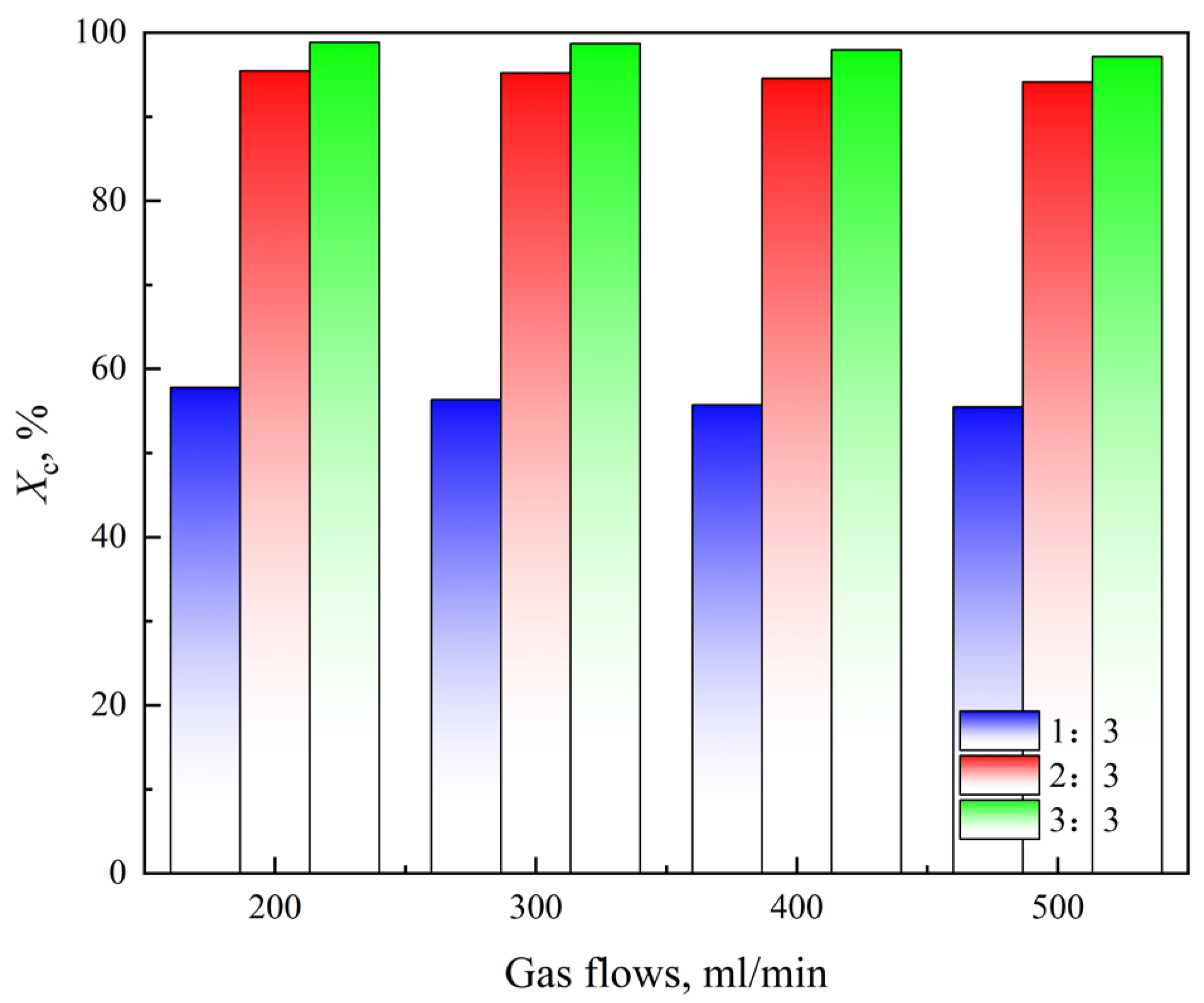
3.1.1. Gas Flow Rate
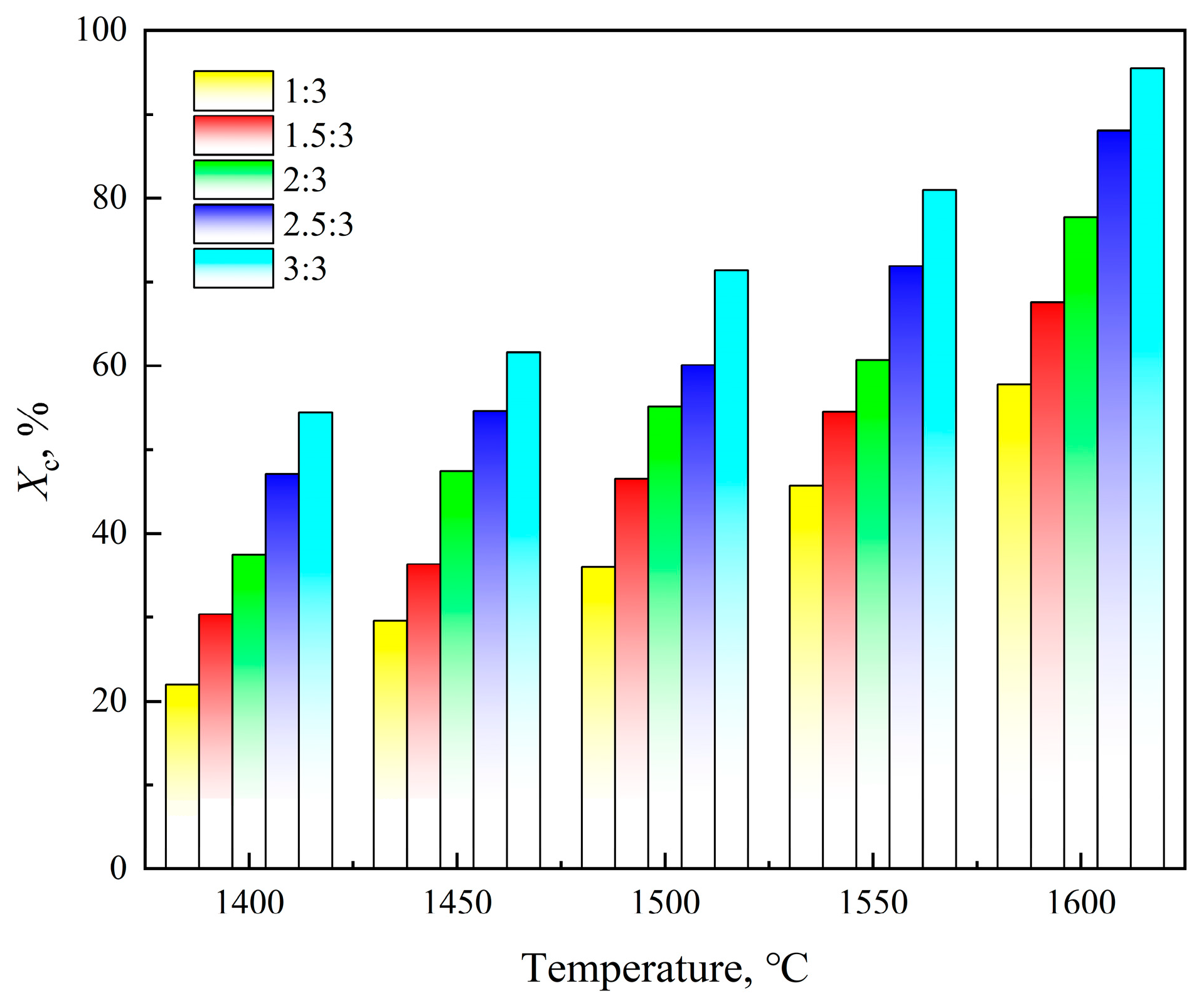
3.1.2. Reaction Temperature
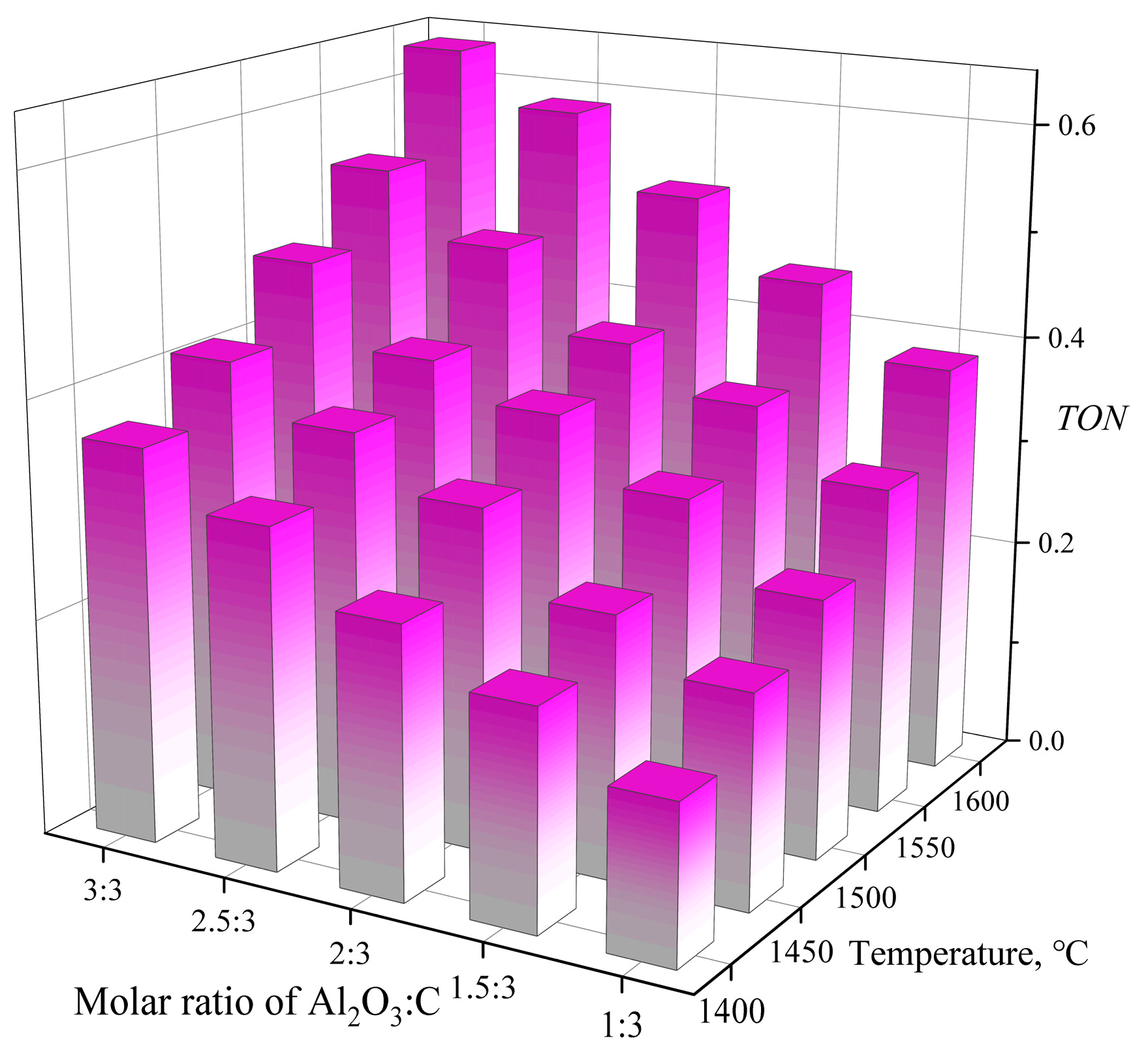
3.1.3. α-Al2O3/C Molar Ratio
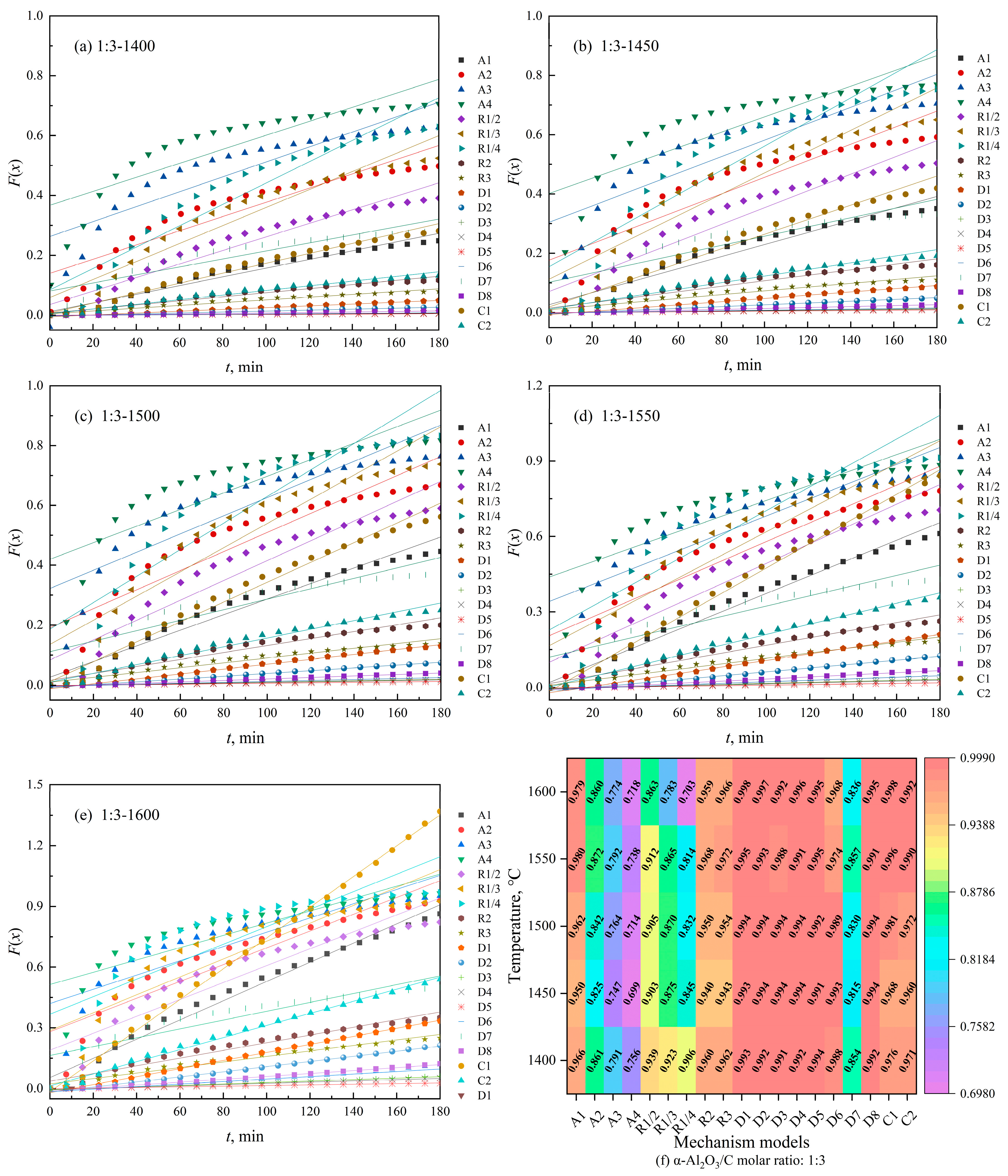
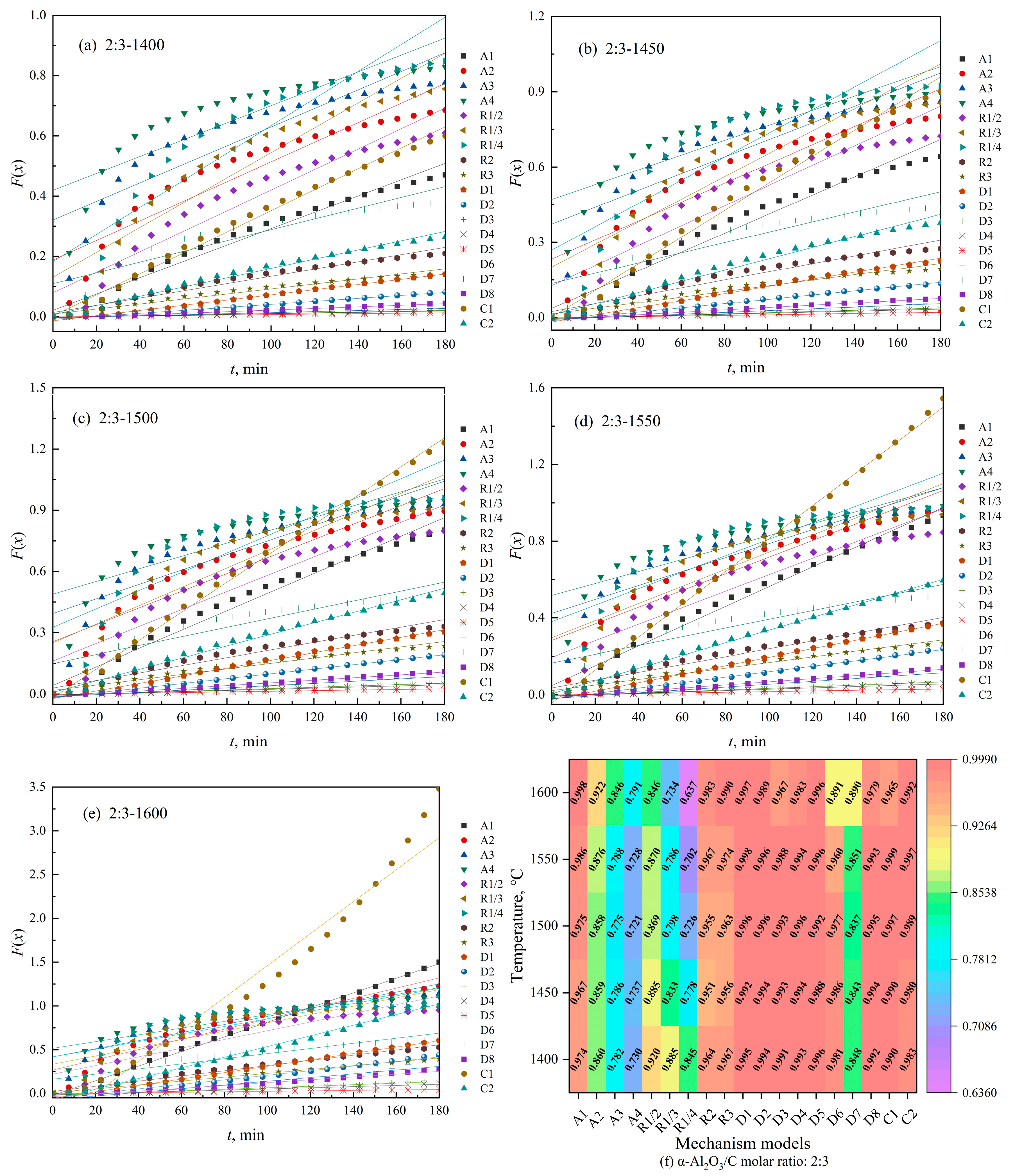
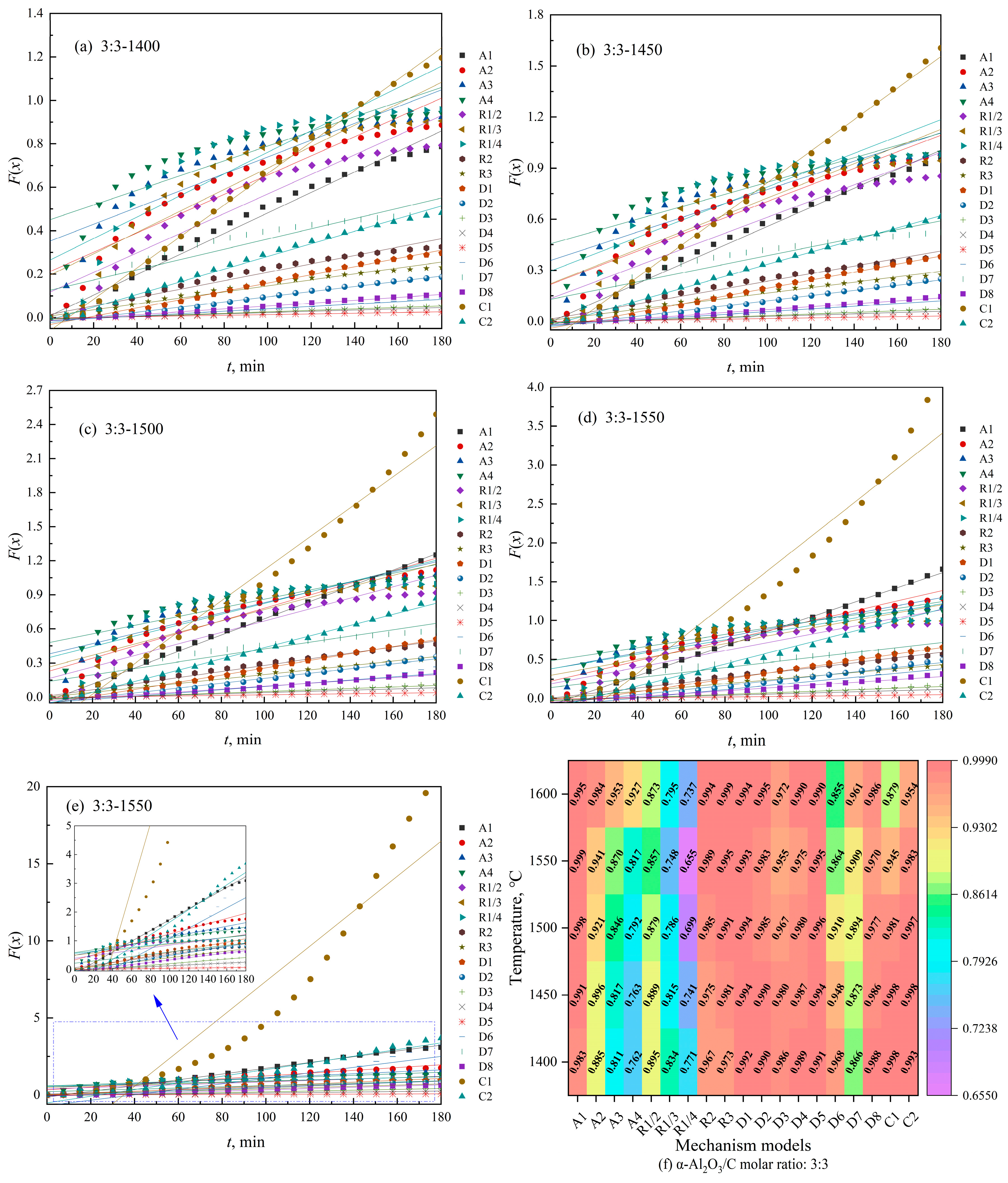
3.2. Kinetic Modeling of Biochar-Based N-Absorption Reaction
3.2.1. Kinetic Model of Biochar-Based N-Absorption Reaction
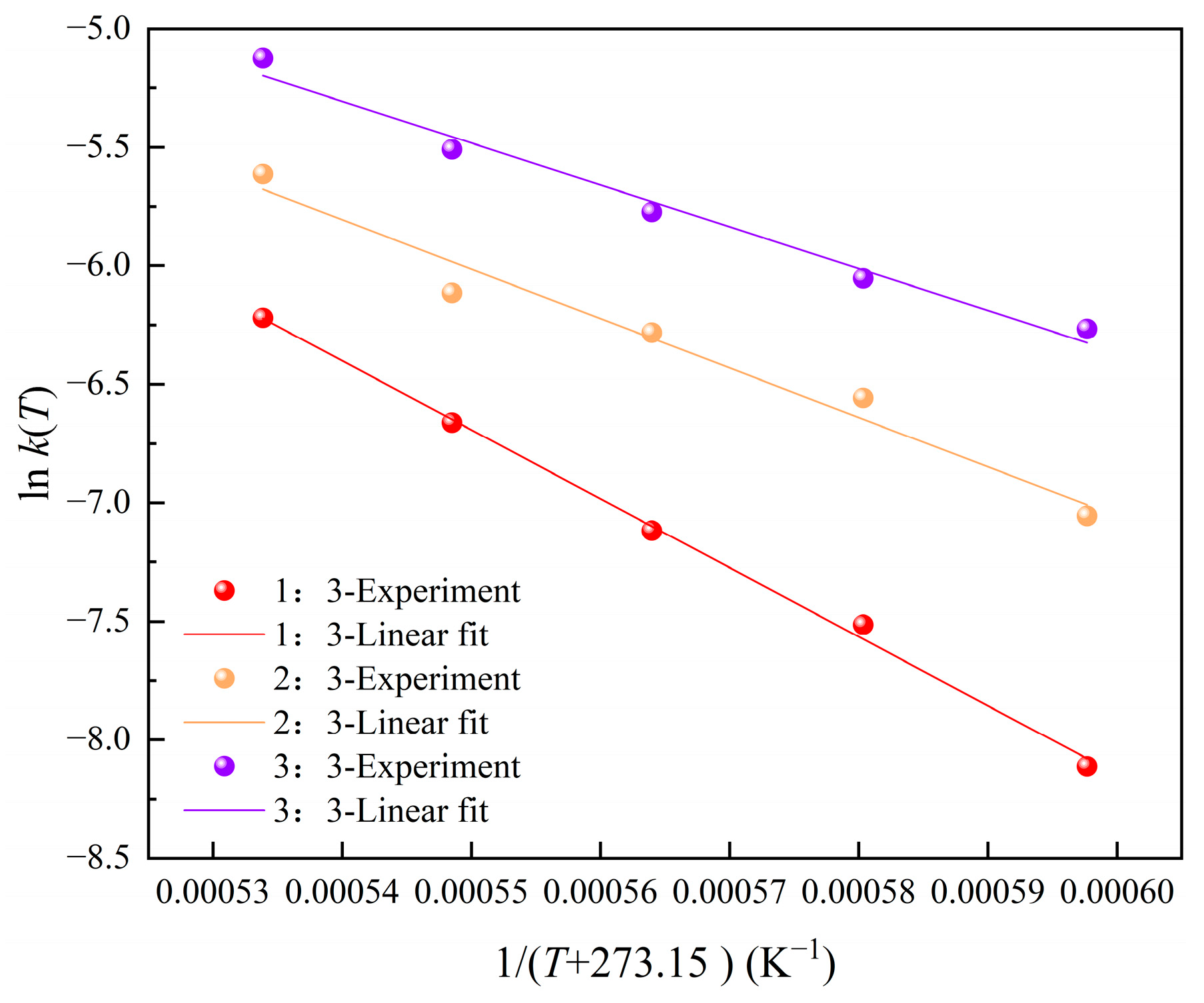
3.2.2. The Activation Energy and the Reaction Rate Coefficient
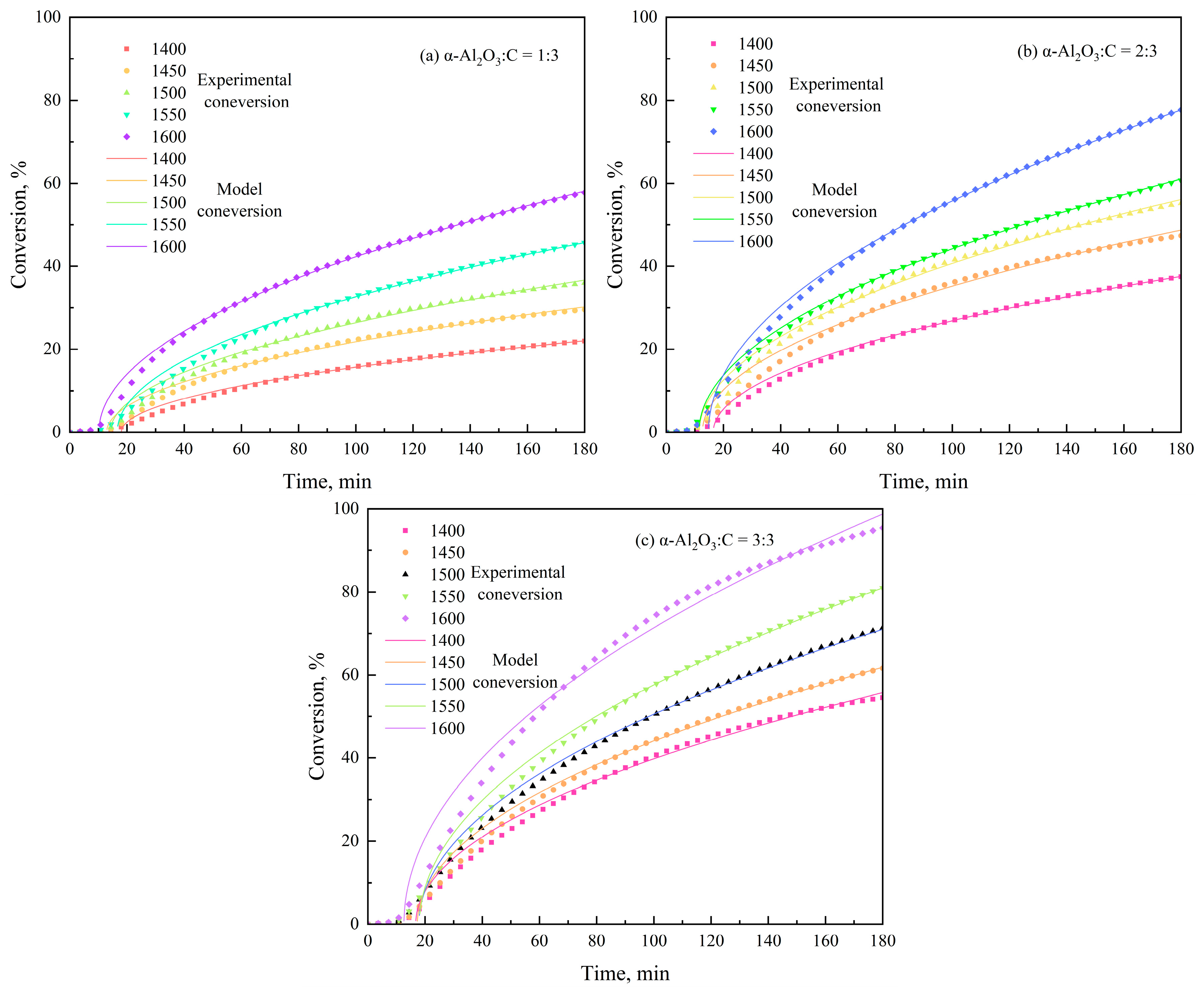
3.3. Interpretation of the Kinetic Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gezerman, A.O. A Critical Assessment of Green Ammonia Production and Ammonia Production Technologies. Kem. U Ind. 2022, 71, 57–66. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Z.; Wang, Y.; Yan, G.; Janz, B.; Wang, X.; Zhan, Y.; Wang, R.; Zheng, X.; Zhou, M.; et al. Characteristics of annual NH(3) emissions from a conventional vegetable field under various nitrogen management strategies. J. Environ. Manag. 2023, 342, 118276. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Yang, Q.; Chen, X.; Liu, G.; Zhao, Y.; Li, L. Performance evaluation of NH3/CO2 cascade refrigeration system with ejector subcooling for low-temperature cycle. Int. J. Refrig. 2022, 136, 162–171. [Google Scholar] [CrossRef]
- Penkuhn, M.; Tsatsaronis, G. Comparison of different ammonia synthesis loop configurations with the aid of advanced exergy analysis. Energy 2017, 137, 854–864. [Google Scholar] [CrossRef]
- Pingkuo, L.; Xue, H. Comparative analysis on similarities and differences of hydrogen energy development in the World’s top 4 largest economies: A novel framework. Int. J. Hydrogen Energy 2022, 47, 9485–9503. [Google Scholar] [CrossRef]
- Lesmana, H.; Zhang, Z.; Li, X.; Zhu, M.; Xu, W.; Zhang, D. NH3 as a transport fuel in internal combustion engines: A technical review. J. Energy Resour. Technol. 2019, 141, 070703. [Google Scholar] [CrossRef]
- Boretti, A.; Castelletto, S. NH3 Prospects in Combustion Engines and Fuel Cells for Commercial Aviation by 2030. ACS Energy Lett. 2022, 7, 2557–2564. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber-Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Vojvodic, A.; Medford, A.J.; Studt, F.; Abild-Pedersen, F.; Khan, T.S.; Bligaard, T.; Nørskov, J.K. Exploring the limits: A low-pressure, low-temperature Haber–Bosch process. Chem. Phys. Lett. 2014, 598, 108–112. [Google Scholar] [CrossRef]
- Rafiqul, I.; Weber, C.; Lehmann, B.; Voss, A. Energy efficiency improvements in ammonia production—Perspectives and uncertainties. Energy 2005, 30, 2487–2504. [Google Scholar] [CrossRef]
- Galvez, M.E.; Halmann, M.; Steinfeld, A. Ammonia production via a two-step Al2O3/AlN thermochemical cycle. 1. Thermodynamic, environmental, and economic analyses. Ind. Eng. Chem. Res. 2007, 46, 2042–2046. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, Y.; Zhang, Q.; Cai, T.; Chen, X.; Liu, D.; Fan, M. Promising zirconia-mixed Al-based nitrogen carriers for chemical looping of NH3: Reduced NH3 decomposition and improved NH3 yield. Fuel 2020, 264, 116821. [Google Scholar] [CrossRef]
- Wang, X.; Su, M.; Zhao, H. Process design and exergy cost analysis of a chemical looping ammonia generation system using AlN/Al2O3 as a nitrogen carrier. Energy 2021, 230, 120767. [Google Scholar] [CrossRef]
- Weng, Q.; Toan, S.; Ai, R.; Sun, Z.; Sun, Z. Ammonia production from biomass via a chemical looping-based hybrid system. J. Clean. Prod. 2021, 289, 125749. [Google Scholar] [CrossRef]
- Forslund, B.; Zheng, J. Carbothermal synthesis of aluminium nitride at elevated nitrogen pressures: Part I Effect of process parameters on conversion rate. J. Mater. Sci. 1993, 28, 3125–3131. [Google Scholar] [CrossRef]
- Forslund, B.; Zheng, J. Carbothermal synthesis of aluminium nitride at elevated nitrogen pressures: Part II Effect of process parameters on particle size and morphology. J. Mater. Sci. 1993, 28, 3132–3136. [Google Scholar] [CrossRef]
- Lefort, P.; Billy, M. Mechanism of AlN formation through the carbothermal reduction of Al2O3 in a flowing N2 atmosphere. J. Am. Ceram. Soc. 1993, 76, 2295–2299. [Google Scholar] [CrossRef]
- Gálvez, M.; Hischier, I.; Frei, A.; Steinfeld, A. Ammonia Production via a Two-Step Al2O3/AlN Thermochemical Cycle. 3. Influence of the Carbon Reducing Agent and Cyclability. Ind. Eng. Chem. Res. 2008, 47, 2231–2237. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Gao, Y.; Chen, X.; Liu, D.; Fan, M. High-performance mesoporous (AlN/Al2O3) for enhanced NH3 yield during chemical looping ammonia generation technology. Int. J. Hydrogen Energy 2020, 45, 9903–9913. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, Q.; Wu, Y.; Liu, D. Using Coal Coke for N-Sorption with an Al-based Nitrogen Carrier during Chemical Looping Ammonia Generation. Energy Fuels 2020, 34, 12527–12534. [Google Scholar] [CrossRef]
- Komeya, K.; Mitsuhashi, E.; Meguro, T. Synthesis of AlN Powder by Carbothermal Reduction-Nitridation Method Effect of Additives on Reaction Rate. J. Ceram. Soc. Jpn. 1993, 101, 377–382. [Google Scholar] [CrossRef]
- Variny, M.; Varga, A.; Rimár, M.; Janošovský, J.; Kizek, J.; Lukáč, L.; Jablonský, G.; Mierka, O. Advances in Biomass Co-Combustion with Fossil Fuels in the European Context: A Review. Processes 2021, 9, 100. [Google Scholar] [CrossRef]
- Kang, Q.; Appels, L.; Tan, T.; Dewil, R. Bioethanol from Lignocellulosic Biomass: Current Findings Determine Research Priorities. Sci. World J. 2014, 2014, 298153. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Yang, C.D.; Liu, J.J.; Ying, H.C.; Lu, S.G. Soil pore structure changes induced by biochar affect microbial diversity and community structure in an Ultisol. Soil Tillage Res. 2022, 224, 10. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Gao, J.; Zhao, J.; Duan, W. Effect of pyrolysis parameters on the biochar reactivity in the N-absorption reaction of chemical looping ammonia generation. Energy 2024, 310, 133321. [Google Scholar] [CrossRef]
- Galvez, M.E.; Frei, A.; Halmann, M.; Steinfeld, A. Ammonia production via a two-step Al2O3/AlN thermochemical cycle. 2. Kinetic analysis. Ind. Eng. Chem. Res. 2007, 46, 2047–2053. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Wang, H.; Wu, J.; Tao, S. Selecting nitrogen carriers used for chemical looping ammonia generation of biomass and H2O by thermodynamic method. Int. J. Hydrogen Energy 2023, 48, 4035–4051. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y.; Zhang, Q.; Chen, X.; Jiang, G.; Liu, D. N-desorption or NH3 generation of TiO2-loaded Al-based nitrogen carrier during chemical looping ammonia generation technology. Int. J. Hydrogen Energy 2018, 43, 16589–16597. [Google Scholar] [CrossRef]
- Duan, W.; Yu, Q.; Liu, J.; Wu, T.; Yang, F.; Qin, Q. Experimental and kinetic study of steam gasification of low-rank coal in molten blast furnace slag. Energy 2016, 111, 859–868. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Wang, K.; Xie, H.; Qin, Q. Kinetic characterizations of biomass char CO2 -gasification reaction within granulated blast furnace slag. Int. J. Hydrogen Energy 2017, 42, 20520–20528. [Google Scholar] [CrossRef]
- Doǧu, T. The importance of pore structure and diffusion in the kinetics of gas-solid non-catalytic reactions: Reaction of calcined limestone with SO2. Chem. Eng. J. 1981, 21, 213–222. [Google Scholar] [CrossRef]
- Bar-Ziv, E.; Kantorovich, I.I. Mutual effects of porosity and reactivity in char oxidation. Prog. Energy Combust. Sci. 2001, 27, 667–697. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, J.-I.; Vithanage, M.; Park, Y.-K.; Lee, J.; Kwon, E.E. Modification of biochar properties using CO2. Chem. Eng. J. 2019, 372, 383–389. [Google Scholar] [CrossRef]



| Ultimate Analysis (d.b.) | wt% | Pore Structure | |
|---|---|---|---|
| C | 96.5 | SBET (m2/g) | 341.0305 |
| H | 1.2 | Smic (m2/g) | 308.4343 |
| O (by difference) | 1.84 | Sext (m2/g) | 32.5962 |
| N | 0.45 | Dave (nm) | 1.8016 |
| S | 0.01 | Vmic (cm3/g) | 0.1213 |
| Vtotal (cm3/g) | 0.1536 | ||
| Vmic/Vtotal | 0.7897 |
| Code | Reaction Model | Differential f(x) | Integral F(x) |
|---|---|---|---|
| Am | Nucleus production model | ||
| A1 | m = 1 | 1 − x | −ln(1 − x) |
| A2 | m = 2 | 2(1 − x)[−ln(1 − x)]1/2 | [−ln(1 − x)]1/2 |
| A3 | m = 3 | 3(1 − x)[−ln(1 − x)]2/3 | [−ln(1 − x)]1/3 |
| A4 | m = 4 | 4(1 − x)[−ln(1 − x)]1/4 | [−ln(1 − x)]1/4 |
| Rm | Shrinking core model | ||
| R1/2 | m = 1/2 | (1/2)(1 − x)−1 | 1 − (1 − x)2 |
| R1/3 | m = 1/3 | (1/3)(1 − x)−2 | 1 − (1 − x)3 |
| R1/4 | m = 1/4 | (1/4)(1 − x)−3 | 1 − (1 − x)4 |
| R2 | m = 2 | 2(1 − x)1/2 | 1 − (1 − x)1/2 |
| R3 | m = 3 | 3(1 − x)2/3 | 1 − (1 − x)1/3 |
| Dm | Dimensional diffusion model | ||
| D1 | Dimensional diffusion | 1/2x−1 | x2 |
| D2 | Two-dimensional diffusion | [−ln(1 − x)]−1 | x + (1 − x)ln(1 − x) |
| D3 | Three-dimensional diffusion | (3/2)(1 − x)2/3[1 − (1 − x)1/3]−1 | [1 − (1 − x)1/3]2 |
| D4 | Three-dimensional diffusion | (3/2)[(1 − x)−1/3 − 1]−1 | 1 − 2/3x − (1 − x)2/3 |
| D5 | 3-D (Anti-Jander) | (3/2)(1 + x)2/3[(1 + x)1/3 − 1]−1 | [(1 + x)1/3 − 1]2 |
| D6 | 3-D (ZLT) | (3/2)(1 − x)4/3[(1 − x)−1/3 − 1]−1 | [(1 − x)−1/3 − 1]2 |
| D7 | 3-D (Jander) | 6(1 − x)2/3[1 − (1 − x)1/3]1/2 | [1 − (1 − x)1/3]1/2 |
| D8 | 2-D (Jander) | (1 − x)1/2[1 − (1 − x)1/2]−1 | [1 − (1 − x)1/2]2 |
| Cn | Phase boundary reaction | ||
| C1 | Reaction order: n = 2 | (1 − x)2 | (1 − x)−1 − 1 |
| C2 | Reaction order: n = 3/2 | 2(1 − x)3/2 | (1 − x)−1/2 − 1 |
| α-Al2O3/C Molar Ratio | Intercept | Slope | Ea (kJ/mol) | k0 (min−1) | R2 |
|---|---|---|---|---|---|
| 1:3 | 9.31 | −29,096.66 | 241.91 | 11,061.43 | 0.99754 |
| 2:3 | 5.45 | −20,850.46 | 173.35 | 233.58 | 0.97167 |
| 3:3 | 4.23 | −17,653.34 | 146.77 | 68.49 | 0.98175 |
| α-Al2O3/C Molar Ratio | Kinetic Equations of the Biochar-Based N-Absorption Reaction |
|---|---|
| 1:3 | |
| 2:3 | |
| 3:3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yu, Q.; Xie, H.; Gao, J.; Zhao, J. Experimental and Kinetic Study of Biochar in N-Absorption Reaction of Chemical Looping Ammonia Generation. Processes 2024, 12, 2870. https://doi.org/10.3390/pr12122870
Liu Z, Yu Q, Xie H, Gao J, Zhao J. Experimental and Kinetic Study of Biochar in N-Absorption Reaction of Chemical Looping Ammonia Generation. Processes. 2024; 12(12):2870. https://doi.org/10.3390/pr12122870
Chicago/Turabian StyleLiu, Zhongyuan, Qingbo Yu, Huaqing Xie, Jinchao Gao, and Jiatai Zhao. 2024. "Experimental and Kinetic Study of Biochar in N-Absorption Reaction of Chemical Looping Ammonia Generation" Processes 12, no. 12: 2870. https://doi.org/10.3390/pr12122870
APA StyleLiu, Z., Yu, Q., Xie, H., Gao, J., & Zhao, J. (2024). Experimental and Kinetic Study of Biochar in N-Absorption Reaction of Chemical Looping Ammonia Generation. Processes, 12(12), 2870. https://doi.org/10.3390/pr12122870






