Experimental and Kinetic Study of Biochar in N-Absorption Reaction of Chemical Looping Ammonia Generation
Abstract
1. Introduction
2. Experiments
2.1. Feedstock
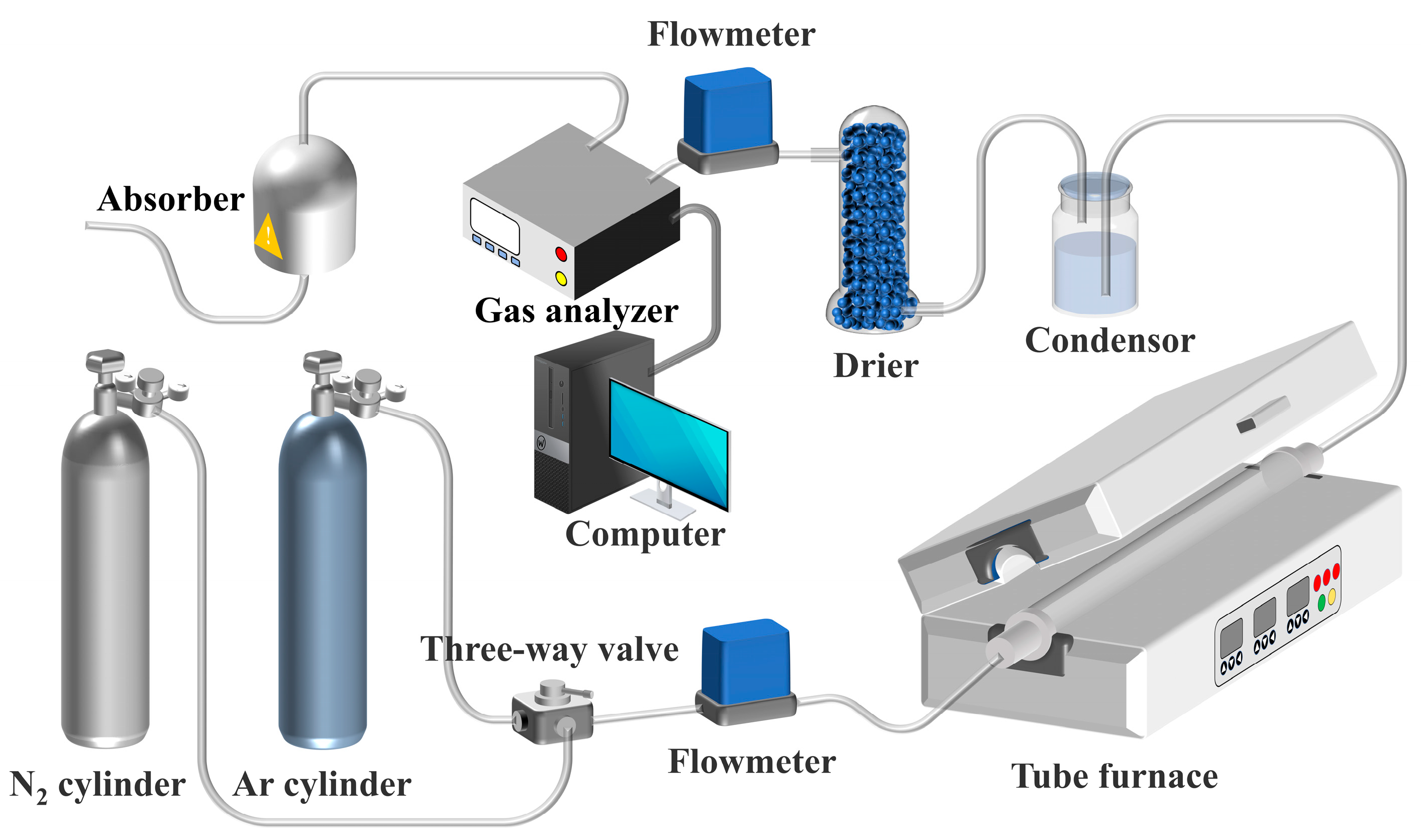
2.2. Experiment Process
2.3. Kinetic Calculation
3. Results and Discussion
3.1. Aspects Influencing Biochar Conversion Efficiency
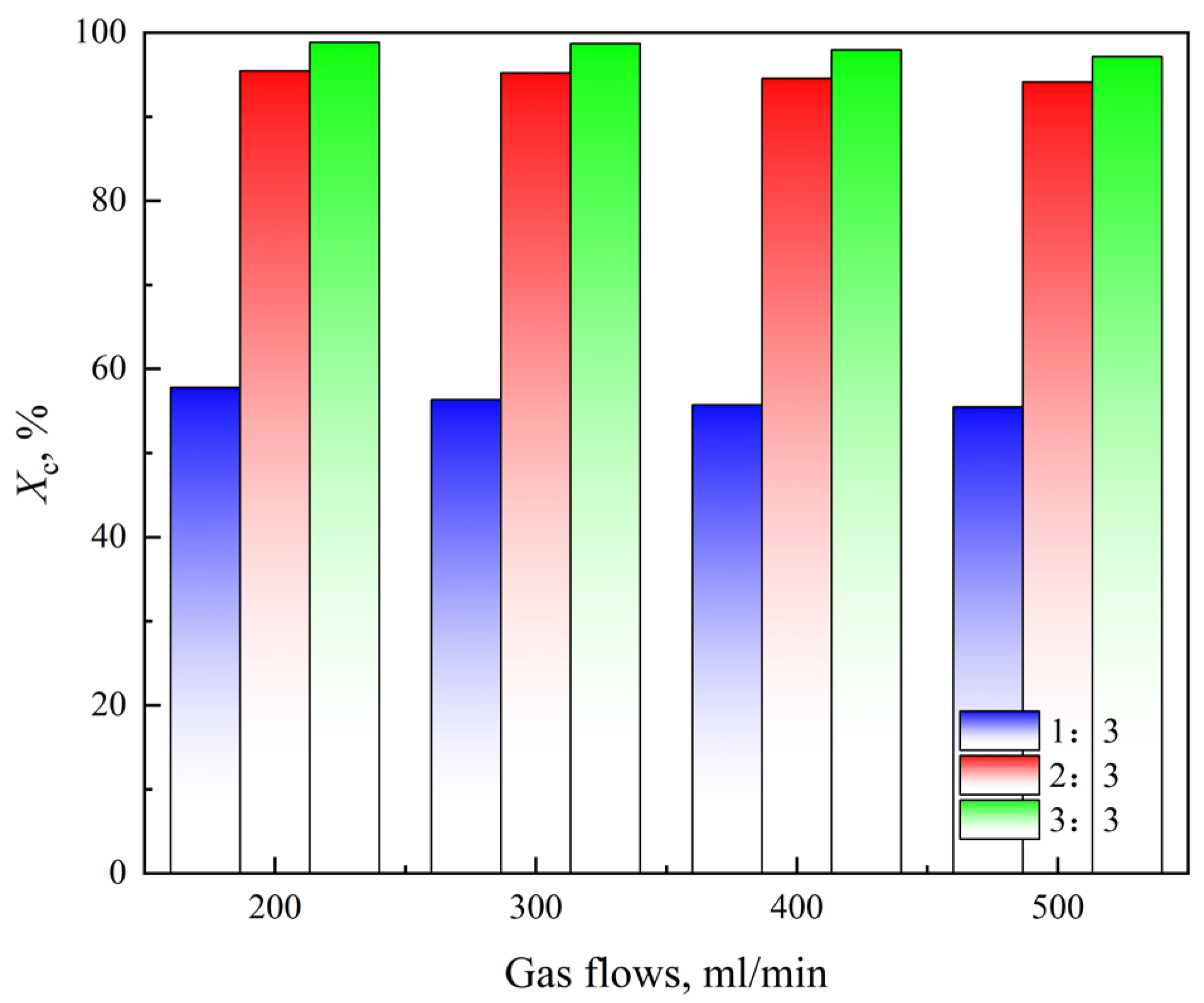
3.1.1. Gas Flow Rate
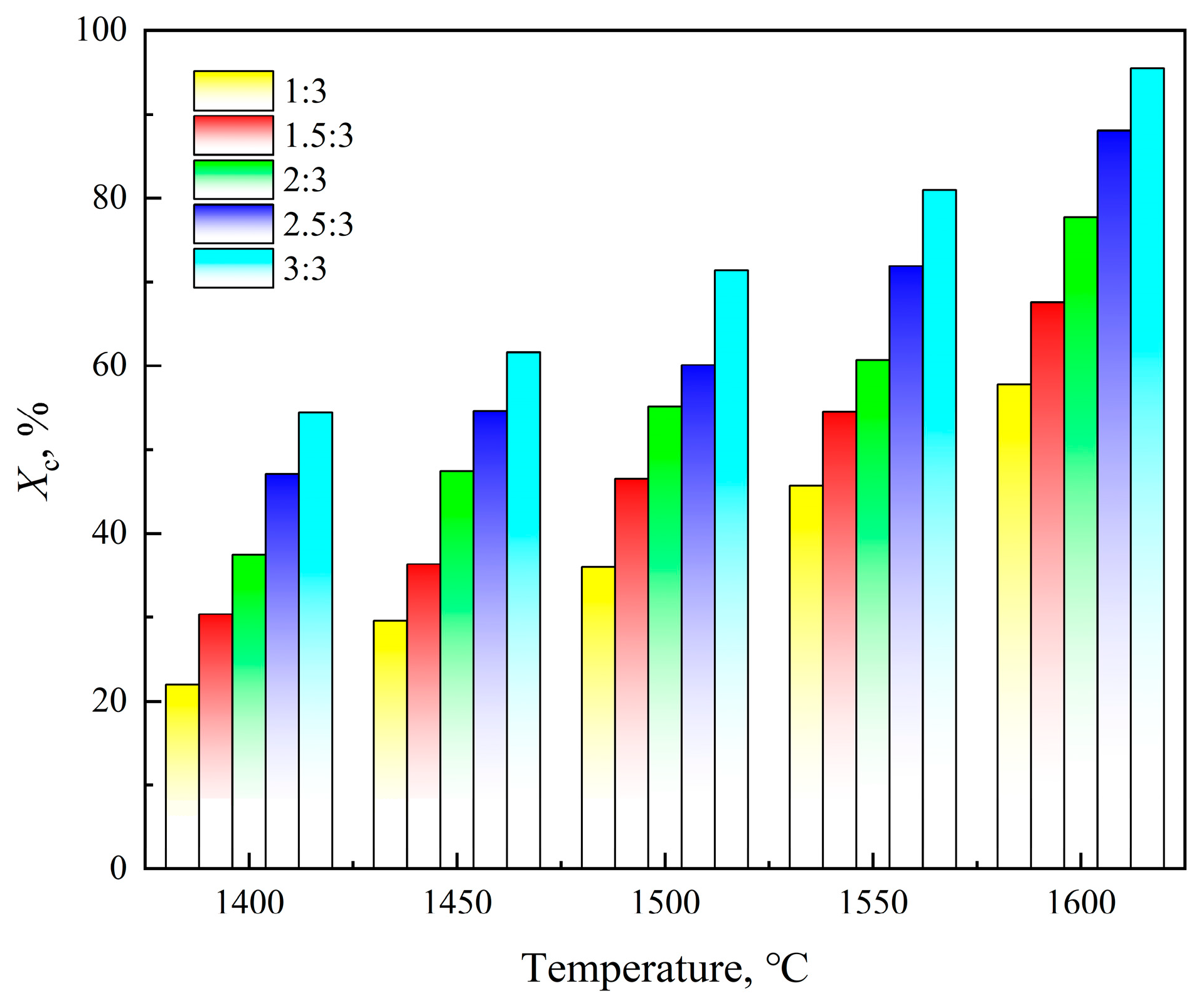
3.1.2. Reaction Temperature
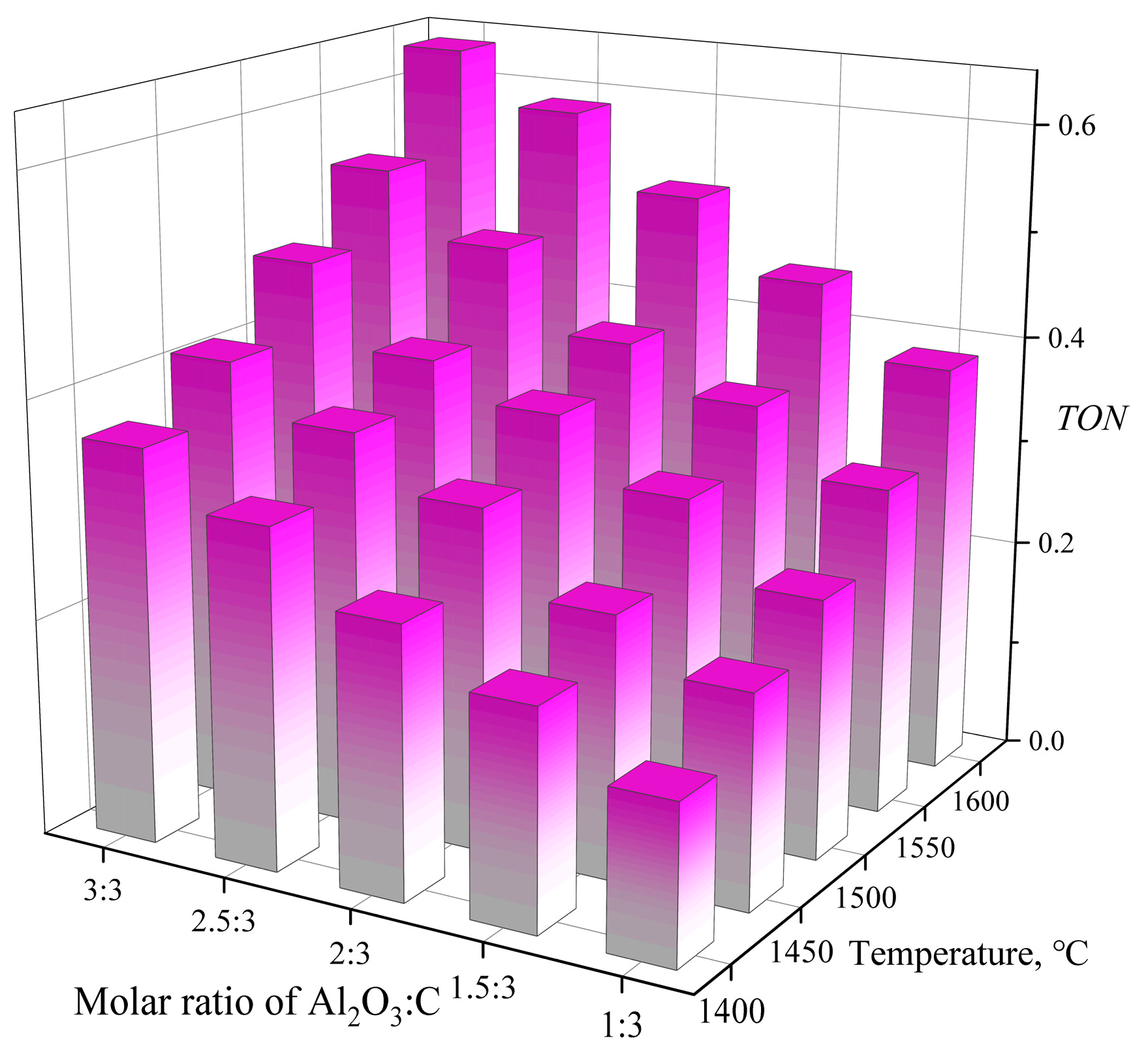
3.1.3. α-Al2O3/C Molar Ratio
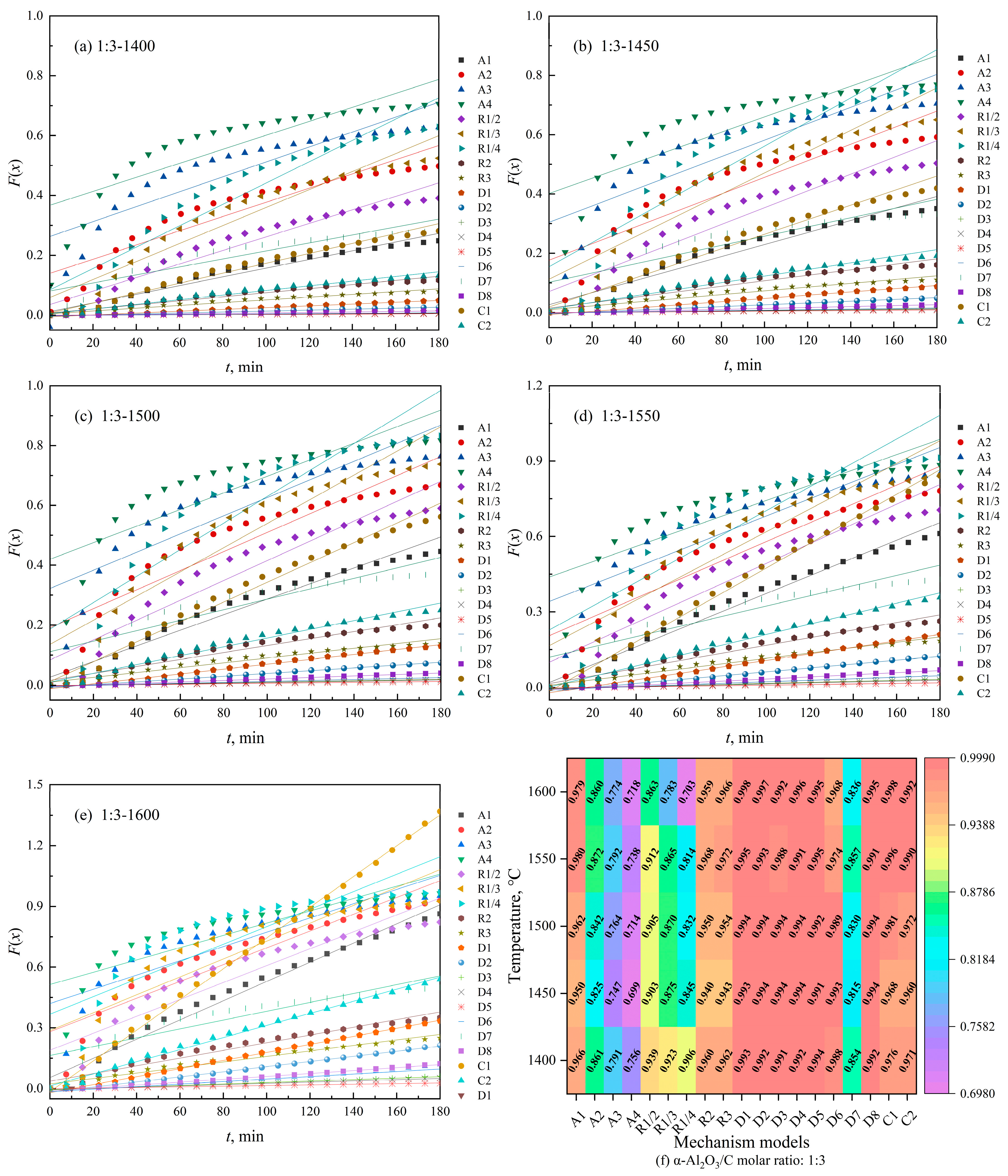
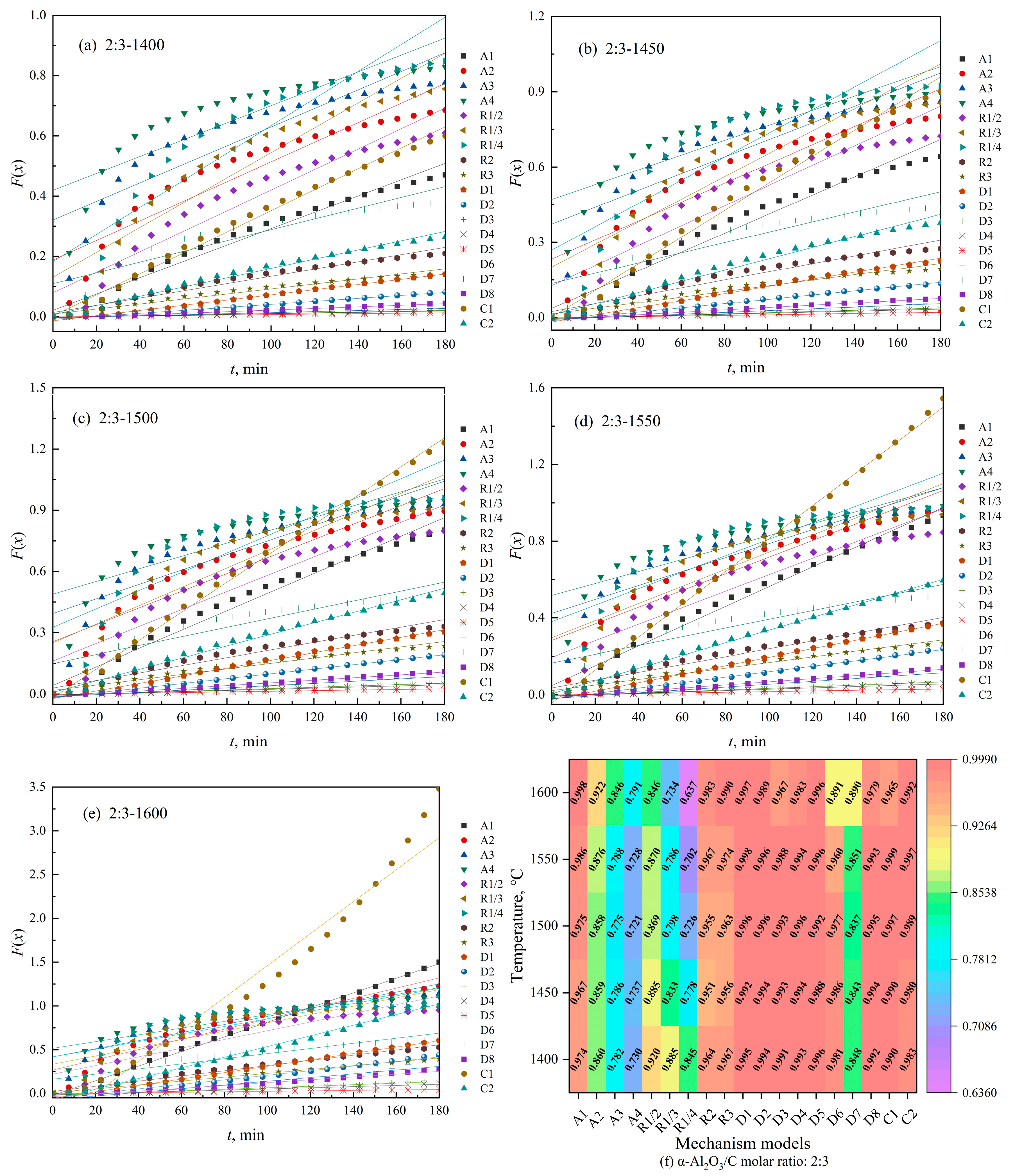
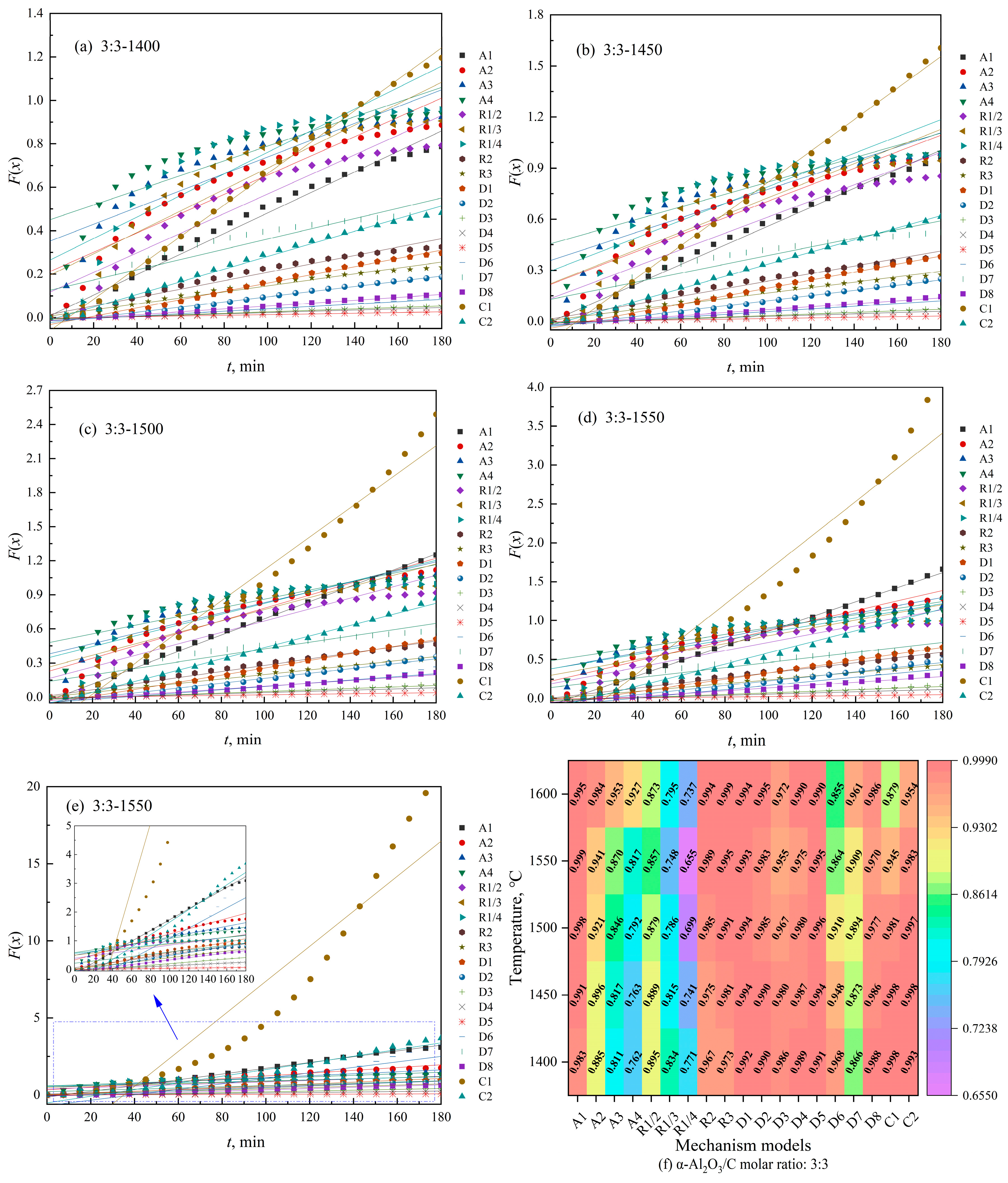
3.2. Kinetic Modeling of Biochar-Based N-Absorption Reaction
3.2.1. Kinetic Model of Biochar-Based N-Absorption Reaction
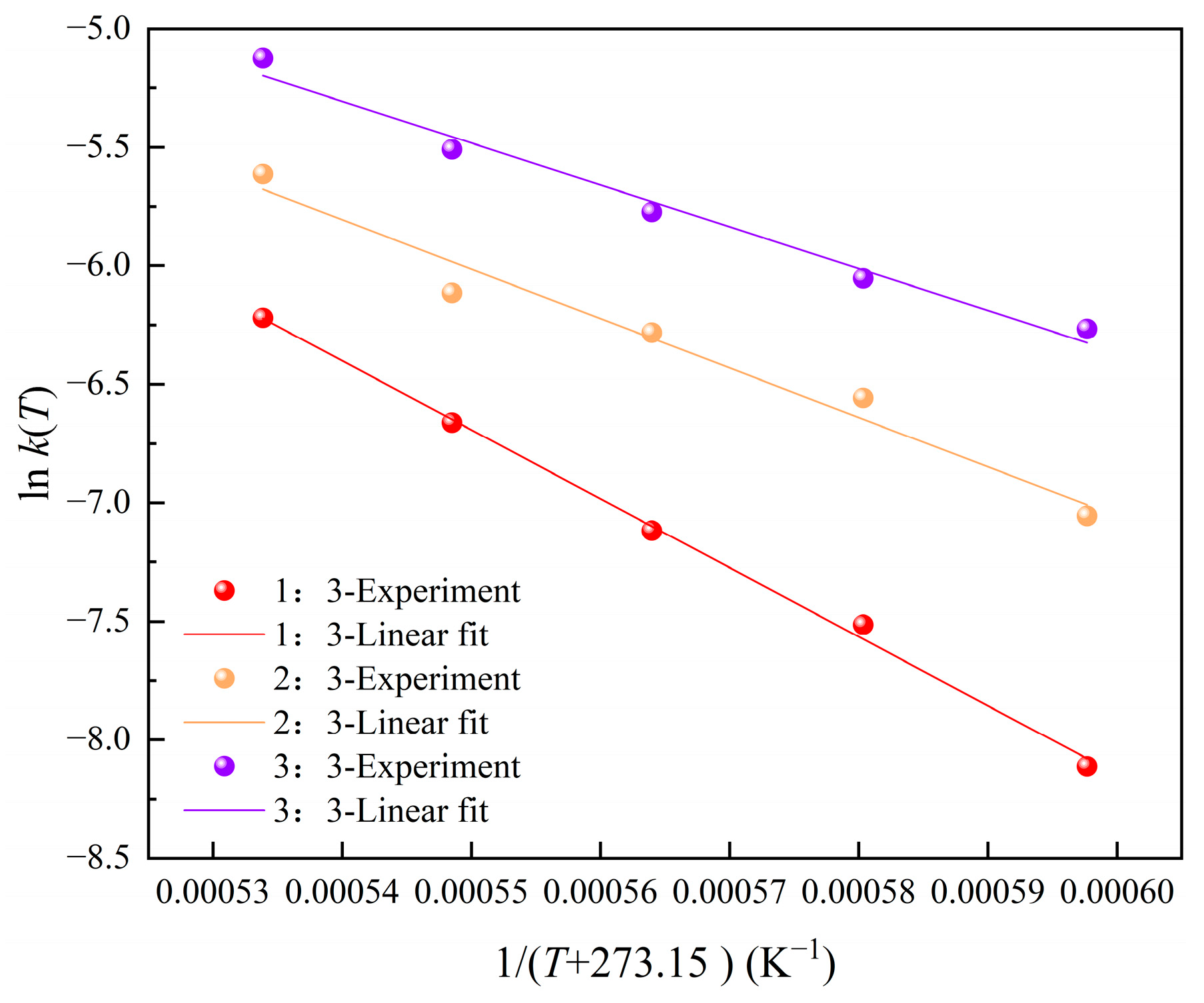
3.2.2. The Activation Energy and the Reaction Rate Coefficient
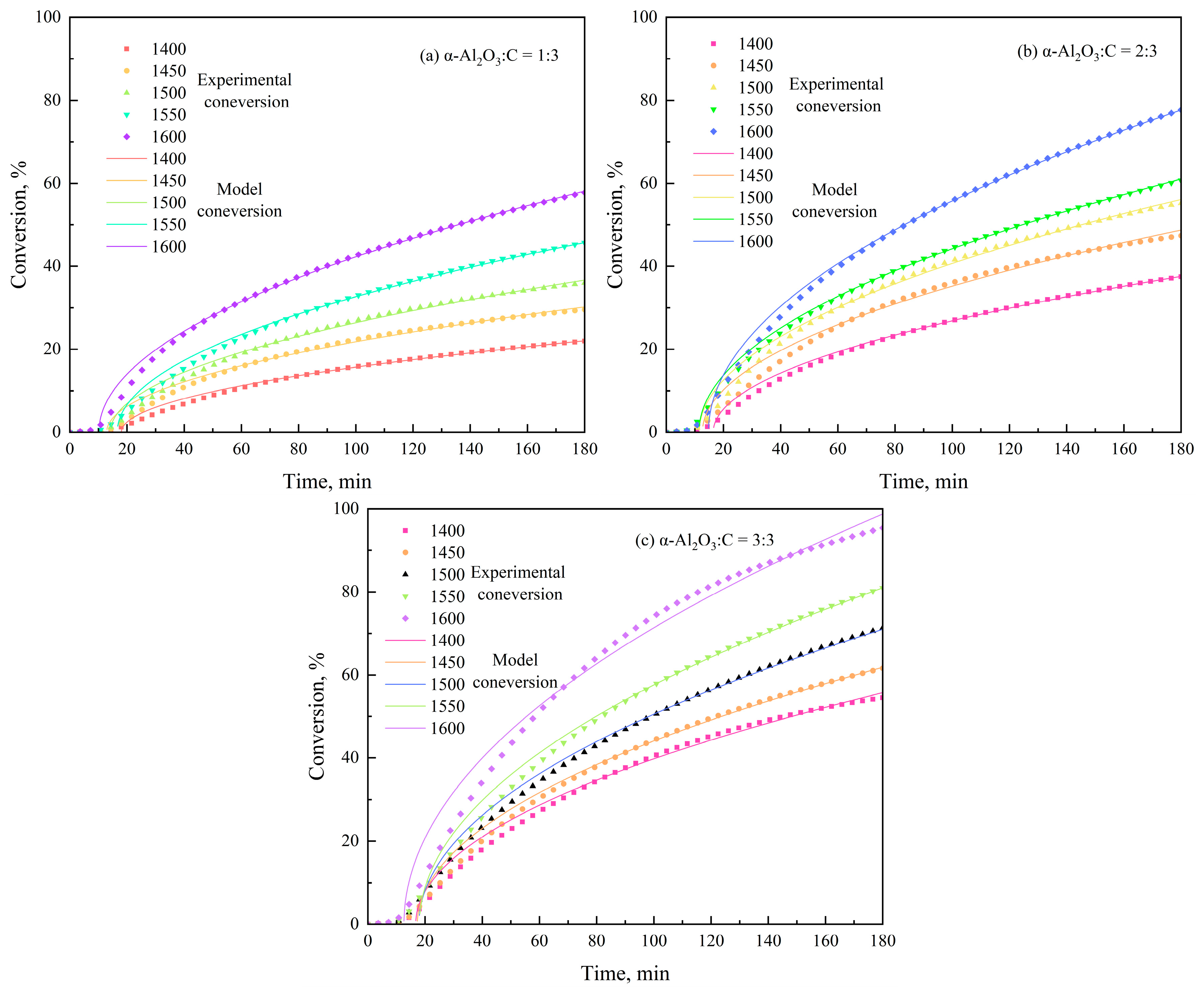
3.3. Interpretation of the Kinetic Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gezerman, A.O. A Critical Assessment of Green Ammonia Production and Ammonia Production Technologies. Kem. U Ind. 2022, 71, 57–66. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Z.; Wang, Y.; Yan, G.; Janz, B.; Wang, X.; Zhan, Y.; Wang, R.; Zheng, X.; Zhou, M.; et al. Characteristics of annual NH(3) emissions from a conventional vegetable field under various nitrogen management strategies. J. Environ. Manag. 2023, 342, 118276. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Yang, Q.; Chen, X.; Liu, G.; Zhao, Y.; Li, L. Performance evaluation of NH3/CO2 cascade refrigeration system with ejector subcooling for low-temperature cycle. Int. J. Refrig. 2022, 136, 162–171. [Google Scholar] [CrossRef]
- Penkuhn, M.; Tsatsaronis, G. Comparison of different ammonia synthesis loop configurations with the aid of advanced exergy analysis. Energy 2017, 137, 854–864. [Google Scholar] [CrossRef]
- Pingkuo, L.; Xue, H. Comparative analysis on similarities and differences of hydrogen energy development in the World’s top 4 largest economies: A novel framework. Int. J. Hydrogen Energy 2022, 47, 9485–9503. [Google Scholar] [CrossRef]
- Lesmana, H.; Zhang, Z.; Li, X.; Zhu, M.; Xu, W.; Zhang, D. NH3 as a transport fuel in internal combustion engines: A technical review. J. Energy Resour. Technol. 2019, 141, 070703. [Google Scholar] [CrossRef]
- Boretti, A.; Castelletto, S. NH3 Prospects in Combustion Engines and Fuel Cells for Commercial Aviation by 2030. ACS Energy Lett. 2022, 7, 2557–2564. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber-Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Vojvodic, A.; Medford, A.J.; Studt, F.; Abild-Pedersen, F.; Khan, T.S.; Bligaard, T.; Nørskov, J.K. Exploring the limits: A low-pressure, low-temperature Haber–Bosch process. Chem. Phys. Lett. 2014, 598, 108–112. [Google Scholar] [CrossRef]
- Rafiqul, I.; Weber, C.; Lehmann, B.; Voss, A. Energy efficiency improvements in ammonia production—Perspectives and uncertainties. Energy 2005, 30, 2487–2504. [Google Scholar] [CrossRef]
- Galvez, M.E.; Halmann, M.; Steinfeld, A. Ammonia production via a two-step Al2O3/AlN thermochemical cycle. 1. Thermodynamic, environmental, and economic analyses. Ind. Eng. Chem. Res. 2007, 46, 2042–2046. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, Y.; Zhang, Q.; Cai, T.; Chen, X.; Liu, D.; Fan, M. Promising zirconia-mixed Al-based nitrogen carriers for chemical looping of NH3: Reduced NH3 decomposition and improved NH3 yield. Fuel 2020, 264, 116821. [Google Scholar] [CrossRef]
- Wang, X.; Su, M.; Zhao, H. Process design and exergy cost analysis of a chemical looping ammonia generation system using AlN/Al2O3 as a nitrogen carrier. Energy 2021, 230, 120767. [Google Scholar] [CrossRef]
- Weng, Q.; Toan, S.; Ai, R.; Sun, Z.; Sun, Z. Ammonia production from biomass via a chemical looping-based hybrid system. J. Clean. Prod. 2021, 289, 125749. [Google Scholar] [CrossRef]
- Forslund, B.; Zheng, J. Carbothermal synthesis of aluminium nitride at elevated nitrogen pressures: Part I Effect of process parameters on conversion rate. J. Mater. Sci. 1993, 28, 3125–3131. [Google Scholar] [CrossRef]
- Forslund, B.; Zheng, J. Carbothermal synthesis of aluminium nitride at elevated nitrogen pressures: Part II Effect of process parameters on particle size and morphology. J. Mater. Sci. 1993, 28, 3132–3136. [Google Scholar] [CrossRef]
- Lefort, P.; Billy, M. Mechanism of AlN formation through the carbothermal reduction of Al2O3 in a flowing N2 atmosphere. J. Am. Ceram. Soc. 1993, 76, 2295–2299. [Google Scholar] [CrossRef]
- Gálvez, M.; Hischier, I.; Frei, A.; Steinfeld, A. Ammonia Production via a Two-Step Al2O3/AlN Thermochemical Cycle. 3. Influence of the Carbon Reducing Agent and Cyclability. Ind. Eng. Chem. Res. 2008, 47, 2231–2237. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Gao, Y.; Chen, X.; Liu, D.; Fan, M. High-performance mesoporous (AlN/Al2O3) for enhanced NH3 yield during chemical looping ammonia generation technology. Int. J. Hydrogen Energy 2020, 45, 9903–9913. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, Q.; Wu, Y.; Liu, D. Using Coal Coke for N-Sorption with an Al-based Nitrogen Carrier during Chemical Looping Ammonia Generation. Energy Fuels 2020, 34, 12527–12534. [Google Scholar] [CrossRef]
- Komeya, K.; Mitsuhashi, E.; Meguro, T. Synthesis of AlN Powder by Carbothermal Reduction-Nitridation Method Effect of Additives on Reaction Rate. J. Ceram. Soc. Jpn. 1993, 101, 377–382. [Google Scholar] [CrossRef][Green Version]
- Variny, M.; Varga, A.; Rimár, M.; Janošovský, J.; Kizek, J.; Lukáč, L.; Jablonský, G.; Mierka, O. Advances in Biomass Co-Combustion with Fossil Fuels in the European Context: A Review. Processes 2021, 9, 100. [Google Scholar] [CrossRef]
- Kang, Q.; Appels, L.; Tan, T.; Dewil, R. Bioethanol from Lignocellulosic Biomass: Current Findings Determine Research Priorities. Sci. World J. 2014, 2014, 298153. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Yang, C.D.; Liu, J.J.; Ying, H.C.; Lu, S.G. Soil pore structure changes induced by biochar affect microbial diversity and community structure in an Ultisol. Soil Tillage Res. 2022, 224, 10. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Gao, J.; Zhao, J.; Duan, W. Effect of pyrolysis parameters on the biochar reactivity in the N-absorption reaction of chemical looping ammonia generation. Energy 2024, 310, 133321. [Google Scholar] [CrossRef]
- Galvez, M.E.; Frei, A.; Halmann, M.; Steinfeld, A. Ammonia production via a two-step Al2O3/AlN thermochemical cycle. 2. Kinetic analysis. Ind. Eng. Chem. Res. 2007, 46, 2047–2053. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Wang, H.; Wu, J.; Tao, S. Selecting nitrogen carriers used for chemical looping ammonia generation of biomass and H2O by thermodynamic method. Int. J. Hydrogen Energy 2023, 48, 4035–4051. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y.; Zhang, Q.; Chen, X.; Jiang, G.; Liu, D. N-desorption or NH3 generation of TiO2-loaded Al-based nitrogen carrier during chemical looping ammonia generation technology. Int. J. Hydrogen Energy 2018, 43, 16589–16597. [Google Scholar] [CrossRef]
- Duan, W.; Yu, Q.; Liu, J.; Wu, T.; Yang, F.; Qin, Q. Experimental and kinetic study of steam gasification of low-rank coal in molten blast furnace slag. Energy 2016, 111, 859–868. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Wang, K.; Xie, H.; Qin, Q. Kinetic characterizations of biomass char CO2 -gasification reaction within granulated blast furnace slag. Int. J. Hydrogen Energy 2017, 42, 20520–20528. [Google Scholar] [CrossRef]
- Doǧu, T. The importance of pore structure and diffusion in the kinetics of gas-solid non-catalytic reactions: Reaction of calcined limestone with SO2. Chem. Eng. J. 1981, 21, 213–222. [Google Scholar] [CrossRef]
- Bar-Ziv, E.; Kantorovich, I.I. Mutual effects of porosity and reactivity in char oxidation. Prog. Energy Combust. Sci. 2001, 27, 667–697. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, J.-I.; Vithanage, M.; Park, Y.-K.; Lee, J.; Kwon, E.E. Modification of biochar properties using CO2. Chem. Eng. J. 2019, 372, 383–389. [Google Scholar] [CrossRef]



| Ultimate Analysis (d.b.) | wt% | Pore Structure | |
|---|---|---|---|
| C | 96.5 | SBET (m2/g) | 341.0305 |
| H | 1.2 | Smic (m2/g) | 308.4343 |
| O (by difference) | 1.84 | Sext (m2/g) | 32.5962 |
| N | 0.45 | Dave (nm) | 1.8016 |
| S | 0.01 | Vmic (cm3/g) | 0.1213 |
| Vtotal (cm3/g) | 0.1536 | ||
| Vmic/Vtotal | 0.7897 |
| Code | Reaction Model | Differential f(x) | Integral F(x) |
|---|---|---|---|
| Am | Nucleus production model | ||
| A1 | m = 1 | 1 − x | −ln(1 − x) |
| A2 | m = 2 | 2(1 − x)[−ln(1 − x)]1/2 | [−ln(1 − x)]1/2 |
| A3 | m = 3 | 3(1 − x)[−ln(1 − x)]2/3 | [−ln(1 − x)]1/3 |
| A4 | m = 4 | 4(1 − x)[−ln(1 − x)]1/4 | [−ln(1 − x)]1/4 |
| Rm | Shrinking core model | ||
| R1/2 | m = 1/2 | (1/2)(1 − x)−1 | 1 − (1 − x)2 |
| R1/3 | m = 1/3 | (1/3)(1 − x)−2 | 1 − (1 − x)3 |
| R1/4 | m = 1/4 | (1/4)(1 − x)−3 | 1 − (1 − x)4 |
| R2 | m = 2 | 2(1 − x)1/2 | 1 − (1 − x)1/2 |
| R3 | m = 3 | 3(1 − x)2/3 | 1 − (1 − x)1/3 |
| Dm | Dimensional diffusion model | ||
| D1 | Dimensional diffusion | 1/2x−1 | x2 |
| D2 | Two-dimensional diffusion | [−ln(1 − x)]−1 | x + (1 − x)ln(1 − x) |
| D3 | Three-dimensional diffusion | (3/2)(1 − x)2/3[1 − (1 − x)1/3]−1 | [1 − (1 − x)1/3]2 |
| D4 | Three-dimensional diffusion | (3/2)[(1 − x)−1/3 − 1]−1 | 1 − 2/3x − (1 − x)2/3 |
| D5 | 3-D (Anti-Jander) | (3/2)(1 + x)2/3[(1 + x)1/3 − 1]−1 | [(1 + x)1/3 − 1]2 |
| D6 | 3-D (ZLT) | (3/2)(1 − x)4/3[(1 − x)−1/3 − 1]−1 | [(1 − x)−1/3 − 1]2 |
| D7 | 3-D (Jander) | 6(1 − x)2/3[1 − (1 − x)1/3]1/2 | [1 − (1 − x)1/3]1/2 |
| D8 | 2-D (Jander) | (1 − x)1/2[1 − (1 − x)1/2]−1 | [1 − (1 − x)1/2]2 |
| Cn | Phase boundary reaction | ||
| C1 | Reaction order: n = 2 | (1 − x)2 | (1 − x)−1 − 1 |
| C2 | Reaction order: n = 3/2 | 2(1 − x)3/2 | (1 − x)−1/2 − 1 |
| α-Al2O3/C Molar Ratio | Intercept | Slope | Ea (kJ/mol) | k0 (min−1) | R2 |
|---|---|---|---|---|---|
| 1:3 | 9.31 | −29,096.66 | 241.91 | 11,061.43 | 0.99754 |
| 2:3 | 5.45 | −20,850.46 | 173.35 | 233.58 | 0.97167 |
| 3:3 | 4.23 | −17,653.34 | 146.77 | 68.49 | 0.98175 |
| α-Al2O3/C Molar Ratio | Kinetic Equations of the Biochar-Based N-Absorption Reaction |
|---|---|
| 1:3 | |
| 2:3 | |
| 3:3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yu, Q.; Xie, H.; Gao, J.; Zhao, J. Experimental and Kinetic Study of Biochar in N-Absorption Reaction of Chemical Looping Ammonia Generation. Processes 2024, 12, 2870. https://doi.org/10.3390/pr12122870
Liu Z, Yu Q, Xie H, Gao J, Zhao J. Experimental and Kinetic Study of Biochar in N-Absorption Reaction of Chemical Looping Ammonia Generation. Processes. 2024; 12(12):2870. https://doi.org/10.3390/pr12122870
Chicago/Turabian StyleLiu, Zhongyuan, Qingbo Yu, Huaqing Xie, Jinchao Gao, and Jiatai Zhao. 2024. "Experimental and Kinetic Study of Biochar in N-Absorption Reaction of Chemical Looping Ammonia Generation" Processes 12, no. 12: 2870. https://doi.org/10.3390/pr12122870
APA StyleLiu, Z., Yu, Q., Xie, H., Gao, J., & Zhao, J. (2024). Experimental and Kinetic Study of Biochar in N-Absorption Reaction of Chemical Looping Ammonia Generation. Processes, 12(12), 2870. https://doi.org/10.3390/pr12122870






