Efficient One-Pot Hydrothermal Synthesis of TiO2 Nanostructures for Reactive Black 5 Dye Removal: Experimental and Theoretical Insights
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reagents
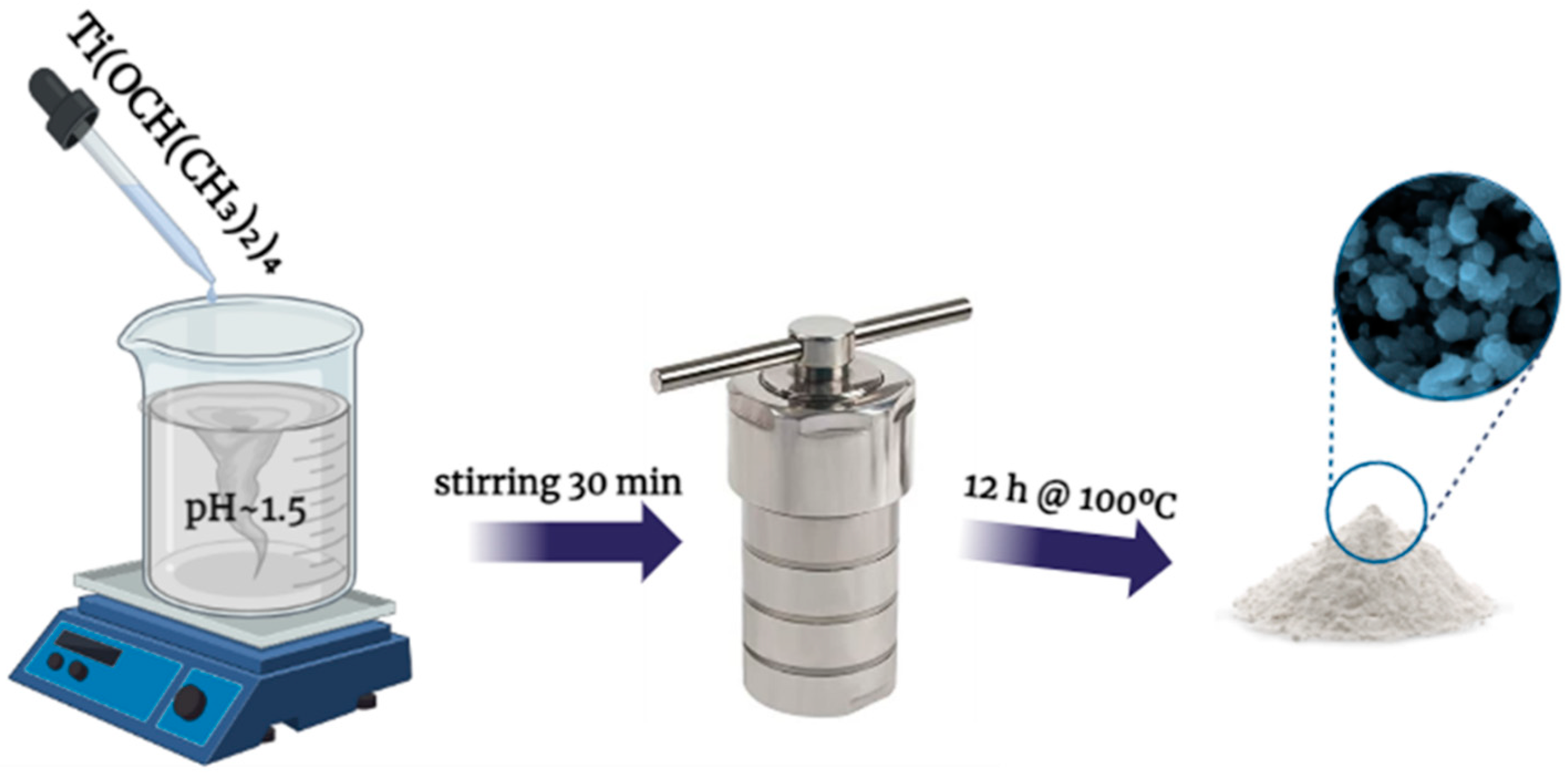
2.1.1. Synthesis of TiO2 (Titania) Nanoparticles
2.1.2. Characterization
2.2. Adsorption Studies
2.3. Theoretical Calculations
3. Results and Discussion
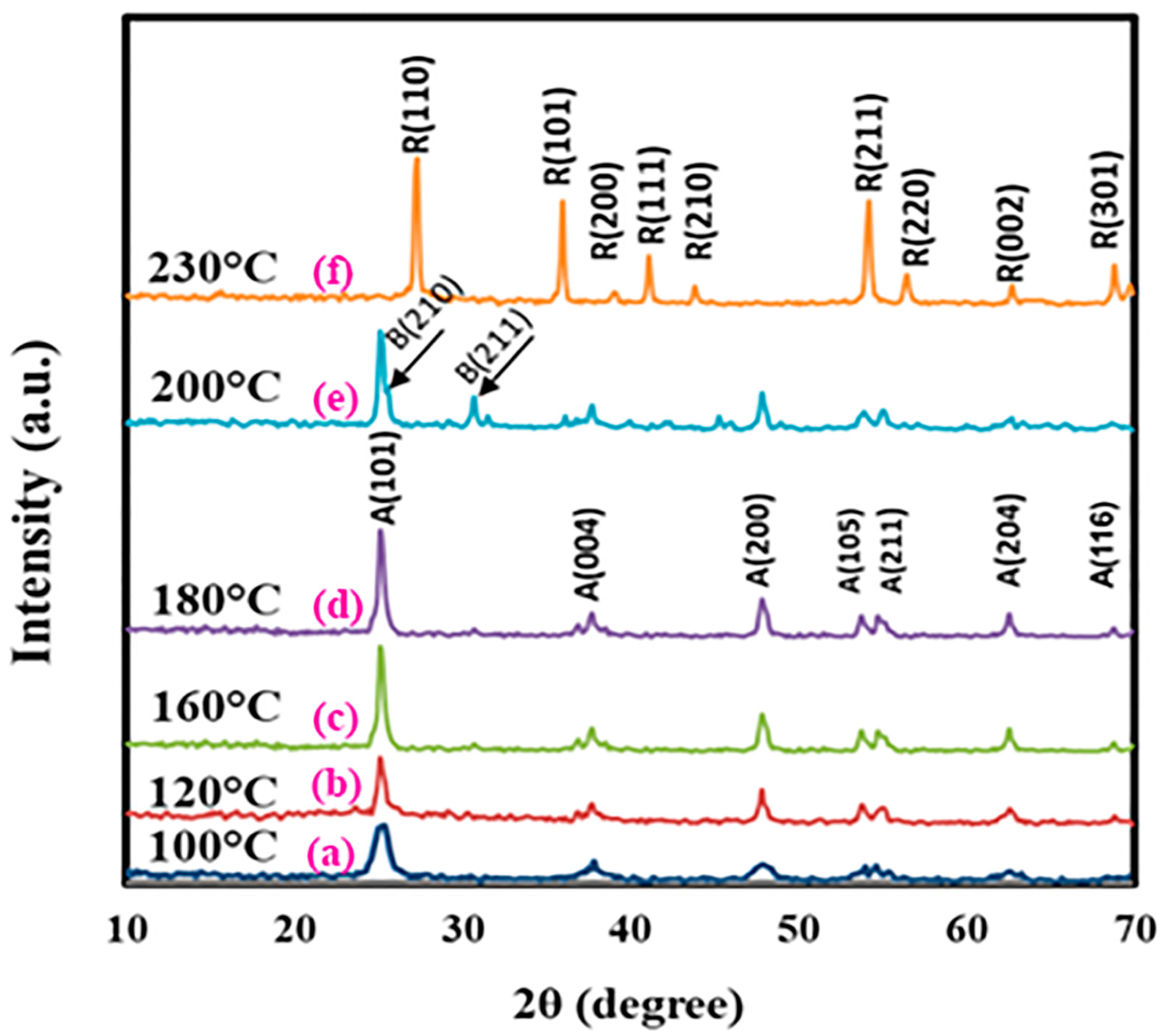
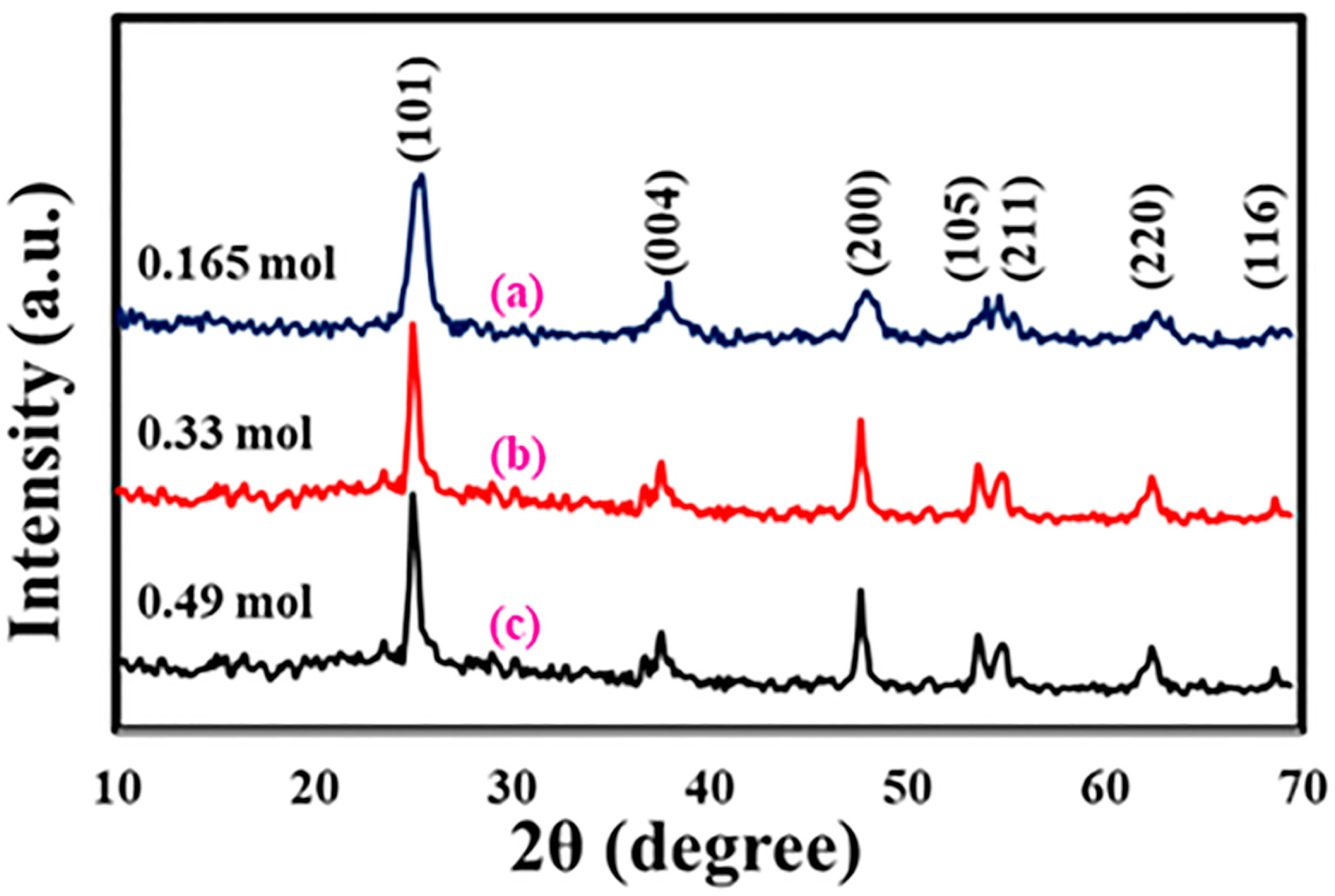
3.1. Fabrication and Analysis of TiO2 Nanostructures
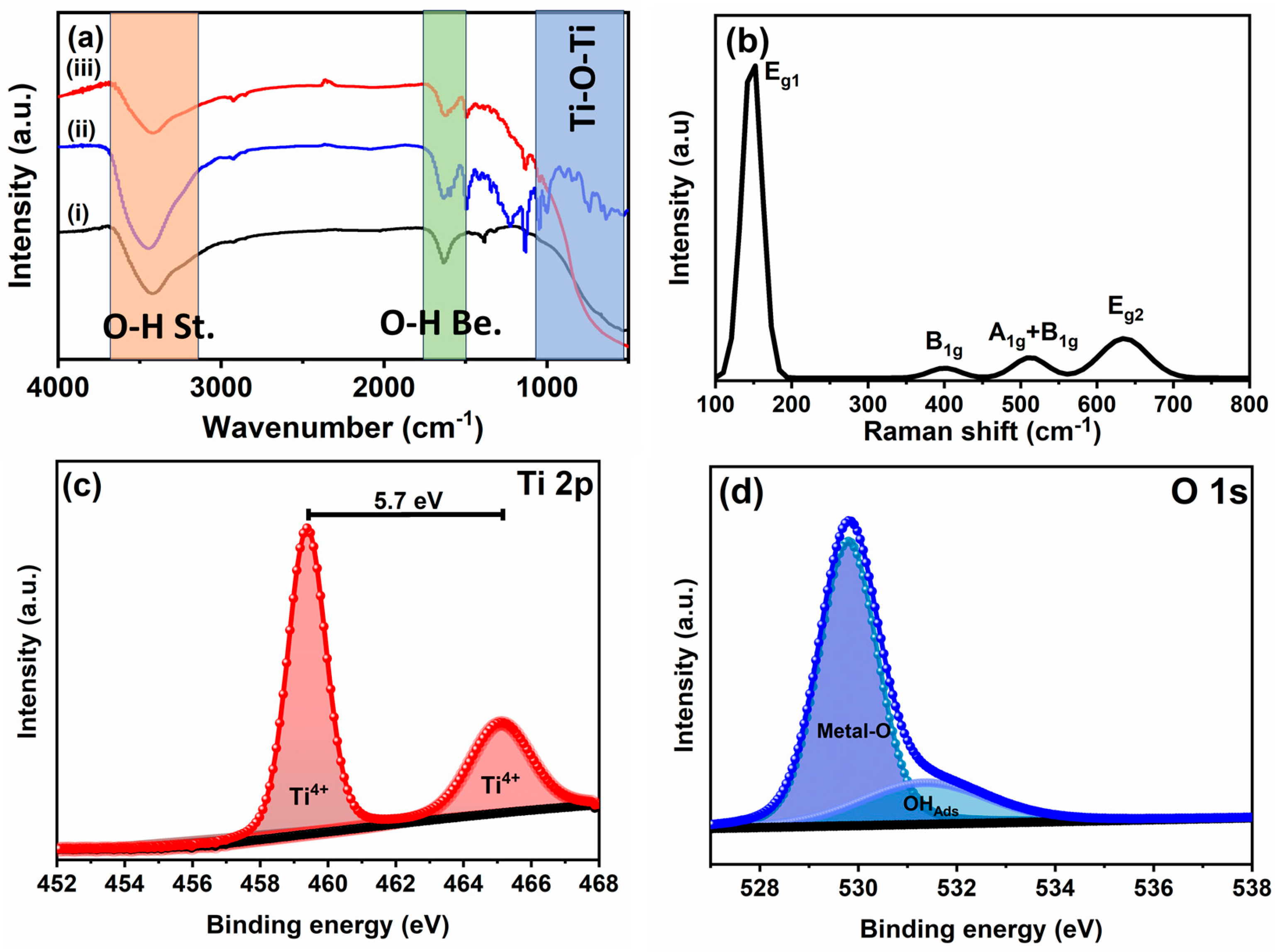
3.1.1. FT-IR, Raman, and XPS Analyses
3.1.2. Morphological Studies
3.1.3. Pore Structure Characterization of Prepared TiO2
3.1.4. Zero-Charge Point (pHpzc) and Isoelectric Point (IEP) Investigation
3.2. Adsorption Investigation
3.2.1. pH Effect
3.2.2. Impact of Ionic Strength
3.2.3. Contact Time and Kinetics
3.2.4. Adsorption Isotherms
3.2.5. Thermodynamic Evaluations
3.2.6. Regeneration of TiO2 Nano-Adsorbent and Its Comparison with Alternative Adsorbents
3.2.7. Theoretical Calculation Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciğeroğlu, Z.; El Messaoudi, N.; Şenol, Z.M.; Başkan, G.; Georgin, J.; Gubernat, S. Clay-based nanomaterials and their adsorptive removal efficiency for dyes and antibiotics: A review. Mater. Today Sustain. 2024, 26, 100735. [Google Scholar] [CrossRef]
- Cavalcante, L.C.; de Carvalho, K.Q.; Bassetti, F.J.; Coral, L.A.A. Kinetic, isothermal and thermodynamic studies of Reactive Black 5 removal using rice husk ashes and powdered activated carbon. Desalination Water Treat. 2024, 320, 100606. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Moustafa, M.M.; Taha, M.M. Hydrothermal tuning of the morphology and particle size of hydrozincite nanoparticles using different counterions to produce nanosized ZnO as an efficient adsorbent for textile dye removal. RSC Adv. 2016, 6, 42180–42195. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008, 42, 3601–3610. [Google Scholar] [CrossRef]
- Prabhakar, N.; Isloor, A.M.; Padaki, M.; Ismail, A.F. Fabrication of TiO2@ZIF-67 metal organic framework composite incorporated PVDF membranes for the removal of hazardous reactive black 5 and Congo red dyes from contaminated water. Chem. Eng. J. 2024, 498, 155270. [Google Scholar] [CrossRef]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical precipitation of heavy metals from acid mine drainage. Water Res. 2002, 36, 4757–4764. [Google Scholar] [CrossRef]
- Sadri Moghaddam, S.; Alavi Moghaddam, M.R.; Arami, M. Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology. J. Hazard. Mater. 2010, 175, 651–657. [Google Scholar] [CrossRef]
- Ajao, V.; Nam, K.; Chatzopoulos, P.; Spruijt, E.; Bruning, H.; Rijnaarts, H.; Temmink, H. Regeneration and reuse of microbial extracellular polymers immobilised on a bed column for heavy metal recovery. Water Res. 2020, 171, 115472. [Google Scholar] [CrossRef]
- Uğan, M.; Onac, C.; Kaya, A.; Köseoğlu, D.; Akdoğan, A. Removal of Reactive Red 195 dye from textile industry wastewater with Deep Eutectic Solvent-based green extraction. J. Mol. Liq. 2024, 398, 124249. [Google Scholar] [CrossRef]
- Bazarin, G.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Borba, C.E.; Trigueros, D.E.G.; Dall’Oglio, I.C. High removal performance of reactive blue 5G dye from industrial dyeing wastewater using biochar in a fixed-bed adsorption system: Approaches and insights based on modeling, isotherms, and thermodynamics study. J. Environ. Chem. Eng. 2024, 12, 111761. [Google Scholar] [CrossRef]
- Mustafa, G.; Munir, R.; Sadia, B.; Younas, F.; Sayed, M.; Muneer, A.; Sardar, M.F.; Albashe, G.; Noreen, S. Synthesis of polymeric ferrite composites (Ni-CoFe2O4/Chitosan, Zn-NiFe2O4/Starch, Co-NiZnFe2O4/Polyaniline, Ni doped CrZnFe2O4/Alginate, and Cr doped ZnCoFe2O4/PVA) for the removal of reactive golden yellow-160 dye from wastewater. J. Environ. Chem. Eng. 2024, 12, 112581. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; Hu, H.; Xie, B.; Du, Y.; Zhu, Q. Quaternized hollow TiO2-enhanced the dye adsorption capacity and photogenerated carrier separation for efficient reactive dye removal. Appl. Surf. Sci. 2024, 644, 158764. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Wu, R.; Musa, S.A.; Agha, H.M.; ALOthman, Z.A.; Jawad, A.H.; Algburi, S. Bisphenol-A-diglycidyl ether modified chitosan/nano-SiO2 via hydrothermal process: A statistical modeling and adsorption mechanism for reactive orange 16 dye removal. Int. J. Biol. Macromol. 2024, 256, 128267. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, L.; Beyou, E.; El Ghali, A.; Baouab, M.H.V. Comparative studies on the adsorption of metal ions from aqueous solutions using various functionalized graphene oxide sheets as supported adsorbents. J. Hazard. Mater. 2020, 389, 121839. [Google Scholar] [CrossRef] [PubMed]
- Abbas, W.A.; Ramadan, M.; Faid, A.Y.; Abdellah, A.M.; Ouf, A.; Moustafa, N.; Allam, N.K. Photoactive catalysts for effective water microbial purification: Morphology-activity relationship. Environ. Nanotechnol. Monit. Manag. 2018, 10, 87–93. [Google Scholar] [CrossRef]
- Asemi, M.; Ghanaatshoar, M. Hydrothermal growth of one-dimensional Ce-doped TiO2 nanostructures for solid-state DSSCs comprising Mg-doped CuCrO2. J. Mater. Sci. 2017, 52, 489–503. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, M.; Zhang, Z.; Tang, T.; Wang, T. Preparation of TiO2 nanotubes/reduced graphene oxide binary nanocomposites enhanced photocatalytic properties. J. Mater. Sci. Mater. Electron. 2017, 28, 9416–9422. [Google Scholar] [CrossRef]
- Amiri, R.; Bourezgui, A.; Djeridi, W.; Dappozze, F.; Houas, A.; Guillard, C.; Elsellami, L. Surface modification of TiO2 with a less expensive metal (iron) to exploit solar energy in photocatalysis: An ecological and economical solution. Int. J. Hydrogen Energy 2024, 51, 638–647. [Google Scholar] [CrossRef]
- Trommetter, G.; Mendret, J.; Khaska, S.; La Salle, C.; Brosillon, S.; Goetz, V.; Plantard, G. Removal efficiencies for 52 pesticides and pharmaceuticals from wastewater effluent by coupling solar heterogeneous photo-oxidation with TiO2 and infiltration in saturated soil column. Sci. Total Environ. 2024, 956, 177278. [Google Scholar] [CrossRef]
- Chen, Z.; Zhuo, H.; Hu, Y.; Zhong, L.; Peng, X.; Jing, S.; Liu, Q.; Zhang, X.; Liu, C.; Sun, R. Self-Biotemplate Preparation of Hierarchical Porous Carbon with Rational Mesopore Ratio and High Oxygen Content for an Ultrahigh Energy-Density Supercapacitor. ACS Sustain. Chem. Eng. 2018, 6, 7138–7150. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Ali, E.I.; Zakaria, E.S. Tunable auto-combustion preparation of TiO2 nanostructures as efficient adsorbents for the removal of an anionic textile dye. RSC Adv. 2017, 7, 8034–8050. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Reucroft, P.J.; Yang, F.; Dozier, A. Growth of TiO2 nanorods by metalorganic chemical vapor deposition. J. Cryst. Growth 2003, 256, 83–88. [Google Scholar] [CrossRef]
- Naghibi, S.; Faghihi Sani, M.A.; Madaah Hosseini, H.R. Application of the statistical Taguchi method to optimize TiO2 nanoparticles synthesis by the hydrothermal assisted sol-gel technique. Ceram. Int. 2014, 40, 4193–4201. [Google Scholar] [CrossRef]
- Hakki, H.K.; Allahyari, S.; Rahemi, N.; Tasbihi, M. Surface properties, adherence, and photocatalytic activity of sol–gel dip-coated TiO2–ZnO films on glass plates. Comptes Rendus Chim. 2019, 22, 393–405. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Abdallah, S. Facile controllable hydrothermal route for a porous CoMn2O4 nanostructure: Synthesis, characterization, and textile dye removal from aqueous media. RSC Adv. 2016, 6, 84050–84067. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Ahmed, I.S.; Hendy, H.S. A facile one-pot hydrothermal synthesis of hematite (α-Fe2O3) nanostructures and cephalexin antibiotic sorptive removal from polluted aqueous media. J. Mol. Liq. 2018, 271, 844–856. [Google Scholar] [CrossRef]
- Refat, N.M.; Nassar, M.Y.; Sadeek, S.A. A controllable one-pot hydrothermal synthesis of spherical cobalt ferrite nanoparticles: Synthesis, characterization, and optical properties. RSC Adv. 2022, 12, 25081–25095. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, H.-T.; Liu, J.-H.; Yang, C.-P.; Jing, Q.-S.; Zhang, Y.-X.; Yang, X.-K.; Huang, K.-J. Hydrothermal preparation and electrochemical sensing properties of TiO2-graphene nanocomposite. Colloids Surfaces B Biointerfaces 2011, 83, 78–82. [Google Scholar] [CrossRef]
- Nagaraju, G.; Ebeling, G.; Gonçalves, R.V.; Teixeira, S.R.; Weibel, D.E.; Dupont, J. Controlled growth of TiO2 and TiO2–RGO composite nanoparticles in ionic liquids for enhanced photocatalytic H2 generation. J. Mol. Catal. A Chem. 2013, 378, 213–220. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Marques, M.; Teles, L.K. An approximation to density functional theory for an accurate calculation of band-gaps of semiconductors. Phys. Rev. B 2008, 78, 125116. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accnrate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef] [PubMed]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of Crystals with the Quasi-Newton Method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Delley, B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B–Condens. Matter Mater. Phys. 2002, 66, 155125. [Google Scholar] [CrossRef]
- Tolba, S.A.; Gameel, K.M.; Ali, B.A.; Almossalami, H.A.; Allam, N.K. The DFT+U: Approaches, Accuracy, and Applications. In Density Functional Calculations-Recent Progresses of Theory and Application; IntechOpen: London, UK, 2018; pp. 3–30. [Google Scholar] [CrossRef]
- Kinsinger, N.M.; Wong, A.; Li, D.; Villalobos, F.; Kisailus, D. Nucleation and Crystal Growth of Nanocrystalline Anatase and Rutile Phase TiO2 from a Water-Soluble Precursor. Cryst. Growth Des. 2010, 10, 5254–5261. [Google Scholar] [CrossRef]
- Dahiya, S.; Shoran, S.; Sharma, D.; Rao, V.; Chaudhary, S.; Nehra, S.; Sharma, A. Bioengineered sustainable phytofabrication of anatase TiO2 -adorned g-C3N4 nanocomposites and unveiling their photocatalytic potential towards advanced environmental remediation. Chemosphere 2024, 362, 142456. [Google Scholar] [CrossRef]
- Qahtan, T.F.; Owolabi, T.O.; Saleh, T.A. X-ray photoelectron spectroscopy of surface-treated TiO2 mesoporous film by 500 eV argon ion beam. J. Mol. Liq. 2024, 393, 123556. [Google Scholar] [CrossRef]
- Carrott, P.J.M.; Roberts, R.A.; Sing, K.S.W. Adsorption of nitrogen by porous and non-porous carbons. Carbon. N. Y 1987, 25, 59–68. [Google Scholar] [CrossRef]
- Kosmulski, M. pH-dependent surface charging and points of zero charge. IV. Update and new approach. J. Colloid. Interface Sci. 2009, 337, 439–448. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Amin, A.S.; Ahmed, I.S.; Abdallah, S. Sphere-like Mn2O3 nanoparticles: Facile hydrothermal synthesis and adsorption properties. J. Taiwan. Inst. Chem. Eng. 2016, 64, 79–88. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Proceedings of the International Conference on Water Pollution Symposium; Pergamon Press: Oxford, UK, 1962. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. De Gruyter 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Halsey, G.D. The Role of Surface Heterogeneity in Adsorption. In Advances in Catalysis; Frankenburg, W.G., Komarewsky, V.I., Rideal, E.K., Eds.; Academic Press: Cambridge, MA, USA, 1952; Volume 4, pp. 259–269. [Google Scholar]
- Enache, A.-C.; Predeanu, G.; Cojocaru, C.; Samoila, P.; Ciornea, V.; Apolzan, R.; Harabagiu, V. Adsorption of Brilliant Green Dye onto a Mercerized Biosorbent: Kinetic, Thermodynamic, and Molecular Docking Studies. Molecules 2023, 28, 4129. [Google Scholar] [CrossRef]
- Ghaedi, M.; Jah, A.H.; Khodadoust, S.; Sahraei, R.; Daneshfar, A.; Mihandoost, A.; Purkait, M.K. Cadmium telluride nanoparticles loaded on activated carbon as adsorbent for removal of sunset yellow. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 22–27. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Ma, J.; Jia, Y.; Jing, Y.; Yao, Y.; Sun, J. Kinetics and thermodynamics of methylene blue adsorption by cobalt-hectorite composite. Dyes Pigments 2012, 93, 1441–1446. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Pinto, R.B.; Lima, E.C.; Calvete, T.; Amavisca, C.V.; Royer, B.; Cunha, M.L.; Fernandes, T.H.; Pinto, I.S. Removal of remazol black B textile dye from aqueous solution by adsorption. Desalination 2011, 269, 92–103. [Google Scholar] [CrossRef]
- Khosravi, M.; Azizian, S. Synthesis of Fe3O4 flower-like hierarchical nanostructures with high adsorption performance toward dye molecules. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 482, 438–446. [Google Scholar] [CrossRef]
- González, J.A.; Villanueva, M.E.; Piehl, L.L.; Copello, G.J. Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes: Adsorption and desorption study. Chem. Eng. J. 2015, 280, 41–48. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ohno, T. A hybrid density functional study on the electron and hole trap states in anatase titanium dioxide. Phys. Chem. Chem. Phys. 2012, 14, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Deák, P.; Aradi, B.; Frauenheim, T. Polaronic effects in TiO2 calculated by the HSE06 hybrid functional: Dopant passivation by carrier self-trapping. Phys Rev B–Condens Matter Mater Phys 2011, 83, 155207. [Google Scholar] [CrossRef]
- Deák, P.; Aradi, B.; Frauenheim, T. Oxygen deficiency in TiO2: Similarities and differences between the Ti self-interstitial and the O vacancy in bulk rutile and anatase. Phys. Rev. B–Condens. Matter Mater. Phys. 2015, 92, 045204. [Google Scholar] [CrossRef]









| Dye | Reactive Black 5 (RB5) |
 | |
| Molecular formula | C26H21N5Na4O19S6 |
| Molecular weight | 991.82 |
| λmax | 598 nm |
| Type | Anionic dye |
| Chemical class | Azo derivative compound |
| Solubility | Water-soluble |
| C.I. number | Remazol Black B |
| SBET m2 g−1 | Smeso m2 g−1 | Smacro m2 g−1 | Smicro/Smeso | Vtot. cm3 g−1 | Vmacro cm3 g−1 | Vmeso cm3 g−1 | Vavar. nm | Pore Size, nm | |
|---|---|---|---|---|---|---|---|---|---|
| BJH | DFT | ||||||||
| 379.5 | 335.7 | 43.8 | 0.26 | 0.62 | 0.13 | 0.49 | 13.73 | 21.67 | 31.68 |
| Pseudo-Second-Order Model for RB5 | |||||
|---|---|---|---|---|---|
| C0 [mg L−1] | K2 [g mg−1min−1] | qe(cal) [mg g−1] | r12 | h [g mg−1min−1] | qe(exp) [mg g−1] |
| 100 | 0.0004 | 49.75 | 0.999 | 28,042.52 | 49.92 |
| 150 | 0.00023 | 66.29 | 0.986 | 6.01 | 64.62 |
| 200 | 0.00014 | 85.71 | 0.998 | 29.97 | 84.15 |
| 250 | 8.3 × 10−5 | 110.09 | 0.994 | 8.71 | 109.54 |
| Adsorption Model Isotherm | Parameters | Value |
|---|---|---|
| Langmuir | KL (L mol−1) | 6.18 × 10−3 |
| qm(cal) (mg g−1) | 109.7 | |
| r12 | 0.999 | |
| RL | 0.246 | |
| qe(exp) (mg g−1) | 109.5 | |
| Freundlich | KF [(mg g−1).(L mg−1)1/n] | 51.46 |
| qm(cal) (mg g−1) | 190.53 | |
| r22 | 0.905 | |
| qe,(exp) (mg g−1) | 109.5 | |
| n | 6.79 |
| Approach | Temperature (K) | Kad | ∆Gad kJ mol−1 | ∆Sad [J mol−1 K−1] | ∆Had [kJ mol−1] | Ea [kJ mol−1] | S* |
|---|---|---|---|---|---|---|---|
| 298 | 7.57 104 | −27.834 | 51.464 | −12.503 | 25.33 | 55 10−3 | |
| 308 | 4.82 104 | −27.612 | 49.073 | ||||
| 318 | 4.95 104 | −28.5793 | 50.570 | ||||
| 328 | 3.12 104 | −28.219 | 47.931 | ||||
| 298 | 3.89 105 | −31.89 | 65.075 | −12.494 | 25.33 | 55 10−3 | |
| 308 | 105 | −32.50 | 62.687 | ||||
| 318 | 2.92 105 | −33.28 | 64.174 | ||||
| 328 | 2.80 105 | −34.20 | 61.536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassar, M.Y.; Taha, M.M.; Tolba, S.; Masoud, E.M.; Adam, M.S.S.; Khalil, A. Efficient One-Pot Hydrothermal Synthesis of TiO2 Nanostructures for Reactive Black 5 Dye Removal: Experimental and Theoretical Insights. Processes 2024, 12, 2786. https://doi.org/10.3390/pr12122786
Nassar MY, Taha MM, Tolba S, Masoud EM, Adam MSS, Khalil A. Efficient One-Pot Hydrothermal Synthesis of TiO2 Nanostructures for Reactive Black 5 Dye Removal: Experimental and Theoretical Insights. Processes. 2024; 12(12):2786. https://doi.org/10.3390/pr12122786
Chicago/Turabian StyleNassar, Mostafa Y., Manar M. Taha, Sarah Tolba, Emad M. Masoud, Mohamed Shaker S. Adam, and Ahmed Khalil. 2024. "Efficient One-Pot Hydrothermal Synthesis of TiO2 Nanostructures for Reactive Black 5 Dye Removal: Experimental and Theoretical Insights" Processes 12, no. 12: 2786. https://doi.org/10.3390/pr12122786
APA StyleNassar, M. Y., Taha, M. M., Tolba, S., Masoud, E. M., Adam, M. S. S., & Khalil, A. (2024). Efficient One-Pot Hydrothermal Synthesis of TiO2 Nanostructures for Reactive Black 5 Dye Removal: Experimental and Theoretical Insights. Processes, 12(12), 2786. https://doi.org/10.3390/pr12122786







