Molecular Simulation Study on the Adsorption Mechanisms of Microbial Components and Metabolic Products on Activated Carbon in HVAC Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Activated Carbon Model
2.2. Peptidoglycan and p-Xylene Models
2.3. Molecular Simulation Process
3. Results
3.1. Adsorption Sites and Radial Distribution Function (RDF)
- where g(r) represents the probability density of molecules at a distance r from the carbon atoms on activated carbon;
- n is the number of molecules in the range of distance r to r + dr;
- ρ is the density of the molecules.
3.2. Average Adsorption Heat and Energy Distribution
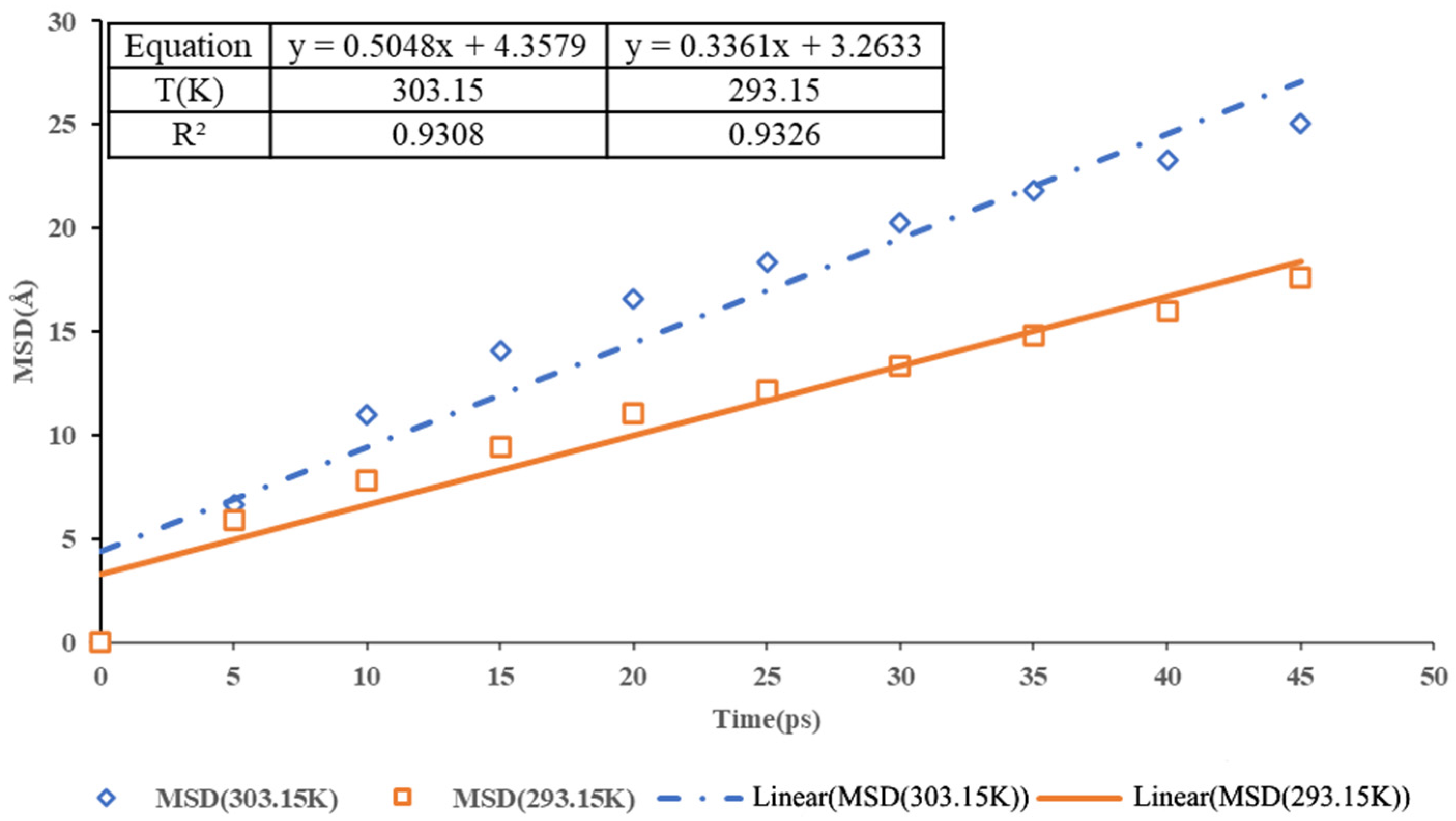
3.3. Diffusion Coefficient
- where Dα is the diffusion coefficient;
- []2 is the mean square displacement of the atoms in the structure.

4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumari, M.; Chaudhary, G.R.; Chaudhary, S.; Umar, A. Transformation of solid plastic waste to activated carbon fibres for wastewater treatment. Chemosphere 2022, 294, 133692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qi, F.; Li, H.; Shi, Z. The activation of carbon materials to control airborne pathogenic bacteria in the pig house by efficient adsorption. Biosyst. Eng. 2023, 236, 71–78. [Google Scholar] [CrossRef]
- Al-Fawzan, F.F. An Eco-Friendly Procedure for Preparation of Activated Carbon as a Capture Material of Heavy Metals in Wastewater. Sci. Adv. Mater. 2020, 12, 1157–1163. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, S.; Cao, G.; Chong, M.; Bao-Jie, H. Distribution characteristics, growth, reproduction and transmission modes and control strategies for microbial contamination in HVAC systems: A literature review. Energy Build. 2018, 177, 77–95. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, K.; Li, H.; Cao, G.; Wu, D.; Shi, Y. Exploring the potential relationship between indoor air quality and the concentration of airborne culturable fungi: A combined experimental and neural network modeling study. Environ. Sci. Pollut. Res. 2018, 25, 3510–3517. [Google Scholar] [CrossRef]
- Nevalainen, A.; Taubel, M.; Hyvarinen, A. Indoor fungi: Companions and contaminants. Indoor Air 2015, 25, 125–156. [Google Scholar] [CrossRef]
- Forthomme, A.; Joubert, A.; Andrès, Y.; Simon, X.; Duquenne, P.; Bemer, D.; Le Coq, L. Microbial aerosol filtration: Growth and release of a bacteria–fungi consortium collected by fibrous filters in different operating conditions. J. Aerosol Sci. 2014, 72, 32–46. [Google Scholar] [CrossRef]
- Lv, Y.; Hu, G.; Wang, C.; Yuan, W.; Wei, S.; Gao, J.; Wang, B.; Song, F. Actual measurement, hygrothermal response experiment and growth prediction analysis of microbial contamination of central air conditioning system in Dalian, China. Sci. Rep. 2017, 7, 44190. [Google Scholar] [CrossRef]
- Haines, S.R.; Hall, E.C.; Marciniak, K.; Misztal, P.K.; Goldstein, A.H.; Adams, R.I.; Dannemiller, K.C. Microbial growth and volatile organic compound (VOC) emissions from carpet and drywall under elevated relative humidity conditions. Microbiome 2021, 9, 209. [Google Scholar]
- Danko, D.; Bezdan, D.; Afshin, E.E.; Ahsanuddin, S.; Bhattacharya, C.; Butler, D.J.; Chng, K.R.; Donnellan, D.; Hecht, J.; Jackson, K.; et al. A global metagenomic map of urban microbiomes and antimicrobial resistance. Cell 2021, 184, 3376. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Estensmo, E.F.; Morgado, L.N.; Maurice, S.; Engh, I.B.; Skrede, I.; Kauserud, H. Analysing indoor mycobiomes through a large-scale citizen science study in Norway. Mol. Ecol. 2021, 30, 2689–2705. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhou, X.; Zhu, Y. Microbiome and antibiotic resistome in household dust from Beijing, China. Environ. Int. 2020, 139, 105702. [Google Scholar] [CrossRef] [PubMed]
- Ezeonu, I.M.; Price, D.L.; Simmons, R.B.; Crow, S.A.; Ahearn, D.G. Fungal production of volatiles during growth on fiberglass. Appl. Environ. Microbiol. 1994, 60, 4172–4173. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Stephens, B. Microbiology of the built environment. Nat. Rev. Microbiol. 2018, 16, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.J.; Beaucham, C. Dominant microbial volatile organic compounds in 23 US homes. Chemosphere 2013, 90, 977–985. [Google Scholar] [CrossRef]
- Vollmer, W.; Blanot, D.; de Pedro, M.A. Peptidoglycan structure and architecture. Fems Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef]
- Egan, A.; Errington, J.; Vollmer, W. Regulation of peptidoglycan synthesis and remodelling. Nat. Rev. Microbiol. 2020, 18, 446–460. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Pasquina-Lemonche, L.; Burns, J.; Turner, R.D.; Kumar, S.; Tank, R.; Mullin, N.; Wilson, J.S.; Chakrabarti, B.; Bullough, P.A.; Foster, S.J.; et al. The architecture of the Gram-positive bacterial cell wall. Nature 2020, 582, 294–297. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Kim, S.J.; Chang, J.; Singh, M. Peptidoglycan architecture of Gram-positive bacteria by solid-state NMR. Biochim. Biophys. Acta-Biomembr. 2015, 1848 Pt B, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.; Probian, C.; Wilkes, H.; Harder, J. Highly enriched Betaproteobacteria growing anaerobically with p-xylene and nitrate. FEMS Microbiol. Ecol. 2010, 71, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Chalkos, D.; Karamanoli, K.; Vokou, D. Monoterpene Enrichments Have Positive Impacts on Soil Bacterial Communities and the Potential of Application in Bioremediation. Plants 2021, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Perkowski, J.; Stuper, K.; Buśko, M.; Góral, T.; Kaczmarek, A.; Jeleń, H. Differences in metabolomic profiles of the naturally contaminated grain of barley, oats and rye. J. Cereal Sci. 2012, 56, 544–551. [Google Scholar] [CrossRef]
- Zhou, S.; Guo, C.; Wu, Z.; Wang, M.; Wang, Z.; Wei, S.; Li, S.; Lu, X. Edge-functionalized nanoporous carbons for high adsorption capacity and selectivity of CO2 over N2. Appl. Surf. Sci. 2017, 410, 259–266. [Google Scholar] [CrossRef]
- Birkett, G.R.; Do, D.D. Simulation study of water adsorption on carbon black: The effect of graphite water interaction strength. J. Phys. Chem. C 2007, 111, 5735–5742. [Google Scholar] [CrossRef]
- Liang, X.Y.; Chi, J.J.; Yang, Z. The influence of the functional group on activated carbon for, acetone adsorption property by molecular simulation study. Microporous Mesoporous Mater. 2018, 262, 77–88. [Google Scholar] [CrossRef]
- Amirjalayer, S.; Tafipolsky, M.; Schmid, R. Molecular dynamics simulation of benzene diffusion in MOF-5: Importance of lattice dynamics. Angew. Chem.-Int. Ed. 2007, 46, 463–466. [Google Scholar] [CrossRef]
- Veclani, D.; Melchior, A. Adsorption of ciprofloxacin on carbon nanotubes: Insights from molecular dynamics simulations. J. Mol. Liq. 2020, 298, 111977. [Google Scholar] [CrossRef]
- Niu, W.; Nie, W.; Yuan, M.; Bao, Q.; Zhou, W.; Yan, J.; Yu, F.; Liu, C.; Sun, N.; Xue, Q. Study of the microscopic mechanism of lauryl glucoside wetting coal dust: Environmental pollution prevention and control. J. Hazard. Mater. 2021, 412, 125223. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Sangsuradet, S.; Worathanakul, P. Simulation of CO2 Adsorption to Enhance Adsorbent Material Efficiency. Key Eng. Mater. 2018, 777, 251–255. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.L.; Tsimnadis, K.; Sebos, I.; Charabi, Y. Investigating the Effect of Pore Size Distribution on the Sorption Types and the Adsorption-Deformation Characteristics of Porous Continua: The Case of Adsorption on Carbonaceous Materials. Crystals 2024, 14, 742. [Google Scholar] [CrossRef]
- Nazarizadeh, P.; Akbarzadeh, A.R.; Pazouki, M. Wastewater purification from Rhodamine B and Gemifeloxacine by graphene oxide/pectin/ferrite nanocomposite: A novel molecular dynamics simulation for experimental contaminants removing. Water Environ. Res. 2023, 95, e10921. [Google Scholar] [CrossRef]
- Liu, Y.L.; Li, N.; Cui, X.; Yan, W.; Su, J.; Jin, L. A Review on the Morphology and Material Properties of the Gas Separation Membrane: Molecular Simulation. Membranes 2022, 12, 1274. [Google Scholar] [CrossRef]
- Nwobodo, I.C.; Louis, H.; Unimuke, T.O.; Ikenyirimba, O.J.; Iloanya, A.C.; Mathias, G.E.; Osabor, V.N.; Ahuekwe, E.F.; Adeyinka, A.A. Molecular Simulation of the Interaction of Diclofenac with Halogen (F, Cl, Br)-Encapsulated Ga12As12 Nanoclusters. ACS Omega 2023, 8, 17538–17551. [Google Scholar] [CrossRef]
- Wang, M.Y.; Sheng, Y. Molecular simulation to analyze the influence of ultrafine particles on activated carbon adsorbing low concentration toluene. Build. Environ. 2022, 213, 108875. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, C.; Lu, R.; Wang, X.; Sun, C.; Zou, Z. Volatile organic compounds in children’s bedrooms, Shanghai, China: Sources and influential factors. Atmos. Pollut. Res. 2023, 14, 101751. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, M.; Dong, Q. Gas-particle two-phase adsorption of toluene and ultrafine particles on activated carbon studied by molecular simulation. Sci. Total Environ. 2023, 891, 164591. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Gelb, L.D.; Gubbins, K.E. Pore Size Distributions in Porous Glasses: A Computer Simulation Study. Langmuir 1999, 15, 305–308. [Google Scholar] [CrossRef]
- Morris, J.R.; Contescu, C.I.; Chisholm, M.F.; Cooper, V.R.; Guo, J.; He, L.; Ihm, Y.; Mamontov, E.; Melnichenko, Y.B.; Olsen, R.J.; et al. Modern approaches to studying gas adsorption in nanoporous carbons. J. Mater. Chem. A 2013, 1, 9341–9350. [Google Scholar] [CrossRef]
- Huang, Y.; Cannon, F.S.; Guo, J.S.; Watson, J.K.; Wathews, J.P. Atomistic modelling insight into the structure of lignite-based activated carbon and benzene sorption behavior. RSC Adv. 2016, 6, 56623–56637. [Google Scholar] [CrossRef]
- Huang, Y.; Cannon, F.S.; Watson, J.K.; Reznik, B.; Mathews, J.P. Activated carbon efficient atomistic model construction that depicts experimentally-determined characteristics. Carbon 2015, 83, 1–14. [Google Scholar] [CrossRef]
- Kim, S.J.; Singh, M.; Preobrazhenskaya, M.; Schaefer, J. Staphylococcus aureus Peptidoglycan Stem Packing by Rotational-Echo Double Resonance NMR Spectroscopy. Biochemistry 2013, 52, 3651–3659. [Google Scholar] [CrossRef][Green Version]
- Pokhrel, R.; Shakya, R.; Baral, P.; Chapagain, P. Molecular Modeling and Simulation of the Peptidoglycan Layer of Gram-Positive Bacteria Staphylococcus aureus. J. Chem. Inf. Model. 2022, 62, 4955–4962. [Google Scholar] [CrossRef]
- Meroueh, S.O.; Bencze, K.Z.; Hesek, D.; Lee, M.; Fisher, J.D.; Stemmler, T.L.; Mobashery, S. Three-dimensional structure of the bacterial cell wall peptidoglycan. Proc. Natl. Acad. Sci. USA 2006, 103, 4404–4409. [Google Scholar] [CrossRef]
- Li, S.; Song, K.L.; Zhao, D.F.; Rugarabamu, J.R.; Diao, R.; Gu, Y.Y. Molecular simulation of benzene adsorption on different activated carbon under different temperatures. Microporous Mesoporous Mater. 2020, 302, 110220. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Yao, J. Aerosolization of fungal spores in indoor environments. Sci. Total Environ. 2022, 820, 153003. [Google Scholar] [CrossRef]
- Tsao, H.; Scheikl, U.; Herbold, C.; Indra, A.; Walochnik, J.; Horn, M. The cooling tower water microbiota: Seasonal dynamics and co-occurrence of bacterial and protist phylotypes. Water Res. 2019, 159, 464–479. [Google Scholar] [CrossRef]
- Dimitroulopoulou, S.; Dudzińska, M.R.; Gunnarsen, L.; Hägerhed, L.; Maula, H.; Singh, R.; Toyinbo, O.; Haverinen-Shaughnessy, U. Indoor air quality guidelines from across the world: An appraisal considering energy saving, health, productivity, and comfort. Environ. Int. 2023, 178, 108127. [Google Scholar] [CrossRef] [PubMed]
- Poling, B.E. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Peng, Z.; Liu, S.; Li, X. Molecular Simulation Study on the Adsorption Mechanisms of Microbial Components and Metabolic Products on Activated Carbon in HVAC Systems. Processes 2024, 12, 2763. https://doi.org/10.3390/pr12122763
Zhang G, Peng Z, Liu S, Li X. Molecular Simulation Study on the Adsorption Mechanisms of Microbial Components and Metabolic Products on Activated Carbon in HVAC Systems. Processes. 2024; 12(12):2763. https://doi.org/10.3390/pr12122763
Chicago/Turabian StyleZhang, Ge, Zhiyuan Peng, Shuai Liu, and Xiaochen Li. 2024. "Molecular Simulation Study on the Adsorption Mechanisms of Microbial Components and Metabolic Products on Activated Carbon in HVAC Systems" Processes 12, no. 12: 2763. https://doi.org/10.3390/pr12122763
APA StyleZhang, G., Peng, Z., Liu, S., & Li, X. (2024). Molecular Simulation Study on the Adsorption Mechanisms of Microbial Components and Metabolic Products on Activated Carbon in HVAC Systems. Processes, 12(12), 2763. https://doi.org/10.3390/pr12122763






