Thermochemical Conversion of Biomass into 2nd Generation Biofuel
Abstract
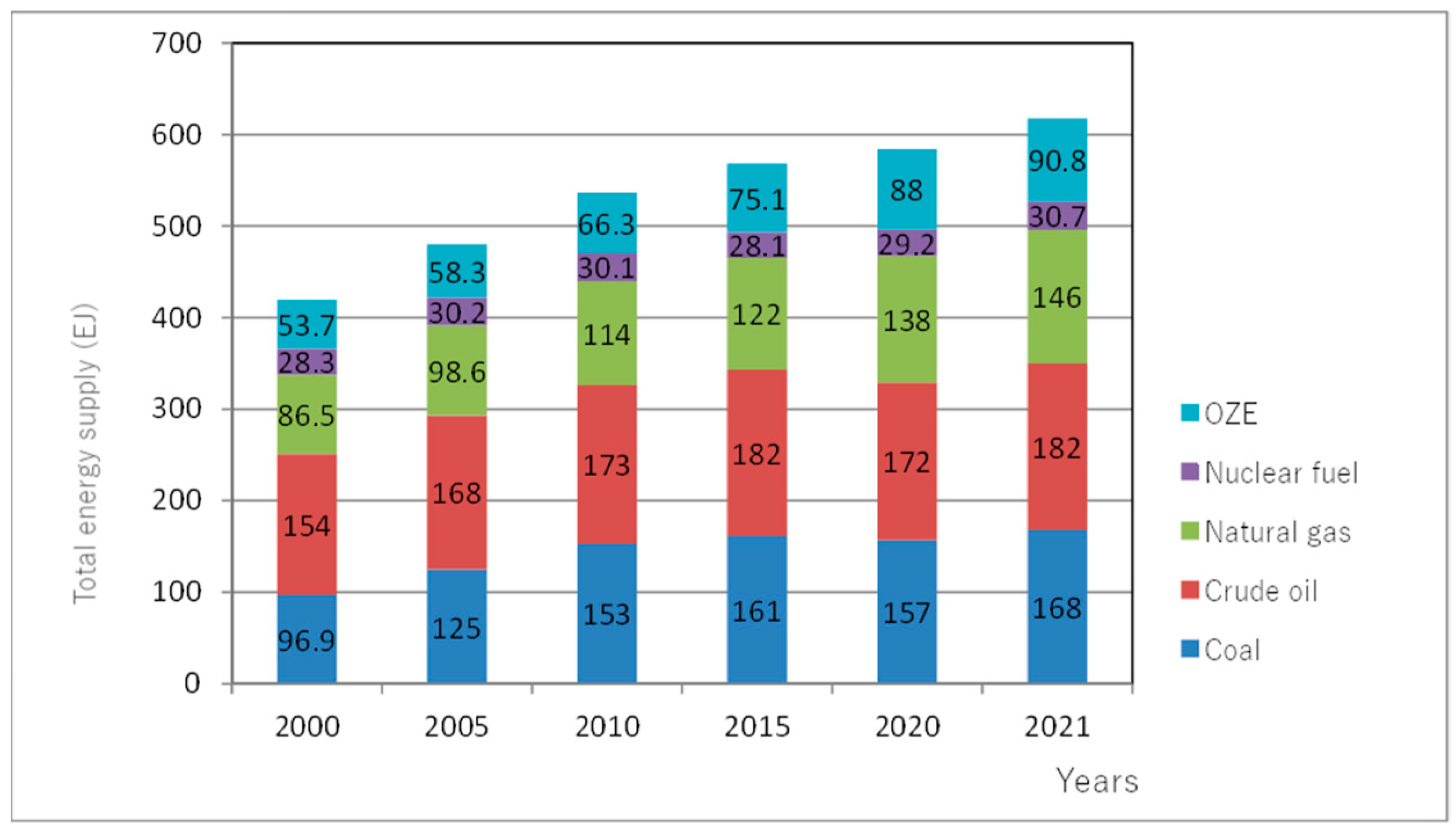
1. Introduction
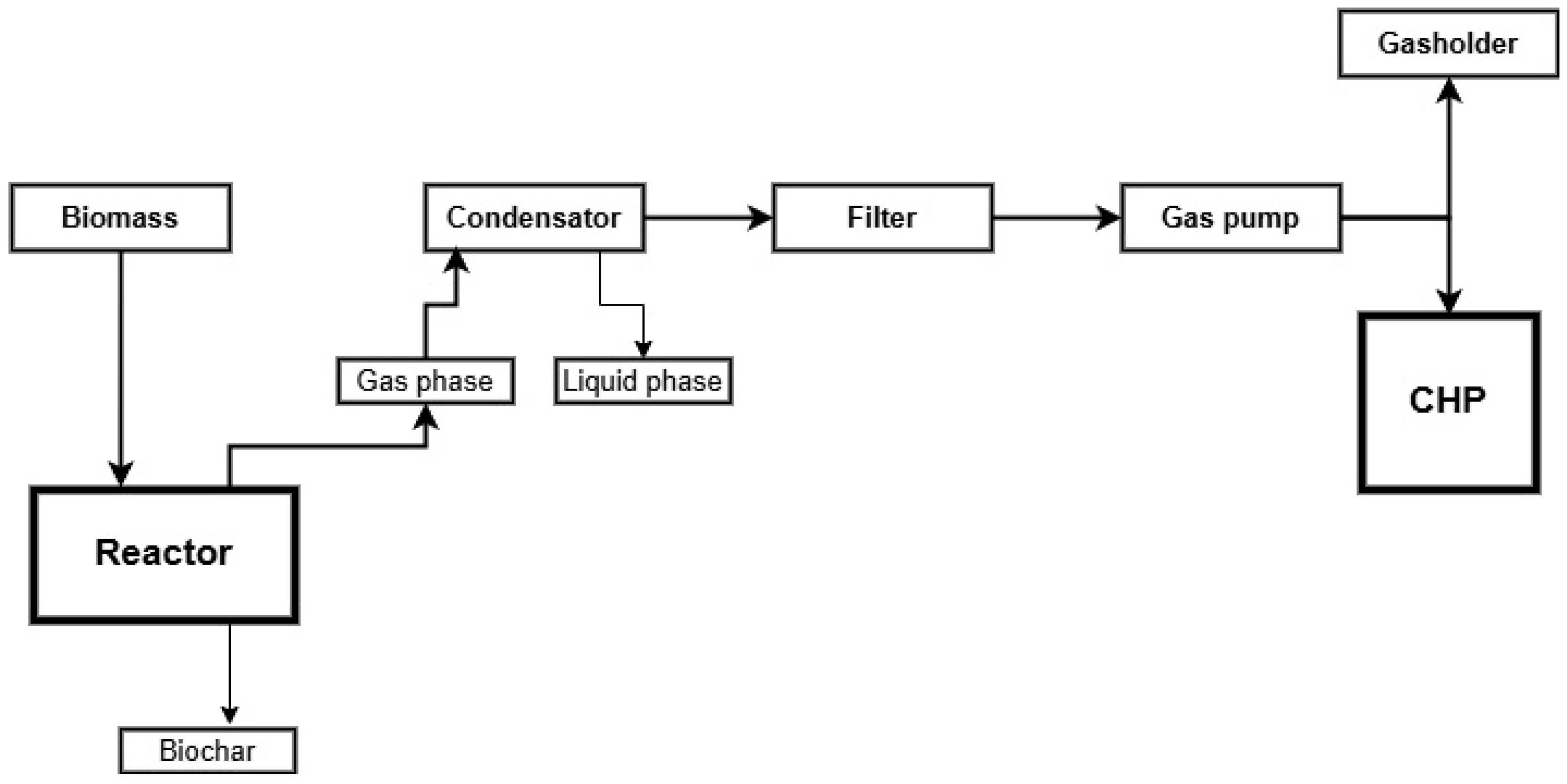
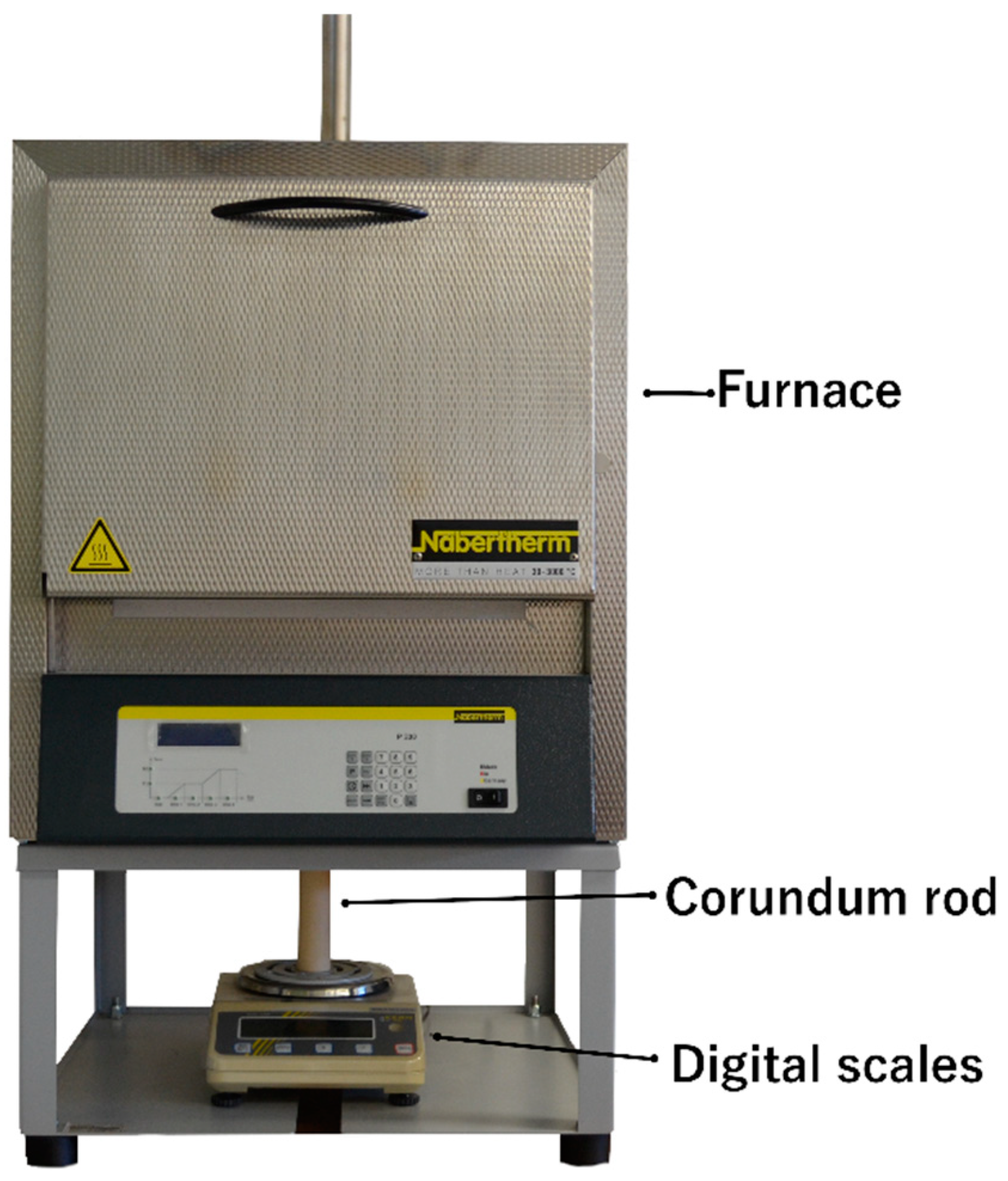
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamperidou, V.; Terzopoulou, P.; Barboutis, I. Marginal lands providing tree-crop biomass as feedstock for solid biofuels. Biofuels Bioproducsts Biorefining 2021, 15, 1395–1405. [Google Scholar] [CrossRef]
- IEA. Bioenergy Annual Report. 2022. Available online: https://www.ieabioenergy.com/wp-content/uploads/2023/05/Annual-Report-2022.pdf (accessed on 12 July 2024).
- Informačné Listy o Európskej Únii. Available online: https://www.europarl.europa.eu/erpl-app-public/factsheets/pdf/sk/FTU_2.4.9.pdf (accessed on 30 August 2024).
- Bioenergy & Biofuels. Available online: https://www.irena.org/Energy-Transition/Technology/Bioenergy-and-biofuels (accessed on 18 June 2024).
- Ján, G. Konverzia Rastlinnej Biomasy na Energiu (Conversion of Plant Biomass to Energy). Naše Pole, 5 November 2020. ISSN 1335-2466. Available online: https://nasepole.sk/konverzia-rastlinnej-biomasy-na-energiu/ (accessed on 4 June 2024).
- WBA. Global Bioenergy Statistics. 2023. Available online: https://www.worldbioenergy.org/uploads/231219%20GBS%20Report.pdf (accessed on 7 June 2024).
- WBA. Global Bioenergy Statistics. 2022. Available online: https://www.worldbioenergy.org/uploads/221223%20WBA%20GBS%202022.pdf (accessed on 9 July 2024).
- Ondro, T.; Vitázek, I.; Húlan, T.; Lawson, M.; Csáki, Š. Non-isothermal kinetic analysis of the thermal decomposition of spruce wood in air atmosphere. Res. Agric. Eng. 2018, 64, 41–46. [Google Scholar] [CrossRef]
- Piteľ, J.; Mižáková, J.; Hošovský, A. Biomass combustion control and stabilization using low-cost sensors. Adv. Mech. Eng. 2013, 5, 685157. [Google Scholar] [CrossRef]
- Mižáková, J.; Piteľ, J.; Hošovský, A.; Kolarčík, M.; Ratnayake, M. Using special filter with membership function in biomass combustion process control. Appl. Sci. 2018, 8, 1279. [Google Scholar] [CrossRef]
- Álvarez, A.; Pizarro, C.; García, R.; Bueno, J.L.; Lavín, A.G. Determination of kinetic parameters for biomass combustion. Bioresour. Technol. 2016, 216, 36–43. [Google Scholar] [CrossRef]
- Khovanskyi, S.; Pavlenko, I.; Pitel, J.; Mizakova, J.; Ochowiak, M.; Grechka, I. Solving the coupled aerodynamic and thermal problem for modeling the air distribution devices with perforated plates. Energies 2019, 12, 3488. [Google Scholar] [CrossRef]
- Holubčík, M.; Jandačka, J.; Kantová, N. Impact of the wood geometric parameters on the particulate matter production in small heat source. In Proceedings of the AIP Conference, Ho Chi Minh, Vietnam, 29–30 April 2018. [Google Scholar]
- Safi, M.J.; Mishra, I.M.; Prasad, B. Global degradation kinetics of pine needles in air. Thermochim. Acta 2004, 412, 155–162. [Google Scholar] [CrossRef]
- Orfão, J.J.M.; Antunes, F.J.A.; Figueiredo, J.L. Pyrolysis kinetics of lignocellulosic materials—Three independent reactions model. Fuel 1999, 78, 349–358. [Google Scholar] [CrossRef]
- Vitázek, I.; Ondro, T.; Sunitrová, I.; Majdan, R.; Šotnar, M. Thermoanalytical investigation of selected fuel during isothermal heating. Agron. Res. 2019, 17, 2455–2459. [Google Scholar] [CrossRef]
- Bilbao, R.; Mastral, J.F.; Aldea, M.E.; Ceamanos, J. Kinetic study for the thermal decomposition of cellulose and pine sawdust in an air atmosphere. J. Anal. Appl. Pyrol. 1997, 39, 53–64. [Google Scholar] [CrossRef]
- Fang, M.X.; Shen, D.K.; Li, Y.X.; Yu, C.J.; Luo, Z.Y.; Cen, K.F. Kinetic study on pyrolysis and combustion of wood under different oxygen concentrations by using TG-FTIR analysis. J. Anal. Appl. Pyrol. 2006, 77, 22–27. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Luo, K.H.; Bridgwater, A.V.; Fang, M.X. Kinetic study on thermal decomposition of woods in oxidative environment. Fuel 2009, 88, 1024–1030. [Google Scholar] [CrossRef]
- Liu, N.A.; Fan, W.; Dobashi, R.; Huang, L. Kinetic modeling of thermal decomposition of natural cellulosic materials in air atmosphere. J. Anal. Appl. Pyrol. 2002, 62, 303–325. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, B.; Ji, Y.; Xu, F.; Zong, P.; Zhang, J.; Tian, Y. Thermal decomposition of castor oil, corn starch, soy protein, lignin, xylan, and cellulose during fast pyrolysis. Bioresour. Technol. 2019, 278, 287–295. [Google Scholar] [CrossRef]
- Crestini, C.; Lange, H.; Sette, M.; Argyropoulos, D.S. On the structure of softwood kraft lignin. Green Chem. 2017, 19, 4104–4121. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Brodin, I.; Sjöholm, E.; Gallerstedt, G. The behavior of kraft lignin during thermal treatment. J. Anal. Appl. Pyrolysis 2010, 87, 70–77. [Google Scholar] [CrossRef]
- European Biochar Certificate—Guidelines for a Sustainable Production of Biochar; Ithaka Institute for Carbon Strategies: Arbaz, Switzerland, 2012; Version 8.2E of 19th April 2019. [CrossRef]
- Chargrow. How to Use Biochar: Where It Should and Should Not Be Placed. 2018. Available online: https://char-grow.com/how-to-use-biochar (accessed on 26 June 2024).
- Schmidt, H.P.; Wilson, K. The 55 Uses of Biochar; The Biochar Journal: Arbaz, Switzerland, 2014; ISSN 2297-1114. Available online: www.biochar-journal.org/en/ct/2 (accessed on 4 June 2024).
- Zheng, W.; Guo, M.; Chow, T.; Bennet, D.N.; Rajagopalan, N. Sorption properties of greenwaste biochar for two triazine pesticides. J. Hazard. Mater. 2010, 181, 121–126, ISSN 0304-3894. [Google Scholar] [CrossRef]
- Day, D.; Evans, R.J.; Lee, J.W.; Reicosky, D. Economical CO2, SOx, and NOx capture from fossil-fuel utilization with combined renewable hydrogen production and large-scale carbon sequestration. Energy 2005, 30, 2558–2579. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387, ISSN 1540-9309. [Google Scholar] [CrossRef]
- Hansson, K.-M.; Samuelsson, J.; Tullin, C.; Åmand, L.-E. Formation of HNCO, HCN, and NH3 from the pyrolysis of bark and nitrogen-containing model compounds. Combust. Flame 2004, 137, 265–277. [Google Scholar] [CrossRef]
- Wang, X.; Si, J.; Tan, H.; Ma, L.; Pourkashanian, M.; Xu, T. Nitrogen, sulfur, and chlorine transformations during the pyrolysis of straw. Energy Fuels 2010, 24, 5215–5221. [Google Scholar] [CrossRef]
- Leijenhorst, E.J.; Wolters, W.; Van De Beld, L.; Prins, W. Inorganic element transfer from biomass to fast pyrolysis oil: Review and experiments. Fuel Process. Technol. 2016, 149, 96–111. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Nannou, T.; Anguilano, L.; Krzyżyńska, R.; Ghazal, H.; Spencer, N.; Jouhara, H. Potentials of pyrolysis processes in the waste management sector. Energy Procedia 2017, 123, 387–394. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Zheng, S.; Jiang, A. Optimizing combustion of coal fired boilers for reducing NOx emission using Gaussian Process. Energy 2018, 153, 149–158. [Google Scholar] [CrossRef]
- Benedetti, V.; Patuzzi, F.; Baratieri, M. Gasification Char as a Potential Substitute of Activated Carbon in Adsorption Applications. Energy Procedia 2017, 105, 712–717. [Google Scholar] [CrossRef]
- Lu, P.; Huang, Q.; Chi, Y.; Yan, J. Preparation of high catalytic activity biochar from biomass waste for tar conversion. J. Anal. Appl. Pyrolysis 2017, 127, 47–56. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- European Biochar Certificate—Guidelines for a Sustainable Production of Biochar; Carbon Standards International: Frick, Switzerland, 2023; Version 10.3 from 5 April 2022; Available online: https://www.european-biochar.org (accessed on 2 July 2024).
- Demo, M.; Húska, D.; Tóthová, M. Vŕba (Salix L.) Ako Zdroj Biomasy pre Energetické Účely, 2nd ed.; Slovenská Poľnohospodárska Univerzita v Nitre: Nitra, Slovakia, 2013; ISBN 978-80-552-1021-6. [Google Scholar]
- STN ISO 1171: 2003 (44 1378); Tuhé Palivá. Stanovenie Popola. Slovenský Ústav Technickej Normalizácie: Bratislava, Slovakia, 2003.
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- STN EN 15148: 2010 (65 7411); Tuhé Biopalivá. Stanovenie Obsahu Prchavých Látok. Slovenský Ústav Technickej Normalizácie: Bratislava, Slovakia, 2010.
- STN EN 14775: 2010 (65 7408); Tuhé Biopalivá. Stanovenie Obsahu Popola. Slovenský Ústav Technickej Normalizácie: Bratislava, Slovakia, 2010.
- Farah, A.; Abdul, S.; Abd Razak, S.; Santhana, K.; Haspina, S.; Mohd Nasrullah, A.W. Biochar production techniques utilizing biomass waste-derived materials and environmental applications—A review. J. Hazard. Mater. Adv. 2022, 7, 100134, ISSN 2772-4166. [Google Scholar] [CrossRef]
- Kwapinski, W.; Byrne, C.M.P.; Kryachko, E.; Wolfram, P.; Adley, C.; Leahy, J.J.; Novotny, E.H.; Hayes, M.H. Biochar from Biomass and Waste. Waste Biomass 2010, 1, 177–189. [Google Scholar] [CrossRef]
- Maniraj, J.; Ramesh, M.; Kumar, S.G.; Sahayaraj, A.F. Introduction of Biochar: Sources, Composition, and Recent Updates. In Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2023; ISBN 978-981-99-5238-0. [Google Scholar] [CrossRef]
- Bird, M.; Keitel, K.; Meredith, W. Analysis of biochars for C, H, N, O, and S by elementar analyser. In Biochar—A Guide to Analytical Methods; CSIRO Publishing: Clayton South, Australia, 2017; pp. 39–50. ISBN 9781486305117. [Google Scholar]
- Mašek, O.; Budarin, V.; Gronnow, M.; Crombie, K.; Brownsort, P.; Fitzpatrick, E.; Hurst, P. Microwave and Slow Pyrolysis Biochar—Comparison of Physical and Functional Properties. J. Anal. Appl. Pyrolysis 2012, 100, 41–48. [Google Scholar] [CrossRef]
- Rasa, K.; Heikkinen, J.; Hannula, M.; Arstila, K.; Kulju, S.; Hyväluoma, J. How and why does willow biochar increase a clay soil water retention capacity? Biomass Bioenergy 2018, 119, 346–353, ISSN 0961-9534. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, X.; Alamu, S.O.; Brown, K.; Lee, S.W.; Kang, D.-H. Qualities and Quantities of Poultry Litter Biochar Characterization and Investigation. Energies 2024, 17, 2885. [Google Scholar] [CrossRef]
- Vitázek, I.; Šotnar, M.; Hrehová, S.; Darnadyová, K.; Mareček, J. Isothermal Kinetic Analysis of the Thermal Decomposition of Wood Chips from an Apple Tree. Processes 2021, 2, 195. [Google Scholar] [CrossRef]
- Lokwahwar, P.; Yulin, H.; Greg, N. Critical review of the role of ash content and composition in biomass pyrolysis. Front. Fuels 2024, 2, 1378361. [Google Scholar] [CrossRef]





| Biomass | N * (mass. %) | S * (mass. %) | Cl * (mass. %) |
|---|---|---|---|
| Wheat straw | 0.98 | 0.07 | 0.450 |
| Corn husks | 0.42 | 0.04 | 0.426 |
| Sunflower | 0.5 | 0.1 | 0.856 |
| Cotton stalks | 0.92 | 0.11 | 0.159 |
| Wood from fruit trees | 0.62 | 0.06 | 0.049 |
| Time Interval | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Impact period, minute | 60 | 120 | 60 | 60 | 60 | 60 |
| Temperature, °C | 20–105 | 105 | 105–500 | 500 | 500–815 | 815 |
| Biomass | Express | Inger | Sven |
|---|---|---|---|
| Average gas flow [m3/h] | 4.54 | 8.73 | 3.07 |
| Production of biochar from 1 kg biomass | 0.252 | 0.106 | 0.256 |
| Biomass | N % | C % | H % | S % |
|---|---|---|---|---|
| Sven | 1.3 | 79.3 | 2.6 | 0.2 |
| Express | 1.0 | 78.0 | 2.7 | 0.2 |
| Inger | 1.0 | 78.3 | 2.7 | 0.3 |
| Biomass | Express | Inger | Sven |
|---|---|---|---|
| Moisture content wet basis, % | 2.001 | 2.428 | 1.674 |
| Ash content in dry matter, pps, % | 7.210 | 6.008 | 7.279 |
| Volatile matter content in dry matter, phs, % | 92.790 | 93.992 | 92.721 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giertl, T.; Vitázek, I.; Gaduš, J.; Kollárik, R.; Przydatek, G. Thermochemical Conversion of Biomass into 2nd Generation Biofuel. Processes 2024, 12, 2658. https://doi.org/10.3390/pr12122658
Giertl T, Vitázek I, Gaduš J, Kollárik R, Przydatek G. Thermochemical Conversion of Biomass into 2nd Generation Biofuel. Processes. 2024; 12(12):2658. https://doi.org/10.3390/pr12122658
Chicago/Turabian StyleGiertl, Tomáš, Ivan Vitázek, Ján Gaduš, Rastislav Kollárik, and Grzegorz Przydatek. 2024. "Thermochemical Conversion of Biomass into 2nd Generation Biofuel" Processes 12, no. 12: 2658. https://doi.org/10.3390/pr12122658
APA StyleGiertl, T., Vitázek, I., Gaduš, J., Kollárik, R., & Przydatek, G. (2024). Thermochemical Conversion of Biomass into 2nd Generation Biofuel. Processes, 12(12), 2658. https://doi.org/10.3390/pr12122658






