1. Introduction
The ‘water-energy nexus’ has become a topic of increasing attention for the scientific, technological, and policy communities during the last decades. The fact that some innovative technologies can solve the problems with water cleanness and quality, but their energetic demands are higher than the original technology, drives inventors to consider more seriously the complexity of water treatment technologies, especially their energetic requirements. Renewable energy, especially the energy of the sun and wind, is characterized by variable production. But current technological progress, e.g., smart systems for energy storage (battery systems, hydrogen production, etc.), enables coupling renewable energy sources even with water treatment technologies that need to be continually powered. Renewable energy thus can be used in water pumping, desalination, disinfection, decontamination, wastewater treatment, phosphorus removal, and many other processes leading to the gain of clean water [
1].
The insoluble suspended solid particles (SP) frequently cause turbidity or opacity and belong to widespread waterborne contamination, especially in surface water [
2,
3]. High levels of impurities degrade the quality of water, and, in the case of drinking water sources, it elevates the costs of its purification. Suspended matter does not have to be dangerous to public health, except in cases like the occurrence of pathogenic germs or co-contamination with toxic substances [
4]; nevertheless, SP deteriorates at least the organoleptic water properties. Moreover, they can create deposits and cause clogging in water delivery system pipes and fixtures [
5]. Depending on the nature and level of SP contamination, various methods and technologies can be used for their separation [
6,
7], but not all of them are sufficiently sustainable, cost-effective, suitable for decentralization, or connectable with renewable energy sources.
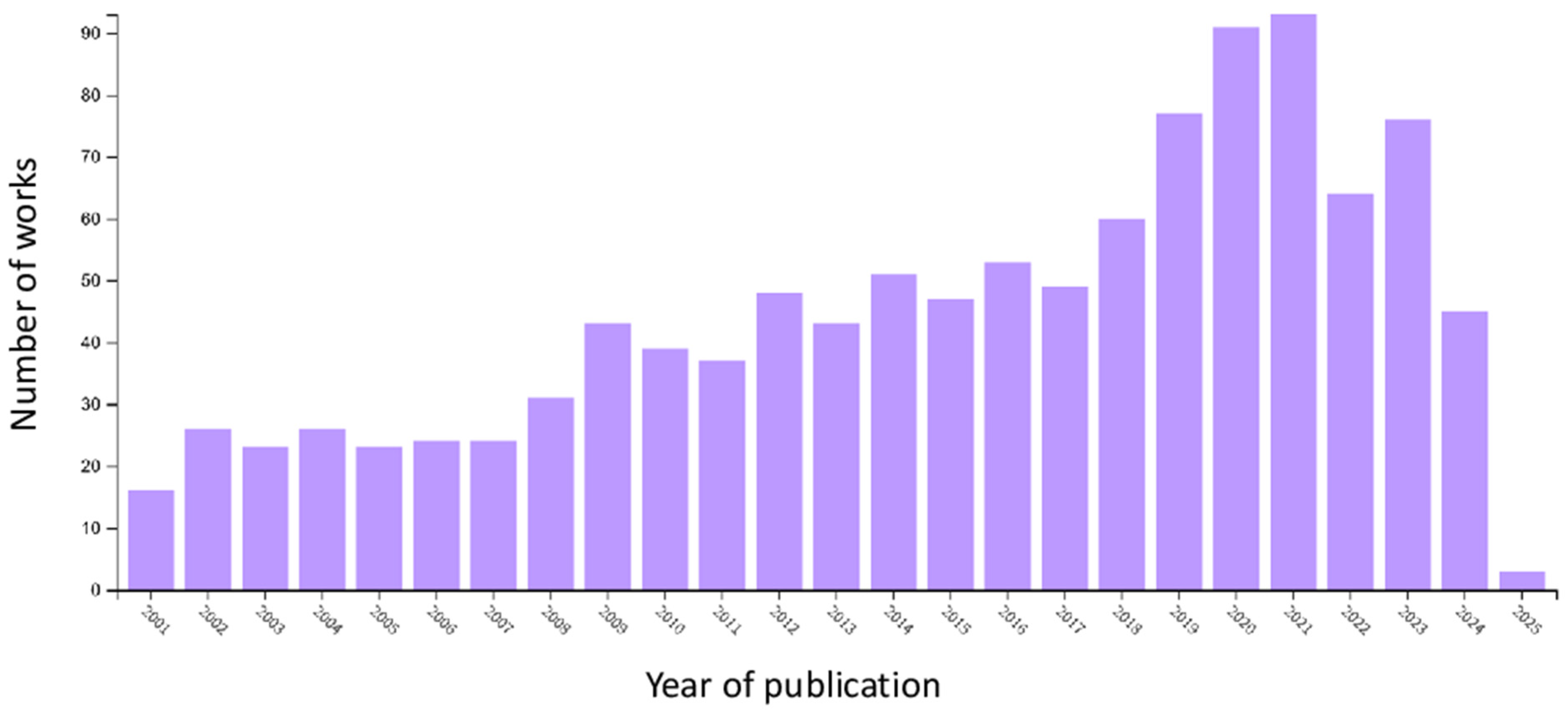
Available literature sources on suspended particles/solids in water and methods for their elimination were analyzed using the Clarivate Web of Science database tools. A search in their “Core Collection” with the phrase “remov* suspended solid* particle* water*” in the summer of 2024 has yielded about 1300 records. Nearly all the papers were published during the last four decades (from 1990 to 2024) with an increasing tendency and reaching a maximum in the years 2020 and 2021 (up to 90 works per year;
Figure 1). According to the categories or scientific disciplines, most of the works fall under environmental sciences, water resources, and environmental, chemical, civil, or agricultural engineering and their multidisciplinary combinations. More than 90% of the works represent research articles, and only 33 works (approx. 2.5%) are reviews.
A similar search in “All databases” yielded nearly 6000 entries, with more than 3000 articles and 78 reviews. More than 35% of the records are registered patents on methods, techniques, technologies, or instrumentation meant for water treatment. Still, in many cases, they are not sufficiently relevant to the topic of SP separation.
As shown in the literature and known from current standard practice, there are three main categories of water treatment methods [
8,
9,
10]: physical, including settling/sedimentation, filtration, reverse osmosis, adsorption, or photo- (UV) treatments; chemical, including coagulation and flocculation, electrochemical treatments, ion exchange, or oxidation/reduction; and biological approaches like phytoremediation (rhizofiltration, constructed wetlands), microbial biodegradation, or digestion in bioreactors. Besides physical methods like filtration, chemical coagulation/flocculation is more or less conventional and widely used due to its high efficiency and relative cost-effectiveness. However, chemical treatment may be unfriendly to the environment and human health due to the need for synthetic chemicals. On the other hand, biological methods or hybrid technologies combining different approaches attempting to overcome the disadvantages of conventional methods have been intensively studied and developed for the last few decades [
11]. Besides their ecological safety, the attention is also devoted to minimizing their energetic demands concerning the current development of renewable energy sources, which could be used to power them up.
Humankind needs reliable and sustainable technologies to purify surface water and thus increase the number of potential drinking water sources due to the lack of clean groundwater in many regions worldwide. Decentralized semi-autonomous or fully autonomous facilities, like desalination systems [
12] or small (waste)water treatment plants [
13,
14] powered by a locally available renewable energy source, can thus comprise an advantageous solution not only for developing countries. On the other hand, even other areas of human activity, like agriculture or industry, nowadays tend to save water sources by recycling and reusing their wastewater. Still, its direct re-use without at least minimal treatment is usually impossible. This review aims to compare various water treatment processes with respect to SP removal and to focus on economically viable and eco-friendly approaches.
2. Definition of Suspended Particles
Suspended matter participates significantly in the functioning of surface aquatic ecosystems. According to the concentration, nature, and type of the suspended particles, it takes part in processes like light absorption, temperature regulation, oxygen regulation, primary production, decomposition, and nutrient cycling or contaminant binding. Moreover, the concentration and composition of suspended matter are subject to dynamic change by both internal processes such as sedimentation, resuspension, (dis)aggregation, biomass growth, or decomposition, and external factors like atmospheric deposition, shore erosion and land runoff, or chemical precipitation [
15,
16,
17].
The raw surface water can contain a broad spectrum of suspended solid matter. Inorganic solids include gravel, sand, silt, clay, and dissolved minerals. Organic particles represent living microorganisms, like (phyto)plankton and bacteria, viruses [
18], and a wide range of organic matter of both natural (dead biomass, large complexes or molecules like humic substances, proteins, and polysaccharides) and anthropogenic origin, including micro- and nanoplastics [
19,
20,
21,
22,
23]. Moreover, the particulate matter can be co-contaminated by dissolved pollutants like persistent residues of pesticides, dyes [
24,
25], oils [
26], and many other organics. Inorganic and organic particles can also collide and stick together to form composite particles, so-called flocks [
2,
27]. When considering the treated water, for instance, from a drinking water treatment plant, the spectrum of SP can be enlarged by a filter material (e.g., sand or activated carbon particles from charcoal), coagulation and flocculation products (iron, aluminum, or organic flocs), or microbes from biofilters [
28].
Some of the particles are stable (like inorganic silt or clay), some are “non-degradable” (microplastics), and others can be precipitable (metals and other ions) or bio-degradable (microorganisms and bio-molecules) [
29]. Solids of high density tend to settle down quickly, and lightweight particles, especially those with higher organic content, can float or remain suspended in the water column for a long time, thus reducing water transparency.
According to size, Tambo and Kamei [
29] classified the suspended solids in water into three groups—suspensions with particle size over 1 µm, colloids with particle size between 1 nm and 1 µm, and solutes (soluble matter) with size smaller than 1 nm. Big solid particles can usually be successfully separated by rough and well-known industrial-sized techniques like filtration in a deep bed. On the other hand, colloidal nanoparticles represent a challenge, calling for more sophisticated technologies to convert them into a more easily separable form.
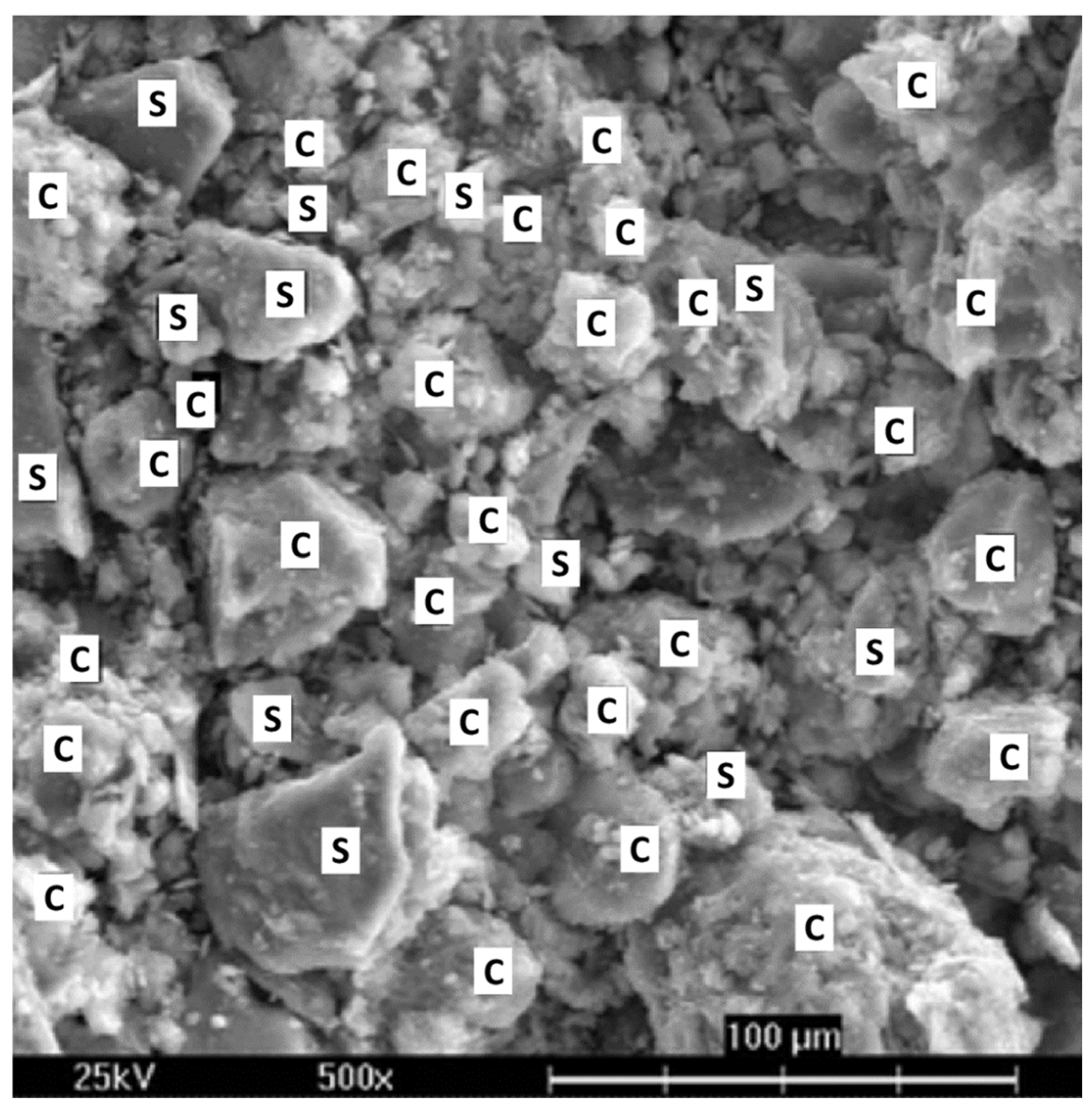
Considering the shape of SP, many instruments and calculations rely on the assumption of spherical particles [
30,
31,
32], which is valid mainly for microbial cells. Nevertheless, real SP can be found even in diverse non-spherical shapes—rods, flakes, rectangles, fibers, and combinations (
Figure 2). Their morphology affects, e.g., the settling rates and can be essential for enhancing the solid–liquid separation effectiveness.
Besides (but with) their nature, shape, and size, even other physico-chemical properties of SP play essential roles in their extractability, like the electrokinetic charge of their surface. So-called “ζ-potential” (zeta potential), enabling the existence of an “electric double layer” on the particle surface, is an essential property of solid particles, especially the tiny colloids. Knowing its value can help select the proper method to disturb the attractivity between the particle surface and water molecules or free ions in the surroundings and thus separate the particles.
3. Methods of SP Quantification
The suspended particles’ nature, size, shape, and optical properties (e.g., color or reflectivity [
2]) are important factors affecting their proper separation and even their correct quantification.
3.1. Optical Turbidity Measurements
The turbidity measurement is the most common way to quantify SP suspended in a water column. This quantification method is based on an optical approach. Suspended SPs in water samples cause the absorption and scattering of a passing radiation beam, and the changes in radiation intensities are recorded by a photodetector (or a series of detectors), usually set at a right angle to the source beam (nephelometry). Recorded values are then recalculated to turbidity units compared to a reference sample. Turbidity is expressed in nephelometric turbidity units (NTU) or formazine turbidity units (FTU, if compared to the formazine turbidity standard solutions). The measurement can be carried out in visible light, far-red, or near-infrared spectra according to the device type and settings. A comprehensive overview of turbidity measurement methods and instruments can be found in Matos et al. [
33]. For a homogeneous SP population, the turbidity value can be recalculated into concentration units using a calibration curve.
Due to technological progress, turbidity measurements can be carried out in situ and in continuous mode to provide real-time data and refer more or less reliably to the actual SP concentration in the water column. A disadvantage of such turbidity measurement is that it can underestimate the total content of solids in water if there are quickly settling particles of higher density [
34] or overestimate it if the water column is accidentally contaminated by re-suspended sediment particles due to a momentary change in hydraulic properties of water flow (e.g., during flushes).
The current legislation in many countries worldwide (including the European Union, North and South America [
35], or some African countries [
36]) adopted the World Health Organization recommendation that the turbidity of drinking water, in general, should not exceed 5 NTU (or 1 NTU on the outflow from a water treatment plant).
3.2. Gravimetric Measurements
Another way to quantify the SP in water samples is to assess their content gravimetrically. An aliquot of a water sample is evaporated (usually oven-dried at 103–105 °C), and the residuum in a vessel represents the total solids weight. Water samples can also be sieved/filtered, and the sieve/filter content is then oven-dried until constant weight. When a set of sieves/filters of different pore sizes is used, the total SP amount corresponds to the sum of the amounts caught by each sieve/filter. The SP content is expressed as weight per volume unit (e.g., mg/L).
Methodologies of gravimetric SP assessment are standardized in many countries according to the current legislation related to water quality assessment (see
Table 1).
According to the standards, the term “total solids” includes “total suspended solids (TSS)” (i.e., the amount of SP defined as particles retainable by a filter with 1 µm (EU and CZ standards) or 1.5 to 2 µm (US standards) pore size, weight assessed after drying at 103–105 °C) and “total dissolved solids (TDS)” (particles that pass through a filter with 1.5 µm (US) or 0.45 µm (EU) pore size, weight after drying usually at 180 °C (US) or 105 to 180 °C (EU)). Moreover, both groups can contain “volatile solids” (predominantly organic solids, detectable as a weight loss on ignition at a temperature of 550 °C) and “fixed solids” (non-volatile) as the rest. However, this classification does not allow us to distinguish precisely between inorganic and organic solids. Although the loss on ignition is mainly due to organic matter combustion, some small portions of minerals can be lost due to decomposition or volatilization, too.
Suspended particles in a water sample can be characterized even by the portion of settleable and non-settleable particles as the difference between the total suspended solids of the whole sample and the total suspended solids of the liquid layer above the sediment after a sedimentation period.
4. Technologies for SP Separation
As stated in the Introduction chapter, several physical, chemical, biological, or hybrid approaches are currently utilized for SP and other contamination removal in water treatment processes. The selection of the proper method (or a sequence of methods) should respect the SP’s nature, size, and concentration, as well as the target purpose of the treated water (drinking water, non-potable utility water, natural water body). The common aim is to separate SP as efficiently as possible, minimizing the sludge amount produced, energy consumed, and operational costs needed, and maintaining high ecotoxicological safety to protect the environment and human health. The most commonly used methods are summarized in
Table 2, and their advantages and disadvantages are discussed further in the following chapters. Considering the economic point of view, the technologies differ significantly in their operation costs and energy (mainly electricity) consumption in relation to the method(s) utilized and facility size.
4.1. Filtration and Other Physical Methods
The most common and frequently used physical technique for SP removal from water is filtration. The filtration mechanism includes straining, impaction, interception, or adhesion and is affected primarily by particle size [
3]. Screens, disks, and centrifugal filters can remove particles bigger than 100 μm in diameter. Filtering technologies suitable for removing small particles, for example, microorganisms smaller than 10 μm, are membrane filtration or slow sand filtration/deep bed filtration [
42]. The smallest particles, including molecules and ions, can be removed by reverse osmosis [
43]. Besides being utilized as a pre-treatment, filtration can even be the last step of water purification after other methods like coagulation/flocculation have been applied.
The filtration can run in gravity without additional energy, but such a process is slow and inefficient. Therefore, the filtration systems usually comprise pumps for feeding the system (filtration by overpressure) or draining the filtered water (filtration by negative pressure). The biggest portion of energy consumption in filtration processes is therefore connected with running the pumps for feeding the system or backwashing the filtration media, and according to Yateh et al. [
66], the common energy input ranges from 0.005 to 0.014 kWh/m
3 of processed water.
The disadvantage of filtration lies in the gradual clogging of the filters/membranes/filtration media. The rate of clogging tightly corresponds with the size and amount of SP in water—a high amount of small SP will lead to quick clogging and the necessity to backwash or even replace the filtration media frequently. This disadvantage can be partly overcome when additional technologies are applied before the primary filtration media, e.g., acoustic (ultrasonic), thermal, magnetic, or electric fields [
44]. Their primary role is to diminish the number of particles reaching the filtration media and maintain the pores free from water passing through. For example, an electric field can interact with particle charges and thus limit the undesirable charge-driven interaction between particles and filtration media material. Similarly, hybrid technologies combining conventional methods (coagulation/flocculation) and advanced methods (ozonation-biological activated carbon filtration) can represent highly efficient pre-treatment, preventing the clogging of filtration systems (e.g., membrane nanofiltration or reverse osmosis), especially by organic material [
11,
45], and therefore enabling the production of high-quality clean water.
4.2. Coagulation and Flocculation Treatments
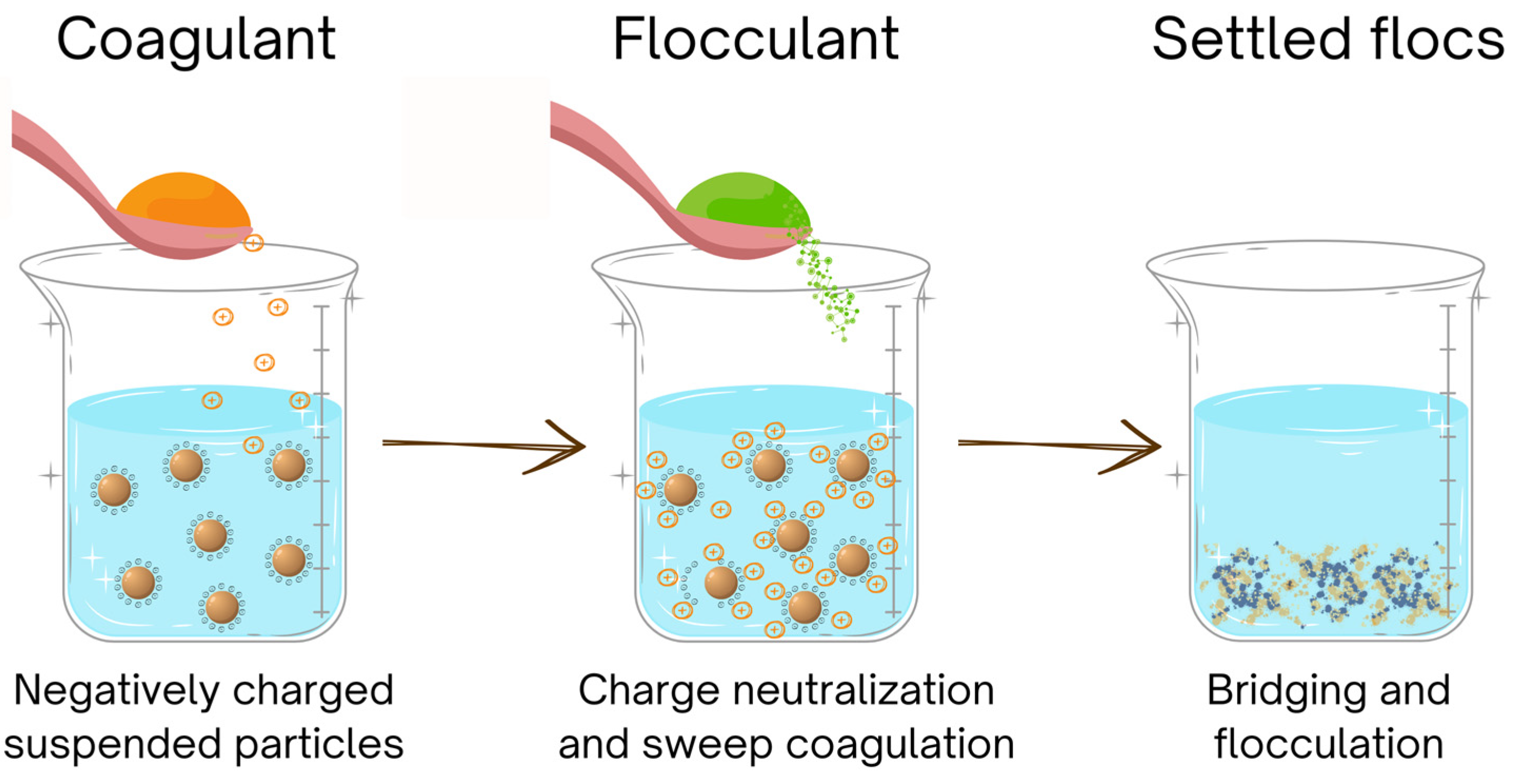
Coagulation and flocculation (CF) are the commonly employed methods in water and wastewater treatment to remove suspended particles, impurities, or, e.g., excessive nutrients (
Figure 3). The methods use various coagulants/flocculants to neutralize the SP surface charge (destabilizing their zeta potential), thus enabling their precipitation into agglomerates. Zeta potentials of both SP and coagulant are usually dependent on pH value. Under optimal conditions, when charges are neutralized, flocculation can occur and includes two other mechanisms—polymer bridging and enmeshment [
24]. Besides pH value and the type and amount of coagulant/flocculant, the performance of CF processes is influenced even by stirring/mixing intensity and speed, as well as the reaction time needed.
The resulting flocks should be either sufficiently big, heavy, and stable to be readily separable by settling and filtration or lightweight floating flocks lifted by gas bubbles, which can be collected from the water surface [
46]. In both cases, the aim is to use low amounts of coagulants/flocculants and to produce as little sludge/sediment/foam as possible, which should be easily dewaterable, degradable, and non-toxic.
The energetic demands of CF processes are tightly connected with all the steps involved, like coagulant distribution and mixing with the treated water, mixture agitation, final flocculation, and treated water/sludge pumping. Grzegorzek et al. [
67] and Yateh et al. [
66] computed the overall electricity consumption of CF processes from 0.004 up to 0.7 kWh/m
3. Similarly, Bukhary et al. [
68] stated that the coagulation and flocculation processes, along with the pumps used to distribute treated water, are the biggest energy consumers in wastewater treatment and, therefore, even producers of emissions, accounting for up to 95% of total energy consumption (up to 0.004 kWh/m
3 in their different experimental setup scenarios). As they summarized, big wastewater treatment plants can consume 0.2 to 0.4 kWh/m
3. Renewable energy can be used to power water systems, including drinking water and wastewater treatment, as well as water pumping and distribution. Using a solar-based design, the net reduction in carbon emissions with and without the provision of battery storage was found to be 450 and 240 tonnes CO
2-eq per year, respectively.
4.2.1. Coagulation
Currently, there are several commonly used coagulation agents, including metal salts, mainly with aluminum or iron ions. Metal ion-based coagulants (polyaluminum chloride, sodium aluminate, iron sulfate, iron chloride, etc.) are highly efficient in removing a wide range of SP, mineral nutrients like phosphorus, and other water impurities. But factors like (i) relatively high costs of their production and handling, (ii) hazardous potential for health (especially aluminum) in case of excessive use, and (iii) usually high amounts of formed undegradable sludge or sediment currently raise concern regarding their use. In cases when it is necessary to remove the sludge, its thickening or dewatering poses challenges [
47,
48]. Additionally, introducing the metal salts into the water leads to elevated chloride and sulfate anion occurrences. Many metal salts are also acidic, often requiring the addition of other chemicals to adjust the coagulation pH to the optimal range for treatment if natural alkalinity is insufficient. Although pH and coagulant concentration are commonly studied parameters, coagulation efficiency depends on other water quality parameters, such as total hardness and ionic strength [
49,
50].
4.2.2. Coagulant and Flocculant Aid
To reduce the required dosage of metal coagulants and keep the process efficient, so-called coagulant and flocculant aid can be used for the enhancement. Commonly used agent aids include synthetic organic polymers, e.g., polyacrylamide or polyDADMAC (polydiallyldimethylammonium chloride) [
51]. These polymers are approved for use in drinking water treatment. PolyDADMAC is often preferred over polyacrylamides (PAMs) because of its lower molecular weight and lower tendency to reduce filtration time. However, these compounds can be problematic because of high production costs, potential toxicity, and resistance to biodegradation. Scientists, therefore, turn their attention to natural substances like chitosan [
19,
52] or materials like charcoal and ash (e.g., silica-rich rice straw ash [
53]). These substances have the potential to overcome the drawbacks of synthetic compounds and keep the high efficiency of CF processes. Additionally, they could reduce the costs of the technology, making it more accessible in more regions, including those in developing countries. Another way is to use more complex mixtures of compounds with coagulation/flocculation capability, like zinc oxide, polyacrylate, and plant biomass-derived tannins (ZOPAT [
24]). As Othmani et al. [
25] stated in the example of polygalacturonic acid and other polysaccharides contained in dry cactus biomass powder, the significant advantage of bio-based coagulants/flocculants can be the amphoteric nature of their molecules (or mixture components) because they can interact with both cationic and anionic SP at the same time.
4.2.3. Alternative Methods for Chemical Coagulation/Flocculation Processes
Electrochemical Processes
Physico-chemical alternatives to chemical coagulation are various electrochemical processes like electrocoagulation, electro-flocculation, electro-oxidation, electro-flotation, and others [
54,
55,
69]. Compared to the chemical CF, their main advantage is that these processes are driven by electric current and can run entirely without (or at least with minimal) addition of any chemicals. Passing electric current between electrodes immersed in the purified water causes (i) partial water electrolysis producing H
+ and OH
− ions, thus creating a highly reactive environment, and (ii) the release of gasses (H
2, O
2, Cl
2, and others) in the form of bubbles, which can significantly enhance the flotation of impurities. Electric potential directly affects all the charged particles, causing their stacking on the electrode surface. Moreover, the material of electrodes (especially in the case of so-called sacrificed metal Fe or Al anodes) can, if corroded, serve as an in situ produced coagulation agent. Currently, available devices for electrochemical water treatment can be powered by renewable energy sources and work more or less autonomously. As Aryanti et al. [
69] summarized in their review, such integration can provide more efficient and faster treatment than conventional methods. Still, some questions usually remain, like suitable size and setup of the device (e.g., concerning the lifetime of electrodes depending on the material used) and the complexity of renewable energy source integration (solar panels, wind turbines, battery packs, hydrogen or microbial fuel cells, and all the infrastructure needed), which could counteract in case of small-scale installations due to high initial and operation costs.
Electrocoagulation (EC), as an electrochemical water treatment process, uses electric current to remove contaminants from water by destabilizing and aggregating suspended solids, toxic metals, oils, and other pollutants, including microplastics [
23]. The process is being widely investigated for applications such as wastewater treatment, industrial effluent remediation, and even desalination. In most applications, the energy consumption ranges from 0.2 to 5 kWh/m
3, contingent on the previously mentioned variables. In the case of water with low to moderate pollutant levels, the energy consumption can be as low as 0.2–1 kWh/m
3. Highly polluted water, such as that coming from industry, can increase the energy consumption to 2–5 kWh/m
3 or higher [
69]. High energetic demands of EC are to some extent counterbalanced with the advantage to run without the addition of any chemicals.
Hydrodynamic Shear Flow and Flotation
A physical alternative to chemical coagulation can be a hydrodynamic shear flow of treated water between static and moving (usually rotating) parts of a shear flow reactor [
53]. The shear forces in vortices and the occurrence of tiny microbubbles enhance the SP agglomeration due to increasing the possibility of particle collisions. Authors calculated the overall energy consumption of the shear mixer to be 0.478 kWh/m
3 and the whole process, including microbubble flotation, to be 1.1 kWh/m
3 as compared to conventional dissolved-air flotation with 0.6 kWh/m
3 for winery wastewater. Even there the higher energy consumption brought an elevated efficiency of the water treatment process.
Flocculation and especially flotation of coagulated SP can be enhanced by physical methods, including bubbling with microbubbles (dissolved air flotation), where the efficiency increases with decreasing size of bubbles [
46,
56]. Bubbles trapped inside or on the surface of SP flocs cause them to float close to the water surface or create a foam.
Advanced Oxidation Processes
Advanced oxidation treatment (AOP) represents a set of methods based on a usually non-specific reaction of SP and other water impurities with in situ occurring radicals like hydroxyl radicals, ozone, hydrogen peroxide, and other oxidative agents. The methods comprise chemical oxidation processes (Fenton reaction, H
2O
2/O
3, and O
3/Fe
2O
3), photochemical processes (UV light/H
2O
2, UV light/O
3, and photo-Fenton system), or photocatalytic processes (like a combination of UV/TiO
2) [
57]. Due to their wide applicability and high throughput, they represent a promising alternative or at least a considerable supplement to conventional technologies, especially in the case when being applied against emerging contaminants like pharmaceuticals or dyes. Sugha and Bhatti [
70] reported a successful application of the UV/H
2O
2 system for methylene blue dye removal, but the energy consumption reached 11 kWh/m
3 in highly polluted water. Han et al. [
71] described an application of UV light with various photosensitive chemicals as an AOP system for treating antibiotic-resistant bacteria and calculated the median energy consumption to be 9.86 kWh/m
3 needed to lower the bacteria population by one order of magnitude.
Besides chemicals, a highly efficient source of oxidative species in water can be even a cold plasma discharge [
58], which has been studied intensively in the last two decades as an effective tool for eliminating pesticides, drugs, and other persistent organics [
59,
60,
61]. Gerrity et al. [
72] reported a successful cold plasma-assisted degradation of various drugs, and the overall energy consumption did not exceed 3 kWh/m
3.
The only disadvantage of AOP methods is that the resulting degradation products may be more toxic than the parent compounds due to changed physico-chemical properties like solubility, hydrophobicity, or bioavailability. Therefore, AOP methods can serve as useful pre-treatment technologies.
As shown by Ikhlaq et al. [
73] on SP and other impurities in wastewater from a car wash station, ozonation can be successfully used in combination with both conventional (sedimentation, filtration) and alternative (rice husk and activated carbon absorption) technologies as a powerful aid with wastewater purification and recycling.
All the equipment (like water and air pumps, ozone generators, etc.) needed for physical (or physico-chemical) alternatives to conventional CF treatment technologies can be powered by renewable energy sources, especially in the case of decentralized small-scale facilities, but a cost–benefit analysis considering the investment costs, treatment efficiency, and even environmental benefits should always be conducted.
4.3. Phytoremediation
Macrophytes, including macroalgae and especially higher vascular plants, both aquatic and terrestrial, represent a valuable tool for biological water treatment. Plants are essential thanks to their ability to absorb contaminants via their roots, accumulate them in the biomass, or transport and utilize them in the above-ground parts. They are highly efficient in removing nutrients (nitrogen, phosphorus), trace elements, metal ions (including toxic/heavy metals), and even many organic compounds, including micropollutants. Moreover, the surface of roots, stems, or leaves can provide a place for bigger suspended particles entrapment or microbial biofilm attachment. Successful phytoremediation strategies are based on hydroponics or aquaponics and include the transplantation of emergent and submersed plants, constructed wetlands, and vegetated floating islands into streams, rivers, and other surface water bodies (
Figure 4) [
62]. Besides the aquatic vegetation, even the terrestrial or littoral vegetation cover can serve as a filter, substantially limiting the water contamination by runoff from banks or shores if planted in a sufficiently wide stripe along the stream [
63], or can at least change the sediment transport and behavior during, e.g., flood events [
64]. Especially in agricultural soils, soil erosion and runoff are substantial sources of SP contamination in water streams and bodies. Proper field management, including the cultivation of riparian vegetation stripes, can substantially improve the quality of water [
15], also shown by He et al. [
65] on a model of shrub stripe and its positive effect on a reduction in debris flow at least until its capacity is filled up.
Compared to chemical or physical methods with immediate effect, phytoremediation is a slow process with a time scale reaching months to years [
62]. Moreover, its performance depends on the inflow SP concentrations and other (potentially toxic) contaminants occurrence [
16], actual climate conditions, and the vegetation properties—(i) species composition, age, and metabolic activity of the plants; (ii) regular maintenance of the vegetation, like the removal of excessive or dead biomass, transplantation of new plants, or protection in case of unfavorable conditions, e.g., during winter; and (iii) the amount and composition of microbial biofilms attached to the surface of immersed plant parts [
16]. Compared with the other methods, phytoremediation approaches need nearly no energetic input during the function. On the other hand, the produced plant biomass can be harvested and used as a renewable energy source if burnt or digested in a suitable facility [
12].
5. Sustainable and Eco-Friendly Technologies Applicable in Real Life
Phytoremediation, as mentioned above in
Section 4.3, certainly belongs to nature-friendly and sustainable water treatment and SP removal technologies, but its slowness disqualifies it from massive spreading. Nevertheless, as summarized by Srivastava et al. [
15], biological approaches connected primarily to the agricultural landscape, like crop rotation, tillage management, vegetative cover conservation, planting of shrubs and trees for alleys, grassed waterways, and wetlands restoration, construction of water and sediment control basins, etc., can significantly help to prevent water contamination with SP and other agriculture-related wastes like excess of nutrients or residues of pesticides. A similar role of vegetation when being incorporated into sustainable urban drainage systems is also described by García-Haba et al. [
21] concerning removing microplastics from urban stormwater runoffs.
Regions with acute clean water scarcity need technologies that (i) can process a sufficient volume of water in a short time to secure a reliable water source, (ii) will be easy to handle, and (iii) will be cost-effective and sustainable with high participation of local sources. These demands turn us back to conventional physico-chemical or hybrid technologies but with the aim to enhance them by replacing synthetic chemical agents or expensive machinery with in situ available natural products and nature-friendly approaches, including powering them up from renewable energy sources, thus enabling the decentralization of such facilities.
Annan et al. [
6] described a cheap and efficient production of sintered ceramic filters for water filtering made of local clay, kaolin, and fine sawdust in Ghana. After sintering at 800 °C, these 1.5 cm thick filters with 10 cm in diameter lowered the SP concentration in river water by 80–90% and the turbidity by nearly 99%, reaching the WHO limit 5 of NTU for drinking water when filtering approx. 50–60 mL of water per hour in gravity.
In the case of coagulation/flocculation processes, newly studied natural coagulants/flocculants (NCFs) may be the right way. Based on laboratory tests and small-scale batch experiments, NCFs seem to have considerable potential, but real applications are still scarce. In 2020, Ang and Mohammad [
8] published an exhaustive review of the current research devoted to discovering and testing the efficiency of a wide range of natural products (predominantly plant extracts and biomass or shellfish waste) as replacements for the synthetic chemicals currently used. As they stated, these NCFs differ in their mode of action compared to synthetic (predominantly inorganic) chemicals because they do not form hydroxide precipitates in water. Those with polymeric structures and charged functional groups can mainly neutralize the SP charge and bridge the particles, leading to floc formation. Their main advantages are renewability, biodegradability, nontoxicity, and relative cost-effectiveness because they can come from waste or by-products originating from, e.g., food processing or agriculture. However, besides the compounds with coagulation/flocculation capability (usually proteins or polysaccharides, like starch [
47]), raw materials from which these NCFs are derived also contain a wide range of non-coagulating compounds (lipids, oils). If these impurities are not removed, they can substantially lower the NCF capability and efficiency and cause secondary contamination instead of purification. As the authors conclude, part of the research should be devoted to efficient NCF extraction and purification processes and assessing ways to enhance their purification capability even more.
Another way of NCF use is their coupling with conventional chemical coagulants, serving as a dual or composite coagulant or coagulant aid. Composite coagulant represents a combination of NCF and other coagulant (or coagulants) working simultaneously. As a coagulant aid, NCFs are used as an auxiliary material to a primary coagulant, usually added later during the purification process. Both these approaches can maintain the high efficiency of CF processes and substantially improve other factors, such as minimizing the consumption of conventional (non-renewable) coagulants and reducing the associated adverse environmental and cost impacts.
Besides their direct use in CF processes, NCF can serve as a potent pre-treatment in hybrid approaches, lowering the costs of the main purifying technology.
6. Disposal of Separated SPs
After being successfully separated from water, the next fate of SP is related to the form of the by-product resulting from the (waste)water treatment process. Together with other water contaminants, SP usually becomes a part of sewage sludge and should be thus exposed to sludge treatment processes, including thickening and dewatering, stabilization, or anaerobic and aerobic digestion [
74], and, according to the current legislation in many countries worldwide, the final treated sludge can be landfilled or incinerated. On the other hand, at this stage, the sludge can be a highly valuable raw material for the circular economy, a renewable energy source due to the biogas production, and a source of alternative material for industrial products, like (bio)polymers or (bio)plastics [
74], or a source of strategic materials like phosphorus [
75]. Moreover, if the well-treated sludge is rich in organic matter and contains no pathogenic organisms [
76] and just negligible amounts of toxic substances (usually toxic metals), it can be applied to agricultural soils as a fertilizer or can be composted and thus create another form of re-usable raw material.
Some sludge treatment processes have usually high energetic demands (like, e.g., incineration), but the other can be profitable (like, e.g., anaerobic digestion connected with biogas production). But the question remains: what size of such a facility makes economic sense when considering the effort to decentralize such technologies and to engage renewable energy sources?
7. Conclusions
Suspended particulate matter in surface water does not usually represent a serious risk to public health but substantially deteriorates the water quality and therefore increases the costs needed for its purification. According to the current literature sources, a number of methods, technologies, and approaches are applicable to SP removal and disposal during water (and wastewater) treatment and purification. As stated in previous chapters, the optimal method or technology should be environmentally friendly, cost-effective, have high throughput, have low secondary waste (sludge) production, and be sustainable concerning local resources, including renewable energy sources for powering up. However, it is difficult to select or define one universal method that could be applied to various types of suspended solid particles. There are conventional approaches, like coagulation processes, that are usually suitable for large industrial applications but are unprofitable for small water treatment facilities. On the other hand, there are also alternative methods and technologies with “eco-friendly” and “bio-friendly” potential, like quick electrochemical processes or slow phytoremediation, where locally available resources can be applied and whose decentralization can even be supported by suitable renewable energy sources development. However, many of them are currently developing or scaling from laboratory jar tests/batch experiments and must be optimized, considering, e.g., their energetic demands, which are currently usually higher as compared to conventional methods. They, therefore, are not yet available as fully functioning and efficient technology usable in real situations. Sufficiency of valid data from their operation under realistic conditions on one side and the interest and support from local authorities or potential industrial partners on the other are the basic assumptions for substantial enhancement of their progress. A similar situation can be seen even in methods of suspended particle disposal. Being usually a part of the sludge, priority should be given to treatment technologies, which enable the re-use of this matter as a renewable energy source or recyclable raw material.
Author Contributions
Supervision, conceptualization, funding acquisition, B.M.; writing—original draft preparation, Š.Z.; writing—review and editing, E.M., K.O., M.P. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Culture of the Czech Republic NAKI III Project No. DH23P03OVV063.
Data Availability Statement
No new data were created in this study.
Acknowledgments
The authors thank Patrik Bayer from Brno University of Technology, Czech Republic, for providing the SEM microphotography of suspended particles.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Olsson, G. Clean Water Using Solar and Wind; IWA Publishing: London, UK, 2018; pp. 1–210. [Google Scholar]
- Bowers, D.G.; Binding, C.E. The optical properties of mineral suspended particles: A review and synthesis. Estuar. Coast. Shelf Sci. 2006, 67, 219–230. [Google Scholar] [CrossRef]
- Huang, J.S.; Fisher, P.R. Survey of suspended solids in irrigation water of ornamental plant nurseries and effects of filtration. J. Irrig. Drain. Eng. 2019, 145, 04019008. [Google Scholar] [CrossRef]
- Toure, A.; Wenbiao, D.; Keita, Z.; Dembele, A. Investigation of the water quality of daily used surface-sources for drinking and irrigation by the population of Segou in the center of Mali. J. Water Health 2019, 17, 338–349. [Google Scholar] [CrossRef]
- Braga, A.S.; Filion, Y. The interplay of suspended sediment concentration, particle size and fluid velocity on the rapid deposition of suspended iron oxide particles in PVC drinking water pipes. Water Res. X 2022, 15, 100143. [Google Scholar] [CrossRef] [PubMed]
- Annan, E.; Konadu, D.; George, K.N.; Yankson, A.A. Fabrication, physico-mechanical properties and turbidity reduction of disk-shaped ceramic water filters made for peri-urban rivers. Sadhana-Acad. Proc. Eng. Sci. 2022, 47, 277. [Google Scholar] [CrossRef]
- Santos, P.V.S.; Libânio, M.; Teixeira, M.C. Chitosan in the treatment of mine spoil rainwater- An approach to protect the aquatic biota. Sci. Total Environ. 2024, 912, 168900. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W. State of the art and sustainability of natural coagulants in water and wastewater treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Katrivesis, F.K.; Karela, A.D.; Papadakis, V.G.; Paraskeva, C.A. Revisiting of coagulation-flocculation processes in the production of potable water. J. Water Process Eng. 2019, 27, 193–204. [Google Scholar] [CrossRef]
- Xu, P.; He, H.; Li, T.; Chen, Y.; Dong, B. Combining full-scale ozonation and biological activated carbon filtration (O3-BAC) with pilot-scale nanofiltration (NF) to control disinfection by-product formation for treatment of Taihu Lake water. Water 2023, 15, 843. [Google Scholar] [CrossRef]
- Amiri, A.; Brewer, C. Biomass as a renewable energy source for water desalination: A review. Desalin. Water. Treat. 2020, 181, 113–122. [Google Scholar] [CrossRef]
- Meliss, M.; Neskakis, A.; Plettner-Marliani, J.; Lange, C.; Hovelmann, A.; Schumacher, J. Waste water recycling supplied by renewable energies—Basic conditions and possible treatment technologies. Renew. Energy 1998, 14, 325–331. [Google Scholar] [CrossRef]
- Schäfer, A.; Hughes, G.; Richards, B. Renewable energy powered membrane technology: A leapfrog approach to rural water treatment in developing countries? Renew. Sustain. Energy Rev. 2014, 40, 542–556. [Google Scholar] [CrossRef]
- Srivastava, S.; Basche, A.; Traylor, E.; Roy, T. The efficacy of conservation practices in reducing floods and improving water quality. Front. Environ. Sci. 2023, 11, 1136989. [Google Scholar] [CrossRef]
- Mulling, B.T.M.; van den Boomen, R.M.; Claassen, T.H.L.; van der Geest, H.G.; Kappelhof, J.; Admiraal, W. Physical and biological changes of suspended particles in a free surface flow constructed wetland. Ecol. Eng. 2013, 60, 10–18. [Google Scholar] [CrossRef]
- Jiang, C.R.; Zhang, S.B.; Wang, J.F.; Xia, X.H. Nitrous oxide (N2O) emissions decrease significantly under stronger light irradiance in riverine water columns with suspended particles. Environ. Sci. Technol. 2023, 57, 19749–19759. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shi, J.; Sharma, E.; Gao, S.; Zhou, X.; Liu, Y.; Sivakumar, M.; Jiang, G. Fate of coronaviruses during the wastewater coagulation with ferric chloride. ACS ES T Water 2023, 3, 3206–3214. [Google Scholar] [CrossRef]
- Eamrat, R.; Rujakom, S.; Pussayanavin, T.; Taweesan, A.; Witthayaphirom, C.; Kamei, T. Optimizing biocoagulant aid from shrimp shells (Litopenaeus vannamei) for enhancing microplastics removal from aqueous solutions. Environ. Technol. Innov. 2024, 33, 103457. [Google Scholar] [CrossRef]
- Zink, L.; Meslo, M.; Wiseman, S.; Pyle, G.G. Daphnia magna digestive activity is differentially altered when exposed to equally turbid waters caused by either suspended sediment or suspended microplastics. Environ. Toxicol. 2023, 39, 2086–2091. [Google Scholar] [CrossRef]
- García-Haba, E.; Hernandez-Crespo, C.; Martin, M.; Andres-Domenech, I. The role of different sustainable urban drainage systems in removing microplastics from urban runoff: A review. J. Clean. Prod. 2023, 411, 137197. [Google Scholar] [CrossRef]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-)plastic particles. Environ. Chem. 2015, 12, 539–550. [Google Scholar] [CrossRef]
- Mateo, S.; Zhang, A.; Piedra, A.; Ruiz, A.; Miranda, R.; Rodríguez, F. Electrocoagulation assessment to remove micropolystyrene particles in wastewater. ACS ES T Water 2024, 4, 3049–3058. [Google Scholar] [CrossRef]
- Ishak, S.A.; Murshed, M.F.; Zainol, M.R.R.M.A.; Kamal, N.H.M. Enhancing floc size and strength with a hybrid polymer of zinc oxide, acrylamide, and tannin in textile wastewater. Water Sci. Technol. 2023, 88, 3057–3083. [Google Scholar] [CrossRef] [PubMed]
- Othmani, B.; Gamelas, J.A.E.; Rasteiro, M.G.; Khadhraoui, M. Characterization of two cactus formulation-based flocculants and investigation on their flocculating ability for cationic and anionic dyes removal. Polymers 2020, 12, 1964. [Google Scholar] [CrossRef]
- Loh, A.; Shankar, R.; Ha, S.Y.; An, J.G.; Yim, U.H. Suspended particles enhance biodegradation of oil in sea. Sci. Total Environ. 2019, 685, 324–331. [Google Scholar] [CrossRef]
- Walling, D.E.; Woodward, J.C. Use of a field-based water elutriation system for monitoring the in-situ particle-size characteristics of fluvial suspended sediment. Water Res. 1993, 27, 1413–1421. [Google Scholar] [CrossRef]
- Gauthier, V.; Barbeau, B.; Millette, R.; Block, J.C.; Prévost, M.; Iwa. Suspended particles in the drinking water of two distribution systems. Water Sci. Technol. Water Supply 2001, 1, 237–245. [Google Scholar] [CrossRef]
- Tambo, N.; Kamei, T. Coagulation and flocculation on water quality matrix. Water Sci. Technol. 1998, 37, 31–41. [Google Scholar] [CrossRef]
- Brinker, A.; Schröder, H.; Rösch, R. A high-resolution technique to size suspended solids in flow-through fish farms. Aquacult. Eng. 2005, 32, 325–341. [Google Scholar] [CrossRef]
- Yu, R.; Chen, H.; Cheng, W.; Chu, M. Simultaneously monitoring the particle size distribution, morphology and suspended solids concentration in wastewater applying digital image analysis (DIA). Environ. Monit. Assess. 2009, 148, 19–26. [Google Scholar] [CrossRef]
- Lopez-Betancur, D.; González-Ramírez, E.; Guerrero-Mendez, C.; Saucedo-Anaya, T.; Rivera, M.; Olmos-Trujillo, E.; Jimenez, S. Evaluation of optimization algorithms for measurement of suspended solids. Water 2024, 16, 1761. [Google Scholar] [CrossRef]
- Matos, T.; Martins, M.; Henriques, R.; Goncalves, L. A review of methods and instruments to monitor turbidity and suspended sediment concentration. J. Water Process Eng. 2024, 64, 105624. [Google Scholar] [CrossRef]
- Selbig, W.R.; Bannerman, R.T. Ratios of total suspended solids to suspended sediment concentrations by particle size. J. Environ. Eng. 2011, 137, 1075–1081. [Google Scholar] [CrossRef]
- Pinto, V.; Heller, L.; Bastos, R. Drinking water standards in South American countries: Convergences and divergences. J. Water Health 2012, 10, 295–310. [Google Scholar] [CrossRef][Green Version]
- Gara, T.; Li, F.; Nhapi, I.; Makate, C.; Gumindoga, W. Health safety of drinking water supplied in Africa: A closer look using applicable water-quality standards as a measure. Expos. Health 2018, 10, 117–128. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 24th ed.; American Water Works Association: Denver, CO, USA, 2022. [Google Scholar]
- ASTM D3977-97; Standard Test Method for Determining Sediment Concentration in Water Samples. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2009.
- EPA 600/4-79.020; Methods for Chemical Analysis of Water and Wastes. U.S. Environmental Protection Agency (US EPA): Washington, DC, USA, 1979.
- EN 872-2005 (ICS 13.060.30); Water Quality—Determination of Suspended Solids—Method by Filtration Through Glass Fiberfibre Filters. International Organization for Standardization (ISO): Geneva, Switzerland, 2005.
- ČSN 75 7346-2024 (ICS 13.060.01; 13.060.45); Water Quality—Determination of Dissolved Substances. International Organization for Standardization (ISO): Geneva, Switzerland, 2024.
- Jung, C.H.; Lee, K.W. Analytic solution on critical suspended particle size and minimum collection efficiency in deep bed filtration. J. Environ. Eng. 2006, 132, 1381–1386. [Google Scholar] [CrossRef]
- Pandey, S.R.; Jegatheesan, V.; Baskaran, K.; Shu, L. Fouling in reverse osmosis (RO) membrane in water recovery from secondary effluent: A review. Rev. Environ. Sci. Bio/Technol. 2012, 11, 125–145. [Google Scholar] [CrossRef]
- Mostafazadeh, A.K.; Zolfaghari, M.; Drogui, P. Electrofiltration technique for water and wastewater treatment and bio-products management: A review. J. Water Process Eng. 2016, 14, 28–40. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Role of coagulation/flocculation as a pretreatment option to reduce colloidal/bio-colloidal fouling in tertiary filtration of textile wastewater: A review and future outlooks. Front. Environ. Sci. 2023, 11, 1142227. [Google Scholar] [CrossRef]
- Ladouceur, J.D.; Narbaitz, R.M.; Lan, C.Q. Dissolved air flotation pretreatment for low-pressure membranes in water treatment: A review of fouling mitigation and product water quality. J. Water Process Eng. 2023, 56, 104391. [Google Scholar] [CrossRef]
- Cornwell, D.A.; Brown, R.A. Replacement of alum and PolyDADMAC with natural polymers—Turbidity removal and residuals reduction impacts. J. Am. Water Works Assoc. 2017, 109, E252–E264. [Google Scholar] [CrossRef]
- Dymaczewski, Z.; Kempa, E.S.; Sozanski, M.M. Coagulation as a structure-forming separation process in water and wastewater treatment. Water Sci. Technol. 1997, 36, 25–32. [Google Scholar] [CrossRef]
- Schneider, O.D.; Weinrich, L.A.; Giraldo, E.; LeChevallier, M.W. Impacts of salt type and concentration on coagulation of humic acid and silica. J. Water Supply Res. Technol.—AQUA 2013, 62, 339–349. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.-Y.; Xu, X.-M.; Xu, W.-Y. The effect of total hardness and ionic strength on the coagulation performance and kinetics of aluminum salts to remove humic acid. Chem. Eng. J. 2010, 160, 150–156. [Google Scholar] [CrossRef]
- Bolto, B.; Xie, Z. The use of polymers in the flotation treatment of wastewater. Processes 2019, 7, 374. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Badot, P.M.; Crini, G. Chitosan for coagulation/flocculation processes—An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Pashaki, S.G.A.; Khojastehpour, M.; Ebrahimi-Nik, M.; Tedesco, S. Potential of ash from agricultural waste as substitute of commercial FeCl3 in primary treatment of landfill leachate. J. Environ. Manag. 2024, 351, 119932. [Google Scholar] [CrossRef] [PubMed]
- AlJaberi, F.Y.; Ahmed, S.A.; Makki, H.F.; Naje, A.S.; Zwain, H.M.; Salman, A.D.; Juzsakova, T.; Viktor, S.; Van, B.; Le, P.-C.; et al. Recent advances and applicable flexibility potential of electrochemical processes for wastewater treatment. Sci. Total Environ. 2023, 867, 161361. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrocoagulation for the treatment of textile industry effluent—A review. J. Environ. Manag. 2013, 128, 949–963. [Google Scholar] [CrossRef]
- Vlotman, D.; Key, D.; Cerff, B.; Bladergroen, B.J. Wastewater treatment using shear enhanced flotation separation technology: A pilot plant study for winery wastewater processing. Processes 2024, 12, 3. [Google Scholar] [CrossRef]
- Khan, J.; Sayed, M.; Khan, S.; Shah, N.; Dionysiou, D.; Boczkaj, G. Advanced oxidation processes for the treatment of contaminants of emerging concern. In Contaminants of Emerging Concern in Water and Wastewater: Advanced Teatment Processes; Hernandez-Maldonado, A.J., Blaney, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 299–365. [Google Scholar] [CrossRef]
- Byeon, Y.; Hong, E.; Yoo, S.; Lho, T.; Yoon, S.; Kim, S.; Yoo, S.; Ryu, S. Ballast water treatment test at pilot-scale using an underwater capillary discharge device. Plasma Chem. Plasma Process. 2017, 37, 1405–1416. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.; Parvulescu, V. Degradation of pharmaceutical compounds in water by non-thermal plasma treatment. Water Res. 2015, 81, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Magureanu, M.; Bradu, C.; Parvulescu, V. Plasma processes for the treatment of water contaminated with harmful organic compounds. J. Phys. D Appl. Phys. 2018, 51, 313002. [Google Scholar] [CrossRef]
- Nau-Hix, C.; Multari, N.; Singh, R.; Richardson, S.; Kulkarni, P.; Anderson, R.; Holsen, T.; Thagard, S. Field demonstration of a pilot-scale plasma reactor for the rapid removal of poly- and perfluoroalkyl substances in groundwater. ACS ES T Water 2021, 1, 680–687. [Google Scholar] [CrossRef]
- Ning, D.L.; Huang, Y.; Pan, R.S.; Wang, F.Y.; Wang, H. Effect of eco-remediation using planted floating bed system on nutrients and heavy metals in urban river water and sediment: A field study in China. Sci. Total Environ. 2014, 485, 596–603. [Google Scholar] [CrossRef]
- Alemu, T.; Bahrndorff, S.; Alemayehu, E.; Ambelu, A. Agricultural sediment reduction using natural herbaceous buffer strips: A case study of the east African highland. Water Environ. J. 2017, 31, 522–527. [Google Scholar] [CrossRef]
- Perignon, M.C.; Tucker, G.E.; Griffin, E.R.; Friedman, J.M. Effects of riparian vegetation on topographic change during a large flood event, Rio Puerco, New Mexico, USA. J. Geophys. Res.-Earth 2013, 118, 1193–1209. [Google Scholar] [CrossRef]
- He, S.; Chen, W.; Wang, D.; Chen, X.; Qi, Y.; Zhao, P.; Li, Y.; Lin, Y.; Jamali, A.A. Experimental investigation of the effects of shrub filter strips on debris flow trapping and interception. Int. J. Sediment Res. 2023, 38, 265–278. [Google Scholar] [CrossRef]
- Yateh, M.; Li, F.; Tang, Y.; Li, C.; Xu, B. Energy consumption and carbon emissions management in drinking water treatment plants: A systematic review. J. Clean. Prod. 2024, 437, 140688. [Google Scholar] [CrossRef]
- Grzegorzek, M.; Wartalska, K.; Kazmierczak, B. Review of water treatment methods with a focus on energy consumption. Int. Commun. Heat Mass 2023, 143, 106674. [Google Scholar] [CrossRef]
- Bukhary, S.; Batista, J.; Ahmad, S. Design aspects, energy consumption evaluation, and offset for drinking water treatment operation. Water 2020, 12, 1772. [Google Scholar] [CrossRef]
- Aryanti, P.; Nugroho, F.; Phalakornkule, C.; Kadier, A. Energy efficiency in electrocoagulation processes for sustainable water and wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 114124. [Google Scholar] [CrossRef]
- Sugha, A.; Bhatti, M. Degradation of methylene blue dye by UV/H2O2 advanced oxidation process: Reaction kinetics, residual H2O2 and specific energy consumption evaluation. Desalination Water Treat. 2022, 274, 297–307. [Google Scholar] [CrossRef]
- Han, J.; Li, W.; Yang, Y.; Zhang, X.; Bao, S.; Zhang, X.; Zhang, T.; Leung, K. UV-based advanced oxidation processes for antibiotic resistance control: Efficiency, influencing factors, and energy consumption. Engineering 2024, 37, 27–39. [Google Scholar] [CrossRef]
- Gerrity, D.; Stanford, B.; Trenholm, R.; Snyder, S. An evaluation of a pilot-scale nonthermal plasma advanced oxidation process for trace organic compound degradation. Water Res. 2010, 44, 493–504. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Fiaz, U.; Rizvi, O.S.; Akram, A.; Qazi, U.Y.; Masood, Z.; Irfan, M.; Al-Sodani, K.A.A.; Kanwal, M.; Ibn Shamsah, S.M.; et al. Catalytic ozonation combined with conventional treatment technologies for the recycling of automobile service station wastewater. Water 2023, 15, 171. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Z.; Ganeshan, P.; Sar, T.; Xu, S.; Rajendran, K.; Sindhu, R.; Binod, P.; Pandey, A.; Zhang, Z.; et al. Anaerobic digestion in global bio-energy production for sustainable bioeconomy: Potential and research challenges. Renew. Sustain. Energy Rev. 2025, 208, 114985. [Google Scholar] [CrossRef]
- Tiwari, S.; Veksha, A.; Chan, W.; Fei, X.; Liu, W.; Lisak, G.; Lim, T. Acidic hydrothermal carbonization of sewage sludge for enhanced alkaline extraction of phosphorus and reduced co-extraction of trace elements. Resour. Conserv. Recycl. 2025, 212, 107936. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Fang, W.; Wang, R.; Wang, X.; Wang, X.; Zheng, G.; Zhou, L. Persistence evaluation of fecal pollution indicators in dewatered sludge and dewatering filtrate of municipal sewage sludge: The impacts of ambient temperature and conditioning treatments. Water Res. 2025, 268, 122641. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).