Maleic Anhydride-Modified Water Hyacinth for Adsorption of Methylene Blue and Methyl Violet
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of EC
2.3. Preparation of MA-EC
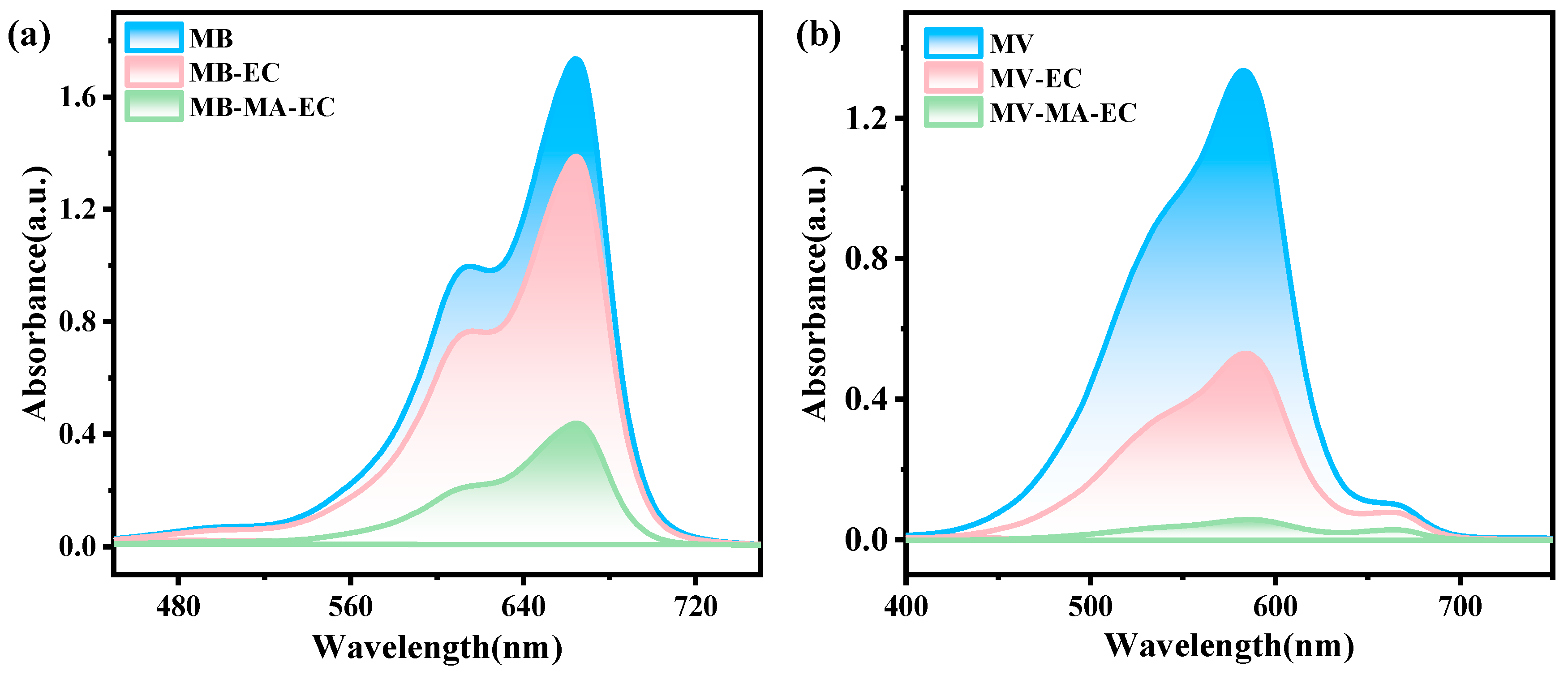
2.4. Adsorption Test
2.5. Characterizations
3. Results and Discussion
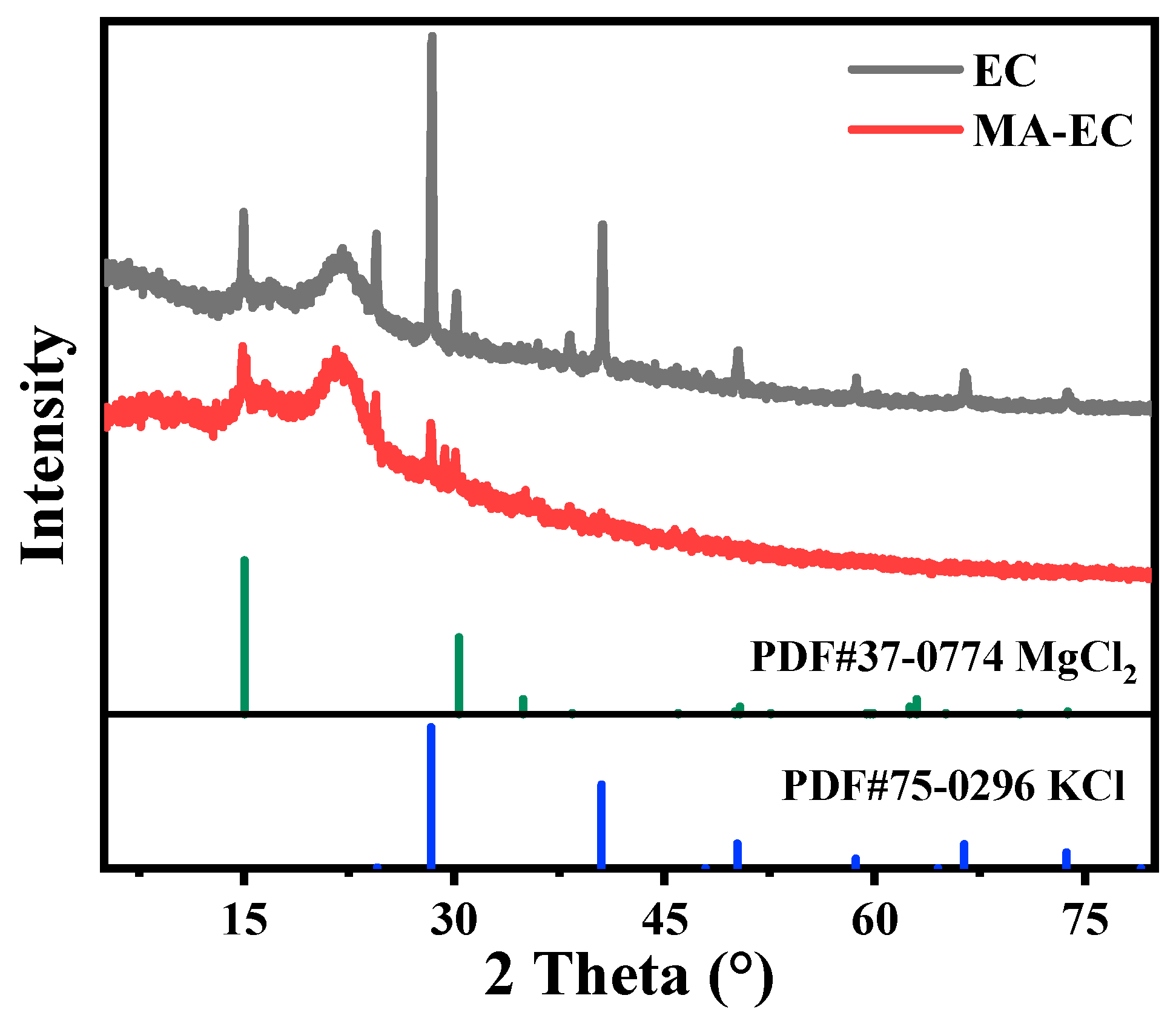
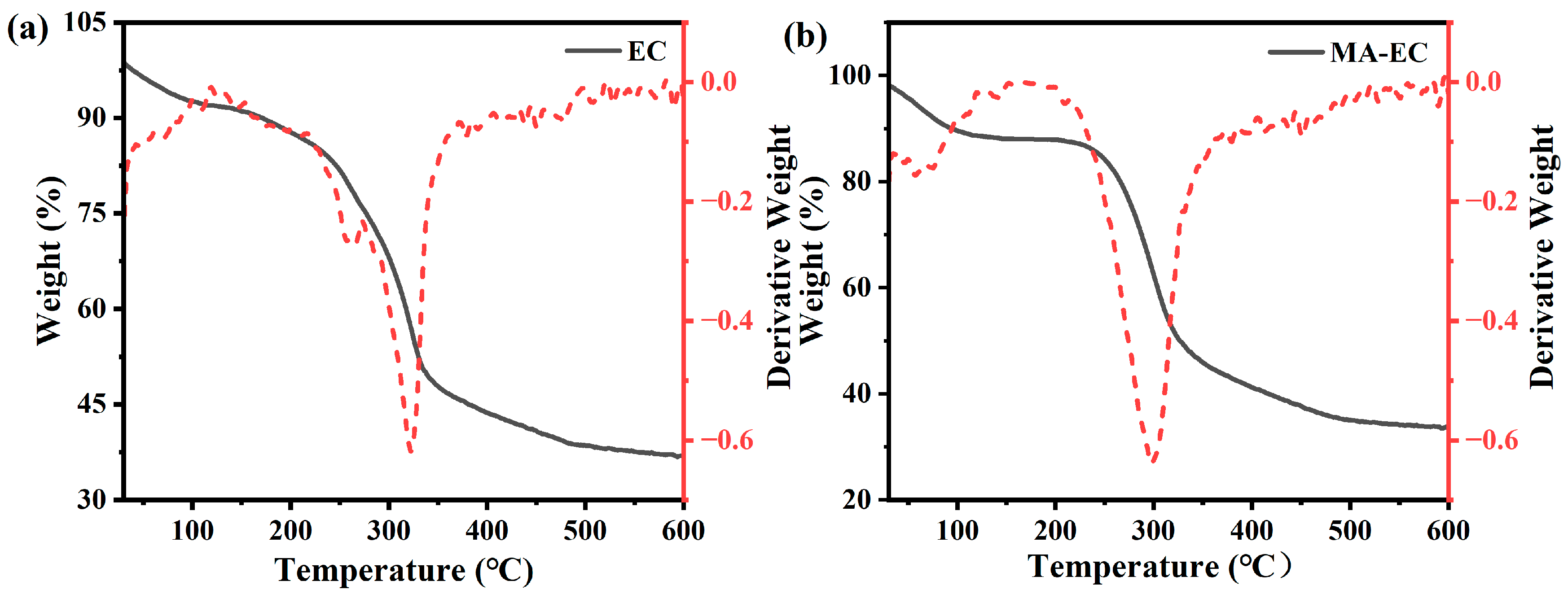
3.1. Characterization of MA-EC and EC
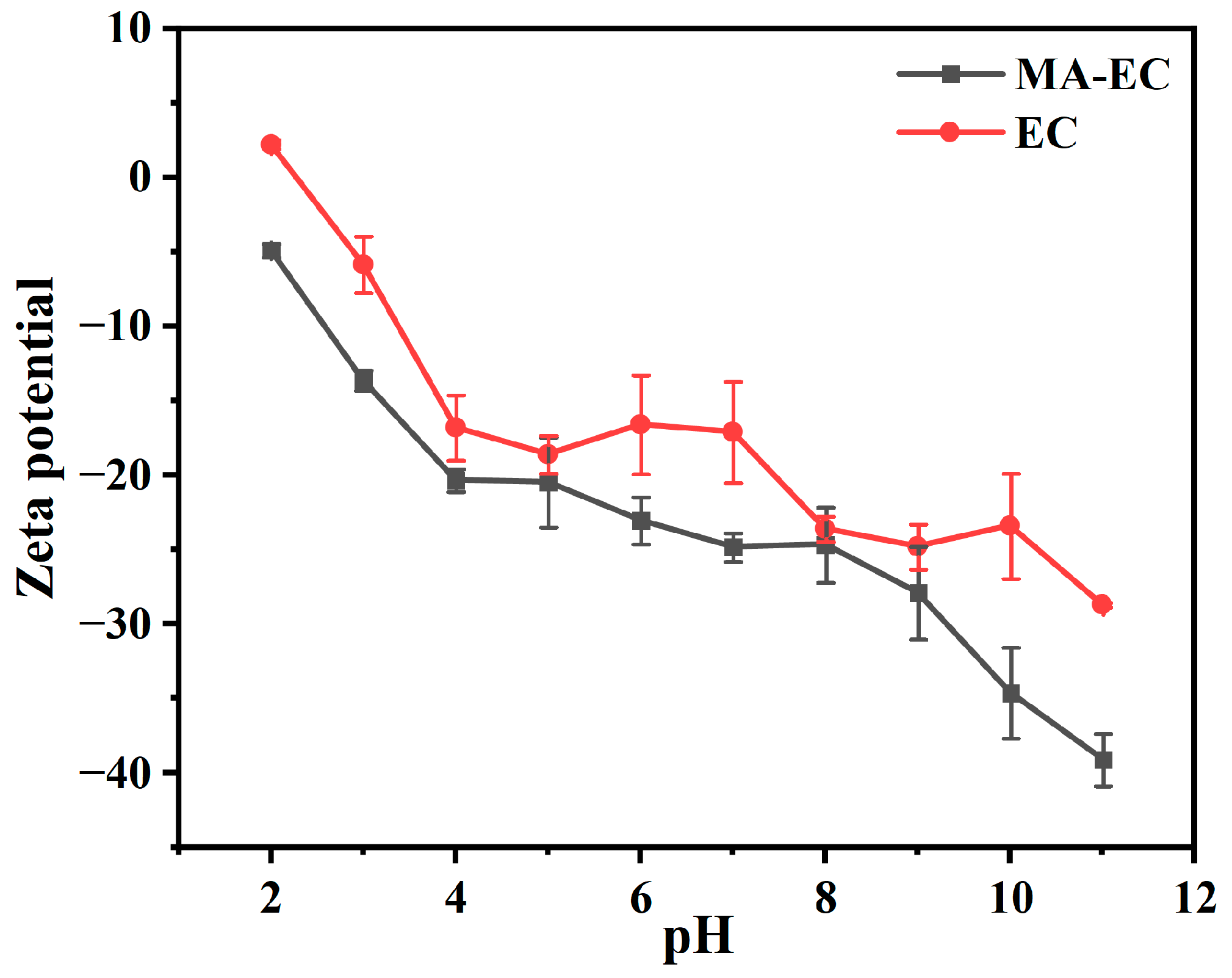
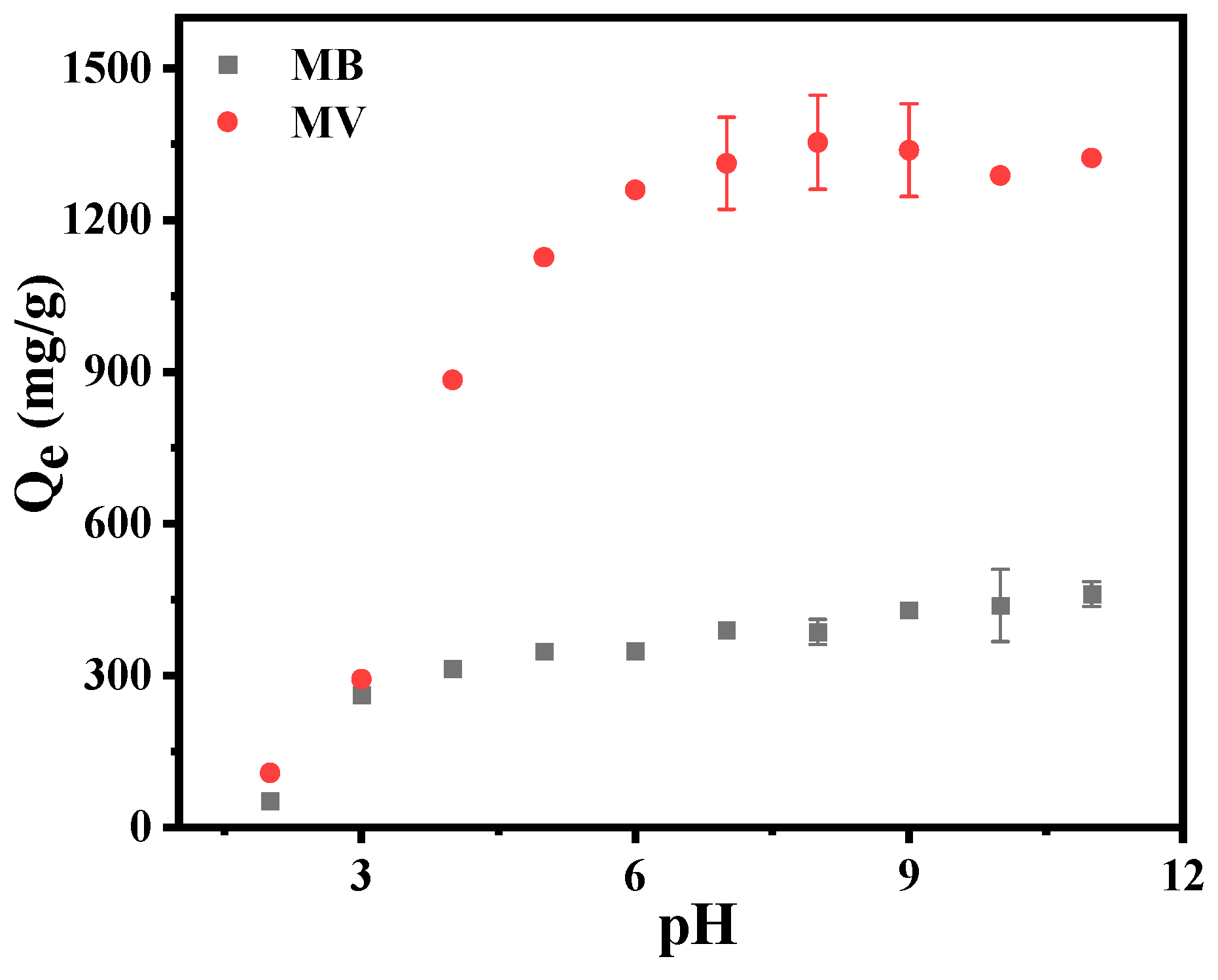
3.2. Influence of pH Value on Adsorption Properties of MA-EC
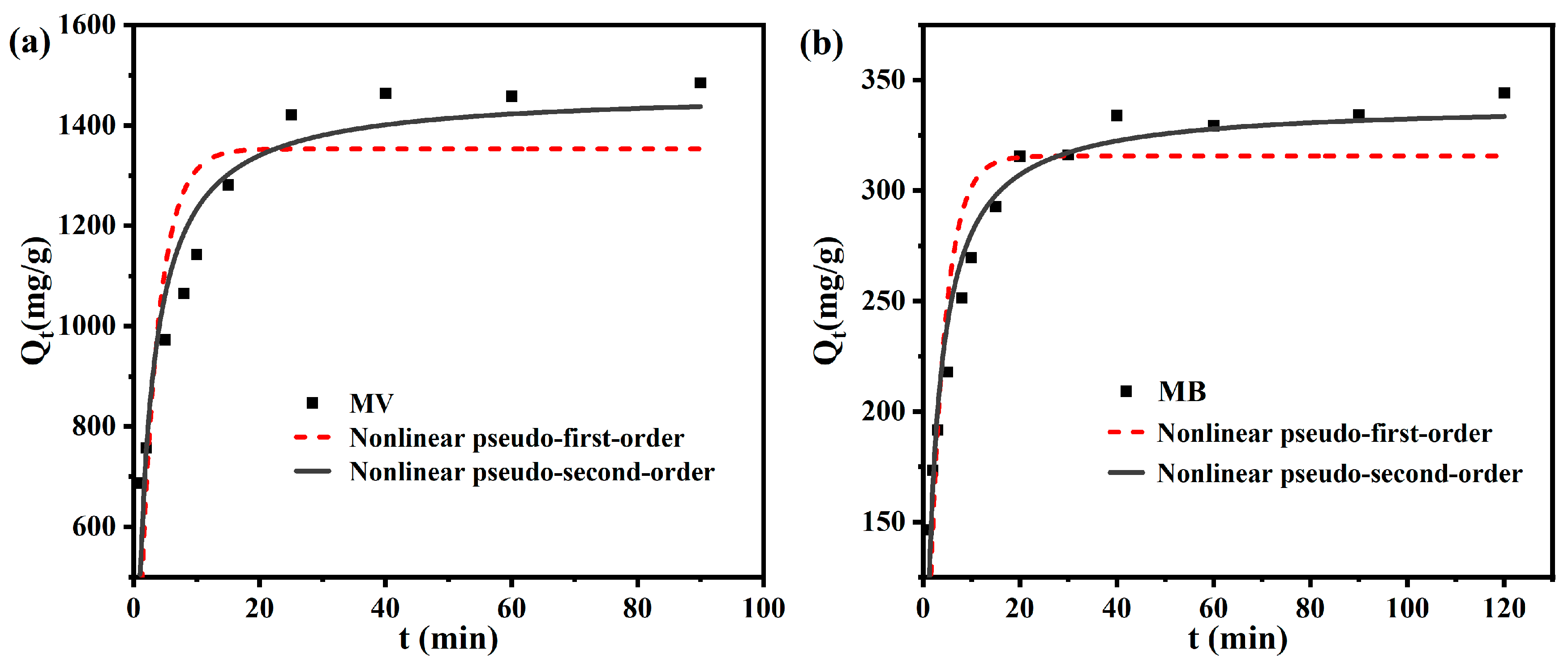
3.3. Adsorption Kinetics
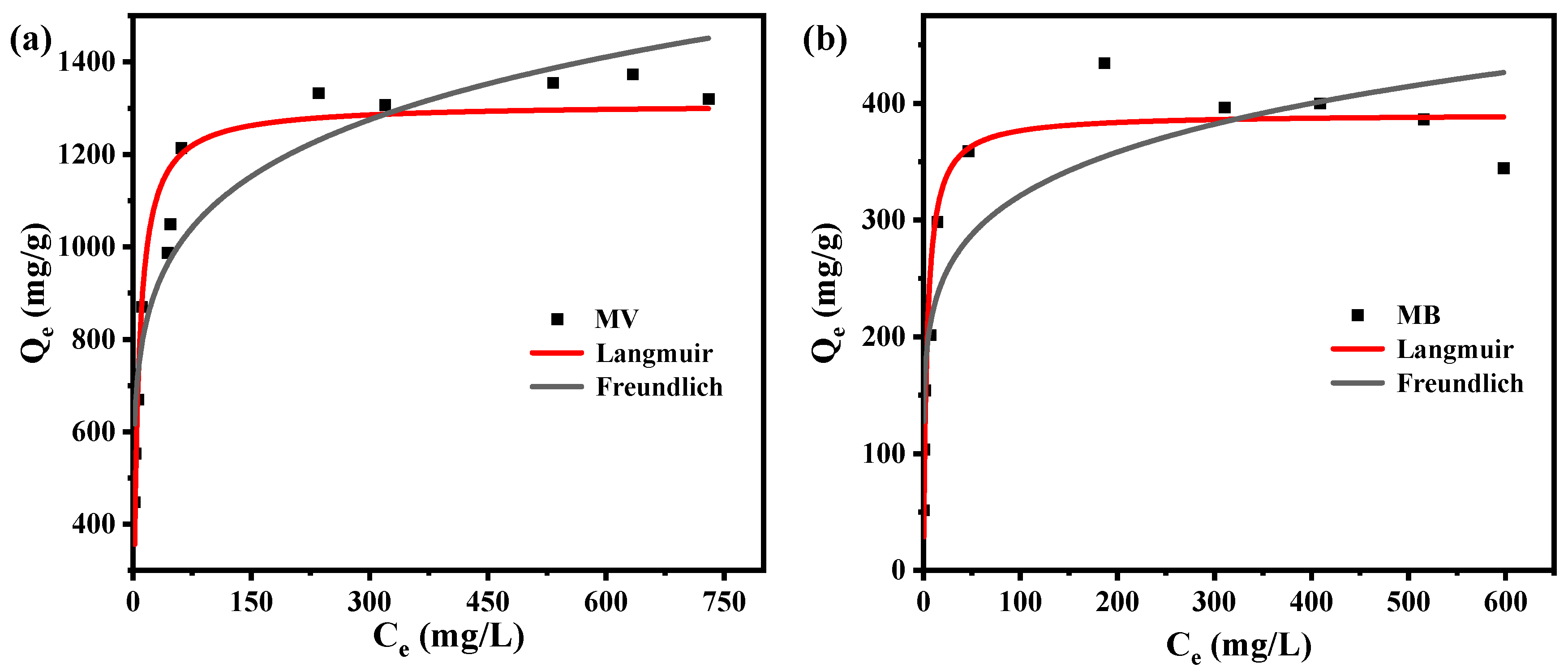
3.4. Adsorption Isotherms
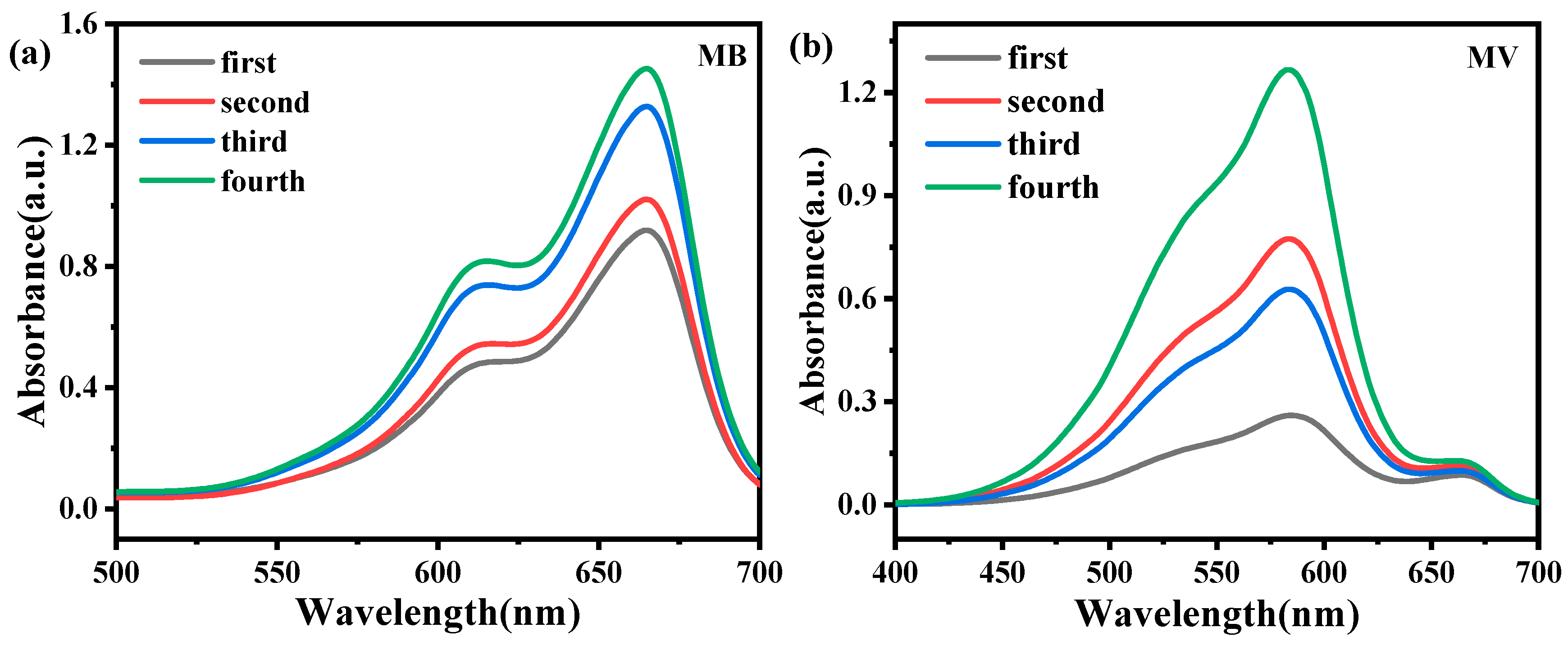
3.5. Cyclic Adsorption Experiment
3.6. Performance Evaluation of Biomass
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, L.; Na, C. Gibbsian interpretation of Langmuir, Freundlich and Temkin isotherms for adsorption in solution. Philos. Mag. Lett. 2022, 102, 239–253. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Adane, T.; Adugna, A.T.; Alemayehu, E. Textile industry effluent treatment techniques. J. Chem. 2021, 2021, 5314404. [Google Scholar] [CrossRef]
- Xie, Y.; Du, G.; Pang, J.; Kong, L.; Lu, L. One-step preparation of magnetic N-doped sodium alginate-based porous carbon and efficient adsorption of bisphenol A. Environ. Sci. Pollut. 2023, 30, 99842–99854. [Google Scholar] [CrossRef]
- Garg, A.; Chopra, L. Dye Waste: A Significant Environmental Hazard. Mater. Today 2022, 48, 1310–1315. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A new Cd (II)-based coordination polymer for efficient photocatalytic removal of organic dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Fındık, S. Sono-assisted Adsorption of Methyl Violet 2B Using a Magnetic Kaolin/TiO2/γ-Fe2O3 Nano Composite. Water Air Soil Pollut. 2024, 235, 514. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Zheng, T.; Tang, T.; Liu, H.; Liu, G.; Zhu, H. Fabrication of Composite Material Based on MOFs and its Adsorption Properties for Methylene Blue Dyes. Chin. J. Mater. Res. 2021, 35, 866–872. [Google Scholar]
- Li, P.; Zhang, J.; Yu, Y.; Jia, W.; Zhao, S. A Collaborative Coagulation Strategy for Algae-laden and Dye-containing Water Treatment. J. Clean. Prod. 2024, 442, 141146. [Google Scholar] [CrossRef]
- Radia, D.; Fouzia, T.; Rachida, R.; Wahib, N.M.; Bentahar, F. Photocatalysis Process to Treat Polluted Water by Azo Dye Cibacron Brilliant Yellow 3G-P. Water Sci. Technol. 2022, 86, 1774–1789. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y.; Wang, Y.; Zhao, X. A Research on the Strengthening Effect of Sludge Charcoal on Activated Sludge Process in Sewage Treatment. Environ. Sci. Pollut. Res. 2024, 31, 5289–5303. [Google Scholar] [CrossRef] [PubMed]
- Mpongwana, N.; Rathilal, S. Exploiting Biofilm Characteristics to Enhance Biological Nutrient Removal in Wastewater Treatment Plants. Appl. Sci. 2022, 12, 7561. [Google Scholar] [CrossRef]
- Gao, Y.; Zeng, J.; Zhu, S.; Liu, Q. Co-modification of Lignocellulosic Biomass by Maleic Anhydride and Ferric Hydroxide for the Highly Efficient Biosorption of Methylene Blue. New J. Chem. 2021, 45, 19678–19690. [Google Scholar] [CrossRef]
- Barik, A.; Rajhans, G.; Sen, S.K.; Raut, S. Agricultural Bio-wastes: A Potent Sustainable Adsorbent for Contaminant Removal. In Handbook of Solid Waste Management; Springer Nature: Singapore, 2022; pp. 571–591. [Google Scholar] [CrossRef]
- Januário, E.F.D.; Vidovix, T.B.; Araujo, L.A.D.; Bergamasco Beltran, L.; Bergamasco, R.; Vieira, A.M.S. Investigation of Citrus Reticulata Peels as an Efficient and Low-cost Adsorbent for the Removal of Safranin Orange Dye. Environ. Technol. 2022, 43, 4315–4329. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Sivamani, S. Biosorption Behavior of Acid Orange 7 Dye onto Cucumis Sativus Peel Biochar and its Effect on Growth of Triticum Aestivum. Environ. Prog. Sustain. Energy 2023, 42, e14106. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Xiao, R.; Wang, M.; Lee, Y.H.; Kang, S.W.; Seo, D.C. Characteristics of Adsorption Behavior of Potentially Toxic Metals by Biochar Derived from Fallen Leaves (Platanus) and its Mechanism. Sustain. Chem. Pharm. 2022, 29, 100776. [Google Scholar] [CrossRef]
- Sebastian, A.; Nangia, A.; Prasad, M.N.V. Cadmium and Sodium Adsorption Properties of Magnetite Nanoparticles Synthesized from Hevea Brasiliensis Muell. Arg. Bark: Relevance in Amelioration of Metal Stress in Rice. J. Hazard. Mater. 2019, 371, 261–272. [Google Scholar] [CrossRef]
- Batool, A.; Valiyaveettil, S. Chemical Transformation of Soya Waste into Stable Adsorbent for Enhanced Removal of Methylene Blue and Neutral Red from Water. J. Environ. Chem. Eng. 2021, 9, 104902. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Ali, H.H.; Nayl, A.A. Modification of Sugarcane Bagasse as a Novel Lignocellulosic Biomass Adsorbent Nanocomposite to Improve Adsorption of Methylene Blue. Cellulose 2023, 30, 5239–5258. [Google Scholar] [CrossRef]
- Yang, P.; Lu, Y.; Zhang, H.; Li, R.; Hu, X.; Shahab, A.; Elnaggar, A.Y.; Alrefaei, A.F.; AlmutairiI, M.H.; Ali, E. Effective Removal of Methylene Blue and Crystal Violet by Low-cost Biomass Derived from Eucalyptus: Characterization, Experiments, and Mechanism Investigation. Environ. Technol. 2024, 33, 103459. [Google Scholar] [CrossRef]
- Lin, H.; Chen, K.; Du, L.; Gao, P.; Zheng, J.; Liu, Y.; Ma, L. Efficient and Selective Adsorption of Methylene Blue and Methyl Violet Dyes by Yellow Passion Fruit Peel. Environ. Technol. 2021, 43, 3519–3530. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.J.; Ding, Y.; Zhuang, Q.J. Invasion and Control of Water Hyacinth (Eichhornia crassipes) in China. J. Zhejiang Univ. Sci. B 2006, 7, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, J.; Fu, Z.; Zhu, L. Water Hyacinth in China: A Sustainability Science-based Management Framework. Environ. Manag. 2007, 40, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Harun, I.; Pushiri, H.; Amirul-Aiman, A.J.; Zulkeflee, Z. Invasive Water Hyacinth: Ecology, Impacts and Prospects for the Rural Economy. Plants 2021, 10, 1613. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Kumar, P. Water hyacinth as a potential biofuel crop. J. Agric. Food Chem. 2010, 9, 112–122. [Google Scholar] [CrossRef]
- Low, K.S.; Lee, C.K.; Tan, K.K. Biosorption of basic dyes by water hyacinth roots. Bioresour. Technol. 1995, 52, 79–83. [Google Scholar] [CrossRef]
- Saltabaş, Ö.; Teker, M.; Konuk, Z. Biosorption of cationic dyes from aqueous solution by water hyacinth roots. Glob. Nest J. 2012, 14, 24–31. [Google Scholar] [CrossRef]
- Tsaniatri, D.A.; Hargono, H. Adsorption of Pb(II) from aqueous solution by acid-modified water hyacinth cellulose. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012010. [Google Scholar] [CrossRef]
- Kumar, P.; Chauhan, M.S. Adsorption of chromium (VI) from the synthetic aqueous solution using chemically modified dried water hyacinth roots. J. Environ. Chem. Eng. 2019, 7, 103218. [Google Scholar] [CrossRef]
- Sevim, F.; Lacin, O.; Ediz, E.F.; Demir, F. Adsorption Capacity, Isotherm, Kinetic, and Thermodynamic Studies on Adsorption Behavior of Malachite Green onto Natural Red Clay. Environ. Prog. Sustain. Energy 2021, 40, e13471. [Google Scholar] [CrossRef]
- Elamin, M.R.; Abdulkhair, B.Y.; Algethami, F.K.; Khezami, L. Linear and nonlinear investigations for the adsorption of paracetamol and metformin from water on acid-treated clay. Sci. Rep. 2021, 11, 13606. [Google Scholar] [CrossRef] [PubMed]
- Bátori, V.; Jabbari, M.; Srivastava, R.K.; Åkesson, D.; Lennartsson, P.R.; Zamani, A.; Taherzadeh, M.J. Synthesis and Characterization of Maleic Anhydride-grafted Orange Waste for Potential Use in Biocomposites. Bioresources 2018, 13, 4986–4997. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, Extraction and Characterization of Chlorella Vulgaris Soluble Polysaccharides and their Applications in AgNPs Biosynthesis and Biostimulation of Plant Growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef] [PubMed]
- Trilokesh, C.; Uppuluri, K.B. Isolation and Characterization of Cellulose Nanocrystals from Jackfruit Peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef]
- Yu, J.X.; Chi, R.A.; He, Z.Y.; Qi, Y.F. Adsorption performances of cationic dyes from aqueous solution on pyromellitic dianhydride modified sugarcane bagasse. Sep. Sci. Technol. 2011, 46, 452–459. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Sun, H.; Ullah, R.; Ali, A.; et al. A review on the Modification of Cellulose and its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q.; Peng, Z. Adsorption of methylene blue by an antibacterial bio-sorbents from ligninsulfonate and tannin. J. Environ. Chem. Eng. 2024, 12, 111807. [Google Scholar] [CrossRef]
- Kang, L.; Mucci, M.; Lürling, M. Influence of Temperature and pH on Phosphate Removal Efficiency of Different Sorbents Used in Lake Restoration. Sci. Total Environ. 2022, 812, 151489. [Google Scholar] [CrossRef]
- Silva, F.; Nascimento, L.; Brito, M.; da Silva, K.; Paschoal Jr, W.; Fujiyama, R. Biosorption of Methylene Blue Dye Using Natural Biosorbents Made from Weeds. Materials 2019, 12, 2486. [Google Scholar] [CrossRef]
- Sorour, F.H.; Aboeleneen, N.M.; Abd El-Monem, N.M.; Ammar, Y.A.; Mansour, R.A. Removal of Malachite Green from Wastewater Using Date Seeds as Natural Adsorbent; Isotherms, Kinetics, Thermodynamic, and Batch Adsorption Process Design. Int. J. Phytoremediat. 2024, 26, 1321–1335. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J.; Sozhan, G. Optimization of the process parameters for the removal of phosphate from drinking water by electrocoagulation. Desalination Water Treat. 2009, 12, 407–414. [Google Scholar] [CrossRef]
- You, X.; Zhou, R.; Zhu, Y.; Bu, D.; Cheng, D. Adsorption of dyes methyl violet and malachite green from aqueous solution on multi-step modified rice husk powder in single and binary systems: Characterization, adsorption behavior and physical interpretations. J. Environ. Chem. Eng. 2022, 430, 128445. [Google Scholar] [CrossRef] [PubMed]
- Kua, T.L.; Kooh, M.R.R.; Dahri, M.K.; Zaidi, N.A.H.M.; Lu, Y.; Lim, L.B.L. Aquatic plant, Ipomoea aquatica, as a potential low-cost adsorbent for the effective removal of toxic methyl violet 2B dye. Appl. Water Sci. 2020, 10, 243. [Google Scholar] [CrossRef]
- Salomón, Y.L.D.O.; Georgin, J.; Franco, D.S.; Netto, M.S.; Grassi, P.; Piccilli, D.G.; Dotto, G.L. Powdered biosorbent from pecan pericarp (Carya illinoensis) as an efficient material to uptake methyl violet 2B from effluents in batch and column operations. Adv. Powder Technol. 2020, 31, 2843–2852. [Google Scholar] [CrossRef]
- Torezan, L.; Bortoluz, J.; Guerra, N.B.; Ferrarini, F.; Bonetto, L.R.; da Silva Teixeira, C.; Carli, L.N. Magnetic chitosan microspheres for the removal of methyl violet 2B from aqueous solutions. J. Dispers. Sci. Technol. 2023, 44, 1170–1182. [Google Scholar] [CrossRef]
- Wu, T.; Yang, G.; Cao, J.; Xu, Z.; Jiang, X. Activation and adsorption mechanisms of methylene blue removal by porous biochar adsorbent derived from eggshell membrane. Chem. Eng. Res. Des. 2022, 188, 330–341. [Google Scholar] [CrossRef]
- Sen, T.K. Adsorptive removal of dye (methylene blue) organic pollutant from water by pine tree leaf biomass adsorbent. Processes 2023, 11, 1877. [Google Scholar] [CrossRef]
- Wei, W.; Shang, N.; Zhang, X.; Liu, W.; Zhang, T.; Wu, M. A green 3-step combined modification for the preparation of biomass sorbent from waste chestnut thorns shell to efficient removal of methylene blue. Bioresour. Technol. 2022, 360, 127593. [Google Scholar] [CrossRef]
- Yan, J.; Lan, G.; Qiu, H.; Chen, C.; Liu, Y.; Du, G.; Zhang, J. Adsorption of heavy metals and methylene blue from aqueous solution with citric acid modified peach stone. Sep. Sci. Technol. 2018, 53, 1678–1688. [Google Scholar] [CrossRef]





| Kinetic Model | Parameter | MV | MB |
|---|---|---|---|
| Qe-exp (mg/g) | 1484.60 | 344.21 | |
| Nonlinear pseudo-first order | Qe-cal (mg/g) | 1353.33 | 315.659 |
| k1 (1/min) | 0.35 | 0.31 | |
| R2 | 0.67 | 0.80 | |
| Nonlinear pseudo-second order | Qe-cal (mg/g) | 1467.83 | 339.39 |
| k2 (g/mg/min) | 0.0004 | 0.0014 | |
| R2 | 0.89 | 0.95 |
| Isotherm Model | Parameter | MV | MB |
|---|---|---|---|
| Freundlich: Qe = KF Ce1/n | KF (mg/g) | 554.83 | 154.54 |
| bF | 6.85 | 6.30 | |
| R2 | 0.8860 | 0.8299 | |
| Langmuir: Qe = Qm KL Ce/(1 + KL Ce) | Qm (mg/g) | 1309.12 | 390.89 |
| KL (L/mg) | 0.18 | 0.26 | |
| R2 | 0.9316 | 0.9355 |
| Adsorbing Material | Dye | Qmax (mg/g) | References |
|---|---|---|---|
| Multi-step modified rice husk | MV | 530.94 | [43] |
| Ipomoea aquatica | MV | 267.90 | [44] |
| Carya illinoensis | MV | 642.00 | [45] |
| Magnetic chitosan microspheres | MV | 128.84 | [46] |
| MA-EC | MV | 1360.73 | This work |
| Eggshell membrane | MB | 110.38 | [47] |
| Pine Tree Leaf | MB | 36.88 | [48] |
| Chestnut thorn shell | MB | 305.81 | [49] |
| Citric acid-modified peach stone | MB | 178.25 | [50] |
| MA-EC | MB | 370.37 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Xu, J.; Wang, X.; Liu, Y. Maleic Anhydride-Modified Water Hyacinth for Adsorption of Methylene Blue and Methyl Violet. Processes 2024, 12, 2290. https://doi.org/10.3390/pr12102290
Shen L, Xu J, Wang X, Liu Y. Maleic Anhydride-Modified Water Hyacinth for Adsorption of Methylene Blue and Methyl Violet. Processes. 2024; 12(10):2290. https://doi.org/10.3390/pr12102290
Chicago/Turabian StyleShen, Liya, Jing Xu, Xinru Wang, and Yuanli Liu. 2024. "Maleic Anhydride-Modified Water Hyacinth for Adsorption of Methylene Blue and Methyl Violet" Processes 12, no. 10: 2290. https://doi.org/10.3390/pr12102290
APA StyleShen, L., Xu, J., Wang, X., & Liu, Y. (2024). Maleic Anhydride-Modified Water Hyacinth for Adsorption of Methylene Blue and Methyl Violet. Processes, 12(10), 2290. https://doi.org/10.3390/pr12102290






