Optimization of CO2 Capture Using a New Aqueous Hybrid Solvent (MDEA-[TBPA][TFA]) with a Low Heat Capacity: Integration of COSMO-RS and RSM Approaches
Abstract
1. Introduction
2. Computational and Experimental Details
2.1. Thermodynamic Properties and Interaction Energy Predictions Using COSMO-RS
2.2. Experimental Section
2.2.1. Materials and Reagents
2.2.2. Apparatus
2.2.3. Preparation of the Absorbent
Synthesis of the Tetrabutylphosphonium Trifluoroacetate Ionic Liquid
Preparation of Aqueous MDEA–[TBP][TFA] Hybrid Solvents
2.2.4. Design of Experiment: RSM-FC-CCD
2.2.5. Thermophysical Characterization of MDEA-[TBP][TFA]
Density and Viscosity
Heat Capacity Measurement
3. Results and Discussion
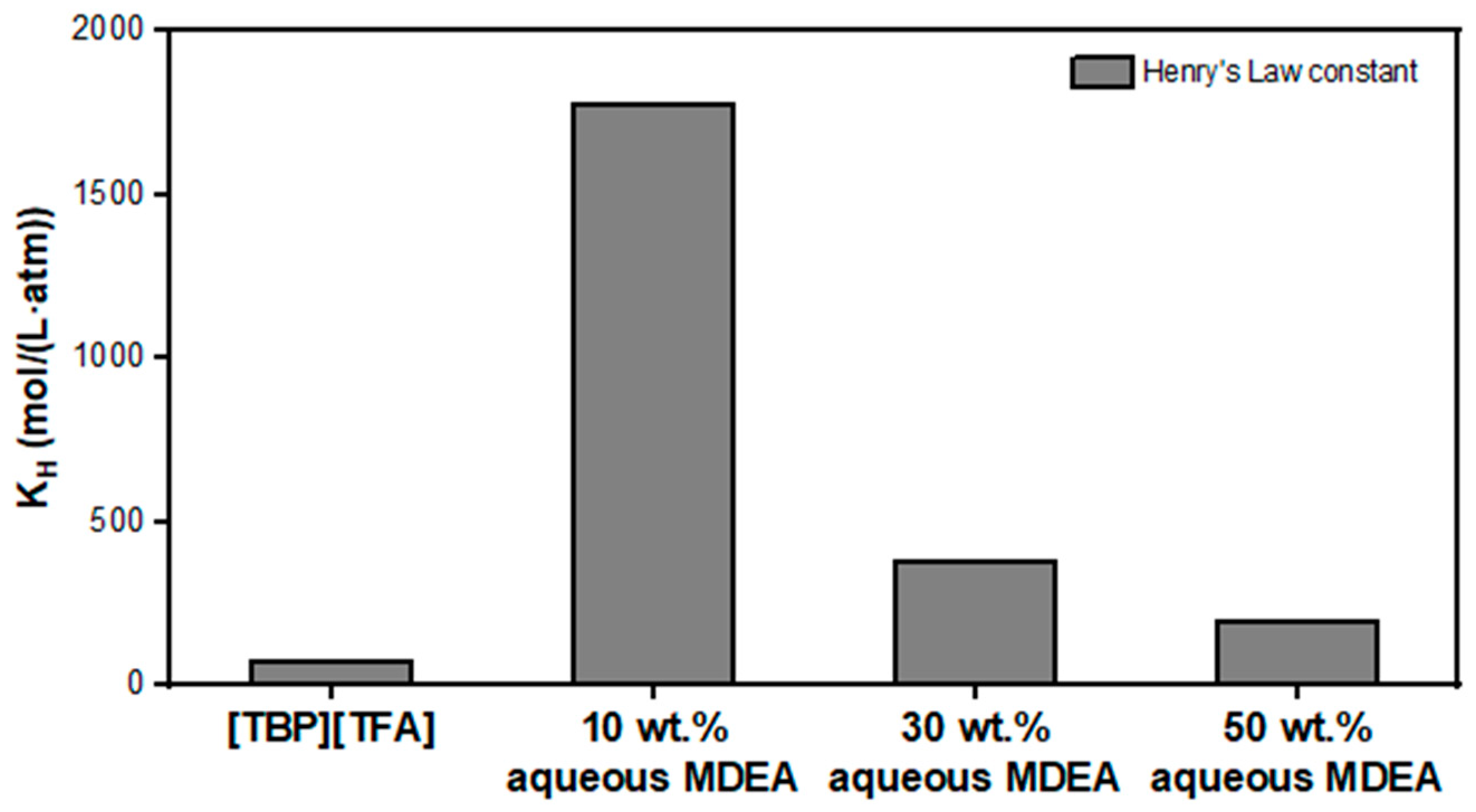
3.1. Prediction of Thermodynamic Properties of Aqueous MDEA and [TBP][TFA] for CO2 Absorption Using COSMO-RS
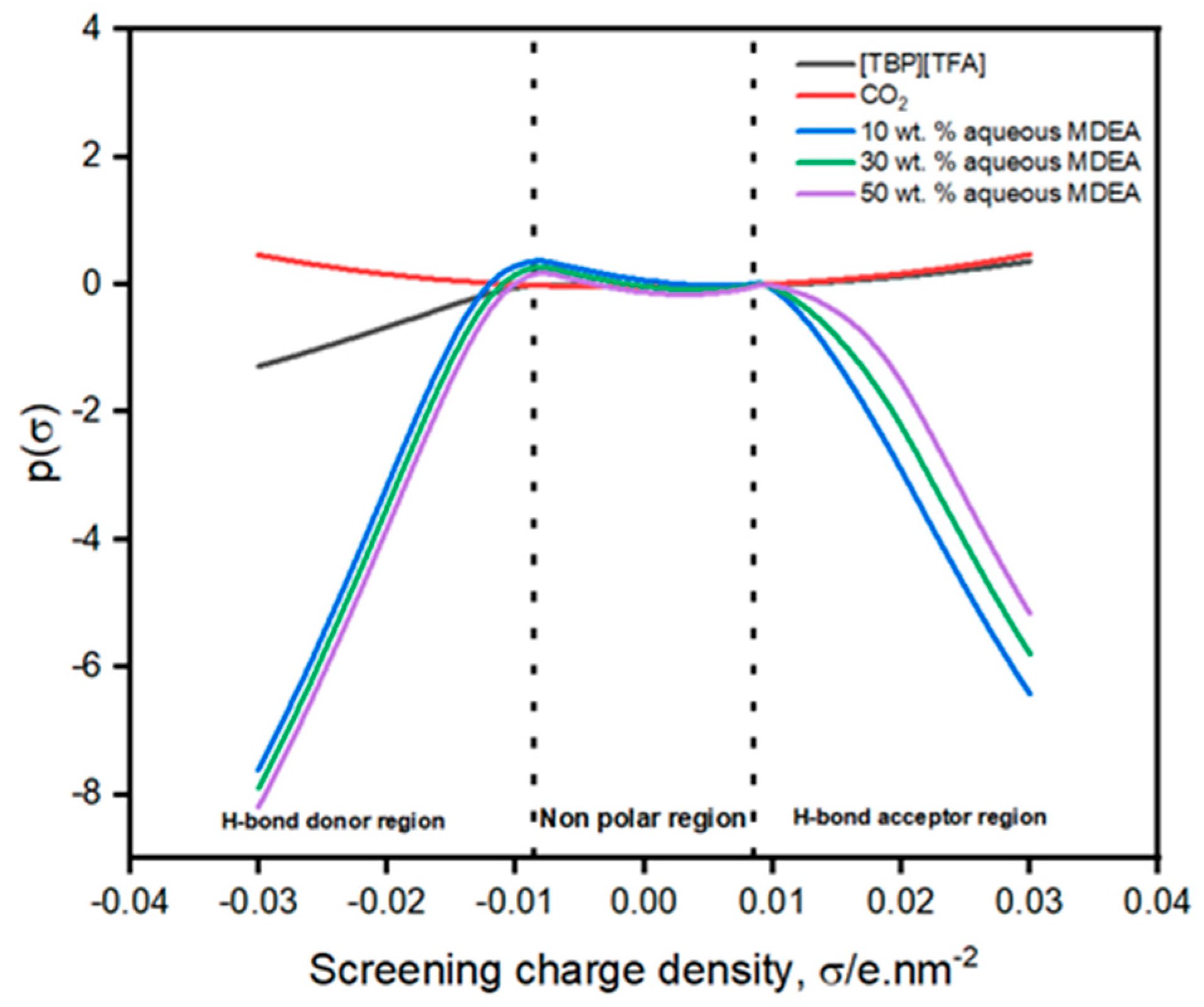
3.2. Predictions of the Sigma Profile, Sigma Potential, and Interaction Energy Using COSMO-RS
3.3. Preparation of the Novel Aqueous MDEA–[TBP][TFA] Hybrid Solvent
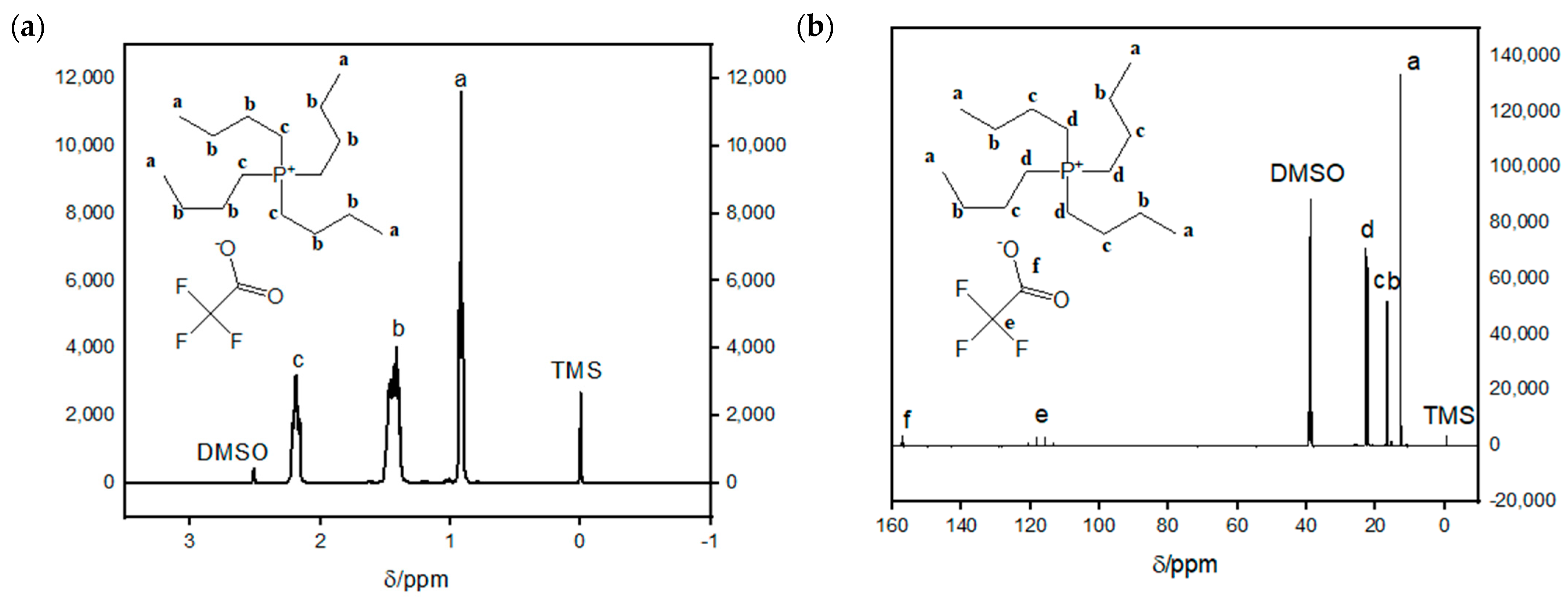
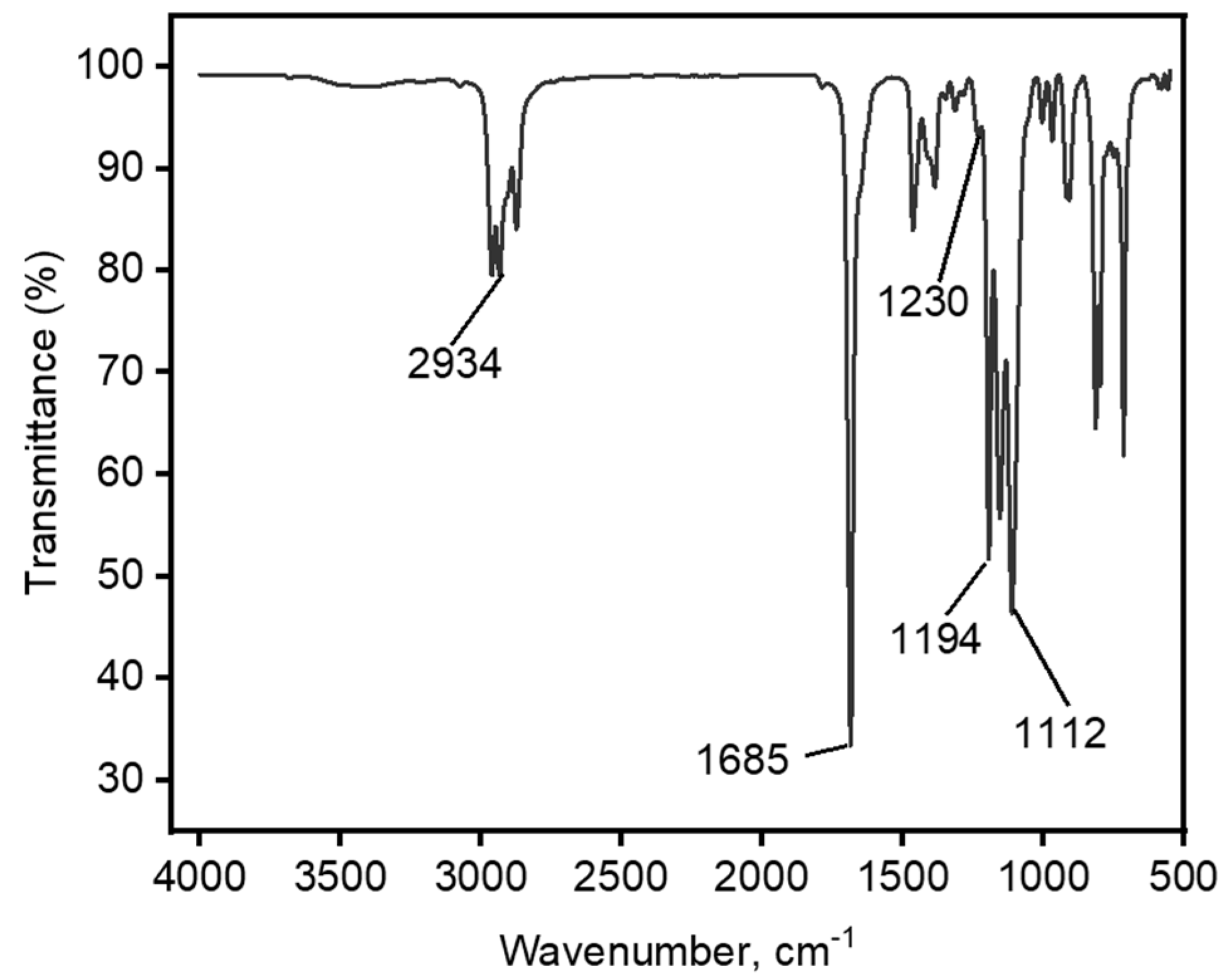
3.3.1. Synthesis and Characterization of [TBP][TFA] Ionic Liquid
3.3.2. Thermophysical Characterization of the Aqueous MDEA-[TBP][TFA] Hybrid Solvent
3.4. CO2 Absorption Study of Aqueous MDEA– [TBP][TFA] Hybrid Solvents Using RSM
3.5. Comparison of CO2 Removal Capacity and Heat Capacity of Hybrid Solvents with Aqueous MDEA and [TBP][TFA] Ionic Liquid
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, K.; Sun, R.; Hochman, G. Do natural gas and renewable energy consumption lead to less CO2 emission? Empirical evidence from a panel of BRICS countries. Energy 2017, 141, 1466–1478. [Google Scholar] [CrossRef]
- De Gouw, J.A.; Parrish, D.D.; Frost, G.J.; Trainer, M. Reduced emissions of CO2, NOx, and SO2 from US power plants owing to switch from coal to natural gas with combined cycle technology. Earth’s Future 2014, 2, 75–82. [Google Scholar] [CrossRef]
- Nugroho, A.A.; Azis, M.M.; Ariyanto, T. An Approach For Selecting CO_2 Removal Technology In Indonesia’s Upstream Natural Gas Industry Using AHP Method. J. Appl. Sci. Eng. 2024, 27, 2559–2570. [Google Scholar]
- Yi, N.; Li, S.; Wang, X.; Kong, F.; Li, X.; Ren, Y.; Liu, H.; Xu, S. Novel amine emissions control methods for CO2 capture based on reducing amine concentration in the scrubbing liquid. Sep. Purif. Technol. 2025, 354, 129295. [Google Scholar] [CrossRef]
- Odunlami, O.; Vershima, D.; Oladimeji, T.; Nkongho, S.; Ogunlade, S.; Fakinle, B. Advanced techniques for the capturing and separation of CO2—A review. Results Eng. 2022, 15, 100512. [Google Scholar] [CrossRef]
- Azzouz, A.; Platon, N.; Nousir, S.; Ghomari, K.; Nistor, D.; Shiao, T.C.; Roy, R. OH-enriched organo-montmorillonites for potential applications in carbon dioxide separation and concentration. Sep. Purif. Technol. 2013, 108, 181–188. [Google Scholar] [CrossRef]
- Mak, J.; Mokhatab, S.; Poe, W. Natural gas treating. In Handbook of Natural Gas Transmission and Processing, 3rd ed.; Gulf Professional Publishing: Houston, TX, USA, 2015; pp. 181–222. [Google Scholar]
- Gautam, A.; Mondal, M.K. Review of recent trends and various techniques for CO2 capture: Special emphasis on biphasic amine solvents. Fuel 2023, 334, 126616. [Google Scholar] [CrossRef]
- Vega, F.; Cano, M.; Camino, S.; Fernández, L.M.G.; Portillo, E.; Navarrete, B. Solvents for carbon dioxide capture. In Carbon Dioxide Chemistry, Capture and Oil Recovery; IntechOpen: London, UK, 2018; Volume 32, pp. 137–144. [Google Scholar]
- Han, S.-C.; Sung, H.; Noh, H.-W.; Mazari, S.A.; Moon, J.-H.; Kim, K.-M. Synergistic effect of blended amines on carbon dioxide absorption: Thermodynamic modeling and analysis of regeneration energy. Renew. Sustain. Energy Rev. 2024, 197, 114362. [Google Scholar] [CrossRef]
- Lawal, A.O.; Idem, R.O. Kinetics of the oxidative degradation of CO2 loaded and concentrated aqueous MEA-MDEA blends during CO2 absorption from flue gas streams. Ind. Eng. Chem. Res. 2006, 45, 2601–2607. [Google Scholar] [CrossRef]
- Shahid, M.Z.; Maulud, A.S.; Bustam, M.A.; Suleman, H.; Halim, H.N.A.; Shariff, A.M. Rate-based modeling for packed absorption column of the MEA–CO2–water system at high-pressure and High-CO2 loading conditions. Ind. Eng. Chem. Res. 2019, 58, 12235–12246. [Google Scholar] [CrossRef]
- Meng, F.; Meng, Y.; Ju, T.; Han, S.; Lin, L.; Jiang, J. Research progress of aqueous amine solution for CO2 capture: A review. Renew. Sustain. Energy Rev. 2022, 168, 112902. [Google Scholar] [CrossRef]
- Kortunov, P.V.; Siskin, M.; Baugh, L.S.; Calabro, D.C. In Situ Nuclear Magnetic Resonance Mechanistic Studies of Carbon Dioxide Reactions with Liquid Amines in Non-aqueous Systems: Evidence for the Formation of Carbamic Acids and Zwitterionic Species. Energy Fuels 2015, 29, 5940–5966. [Google Scholar] [CrossRef]
- Zheng, Y.; El Ahmar, E.; Simond, M.; Ballerat-Busserolles, K.; Zhang, P. CO2 Heat of Absorption in Aqueous Solutions of MDEA and MDEA/Piperazine. J. Chem. Eng. Data 2020, 65, 3784–3793. [Google Scholar] [CrossRef]
- Oexmann, J.; Kather, A.; Linnenberg, S.; Liebenthal, U. Post-combustion CO2 capture: Chemical absorption processes in coal-fired steam power plants. Greenh. Gases Sci. Technol. 2012, 2, 80–98. [Google Scholar] [CrossRef]
- Olugbenga, A.G. Dependence of operational properties on reboiler duty in the removal of CO2 from natural gas with aspen Hysys. Results Eng. 2024, 21, 101755. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, N.; Hong, Y.; Wang, R.; Li, Q.; Li, M.; Wang, L. Tertiary amine based-biphasic solvent using ionic liquids as an activator to advance the energy-efficient CO2 capture. Sep. Purif. Technol. 2025, 355, 129671. [Google Scholar] [CrossRef]
- Vieira, N.S.; Ferreira, M.L.; Castro, P.J.; Araújo, J.M.; Pereiro, A.B. Fluorinated Ionic Liquids as Task-Specific Materials: An Overview of Current Research; IntechOpen: London, UK, 2021. [Google Scholar]
- Yan, H.; Zhao, L.; Bai, Y.; Li, F.; Dong, H.; Wang, H.; Zhang, X.; Zeng, S. Superbase ionic liquid-based deep eutectic solvents for improving CO2 absorption. ACS Sustain. Chem. Eng. 2020, 8, 2523–2530. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef]
- Das, I.; Swami, K.R.; Gardas, R.L. Exploring the potential of ethylenediamine based protic ionic liquids for carbon capture: A study on thermophysical properties and CO2 absorption behavior. Chem. Thermodyn. Therm. Anal. 2024, 15, 100138. [Google Scholar] [CrossRef]
- Renati, P.; Madl, P. What Is the “Hydrogen Bond”? A QFT-QED Perspective. Int. J. Mol. Sci. 2024, 25, 3846. [Google Scholar] [CrossRef]
- Oko, E.; Zacchello, B.; Wang, M.; Fethi, A. Process analysis and economic evaluation of mixed aqueous ionic liquid and monoethanolamine (MEA) solvent for CO2 capture from a coke oven plant. Greenh. Gases Sci. Technol. 2018, 8, 686–700. [Google Scholar] [CrossRef]
- Shen, S.; Yang, Y.-n.; Wang, Y.; Ren, S.; Han, J.; Chen, A. CO2 absorption into aqueous potassium salts of lysine and proline: Density, viscosity and solubility of CO2. Fluid Phase Equilibria 2015, 399, 40–49. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical properties of ionic liquids: Database and evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Fauzi, A.H.M.; Amin, N.A.S. An overview of ionic liquids as solvents in biodiesel synthesis. Renew. Sustain. Energy Rev. 2012, 16, 5770–5786. [Google Scholar] [CrossRef]
- Ma, D.; Zhu, C.; Fu, T.; Ma, Y.; Yuan, X. Synergistic effect of functionalized ionic liquid and alkanolamines mixed solution on enhancing the mass transfer of CO2 absorption in microchannel. Chem. Eng. J. 2021, 417, 129302. [Google Scholar] [CrossRef]
- Hasib-ur-Rahman, M.; Larachi, F. Prospects of using room-temperature ionic liquids as corrosion inhibitors in aqueous ethanolamine-based CO2 capture solvents. Ind. Eng. Chem. Res. 2013, 52, 17682–17685. [Google Scholar] [CrossRef]
- Qian, W.; Xu, Y.; Xie, B.; Ge, Y.; Shu, H. Alkanolamine-based dual functional ionic liquids with multidentate cation coordination and pyrazolide anion for highly efficient CO2 capture at relatively high temperature. Int. J. Greenh. Gas Control. 2017, 56, 194–201. [Google Scholar] [CrossRef]
- Taib, M.M.; Murugesan, T. Solubilities of CO2 in aqueous solutions of ionic liquids (ILs) and monoethanolamine (MEA) at pressures from 100 to 1600 kPa. Chem. Eng. J. 2012, 181, 56–62. [Google Scholar] [CrossRef]
- Xu, F.; Gao, H.; Dong, H.; Wang, Z.; Zhang, X.; Ren, B.; Zhang, S. Solubility of CO2 in aqueous mixtures of monoethanolamine and dicyanamide-based ionic liquids. Fluid Phase Equilibria 2014, 365, 80–87. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, H.; Gao, H.; Olson, W.; Liang, Z. CO2 capture with hybrid absorbents of low viscosity imidazolium-based ionic liquids and amine. Appl. Energy 2019, 235, 311–319. [Google Scholar] [CrossRef]
- Gohndrone, T.R.; Song, T.; DeSilva, M.A.; Brennecke, J.F. Quantification of ylide formation in phosphonium-based ionic liquids reacted with CO2. J. Phys. Chem. B 2021, 125, 6649–6657. [Google Scholar] [CrossRef] [PubMed]
- Balchandani, S.C.; Singh, R.; Mandal, B. Experimental and COSMO-RS analysis of CO2 solubility in novel aqueous blends of 1-butyl-3-methyl-imidazolium tetrafluoroborate activated by 2-aminoethyl piperazine and bis (3-aminopropyl) amine for post combustion carbon capture. J. Environ. Chem. Eng. 2023, 11, 109099. [Google Scholar] [CrossRef]
- Balchandani, S.; Singh, R. Thermodynamic analysis using COSMO-RS studies of reversible ionic liquid 3-aminopropyl triethoxysilane blended with amine activators for CO2 absorption. J. Mol. Liq. 2021, 324, 114713. [Google Scholar] [CrossRef]
- Alioui, O.; Benguerba, Y.; Alnashef, I.M. Investigation of the CO2-solubility in deep eutectic solvents using COSMO-RS and molecular dynamics methods. J. Mol. Liq. 2020, 307, 113005. [Google Scholar] [CrossRef]
- Galiano, F.; Mancuso, R.; Guazzelli, L.; Mauri, M.; Chiappe, C.; Simonutti, R.; Brunetti, A.; Pomelli, C.S.; Barbieri, G.; Gabriele, B. Phosphonium ionic liquid-polyacrylate copolymer membranes for improved CO2 separations. J. Membr. Sci. 2021, 635, 119479. [Google Scholar] [CrossRef]
- Rather, S.-u.; Bamufleh, H.S.; Alhumade, H.; Saeed, U.; Taimoor, A.A.; Sulaimon, A.A.; Alalayah, W.M.; Shariff, A.M. Research Article Theoretical and Experimental Studies of 1-Butyl-3-methylimidazolium Methanesulfonate Ionic Liquid. Gas 2023, 12, 14. [Google Scholar]
- Veza, I.; Spraggon, M.; Fattah, I.R.; Idris, M. Response surface methodology (RSM) for optimizing engine performance and emissions fueled with biofuel: Review of RSM for sustainability energy transition. Results Eng. 2023, 18, 101213. [Google Scholar] [CrossRef]
- Olabinjo, O.O. Response Surface Techniques as an Inevitable Tool in Optimization Process. In Response Surface Methods-Theory, Applications and Optimization Techniques; IntechOpen: London, UK, 2024. [Google Scholar]
- Susaimanickam, A.; Manickam, P.; Joseph, A.A. A comprehensive review on RSM-coupled optimization techniques and its applications. Arch. Comput. Methods Eng. 2023, 30, 4831–4853. [Google Scholar] [CrossRef]
- Ardeshiri, A.; Rashidi, H. Performance of Water-Lean Solvent for Postcombustion Carbon Dioxide Capture in a Process-Intensified Absorber: Experimental, Modeling, and Optimization Using RSM and ML. Ind. Eng. Chem. Res. 2023, 62, 20821–20832. [Google Scholar] [CrossRef]
- Warrag, S.E.; Darwish, A.S.; Adeyemi, I.A.; Hadj-Kali, M.K.; Kroon, M.C.; AlNashef, I.M. Extraction of pyridine from n-alkane mixtures using methyltriphenylphosphonium bromide-based deep eutectic solvents as extractive denitrogenation agents. Fluid Phase Equilibria 2020, 517, 112622. [Google Scholar] [CrossRef]
- Hasib-ur-Rahman, M.; Bouteldja, H.; Fongarland, P.; Siaj, M.; Larachi, F. Corrosion behavior of carbon steel in alkanolamine/room-temperature ionic liquid based CO2 capture systems. Ind. Eng. Chem. Res. 2012, 51, 8711–8718. [Google Scholar] [CrossRef]
- Azhar, F.N.A.; Taha, M.F.; Mat Ghani, S.M.; Ruslan, M.S.H.; Md Yunus, N.M. Experimental and Mathematical Modelling of Factors Influencing Carbon Dioxide Absorption into the Aqueous Solution of Monoethanolamine and 1-Butyl-3-methylimidazolium Dibutylphosphate Using Response Surface Methodology (RSM). Molecules 2022, 27, 1779. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, L.; Ye, Y.; Chen, L.; Qi, Z.; Freund, H.; Sundmacher, K. An Overview of Mutual Solubility of Ionic Liquids and Water Predicted by COSMO-RS. Ind. Eng. Chem. Res. 2012, 51, 6256–6264. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, X.; Xu, B.; Gao, G.; Wu, F.; Li, X.; Zhang, L.; Luo, C. Effect of water on CO2 absorption by a novel anhydrous biphasic absorbent: Phase change behavior, absorption performance, reaction heat, and absorption mechanism. Sep. Purif. Technol. 2023, 325, 124624. [Google Scholar] [CrossRef]
- Wasewar, K. Carbon Dioxide Capture by Ionic Liquids. In Advances in Carbon Capture and Utilization; Pant, D., Kumar Nadda, A., Pant, K.K., Agarwal, A.K., Eds.; Springer: Singapore, 2021; pp. 147–194. [Google Scholar]
- Quaid, T.; Reza, M.T. Carbon Capture from Biogas by Deep Eutectic Solvents: A COSMO Study to Evaluate the Effect of Impurities on Solubility and Selectivity. Clean Technol. 2021, 3, 490–502. [Google Scholar] [CrossRef]
- Khan, S.N.; Hailegiorgis, S.M.; Man, Z.; Shariff, A.M.; Garg, S. Thermophysical properties of aqueous N-methyldiethanolamine (MDEA) and ionic liquids 1-butyl-3-methylimidazolium trifluoromethanesulfonate [bmim][OTf], 1-butyl-3-methylimidazolium acetate [bmim][Ac] hybrid solvents for CO2 capture. Chem. Eng. Res. Des. 2017, 121, 69–80. [Google Scholar] [CrossRef]




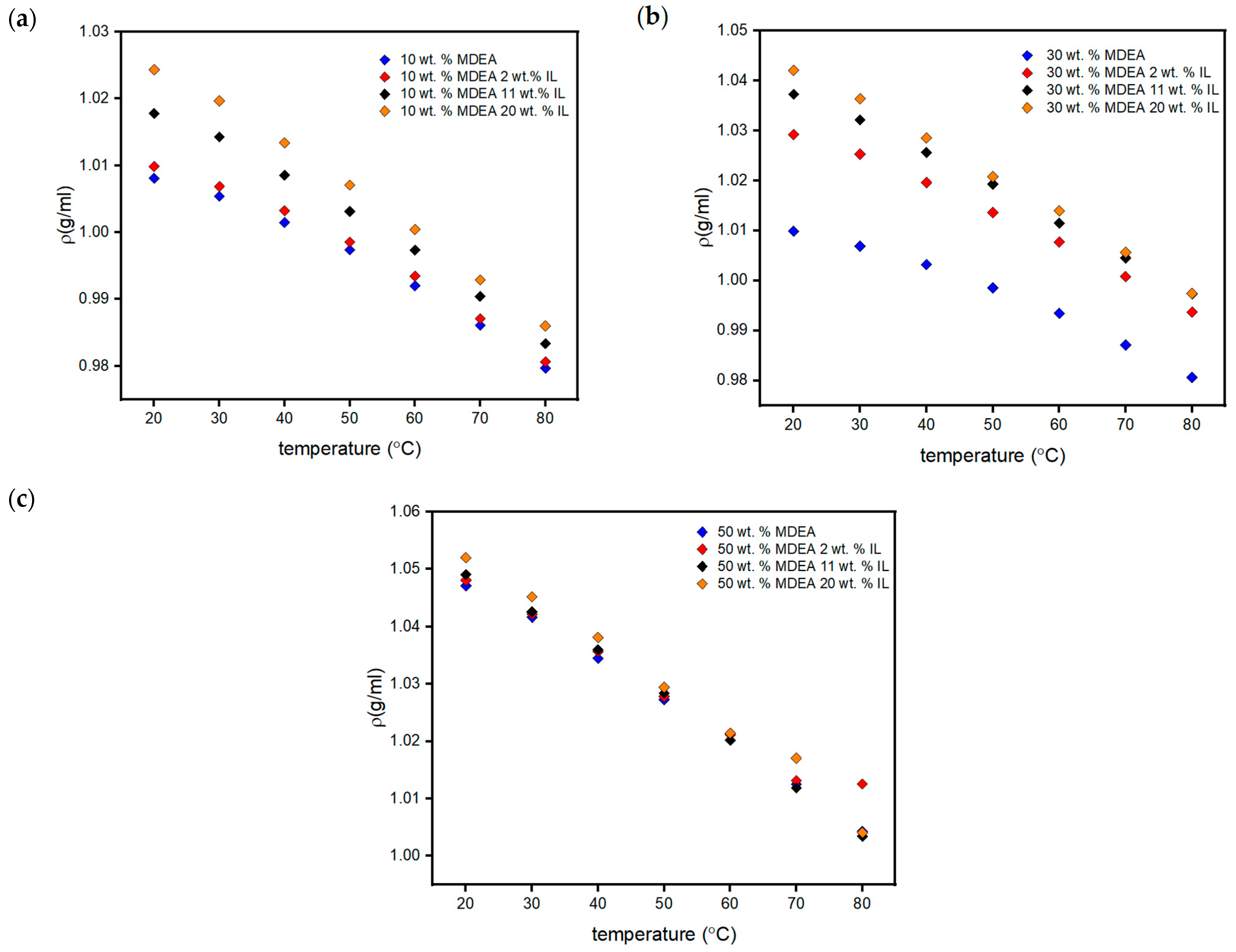

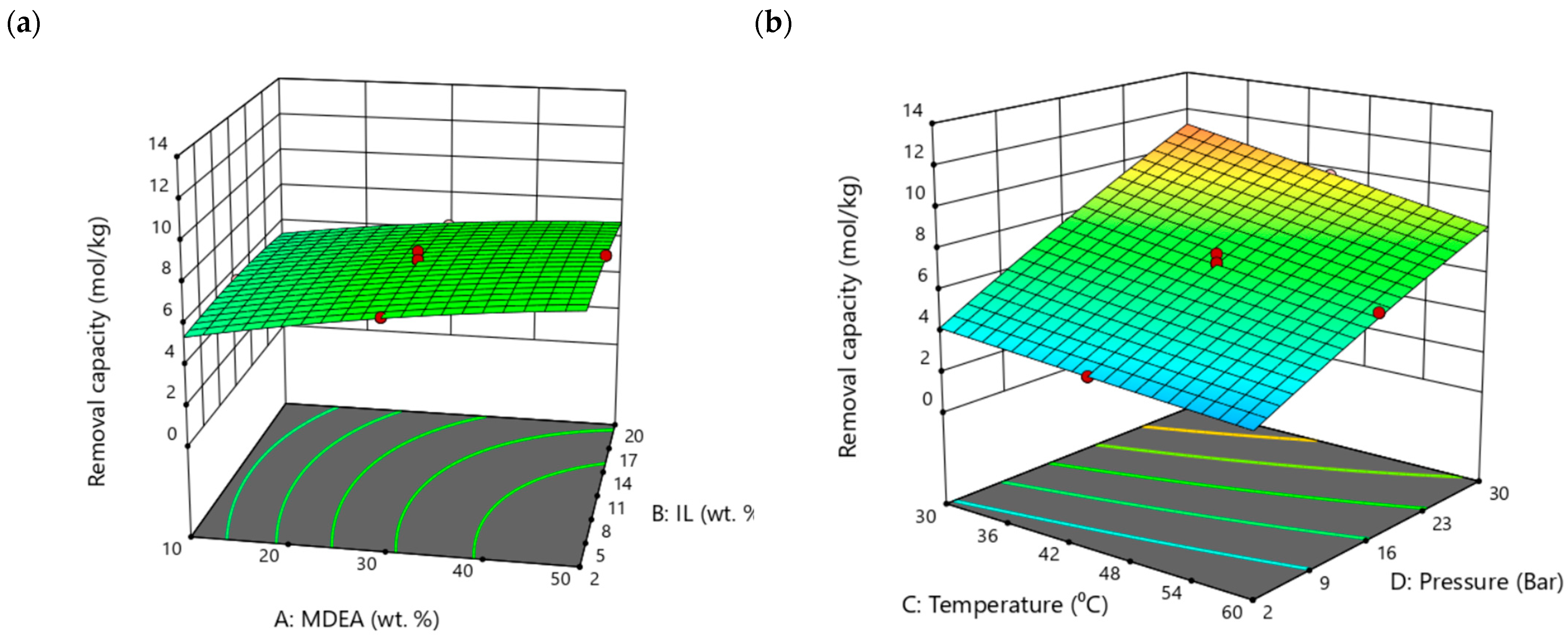
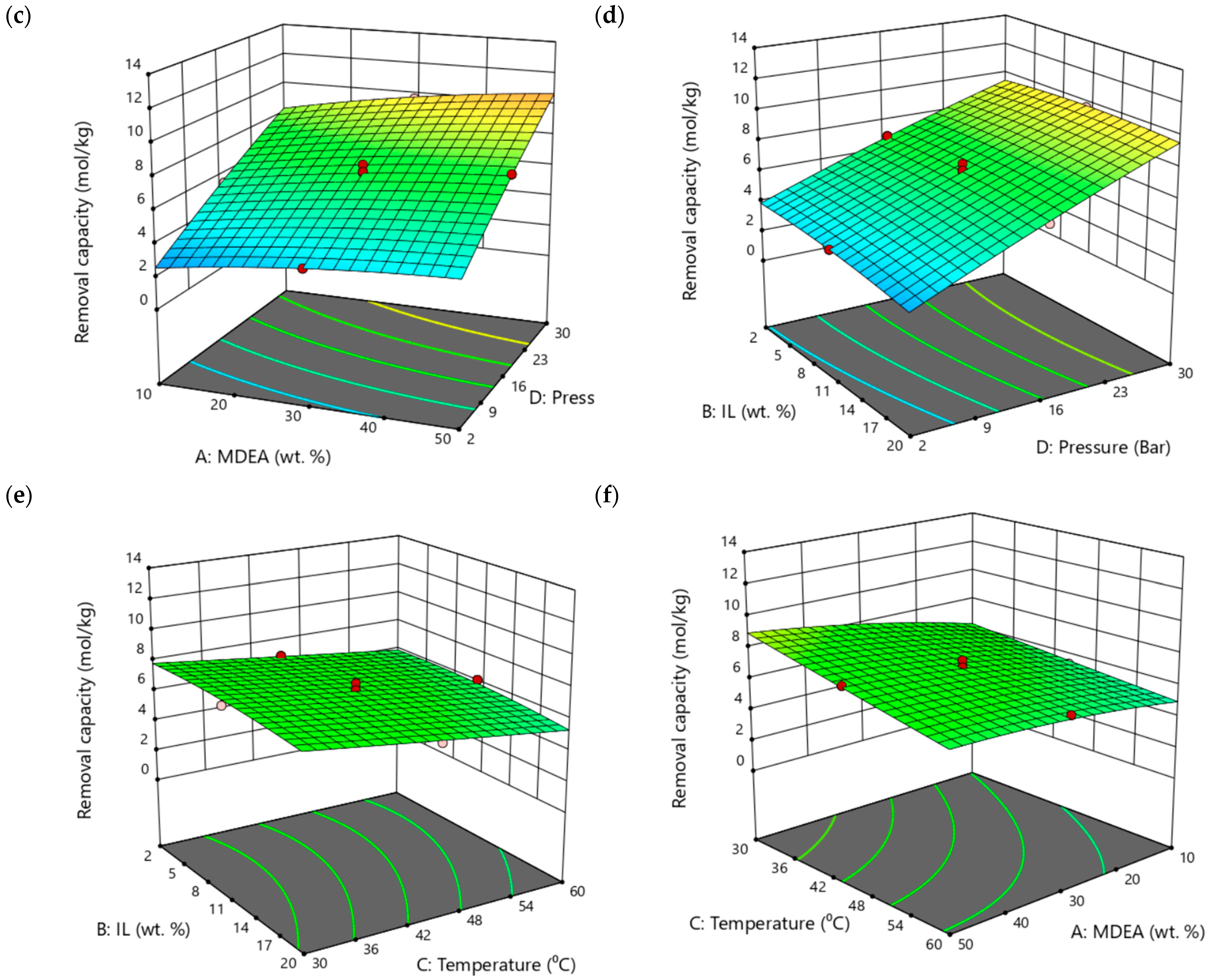
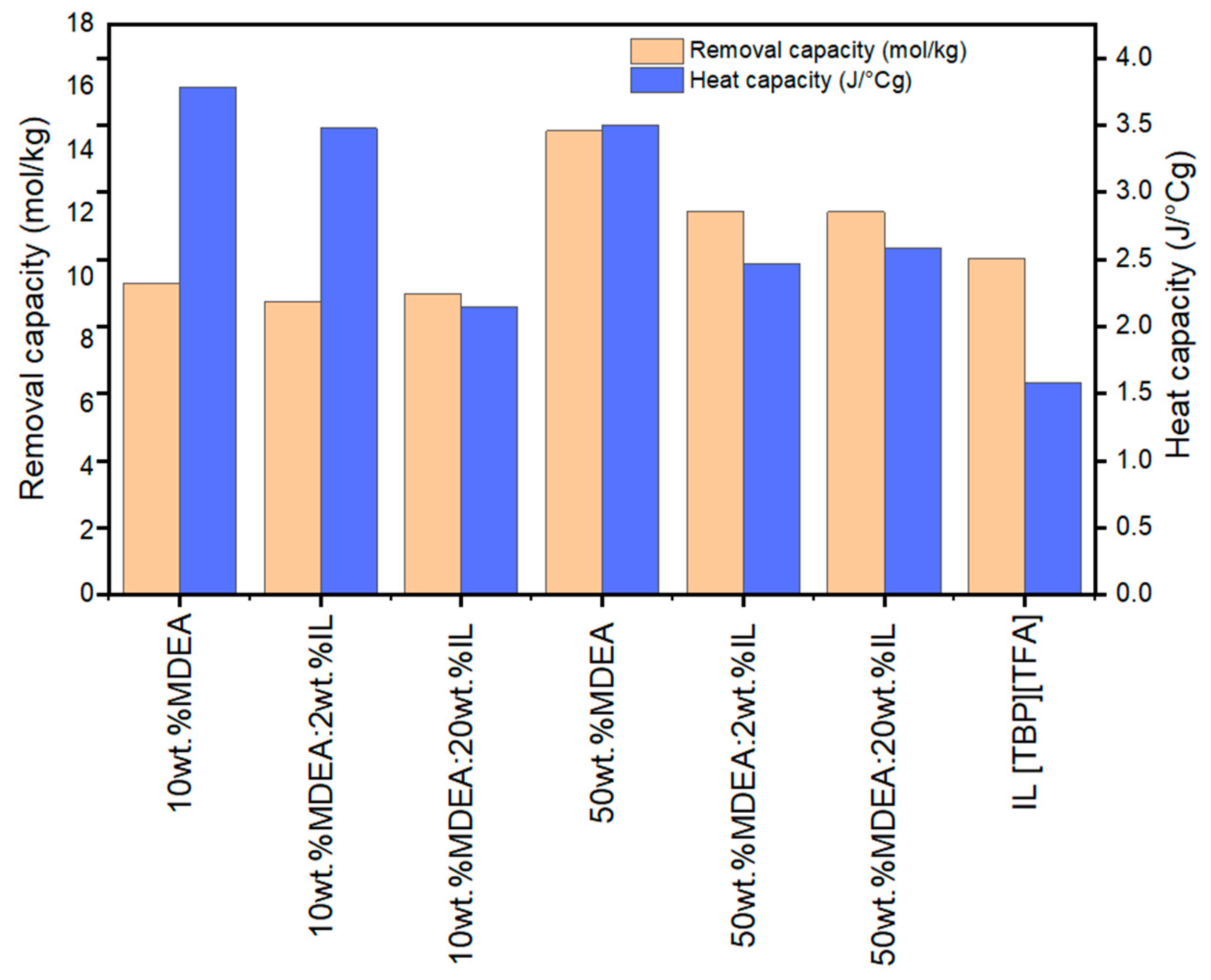
| No. | MDEA (wt.%) | [TBP][TFA] (wt.%) | H2O (wt.%) |
|---|---|---|---|
| 1 | 10 | 0 | 90 |
| 2 | 10 | 2 | 88 |
| 3 | 10 | 11 | 79 |
| 4 | 10 | 20 | 70 |
| 5 | 30 | 0 | 70 |
| 6 | 30 | 2 | 68 |
| 7 | 30 | 11 | 59 |
| 8 | 30 | 20 | 50 |
| 9 | 50 | 0 | 50 |
| 10 | 50 | 2 | 48 |
| 11 | 50 | 11 | 39 |
| 12 | 50 | 20 | 30 |
| Coded Levels of Each Factor in FC-CCD | Factors with Actual Level | |||
|---|---|---|---|---|
| MDEA (wt.%) (x1) | Ionic Liquid (wt.%) (x2) | Temperature (x3) | Pressure (x4) | |
| −1 | 10 | 2 | 30 | 2 |
| 0 | 30 | 11 | 45 | 16 |
| 1 | 50 | 20 | 60 | 30 |
| T (K) | [TBP][TFA] | ||
| Density, ρ (g/mL) | Volume (Å3) | COSMO-Volume (Å3) | |
| 298.15 | 1.0060 | 614.78 | 493.23 |
| 303.15 | 1.0008 | 617.98 | 493.23 |
| 308.15 | 0.9956 | 621.2 | 493.23 |
| 313.15 | 0.9905 | 624.43 | 493.23 |
| 318.15 | 0.9853 | 627.68 | 493.23 |
| 323.15 | 0.9802 | 630.94 | 493.23 |
| 328.15 | 0.9752 | 634.22 | 493.23 |
| 333.15 | 0.9701 | 637.52 | 493.23 |
| T (K) | 10 wt.% aqueous MDEA | ||
| Density, ρ (g/mL) | Volume (Å3) | COSMO-Volume (Å3) | |
| 298.15 | 0.9328 | 500.76 | 390.89 |
| 303.15 | 0.9278 | 503.45 | 390.89 |
| 308.15 | 0.9229 | 506.16 | 390.89 |
| 313.15 | 0.9179 | 508.87 | 390.89 |
| 318.15 | 0.913 | 511.6 | 390.89 |
| 323.15 | 0.9082 | 514.34 | 390.89 |
| 328.15 | 0.9033 | 517.09 | 390.89 |
| 333.15 | 0.8985 | 519.85 | 390.89 |
| T (K) | 30 wt.% aqueous MDEA | ||
| Density, ρ (g/mL) | Volume (Å3) | COSMO-Volume (Å3) | |
| 298.15 | 0.95998 | 836.5 | 660.63 |
| 303.15 | 0.95493 | 840.93 | 660.63 |
| 308.15 | 0.94991 | 845.37 | 660.63 |
| 313.15 | 0.94492 | 849.84 | 660.63 |
| 318.15 | 0.93996 | 854.32 | 660.63 |
| 323.15 | 0.93503 | 858.83 | 660.63 |
| 328.15 | 0.93012 | 863.36 | 660.63 |
| 333.15 | 0.92525 | 867.91 | 660.63 |
| T (K) | 50 wt.% aqueous MDEA | ||
| Density, ρ (g/mL) | Volume (Å3) | COSMO-Volume (Å3) | |
| 298.15 | 0.97159 | 1172.25 | 930.36 |
| 303.15 | 0.96652 | 1178.4 | 930.36 |
| 308.15 | 0.96147 | 1184.59 | 930.36 |
| 313.15 | 0.95646 | 1190.8 | 930.36 |
| 318.15 | 0.95147 | 1197.05 | 930.36 |
| 323.15 | 0.9465 | 1203.32 | 930.36 |
| 328.15 | 0.94157 | 1209.63 | 930.36 |
| 333.15 | 0.93666 | 1215.97 | 930.36 |
| System | EMF kcal/mol | EHB kcal/mol | EvdW kcal/mol | Etotal kcal/mol |
|---|---|---|---|---|
| System I | ||||
| [TBP][TFA] | 11.46 | −1.63 | −20.90 | −11.06 |
| [TBP][TFA] + CO2 | 12.32 | −1.63 | −23.00 | −12.31 |
| 10 wt.% aqueous MDEA | 0.56 | −5.78 | −1.60 | −6.82 |
| 10 wt.% aqueous MDEA + CO2 | 1.41 | −5.78 | −3.71 | −8.07 |
| 30 wt.% aqueous MDEA | 1.10 | −5.65 | −2.93 | −7.49 |
| 30 wt.% aqueous MDEA + CO2 | 1.95 | −5.65 | −5.03 | −8.74 |
| 50 wt.% aqueous MDEA | 1.63 | −5.53 | −4.25 | −8.15 |
| 50 wt.% aqueous MDEA + CO2 | 2.49 | −5.53 | −6.36 | −9.40 |
| System II | ||||
| 10 wt.% aqueous MDEA − [TBP][TFA] + CO2 | 12.88 | −7.41 | −24.60 | −19.14 |
| 30 wt.% aqueous MDEA − [TBP][TFA] + CO2 | 13.41 | −7.28 | −25.93 | −19.80 |
| 50 wt.% aqueous MDEA − [TBP][TFA] + CO2 | 13.95 | −7.16 | −27.25 | −20.46 |
| T | 10 wt.% MDEA | 10 wt.% MDEA 2 wt.% IL | 10 wt.% MDEA 20 wt.% IL | 50 wt.% MDEA | 50 wt.% MDEA 2 wt.% IL | 50 wt.% MDEA 20 wt.% IL | [TBP][TFA] IL |
|---|---|---|---|---|---|---|---|
| °C | J/g °C | ||||||
| 20 | 3.37 | 3.11 | 2.97 | 4.99 | 4.72 | 3.74 | 1.71 |
| 30 | 3.34 | 3.08 | 2.94 | 5.25 | 4.96 | 3.91 | 1.69 |
| 40 | 3.34 | 3.08 | 2.94 | 5.42 | 5.1 | 4.02 | 1.72 |
| 50 | 3.38 | 3.13 | 3.00 | 5.65 | 5.29 | 4.18 | 1.79 |
| 60 | 3.36 | 3.11 | 3.00 | 5.88 | 5.48 | 4.35 | 1.81 |
| 70 | 3.36 | 3.09 | 3.01 | 6.12 | 5.69 | 4.52 | 1.85 |
| 80 | 3.34 | 3.06 | 3.04 | 6.34 | 5.85 | 4.67 | 1.88 |
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Response | |
|---|---|---|---|---|---|
| A: MDEA | B: IL | C: T | D: P | CO2 Removal Capacity | |
| wt.% | wt.% | °C | Bar | Actual Value (mol/kg) | Predicted Value (mol/kg) |
| 30 | 11 | 45 | 16 | 6.61 | 6.88 |
| 30 | 11 | 45 | 16 | 6.92 | 6.88 |
| 30 | 11 | 45 | 30 | 9.66 | 9.78 |
| 50 | 20 | 30 | 30 | 12.12 | 12.14 |
| 50 | 20 | 60 | 2 | 1.50 | 2.12 |
| 10 | 2 | 30 | 30 | 9.26 | 8.84 |
| 30 | 11 | 45 | 16 | 6.98 | 6.88 |
| 30 | 20 | 45 | 16 | 6.23 | 6.38 |
| 30 | 11 | 30 | 16 | 7.54 | 7.9 |
| 10 | 20 | 60 | 2 | 2.18 | 1.71 |
| 30 | 2 | 45 | 16 | 6.92 | 6.87 |
| 50 | 2 | 60 | 30 | 8.88 | 8.86 |
| 50 | 2 | 60 | 2 | 3.87 | 3.71 |
| 30 | 11 | 45 | 2 | 3.61 | 3.6 |
| 50 | 2 | 30 | 2 | 5.43 | 5.47 |
| 30 | 11 | 45 | 16 | 6.97 | 6.88 |
| 30 | 11 | 45 | 16 | 7.42 | 6.88 |
| 10 | 11 | 45 | 16 | 5.40 | 5.62 |
| 10 | 20 | 30 | 30 | 9.52 | 9.45 |
| 30 | 11 | 45 | 16 | 6.70 | 6.88 |
| 50 | 20 | 60 | 30 | 8.69 | 8.45 |
| 10 | 2 | 60 | 30 | 6.61 | 6.79 |
| 50 | 20 | 30 | 2 | 4.51 | 4.1 |
| 30 | 11 | 60 | 16 | 6.13 | 5.89 |
| 50 | 11 | 45 | 16 | 7.68 | 7.57 |
| 10 | 20 | 60 | 30 | 7.02 | 7.18 |
| 10 | 2 | 30 | 2 | 2.81 | 2.82 |
| 10 | 20 | 30 | 2 | 2.04 | 2.25 |
| 50 | 2 | 30 | 30 | 12.11 | 12.04 |
| 10 | 2 | 60 | 2 | 2.33 | 2.49 |
| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
| Model | 218.8 | 14 | 15.63 | 121.61 | <0.0001 | significant |
| A-MDEA | 17.21 | 1 | 17.21 | 133.95 | <0.0001 | |
| B-IL | 1.08 | 1 | 1.08 | 8.42 | 0.011 | |
| C-Temperature | 18.22 | 1 | 18.22 | 141.75 | <0.0001 | |
| D-Pressure | 171.53 | 1 | 171.53 | 1334.77 | <0.0001 | |
| AB | 0.6504 | 1 | 0.6504 | 5.06 | 0.0399 | |
| AC | 2.05 | 1 | 2.05 | 15.94 | 0.0012 | |
| AD | 0.7271 | 1 | 0.7271 | 5.66 | 0.0311 | |
| BC | 0.0438 | 1 | 0.0438 | 0.3406 | 0.5681 | |
| BD | 1.37 | 1 | 1.37 | 10.65 | 0.0052 | |
| CD | 2.96 | 1 | 2.96 | 23.04 | 0.0002 | |
| A2 | 0.208 | 1 | 0.208 | 1.62 | 0.2226 | |
| B2 | 0.1625 | 1 | 0.1625 | 1.26 | 0.2784 | |
| C2 | 0.0006 | 1 | 0.0006 | 0.005 | 0.9444 | |
| D2 | 0.0922 | 1 | 0.0922 | 0.7172 | 0.4104 | |
| Residual | 1.93 | 15 | 0.1285 | |||
| Lack of Fit | 1.53 | 10 | 0.1533 | 1.94 | 0.2399 | not significant |
| Pure Error | 0.3944 | 5 | 0.0789 | |||
| Cor Total | 220.73 | 29 | ||||
| Fit Statistics | ||||||
| Std. Dev. | 0.3585 | R2 | 0.9913 | |||
| Mean | 6.45 | Adjusted R2 | 0.9831 | |||
| C.V. % | 5.55 | Predicted R2 | 0.945 | |||
| Adeq Precision | 41.9434 | |||||
| Condition | Removal Capacity (mol/kg) | Standard Deviation | |
|---|---|---|---|
| Predicted | Actual | ||
| 50:20:30:30 | 12.14 | 11.45 | 0.48 |
| 12.14 | 10.75 | 0.98 | |
| 12.14 | 10.20 | 1.37 | |
| 12.14 | 13.01 | 0.61 | |
| Coefficient of variation (%) | 6.50 | ||
| Absorbent | Removal Capacity (mol/kg) |
|---|---|
| 10 wt.% MDEA | 9.83 |
| 10 wt.% MDEA:2 wt.% IL | 9.26 |
| 10 wt.% MDEA:20 wt.% IL | 9.52 |
| 50 wt.% MDEA | 14.64 |
| 50 wt.% MDEA:2 wt.% IL | 12.11 |
| 50 wt.% MDEA:20 wt.% IL | 12.10 |
| [TBP][TFA] IL | 10.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Rasdi, F.L.; Jeyaseelan, R.; Taha, M.F.; Mohd Razip, M.A.A. Optimization of CO2 Capture Using a New Aqueous Hybrid Solvent (MDEA-[TBPA][TFA]) with a Low Heat Capacity: Integration of COSMO-RS and RSM Approaches. Processes 2024, 12, 2626. https://doi.org/10.3390/pr12122626
Mohd Rasdi FL, Jeyaseelan R, Taha MF, Mohd Razip MAA. Optimization of CO2 Capture Using a New Aqueous Hybrid Solvent (MDEA-[TBPA][TFA]) with a Low Heat Capacity: Integration of COSMO-RS and RSM Approaches. Processes. 2024; 12(12):2626. https://doi.org/10.3390/pr12122626
Chicago/Turabian StyleMohd Rasdi, Fairuz Liyana, Revathi Jeyaseelan, Mohd Faisal Taha, and Mohamad Amirul Ashraf Mohd Razip. 2024. "Optimization of CO2 Capture Using a New Aqueous Hybrid Solvent (MDEA-[TBPA][TFA]) with a Low Heat Capacity: Integration of COSMO-RS and RSM Approaches" Processes 12, no. 12: 2626. https://doi.org/10.3390/pr12122626
APA StyleMohd Rasdi, F. L., Jeyaseelan, R., Taha, M. F., & Mohd Razip, M. A. A. (2024). Optimization of CO2 Capture Using a New Aqueous Hybrid Solvent (MDEA-[TBPA][TFA]) with a Low Heat Capacity: Integration of COSMO-RS and RSM Approaches. Processes, 12(12), 2626. https://doi.org/10.3390/pr12122626





