Real-Time Estimation of CO2 Absorption Capacity Using Ionic Conductivity of Protonated Di-Methyl-Ethanolamine (DMEA) and Electrical Conductivity in Low-Concentration DMEA Aqueous Solutions
Abstract
1. Introduction
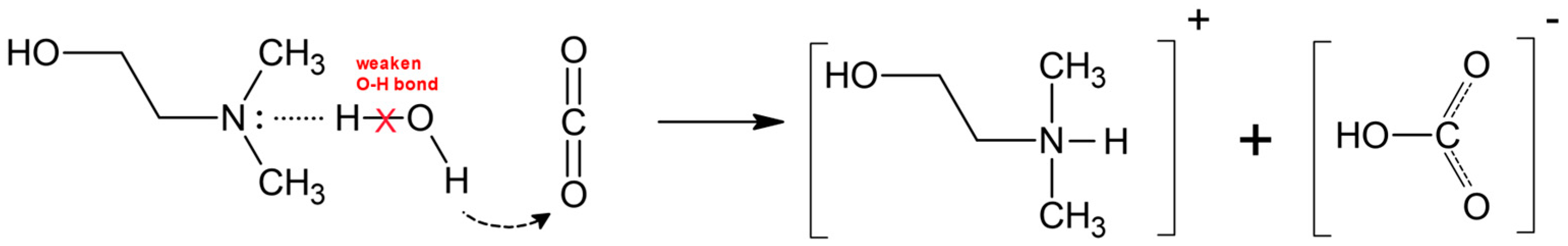
2. CO2 Absorption Mechanism Using DMEA Aqueous Solutions
3. Experimental Methodology and Calculations
3.1. Experimental Setup for CO2 Absorption
3.2. Calculation of Electrical Conductivity (EC) of the CO2-Absorbed DMEA Solutions
3.3. Calculation of Ionic Conductivity of DMEAH+
4. Results and Discussion
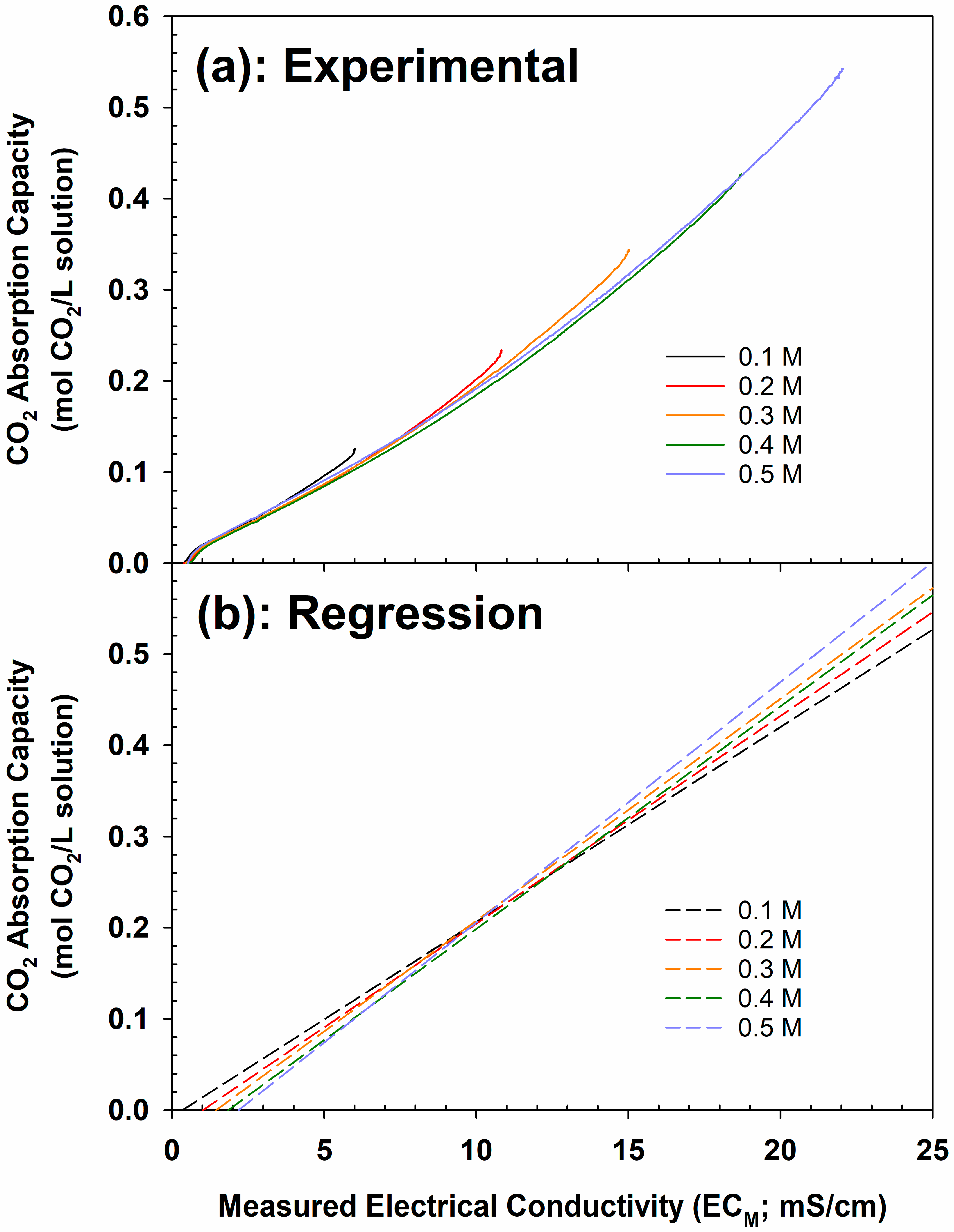
4.1. CO2 Absorption Performance of 0.1–0.5 M DMEA Aqueous Solutions
4.2. Ionic Conductivity of Protonated DMEA (DMEAH+)
4.3. ECC and ECM Variation According to CO2 Absorption in 0.1–0.5 M DMEA Solutions
4.4. Correlation Between CO2 Absorption Capacity and Electrical Conductivity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Carbon Budget. Global Carbon Budget 2023; Copernicus Publications: Göttingen, Germany, 2023. [Google Scholar]
- Hockstad, L.; Hanel, L. Inventory of US Greenhouse Gas Emissions and Sinks. 2018, Environmental System Science Data Infrastructure for a Virtual Ecosystem. Available online: https://data.ess-dive.lbl.gov/datasets/doi:10.15485/1464240 (accessed on 1 November 2018).
- ‘The Era of Global Boiling Has Arrived,’ Says UN Boss, as White House Announces Provisions to Protect Workers from Extreme Heat. Available online: https://cnb.cx/44J1a8k (accessed on 2 September 2024).
- Patel, S.K.S.; Jeon, M.S.; Gupta, R.K.; Jeon, Y.; Kalia, V.C.; Kim, S.C.; Cho, B.K.; Kim, D.R.; Lee, J.-K. Hierarchical macroporous particles for efficient whole-cell immobilization: Application in bioconversion of greenhouse gases to methanol. ACS Appl. Mater. Interfaces 2019, 11, 18968–18977. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Angelidaki, I.; Zhang, Y. In situ biogas upgrading by CO2-to-CH4 bioconversion. Trends Biotechnol. 2021, 39, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, Q.; Qi, Y.; Chen, G.; Song, Y.; Kansha, Y.; Kitamura, Y. Absorption-microalgae hybrid CO2 capture and biotransformation strategy—A review. Int. J. Greenh. Gas Control. 2019, 88, 109–117. [Google Scholar] [CrossRef]
- de Maria, P.D.; Kara, S.; Gallou, F. Biocatalysis in water or in non-conventional media? Adding the CO2 production for the debate. Molecules 2023, 28, 6452. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, L.; Korili, S.A.; Gil, A. Layered double hydroxides for CO2 adsorption at moderate temperatures: Synthesis and amelioration strategies. Chem. Eng. J. 2023, 455, 140551. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, X.; Rao, Y.; Huang, Y. Recent progress of MgO-based materials in CO2 adsorption and conversion: Modification methods, reaction condition, and CO2 hydrogenation. Chin. Chem. Lett. 2024, 35, 108954. [Google Scholar] [CrossRef]
- Moon, H.-S.; Moon, J.-H.; Chun, D.H.; Park, Y.C.; Yun, Y.N.; Sohail, M.; Baek, K.; Kim, H. Synthesis of [Mg2(DOBDC)(DMF)2]@ polystyrene composite and its carbon dioxide adsorption. Microporous Mesoporous Mater. 2016, 232, 161–166. [Google Scholar] [CrossRef]
- Chouikhi, N.; Cecilia, J.A.; Vilarrasa-Garcia, E.; Besghaier, S.; Chlendi, M.; Duro, F.I.F.; Castellon, E.R.; Bagane, M. CO2 adsorption of materials synthesized from clay minerals: A review. Minerals 2019, 9, 514. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Zaharioiu, A.; Oancea, S.; Bucura, F.; Raboaca, M.S.; Filote, C.; Ionete, R.E.; Niculescu, V.C.; Constantinescu, M. Sewage sludge derived materials for CO2 adsorption. Appl. Sci. 2021, 11, 7139. [Google Scholar] [CrossRef]
- Guo, R.-T.; Li, G.-Y.; Liu, Y.; Pan, W.-G. Recent Progress on CO2 Capture Based on Sterically Hindered Amines: A Review. Energy Fuels 2023, 37, 15429–15452. [Google Scholar] [CrossRef]
- Tatarczuk, A.; Tańczyk, M.; Więcław-Solny, L.; Zdeb, J. Pilot plant results of amine-based carbon capture with heat integrated stripper. Appl. Energy 2024, 367, 123416. [Google Scholar] [CrossRef]
- He, W.; Zhang, S.; Zhu, C.; Fu, T.; Ma, Y. CO2 chemical absorption into AMP aqueous solution and mass transfer intensification in cascade sudden expansion microchannels. Chem. Eng. Process. 2022, 181, 109142. [Google Scholar] [CrossRef]
- Ji, C.; Yuan, S.; Huffman, M.; El-Halwagi, M.M.; Wang, Q. Post-combustion carbon capture for tank to propeller via process modeling and simulation. J. CO2 Util. 2021, 51, 101655. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Yu, Y.; Chen, J. Simulation and performance comparison for CO2 capture by aqueous solvents of n-(2-hydroxyethyl) piperazine and another five single amines. Processes 2021, 9, 2184. [Google Scholar] [CrossRef]
- Raznahan, M.M.; Riahi, S.; Mousavi, S.H. A simple, robust and efficient structural model to predict CO2 absorption for different amine solutions: Concern to design new amine compounds. J. Environ. Chem. Eng. 2020, 8, 104572. [Google Scholar] [CrossRef]
- Kayahan, E.; Caprio, U.D.; den Bogaert, A.V.; Khan, M.N.; Bulut, M.; Braeken, L.; Gerven, T.V.; Leblebici, M.E. A new look to the old solvent: Mass transfer performance and mechanism of CO2 absorption into pure monoethanolamine in a spray column. Chem. Eng. Process. Process Intensif. 2023, 184, 109285. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Lim, H.; Kang, J.H.; Park, H.S.; Park, J.; Song, H. Structural investigation of aqueous amine solutions for CO2 capture: CO2 loading, cyclic capacity, absorption–desorption rate, and pKa. J. Environ. Chem. Eng. 2024, 12, 112664. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Qin, Y.; An, A. Model predictive control for the process of mea absorption of CO2 based on the data identification model. Processes 2021, 9, 183. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Shen, M.-T.; Chang, H.; Ho, C.-D. Control of solvent-based post-combustion carbon capture process with optimal operation conditions. Processes 2019, 7, 366. [Google Scholar] [CrossRef]
- López, A.; La Rubia, M.; Pacheco, R.; Sánchez, S.; Navaza, J.; Gómez-Díaz, D. Carbon dioxide absorption by aqueous mixtures of diisopropanolamine and triethanolamine. Chem. Eng. Process. 2016, 110, 73–79. [Google Scholar] [CrossRef]
- BChoi, K.; Kim, S.-M.; Kim, K.-M.; Lee, U.; Choi, J.H.; Lee, J.-S.; Baek, I.H.; Nam, S.C.; Moon, J.-H. Amine blending optimization for maximizing CO2 absorption capacity in a diisopropanolamine–methyldiethanolamine–H2O system using the electrolyte UNIQUAC model. Chem. Eng. J. 2021, 419, 129517. [Google Scholar] [CrossRef]
- Akan, A.P.; Chau, J.; Gullu, G.; Sirkar, K.K. Life Cycle Assessment of Post-Combustion CO2 Capture and Recovery by Hydrophobic Polypropylene Cross-Flow Hollow Fiber Membrane Contactors with Activated Methyldiethanolamine. Atmosphere 2023, 14, 490. [Google Scholar] [CrossRef]
- Xiao, L.; Qiu, Z.; Feng, S.; Duan, X.; Zhao, Z.; Liu, Y.; Ma, L. Carbon dioxide absorption and desorption experiments based on MDEA. Chem. Eng. Process. 2024, 204, 109931. [Google Scholar] [CrossRef]
- Pinto, D.D.; Zahraee, Z.; Buvik, V.; Hartono, A.; Knuutila, H.K. Vapor liquid equilibrium measurements of two promising tertiary amines for CO2 capture. Processes 2019, 7, 951. [Google Scholar] [CrossRef]
- Shiva Nagendra, S.M.; Yasa, P.R.; Narayana, M.V.; Khadirnaikar, S.; Rani, P. Mobile monitoring of air pollution using low cost sensors to visualize spatio-temporal variation of pollutants at urban hotspots. Sustain. Cities Soc. 2019, 44, 520. [Google Scholar] [CrossRef]
- Sofia, D.; Giuliano, A.; Gioiella, F.; Barletta, D.; Poletto, M. Modeling of an air quality monitoring network with high space-time resolution. Comput. Aided Chem. Eng. 2018, 43, 193. [Google Scholar] [CrossRef]
- Alam, A.U.; Clyne, D.; Jin, H.; Hu, N.-X.; Deen, M.J. Fully integrated, simple, and low-cost electrochemical sensor array for in situ water quality monitoring. ACS Sens. 2020, 5, 412. [Google Scholar] [CrossRef]
- Lambrou, T.P.; Anastasiou, C.C.; Panayiotou, C.G.; Polycarpou, M.M. A low-cost sensor network for real-time monitoring and contamination detection in drinking water distribution systems. IEEE Sens. J. 2014, 14, 2765–2772. [Google Scholar] [CrossRef]
- Fulton, S.G.; Stegen, J.C.; Kaufman, M.H.; Dowd, J.; Thompson, A. Laboratory evaluation of open source and commercial electrical conductivity sensor precision and accuracy: How do they compare? PLoS ONE 2023, 18, e0285092. [Google Scholar] [CrossRef]
- Thirstrup, C.; Deleebeeck, L. Review on electrolytic conductivity sensors. IEEE Trans. Instrum. Meas. 2021, 70, 1008222. [Google Scholar] [CrossRef]
- Liu, S.; Gao, H.; Luo, X.; Liang, Z. Kinetics and new mechanism study of CO2 absorption i nto water and tertiary amine solutions by stopped-Flow technique. AIChE J. 2019, 65, 652–661. [Google Scholar] [CrossRef]
- Xiang, J.; Wei, D.; Mao, W.; Liu, T.; Luo, Q.; Huang, Y.; Liang, Z.; Luo, X. Comprehensive kinetic study of carbon dioxide absorption in blended tertiary/secondary amine solutions: Experiments and simulations. Sep. Purif. Technol. 2024, 330, 125310. [Google Scholar] [CrossRef]
- Littel, R.J.; Bos, M.; Knoop, G.J. Dissociation constants of some alkanolamines at 293, 303, 318, and 333 K. J. Chem. Eng. Data 1990, 35, 276–277. [Google Scholar] [CrossRef]
- Kim, S.; Shi, H.; Lee, Y.Y. CO2 absorption mechanism in amine solvents and enhancement of CO2 capture capability in blended amine solvent. Int. J. Greenh. Gas Control. 2016, 45, 181–188. [Google Scholar] [CrossRef]
- Shi, H.; Huang, M.; Wu, Q.; Zheng, L.; Cui, L.; Zhang, S.; Tontiwachwuthikul, P. Study of catalytic CO2 absorption and desorption with tertiary amine DEEA and 1DMA-2p with the aid of solid acid and solid alkaline chemicals. Molecules 2019, 24, 1009. [Google Scholar] [CrossRef]
- Krebs, H.A.; Roughton, F.J.W. Carbonic anhydrase as a tool in studying the mechanism of reactions involving H2CO3, CO2 or HCO3′. Biochem. J. 1948, 43, 550. [Google Scholar] [CrossRef]
- Kladkaew, N.; Idem, R.; Tontiwachwuthikul, P.; Saiwan, C. Studies on corrosion and corrosion inhibitors for amine based solvents for CO2 absorption from power plant flue gases containing CO2, O2 and SO2. Energy Procedia 2011, 4, 1761–1768. [Google Scholar] [CrossRef]
- Han, S.J.; Wee, J.H. Comparison of CO2 absorption performance between methyl-di-ethanolamine and tri-ethanolamine solution systems and its analysis in terms of amine molecules. Greenh. Gases 2021, 11, 445–460. [Google Scholar] [CrossRef]
- Han, S.J.; Wee, J.H. CO2 absorption performance and electrical properties of 2-amino-2-methyl-1-propanol compared to monoethanolamine solutions as primary amine-based absorbents. Energy Fuels 2021, 35, 3197–3207. [Google Scholar] [CrossRef]
- Han, S.J.; Wee, J.H. Estimation of the Amount of CO2 Chemically Absorbed in Real Time by Measuring the Electrical Conductivity Variation of Monoethanol-Amine Aqueous Solutions. Energy Fuels 2023, 37, 19715–19725. [Google Scholar] [CrossRef]
- Prini, R.F.; Harvey, A.H.; Palmer, D.A. Aqueous Systems at Elevated Temperatures and Pressures: Physical Chemistry in Water, Steam and Hydrothermal Solutions; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Rice, E.W.; Bridgewater, L.; American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Debye, P.; Hückel, E. De la theorie des electrolytes. I. abaissement du point de congelation et phenomenes associes. Phys. Z. 1923, 24, 185–206. [Google Scholar]
- Davies, C.; Malpass, V. Ion association and the viscosity of dilute electrolyte solutions. Part 1.—Aqueous inorganic salt solutions. Trans. Faraday Soc. 1964, 60, 2075–2084. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Felippe, A.C.; Bellettini, I.C.; Eising, R.; Minatti, E.; Giacomelli, F.C. Supramolecular complexes formed by the association of poly (ethyleneimine)(PEI), sodium cholate (NaC) and sodium dodecyl sulfate (SDS). J. Braz. Chem. 2011, 22, 1539–1548. [Google Scholar] [CrossRef]
- Corti, H.R.; Trevani, L.N.; Anderko, A. Transport Properties in High Temperature and Pressure Ionic Solutions, in Aqueous Systems at Elevated Temperatures and Pressures; Elsevier: Amsterdam, The Netherlands, 2004; pp. 321–375. [Google Scholar]
- Artemov, V.; Volkov, A.; Sysoev, N.; Volkov, A. On autoionization and pH of liquid water. Dokl. Phys. 2016, 61, 1–4. [Google Scholar] [CrossRef]
- de Myttenaere, A.; Golden, B.; Grand, B.L.; Rossi, F. Mean absolute percentage error for regression models. Neurocomputing 2016, 192, 38–48. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, H.; Liang, Z.; Liu, B.; Tontiwachwuthikul, P.; Hu, X. A comparative kinetics study of CO2 absorption into aqueous DEEA/MEA and DMEA/MEA blended solutions. AIChE J. 2018, 64, 1350–1358. [Google Scholar] [CrossRef]
- Henni, A.; Li, J.; Tontiwachwuthikul, P. Reaction kinetics of CO2 in aqueous 1-amino-2-propanol, 3-amino-1-propanol, and dimethylmonoethanolamine solutions in the temperature range of 298–313 K using the stopped-flow technique. Ind. Eng. Chem. Res. 2008, 47, 2213–2220. [Google Scholar] [CrossRef]
- Tong, C.; Perez, C.C.; Chen, J.; Marcos, J.-C.V.; Neveux, T.; Moullec, Y.L. Measurement and calculation for CO2 solubility and kinetic rate in aqueous solutions of two tertiary amines. Energy Procedia 2013, 37, 2084–2093. [Google Scholar] [CrossRef]
- Liu, B.; Luo, X.; Liang, Z.; Olson, W.; Liu, H.; Idem, R.; Tontiwachwuthikul, P. The development of kinetics model for CO2 absorption into tertiary amines containing carbonic anhydrase. AIChE J. 2017, 63, 4933–4943. [Google Scholar] [CrossRef]
- Kaur, D.P.; Yamada, K.; Park, J.-S.; Sekhon, S.S. Correlation between ion diffusional motion and ionic conductivity for different electrolytes based on ionic liquid. J. Phys. Chem. B 2009, 113, 5381–5390. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, X.; Yang, F.; Zhang, C.; Zhang, Q.; Duan, G.; Jiang, S. Research progress of the ion activity coefficient of polyelectrolytes: A review. Molecules 2023, 28, 2042. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-N.; Shin, H.K.; Hwang, S.; No, K.T. Development of an Infinite Dilution Activity Coefficient Prediction Model for Organic Solutes in Ionic Liquids with Modified Partial Equalization Orbital Electronegativity Method Derived Descriptors. ACS Omega 2021, 6, 15361–15373. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Schade, G.W.; Nielsen, C.J. Real-time monitoring of emissions from monoethanolamine-based industrial scale carbon capture facilities. Environ. Sci. Technol. 2013, 47, 14306–14314. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.W.; Zhou, Q.; Tontiwachiwuthikul, P. Part 4a: Applications of knowledge-based system technology for the CO2 capture process system. Carbon Manag. 2012, 3, 69–79. [Google Scholar] [CrossRef]





| Initial Amine Concentration of DMEA Solution (mol/L) | |||||
|---|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |
| Total CAC (mol CO2/L) | 0.135 | 0.238 | 0.345 | 0.448 | 0.550 |
| Chemical CAC (mol CO2/L) | 0.100 | 0.200 | 0.300 | 0.400 | 0.500 |
| Physical CAC (mol CO2/L) | 0.035 | 0.038 | 0.045 | 0.048 | 0.050 |
| CO2 absorption time (min) | 15.0 | 23.8 | 35.0 | 44.9 | 52.8 |
| Overall CO2 absorption rate (mmol CO2/(L·min)) | 9.0 | 10.0 | 9.9 | 10.0 | 10.4 |
| Ions | Ionic Conductivity (S∙cm2/(mol∙z)) | Absolute Value of Electric Charge (z) | Molar Mass (g/mol) | Reference |
|---|---|---|---|---|
| OH− | 198.6 | 1 | 17.0 | [49] |
| HCO3− | 44.5 | 1 | 61.0 | [50] |
| DMEAH+ | 53.1 | 1 | 90.1 | This work |
| MDEAH+ | 46.5 | 1 | 120.1 | [41] |
| TEAH+ | 37.6 | 1 | 150.1 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.-J.; Han, J.Y.; Wee, J.-H. Real-Time Estimation of CO2 Absorption Capacity Using Ionic Conductivity of Protonated Di-Methyl-Ethanolamine (DMEA) and Electrical Conductivity in Low-Concentration DMEA Aqueous Solutions. Processes 2024, 12, 2495. https://doi.org/10.3390/pr12112495
Han S-J, Han JY, Wee J-H. Real-Time Estimation of CO2 Absorption Capacity Using Ionic Conductivity of Protonated Di-Methyl-Ethanolamine (DMEA) and Electrical Conductivity in Low-Concentration DMEA Aqueous Solutions. Processes. 2024; 12(11):2495. https://doi.org/10.3390/pr12112495
Chicago/Turabian StyleHan, Sang-Jun, Joo Young Han, and Jung-Ho Wee. 2024. "Real-Time Estimation of CO2 Absorption Capacity Using Ionic Conductivity of Protonated Di-Methyl-Ethanolamine (DMEA) and Electrical Conductivity in Low-Concentration DMEA Aqueous Solutions" Processes 12, no. 11: 2495. https://doi.org/10.3390/pr12112495
APA StyleHan, S.-J., Han, J. Y., & Wee, J.-H. (2024). Real-Time Estimation of CO2 Absorption Capacity Using Ionic Conductivity of Protonated Di-Methyl-Ethanolamine (DMEA) and Electrical Conductivity in Low-Concentration DMEA Aqueous Solutions. Processes, 12(11), 2495. https://doi.org/10.3390/pr12112495









