Development of Fruit-Based Carbohydrate Gel for Endurance Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparing the Carbohydrate Gel
2.1.1. Ethical Aspects and Patent Deposit
2.1.2. Sampling
2.1.3. Formulations
2.1.4. Focus Group Testing
2.2. Evaluation of the Carbohydrate Gel
2.2.1. Physico-Chemical Analysis
2.2.2. Rheology: Viscosity
2.2.3. Microbiological Analysis
2.2.4. Sensory Analysis
2.3. Characterization of the Carbohydrate Gel
2.3.1. Proximate Composition
2.3.2. Carbohydrates Glucose, Fructose, and Sucrose
2.3.3. Antioxidant Activity and Total Polyphenols
2.4. Statistical Analysis
3. Results
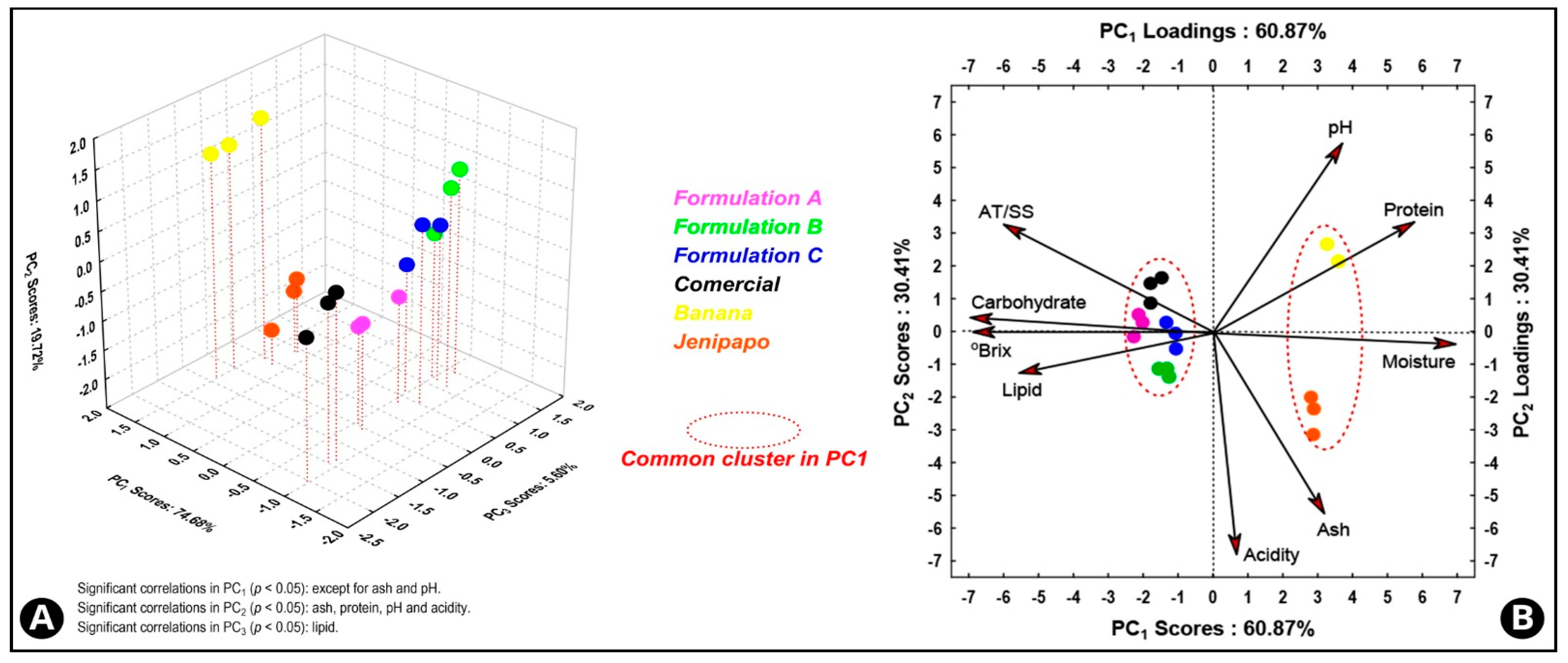
3.1. Physico-Chemical Analysis and Proximate Composition
3.2. Glucose, Fructose, and Sucrose Content, Antioxidant Activity, and Total Polyphenols
3.3. Sensory Analysis: Focus Group and Descriptive Sensory Test
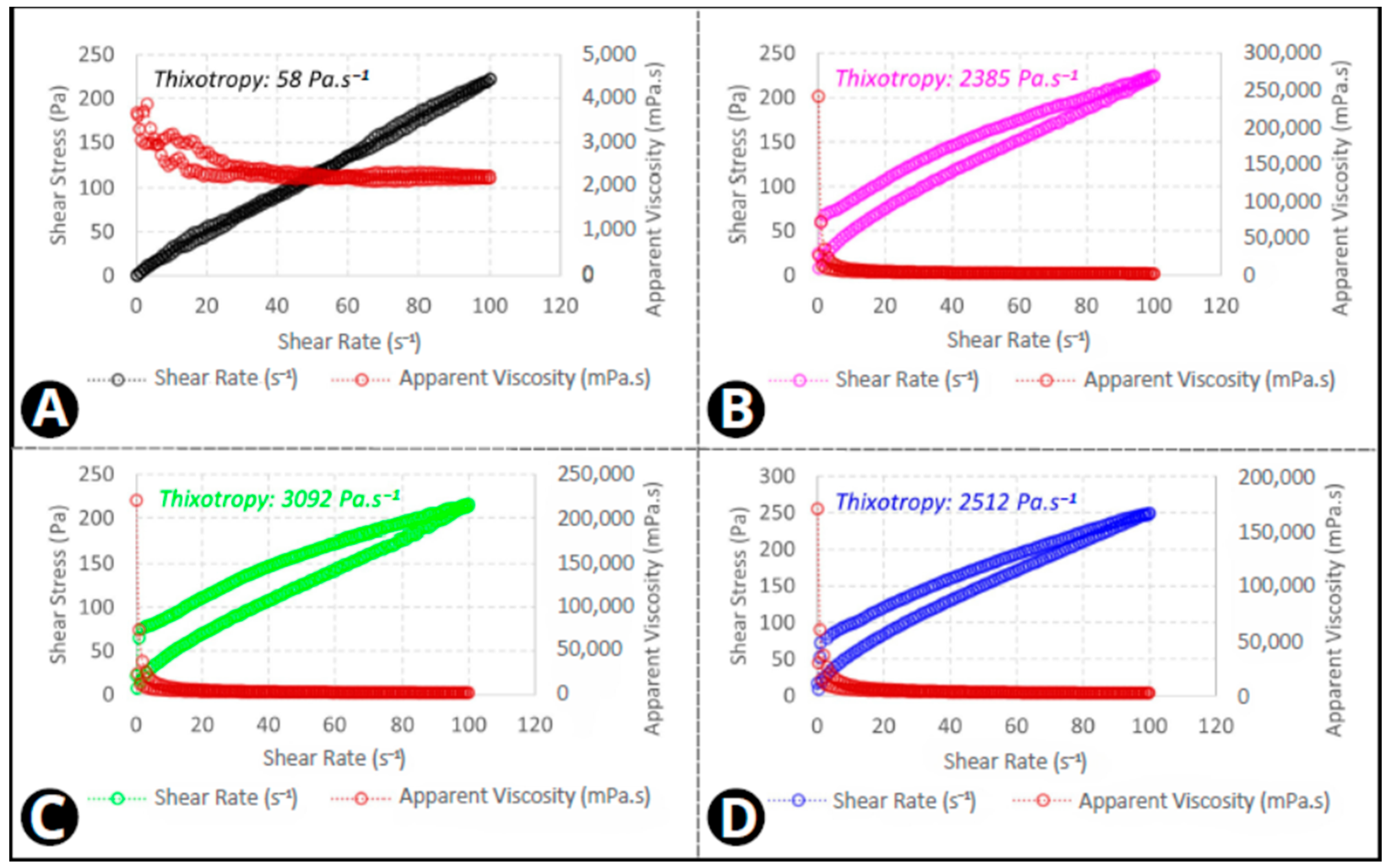
3.4. Rheology
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso, M.R.; Fernández-García, B. Evolution of the use of sports supplements. PharmaNutrition 2020, 14, 100239. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). FDA 101: Dietar Supplements. Dietary Supplements Can Help Improve Health but Can also Have Risks. Get the Facts on Supplements and How the FDA Regulates Them to Help Keep You Safe. 2022. Available online: https://www.fda.gov/consumers/consumer-updates/fda-101-dietary-supplements (accessed on 1 April 2022).
- Légifrance. Decreto n°2023-60 de 03 de Fevereiro de 2023 Relativo L’autorité Compétente en Matière de Réglementation des Auxiliaires Technologiques Pouvant Être Employés Dans la Fabrication des Denrées Alimentaires et en Matière de Réglementation Relative aux Compléments Alimentaires. 2023. Available online: https://www.legifrance.gouv.fr/loda/id/LEGIARTI000047092648/2023-02-05/ (accessed on 1 April 2023).
- Brasil. Ministério da Saúde/Agência Nacional de Vigilância Sanitária/Diretoria Colegiada. Resolução da Diretoria Colegiada-RDC Nº 18, de 27 de abril de 2010. Dispõe Sobre Alimentos para Atletas. 2010. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2010/res0018_27_04_2010.html (accessed on 1 September 2023).
- Naderi, A.; Gobbi, N.; Ali, A.; Berjisian, E.; Hamidvand, A.; Forbes, S.C.; Saunders, B. Carbohydrates and Endurance Exercise: A Narrative Review of a Food First Approach. Nutrients 2023, 15, 1367. [Google Scholar] [CrossRef]
- Silva, M.R.G.; Paiva, T.; Silva, H.H. Chapter 6: The Impact of Sports and Energy Drinks in Performance. In Sports and Energy Drinks; Woodhead Publishing: Sawston, UK, 2019; pp. 183–204. [Google Scholar]
- Arenas-Jal, M.; Suñé-Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Trends in the food and sports nutrition industry: A review. Crit. Rev. Food Sci. Nutr. 2020, 14, 2405–2421. [Google Scholar] [CrossRef]
- Reinhard, C.; Galloway, S.D.R. Carbohydrate intake practices and determinants of food choices during training in recreational, amateur, and professional endurance athletes: A survey analysis. Front. Nutr. 2022, 9, 862396. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.M.; Ross, M.L.; Swann, C.; Rothwell, J.E.; Stevens, C.J. Athlete perceptions of flavored, menthol-enhanced energy gels ingested prior to endurance exercise in the heat. Int. J. Sport. Nutr. 2022, 19, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Suksaard, C.; Kuenpetch, K.; Nokkaew, N. The development of kluai namwa (Musa Sapientum Linn.) energy gel: An alternative ergogenic aid for enhancing endurance running performance. Pak. J. Life Soc. Sci. (PJLSS) 2022, 20, 202–212. [Google Scholar] [CrossRef]
- Assis, R.C.; Monteiro, G.R.; Valentim, A.B.; Maia, C.S.C.; Felipe, S.; Freitas, A.; Ceccatto, V.M.; Alves, C.R. Biological properties of bioactive compounds from the fruit and leaves of the genipap tree (Genipa americana L.): A systematic review. Food Biosci. 2023, 53, 102514. [Google Scholar] [CrossRef]
- Silva, A.; Lédo, A.; Júnior, J. Descritores para Jenipapeiro; Embrapa: Brasília, Brazil, 2020; p. 63. [Google Scholar]
- FDA, U.S. Food & Drug Administration. 2023. Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 26 January 2023).
- Brasil, Ministério do Meio Ambiente. Biodiversidade—MMA Lança Livro Sobre Espécies Nativas com valor Econômico da Região Norte. 2023. Available online: https://www.gov.br/mma/pt-br/assuntos/noticias/mma-lanca-livro-sobre-especies-nativas-com-valor-economico-da-regiao-norte (accessed on 1 January 2023).
- Martínez-Solórzano, G.E.; Rey-Brina, J.C. Bananas (Musa AAA): Importance, production and trade in COVID-19 times. Agron. Mesoam. 2021, 32, 1034–1046. [Google Scholar] [CrossRef]
- Assis, R.C.; Soares, R.L.G.; Siqueira, A.C.P.; Rosso, V.V.; Sousa, P.H.M.; Mendes, A.E.P.; Costa, E.A.; Carneiro, A.P.G.; Maia, C.S.C. Determination of water-soluble vitamins and carotenoids in Brazilian tropical fruits by High Performance Liquid Chromatography. Heliyon 2020, 6, e05307. [Google Scholar] [CrossRef]
- Assis, R.C.; Siqueira, A.C.P.; Oliveira, J.P.S.; Silva, F.L.F.; Matos, W.O.; Gouveia, S.T.; Maia, C.S.C.; Lopes, G.S. Characterization of Mineral Content in Fruits of Northeast Agrobiodiversity of Brazil. Braz. Arch. Biol. Technol. 2022, 65, e22200759. [Google Scholar] [CrossRef]
- Aquino, A.C. Estudo da Ampliação da Escala na Produção de Néctar de Bacuri (Platonia insignis Martius) com Aplicação de Preparações Enzimáticas Comerciais. Master’s Dissertation, Federal University of Ceará, Fortaleza, Brazil, 2012; 145p. [Google Scholar]
- Adler, K.; Salanterä, S.; Zumstein-Shaha, M. Focus group interviews on child, youth, and parent research: An integrative literature review. Int. J. Qual. Methods 2019, 18, 160940691988727. [Google Scholar] [CrossRef]
- Brasil, Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Métodos Físico-Químicos para Análise de Alimentos; Ministério da Saúde: Brasília, Brazil, 2005.
- Andrews, W.H.; Wang, H.; Jacobson, A.; Ge, B.; Zhang, G.; Hammack, T. Salmonella. In BACTERIOLOGICAL Analytical Manual Online; FDA: Silver Spring, MD, USA, 2021; Chapter 5. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-5-salmonella (accessed on 26 October 2023).
- American Public Health Association (APHA). Compendium of Methods for the Microbiological Examination of Foods; APHA: Washinghton, DC, USA, 2015; Available online: https://ajph.aphapublications.org/doi/book/10.2105/MBEF.0222 (accessed on 27 October 2023).
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 3rd ed.; CRC Press LLC: Boca Raton, FL, USA, 1991. [Google Scholar]
- Stone, H.; Sidel, J.L. Sensory Evaluation Practices, 4th ed.Academic Press: Philadelphia, PA, USA, 2012. [Google Scholar]
- Macfie, H.J.; Bratchell, N.; Greenhoff, K.; Vallis, L.V. Designs to balance the effect of order of presentation and firstorder carry-over effects in hall tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- Feddernl, V.; Durante, V.V.O.; Miranda, M.Z.; Mellado, M.L.M.S. Physical and sensory evaluation of wheat and rice bran cookies. Braz. J. Food Technol. 2011, 14, 267–274. [Google Scholar]
- Instituto Adolfo Lutz (IAL). Normas Analíticas do Instituto Adolfo Lutz; IMESP: São Paulo, Brazil, 2005; p. 98. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 13th ed.; Association of Official Analytical Chemists (AOAC): Rockville, MA, USA, 1997; p. 858. [Google Scholar]
- Merrill, A.L.; Watt, B.K. Energy Value of foods: Basis and Derivation (Agriculture Handbook No. 74); United States Epartment of Agriculture: Washington, DC, USA, 1973. Available online: https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Classics/ah74.pdf (accessed on 1 September 2023).
- Araujo Filho, A.A.L.; Sousa, P.H.M.; Vieira, I.G.P.; Fernandes, V.B.; Cunha, F.E.T.; Magalhães, F.E.A.; Silva, L.M.R. Kombucha and kefir fermentation dynamics on cashew nut beverage (Anacardium occidentale L.). Int. J. Gastron. Food Sci. 2023, 33, 100778. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggent, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Oxid. Antioxid. Part. A 1999, 299, 152–178. [Google Scholar]
- Barros, S.L.; Silva, W.P.; Figueirêdo, R.M.F.; Araújo, T.J.; Santos, N.C.; Gomes, J.P. Efeito da adição de diferentes tipos de açúcar sobre a qualidade físico-química de geleias elaboradas com abacaxi e canela. Rev. Principia-Divulg. Científica E Tecnológica Do IFPB 2021, 1, 150. [Google Scholar] [CrossRef]
- Sad, A.A.; Subhan, A.; Amin, R.; Reza, S.; Mahmud, K.; Islam, A.; Tarin, N.N. Development, analysis and sensory evaluation of jelly drinks from orange concentrate. Int. J. Food Sci. Nutr. 2021, 6, 81–86. [Google Scholar]
- Cecchi, H.M. Fundamentos Teóricos e Práticos em Análise de Alimentos, 2nd ed.; Editora UNICAMP: Campinas, Brazil, 2003. [Google Scholar]
- Hearris, M.A.; Pugh, J.N.; Langan-Evans, C.; Mann, S.J.; Burke, L.; Stellingwerff, T.; Gonzalez, J.T.; Morton, J.P. 13C-glucose-fructose labelling reveals comparable exogenous CHO oxidation during exercise when consuming 120 g/h in fluid, gel, jelly chew or co-ingestion. J. Appl. Physiol. 2022, 132, 1394–1406. [Google Scholar] [CrossRef]
- Moreira, B.; Carvalho, L.F.; Santos, T.D.; Oliveira, M.S.; Costa, J.A.V. Microalgal biotechnology for development of energy gel in sports. Int. Food Res. J. 2018, 25, 1942–1947. [Google Scholar]
- Morgado, M.; Vieira, G.; Vasconcellos, J.; Lúcia, M.; Conte-Junior, C.A.; Paola, A.; Alvares, T.S. Development of a beetroot-based nutritional gel containing high content of bioaccessible dietary nitrate and antioxidants. Int. J. Food Sci. Nutr. 2016, 67, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Uchiyama, N.; Kizaki, S.; Mori, E.; Nonaka, T.; Oneda, H. Application of Continuous Glucose Monitoring for Assessment of Individual Carbohydrate Requirement during Ultramarathon Race. Nutrients 2020, 12, 1121. [Google Scholar] [CrossRef] [PubMed]
- Lestari, Y.N.; Farida, E.; Fauzi, N.; Fikri, F.F. Analysis of Physicochemical and Sensory Quality of Chia Seeds Sport Energy Gel (Salvia hispanica, L.) during Storage. In Proceedings of the 5th International Seminar of Public Health and Education, ISPHE 2020, Semarang, Indonesia, 22 July 2020. [Google Scholar]
- The Prevention of Food Adulteration Act & Rules (as on 1.10.2004). 2004. Available online: https://fssai.gov.in/upload/uploadfiles/files/pfa-acts-and-rules.pdf (accessed on 1 September 2024).
- Goldstein, E.R.; Stout, J.R.; Wells, A.J.; Antonio, J.; Vasenina, E.; Fukuda, D.H. Carbohydrate-Protein drink is effective for restoring endurance capacity in masters class athletes after a two-Hour recovery. J. Int. Soc. Sports Nutr. 2023, 20, 2178858. [Google Scholar] [CrossRef]
- Podlogar, T.; Wallis, G.A. New Horizons in Carbohydrate Research and Application for Endurance Athletes. Sports Med. 2022, 52, 5–23. [Google Scholar] [CrossRef]
- Jenner, S.L.; Buckley, G.L.; Belski, R.; Devlin, B.L.; Forsyth, A.K. Dietary Intakes of Professional and Semi-Professional Team Sport Athletes Do Not Meet Sport Nutrition Recommendations—A Systematic Literature Review. Nutrients 2019, 11, 1160. [Google Scholar] [CrossRef]
- Castillo, M.; Lozano-Casanova, M.; Sospedra, I.; Norte, A.; Gutiérrez-Hervás, A.; Martínez-Sanz, J.M. Energy and Macronutrients Intake in Indoor Sport Team Athletes: Systematic Review. Nutrients 2022, 14, 4755. [Google Scholar] [CrossRef]
- Moss, K.; Kreutzer, A.; Graybeal, A.J.; Zhang, Y.; Braun-Trocchio, R.; Porter, R.R.; Shah, M. Nutrient Adequacy in Endurance Athletes. Int. J. Environ. Res. Public Health 2023, 20, 5469. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Rehabilitation nutrition for injury recovery of athletes: The role of macronutrient intake. Nutrients 2020, 12, 2449. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452. [Google Scholar] [CrossRef]
- Qi, X.; Tester, R.F. Is starch or maltodextrin “glucose?”. Starch-Stärke 2018, 70, 1700304. [Google Scholar] [CrossRef]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Z.; Sant’Ana, A.S. Polyphenols in foods: Classification, methods of identification, and nutritional aspects in human health. Adv. Food Nutr. Res. 2021, 98, 1–33. [Google Scholar]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Dzitkowska-Zabielska, M. Polyphenol Supplementation and Antioxidant Status in Athletes: A Narrative Review. Nutrients 2022, 15, 158. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Gaspar, D.; Flores-Félix, J.D.; Falcão, A.; Alves, G.; Silva, L.R. Effects of Functional Phenolics Dietary Supplementation on Athletes’ Performance and Recovery: A Review. Int. J. Mol. Sci. 2022, 23, 4652. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S. Polyphenols: Potential Beneficial Effects of These Phytochemicals in Athletes. Curr. Sports Med. Rep. 2020, 19, 260–265. [Google Scholar] [CrossRef]
- Afzal, M.F.; Khalid, W.; Akram, S.; Khalid, M.A.; Zubair, M.; Kauser, S.; Mohamedahmed, K.A.; Aziz, A.; Siddiqui, S.A. Bioactive profile and functional food applications of banana in food sectors and health: A review. Int. J. Food Prop. 2022, 25, 2286–2300. [Google Scholar] [CrossRef]
- Monteiro, C.L.B. Técnicas de Avaliação Sensorial, 2nd ed.; CEPPA: Paraná, Argentina, 1984; p. 101. [Google Scholar]
- Jaster, H.; Arend, G.D.; Rezzadori, K.; Chaves, V.C.; Reginatto, F.H.; Petrus, J.C.C. Enhancement of antioxidant activity and physicochemical properties of yogurt enriched with concentrated strawberry pulp obtained by block freeze concentration. Food Res. Int. 2018, 104, 119–125. [Google Scholar] [CrossRef]
- Oliveira, W.Q.D.; Wurlitzer, N.J.; Araújo, A.W.D.O.; Comunian, T.A.; Bastos, M.D.S.R.; Oliveira, A.L.D.; Magalhães, H.C.R.; Ribeiro, H.L.; Figueiredo, R.W.D.; Sousa, P.H.M.D. Complex coacervates of cashew gum and gelatin as carriers of green coffee oil: The effect of microcapsule application on the rheological and sensorial quality of a fruit juice. Food Res. Int. 2020, 131, 109047. [Google Scholar] [CrossRef]
- Viana, J.D.R.; Ximenes, S.F.; Souza, A.C.R.; Abreu, F.A.P.; Petrus, J.C.C. Process optimization in the obtention of microfiltered banana (Musa cavendish) juice by response surface methodology. J. Food Process. Preserv. 2021, 45, e15987. [Google Scholar] [CrossRef]
- Lucey, J.A. Formation and Physical Properties of Milk Protein Gels. J. Dairy Sci. 2002, 85, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Karazhiyan, H.; Razavi, S.M.A.; Phillips, G.O.; Fang, Y.; Al-Assaf, S.; Nishinari, K.; Farhoosh, R. Rheological properties of Lepidium sativum seed extract as a function of concentration, temperature and time. Food Hydrocoll. 2009, 23, 2062–2068. [Google Scholar] [CrossRef]
- Hernández, M.J.L. Caracterización reológica de hidrogeles de MCC-NaCMC + almidón: Tixotropía y sinergismo. Doctoral Thesis. 1996. Available online: https://roderic.uv.es/handle/10550/38046 (accessed on 1 January 2024).


| Parameter | Fructose | Glucose | Sucrose |
|---|---|---|---|
| Detection limit [mg·mL−1] | 0.01 | 0.03 | 0.1 |
| Quantification limit [mg·ml−1] | 0.1 | 0.15 | 0.25 |
| Linearity | 155,614x + 96,710 | 137,103x − 7198.3 | 146,832x − 37,666 |
| Correlation coefficient (R2) | 0.9997 | 0.9990 | 0.9995 |
| Retention time [min] | 4.30 | 5.10 | 8.50 |
| pH | Titratable Acidity (TA) | Soluble Solids (SS) | TA/SS Ratio | Moisture (mg/100 g Sample) | Ash (mg/100 g Sample) | Protein (mg/100 g Sample) | Lipid (mg/100 g Sample) | Carbohydrate (mg/100 g Sample) | Energy Value (Kcal) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Formulation A | 3.55 ± 0.01 a | 0.07 ± 0.00 a | 69.67 ± 0.58 a | 966.07 ± 7.23 a | 33.10 ± 1.39 a | 0.73 ± 0.02 a | 0.40 ± 0.04 a | 0.53 ± 0.03 a | 64.96 ± 1.33 a | 266.27 ± 5.35 a |
| Formulation B | 3.57 ± 0.01 a | 0.09 ± 0.01 a | 69.50 ± 0.00 a | 810.23 ± 19.51 b | 38.79 ± 1.51 a | 0.75 ± 0.06 a | 0.35 ± 0.02 a | 0.24 ± 0.02 b | 59.80 ± 1.38 b | 242.80 ± 5.49 b |
| Formulation C | 3.47 ± 0.04 a | 0.10 ± 0.00 c | 69.83 ± 0.58 a | 694.76 ± 26.54 c | 37.56 ± 1.30 a | 0.98 ± 0.07 a | 0.31 ± 0.01 a | 0.38 ± 0.05 a | 60.76 ± 0.00 bc | 247.73 ± 0.00 bc |
| Commercial gel | 3.72 ± 0.08 b | 0.06 ± 0.00 ae | 64.33 ± 0.57 b | 1125.14 ± 29.48 d | 36.15 ± 1.89 a | 0.67 ± 0.16 a | 0.34 ± 0.02 a | 0.26 ± 0.04 b | 62.58 ± 0.00 c | 254.02 ± 0.00 c |
| Genipap | 3.44 ± 0.08 a | 0.12 ± 0.00 d | 25.00 ± 0.00 c | 203.05 ± 14.67 e | 76.71 ± 0.32 b | 1.22 ± 0.12 a | 0.47 ± 0.00 a | 0.15 ± 0.06 c | 21.45 ± 0.00 d | 89.03 ± 0.00 d |
| Banana | 4.33 ± 0.05 c | 0.05 ± 0.00 e | 24.33 ± 0.58 c | 483.90 ± 37.96 f | 76.29 ± 0.32 b | 0.83 ± 0.14 a | 0.76 ± 0.02 b | 0.10 ± 0.01 c | 22.02 ± 0.00 d | 92.02 ± 0.00 d |
| Glucose (mg/g) | Fructose (mg/g) | Sucrose (mg/g) | ABTS (μM Trolox/g) | Total Polyphenols (mg Gallic Acid/ 100 g Sample) | |
|---|---|---|---|---|---|
| Formulation A | 153.12 ± 3.87 a | 60.14 ± 2.10 a | 100.60 ± 2.46 a | 6.39 ± 0.38 a | 105.29 ± 7.37 a |
| Formulation B | 123.93 ± 3.15 b | 42.41 ± 2.32 b | 101.22 ± 0.96 a | 8.15 ± 0.60 ace | 106.01 ± 6.48 a |
| Formulation C | 129.91 ± 4.16 b | 46.29 ± 0.71 b | 139.71 ± 4.81 b | 10.15 ± 1.29 ce | 173.74 ± 7.93 b |
| Commercial gel | 4.12 ± 0.54 c | ND c | ND c | ND d | ND c |
| Genipap | 55.97 ± 6.80 d | 19.11 ± 0.94 d | 29.37 ± 0.86 d | 10.88 ± 2.66 e | 177.73 ± 15.74 b |
| Banana | 48.37 ± 4.52 d | 35.14 ± 1.87 e | ND c | ND d | 45.76 ± 1.79 d |
| Formulation | Taste (%) | Consistency (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V.Wea | W | I | Str | V.Str | V.Flu | Flu | I | D | V.D | |
| A | 0 | 20 | 60 | 20 | 0 | 20 | 80 | 0 | 0 | 0 |
| B | 0 | 0 | 60 | 20 | 20 | 0 | 60 | 40 | 0 | 0 |
| C | 0 | 0 | 40 | 20 | 40 | 20 | 40 | 20 | 20 | 0 |
| D | 0 | 0 | 40 | 40 | 20 | 0 | 0 | 40 | 60 | 0 |
| E | 0 | 0 | 0 | 40 | 60 | 0 | 0 | 60 | 20 | 20 |
| F | 0 | 0 | 0 | 60 | 40 | 0 | 0 | 40 | 0 | 60 |
| Taste | Texture | |||
|---|---|---|---|---|
| Score | AI (%) | Score | AI (%) | |
| Formulation A | 7.11 ± 1.77 a | 78.98 | 7.69 ± 1.33 a | 85.40 |
| Formulation B | 6.65 ± 2.00 a | 73.93 | 7.55 ± 1.39 a | 83.88 |
| Formulation C | 6.75 ± 2.20 a | 75.05 | 7.45 ± 1.64 a | 82.79 |
| Sample | Power Law | |||
| τ0 (Pa) | K (Pa·sn) | N | R2 | |
| Commercial | N/A | 2.79 | 0.9501 | 0.998 |
| Formulation A 2 | 18.78 | 0.5325 | 0.967 | |
| Formulation B 3 | 19.86 | 0.5099 | 0.941 | |
| Formulation C | 20.70 | 0.5350 | 0.969 | |
| Sample | Newton’s Model | |||
| µ (mPa.s) | K (Pa·sn) | N | R2 | |
| Commercial 1 | 2.24 | N/A | N/A | 0.998 |
| Formulation A | 2.58 | 0.823 | ||
| Formulation B | 2.48 | 0.773 | ||
| Formulation C | 2.88 | 0.829 | ||
| Sample | Herschel–Bulkley Model | |||
| τ0 (Pa) | K (Pa·sn) | N | R2 | |
| Commercial | 5.39 | 2.135 | 1.003 | 0.998 |
| Formulation A | 28.21 | 7.862 | 0.696 | 0.969 |
| Formulation B | 64.47 | 1.241 | 1.047 | 0.935 |
| Formulation C 4 | 30.50 | 8.859 | 0.695 | 0.972 |
| Sample | Bingham Model | |||
| τ0 (Pa) | K (Pa·sn) | N | R2 | |
| Commercial | 5.19 | 2.163 | N/A | 0.998 |
| Formulation A | 53.52 | 1.781 | 0.963 | |
| Formulation B | 54.69 | 1.665 | 0.939 | |
| Formulation C | 59.15 | 1.992 | 0.966 | |
| Sample | Casson’s Model | |||
| τ0 (Pa) | K (Pa·sn) | N | R2 | |
| Commercial | 0.45 | 2.005 | N/A | 0.998 |
| Formulation A | 24.37 | 1.024 | 0.959 | |
| Formulation B | 25.61 | 0.9309 | 0.931 | |
| Formulation C | 26.57 | 1.157 | 0.959 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assis, R.; Valentim, A.; Barbosa, I.; Silva, J.; Aquino, A.; Viana, J.; Rabelo, C.; Sousa, P.; Maia, C.; Fernandes, V.; et al. Development of Fruit-Based Carbohydrate Gel for Endurance Athletes. Processes 2024, 12, 2304. https://doi.org/10.3390/pr12102304
Assis R, Valentim A, Barbosa I, Silva J, Aquino A, Viana J, Rabelo C, Sousa P, Maia C, Fernandes V, et al. Development of Fruit-Based Carbohydrate Gel for Endurance Athletes. Processes. 2024; 12(10):2304. https://doi.org/10.3390/pr12102304
Chicago/Turabian StyleAssis, Renata, Ashley Valentim, Isabele Barbosa, Julyana Silva, Andrea Aquino, José Viana, Claisa Rabelo, Paulo Sousa, Carla Maia, Victor Fernandes, and et al. 2024. "Development of Fruit-Based Carbohydrate Gel for Endurance Athletes" Processes 12, no. 10: 2304. https://doi.org/10.3390/pr12102304
APA StyleAssis, R., Valentim, A., Barbosa, I., Silva, J., Aquino, A., Viana, J., Rabelo, C., Sousa, P., Maia, C., Fernandes, V., Vieira, Í., & Alves, C. (2024). Development of Fruit-Based Carbohydrate Gel for Endurance Athletes. Processes, 12(10), 2304. https://doi.org/10.3390/pr12102304








