Experimental Tests on In Situ Combustion Using Dynamic Ignition Simulation System in High-Temperature and High-Pressure Conditions
Abstract
:1. Introduction
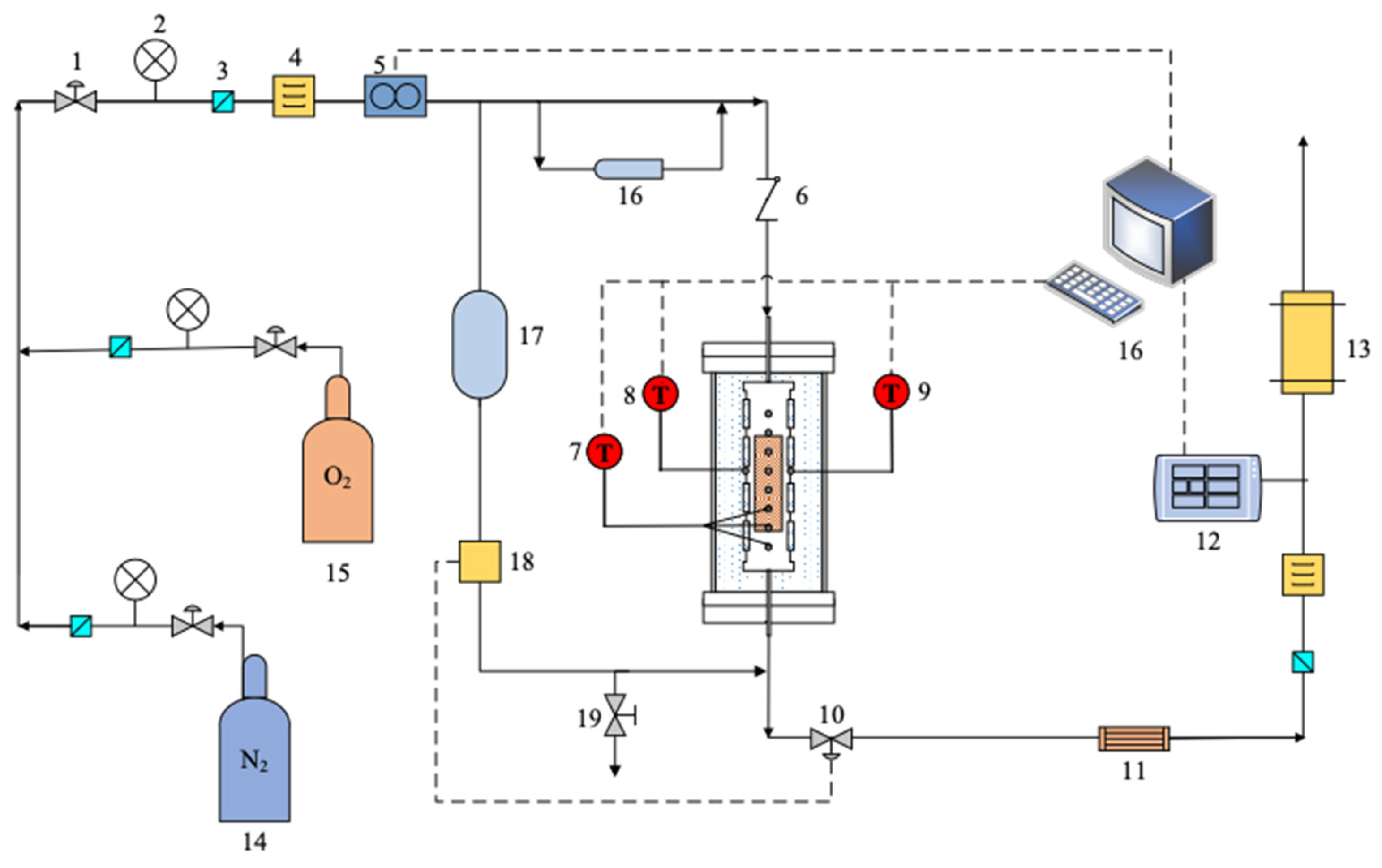
2. System Components and Design
2.1. Ignition Simulation System
2.1.1. Combustion Tube Structure Design
2.1.2. Temperature Control Design
2.1.3. Combustion Tube Sealing and Stability Design
2.2. Online Exhaust Gas Monitoring System
2.3. Injection and Discharge System
3. Experimental Section
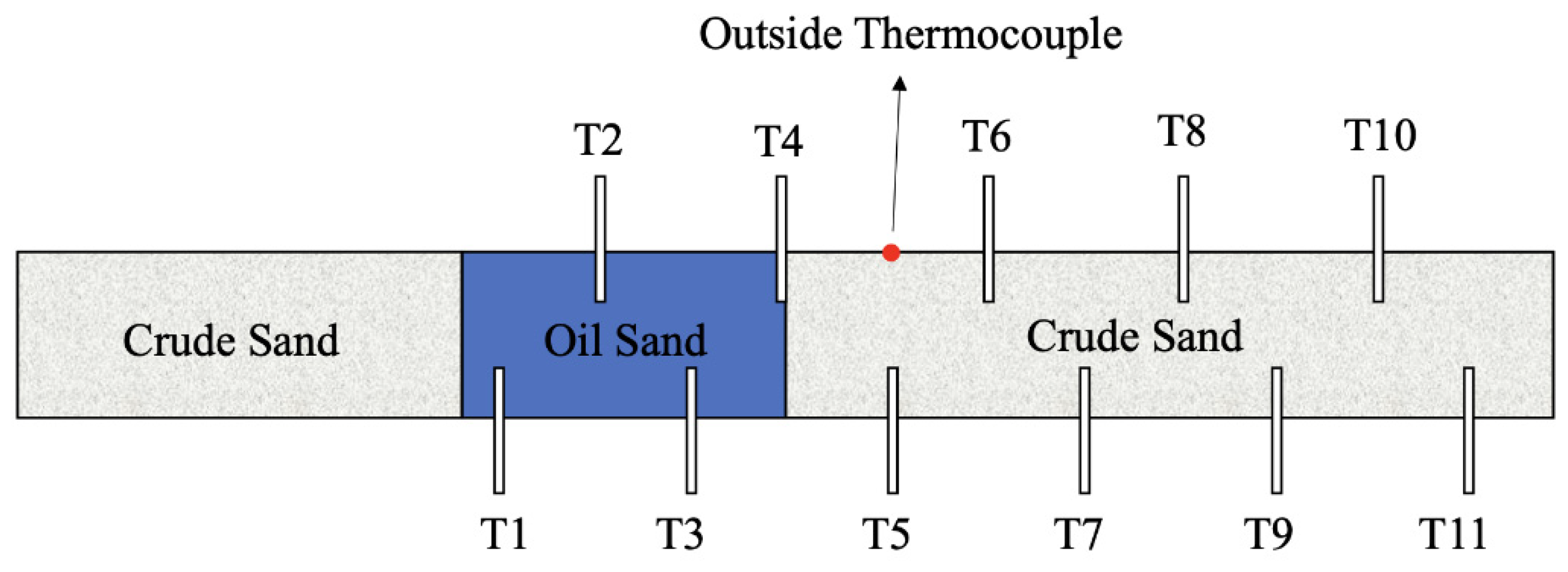
3.1. Materials Preparation
3.2. Experimental Procedure
- Close the outlet valve and inject nitrogen to bring the back pressure to 4 MPa.
- Once the pressure has stabilized, open the outlet valve and adjust the mass flow controller to maintain a flow rate of 300 mL/min. Keep the flow at this rate for 5 min.
- If it is necessary to add liquid additives, place the additives in the liquid cylinder of the liquid injector. Inject nitrogen at a flow rate of 500 mL/min and continue injecting for 5 min. Then, decrease the flow rate to 300 mL/min and switch to air once stabilization is achieved. If there is no need to add liquid additives, switch to using air directly.
- Once the flow rate has stabilized, begin heating and set the heating rate on the PC. Start recording the dynamic parameters of the combustion process.
- When all temperature measurements at the measurement points remain stable for 30 min, close the cylinder valve to stop gas injection. Slowly adjust the pressure relief valve to ensure that the system pressure drops to atmospheric pressure.
- Wait for the device to cool down and then disassemble the core, which is divided into sections at 3 cm intervals, weighed and sealed for later analysis and processing.
3.3. Experimental Test
3.3.1. Blank Heating Experiment
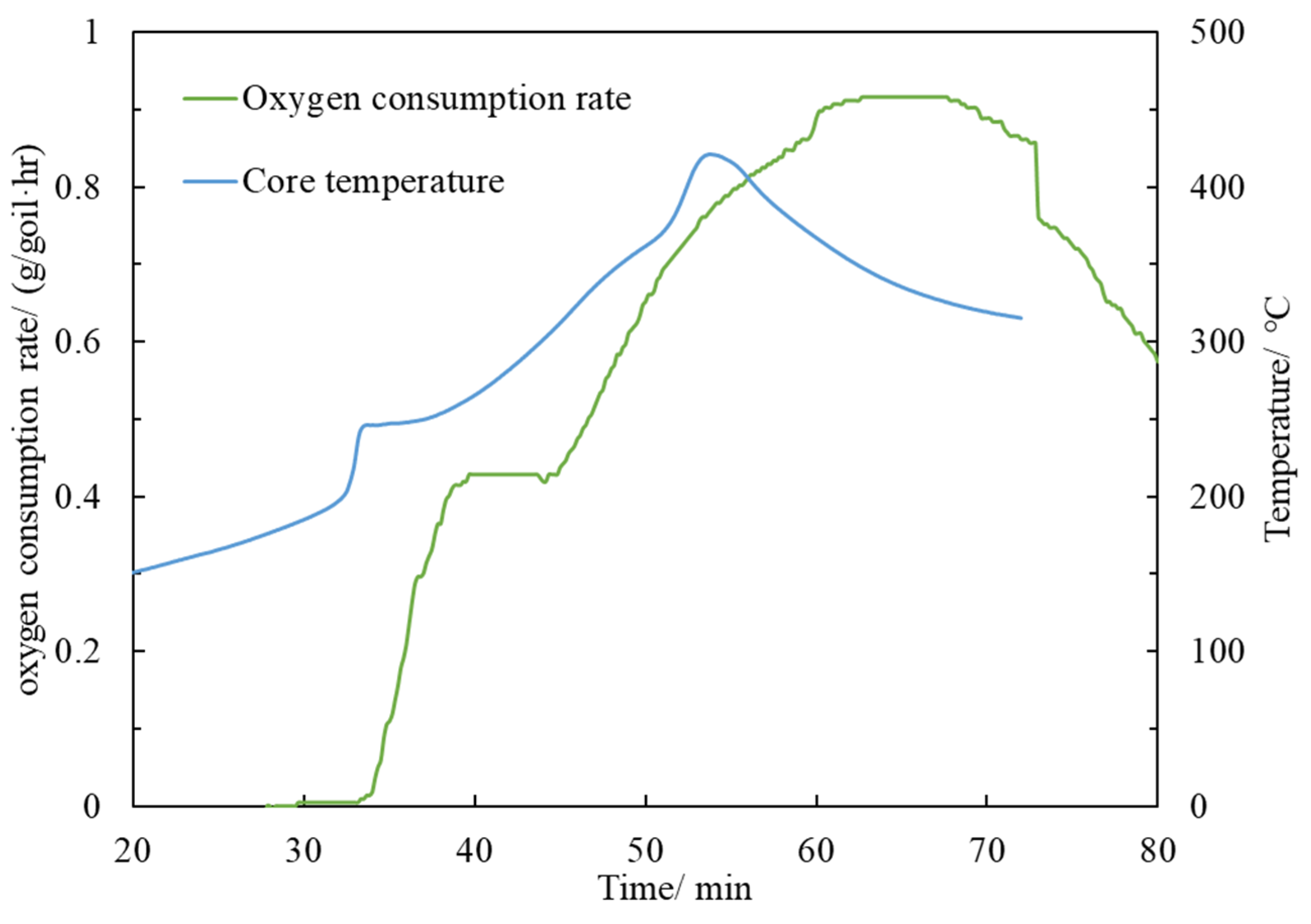
3.3.2. Ignition Test
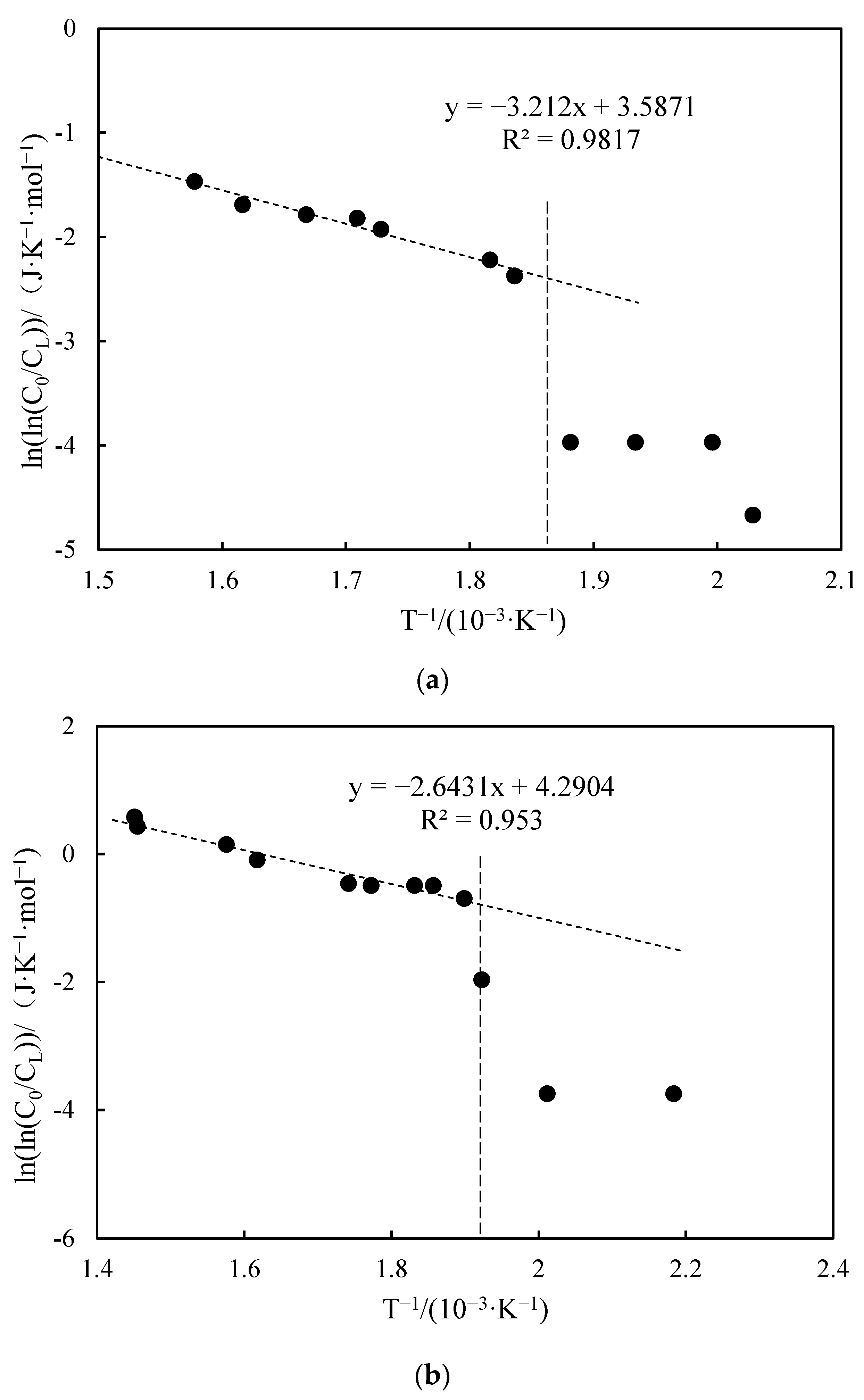
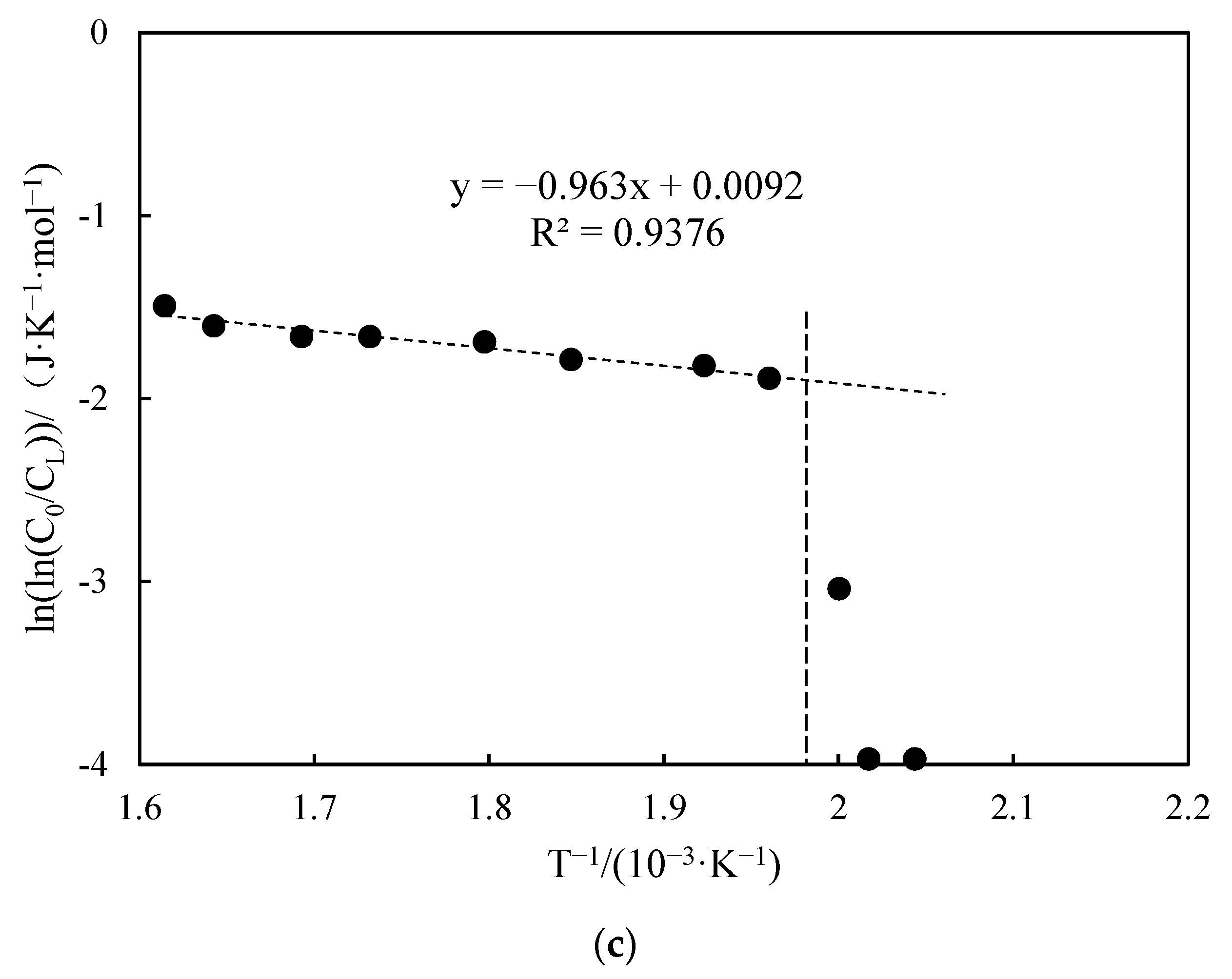
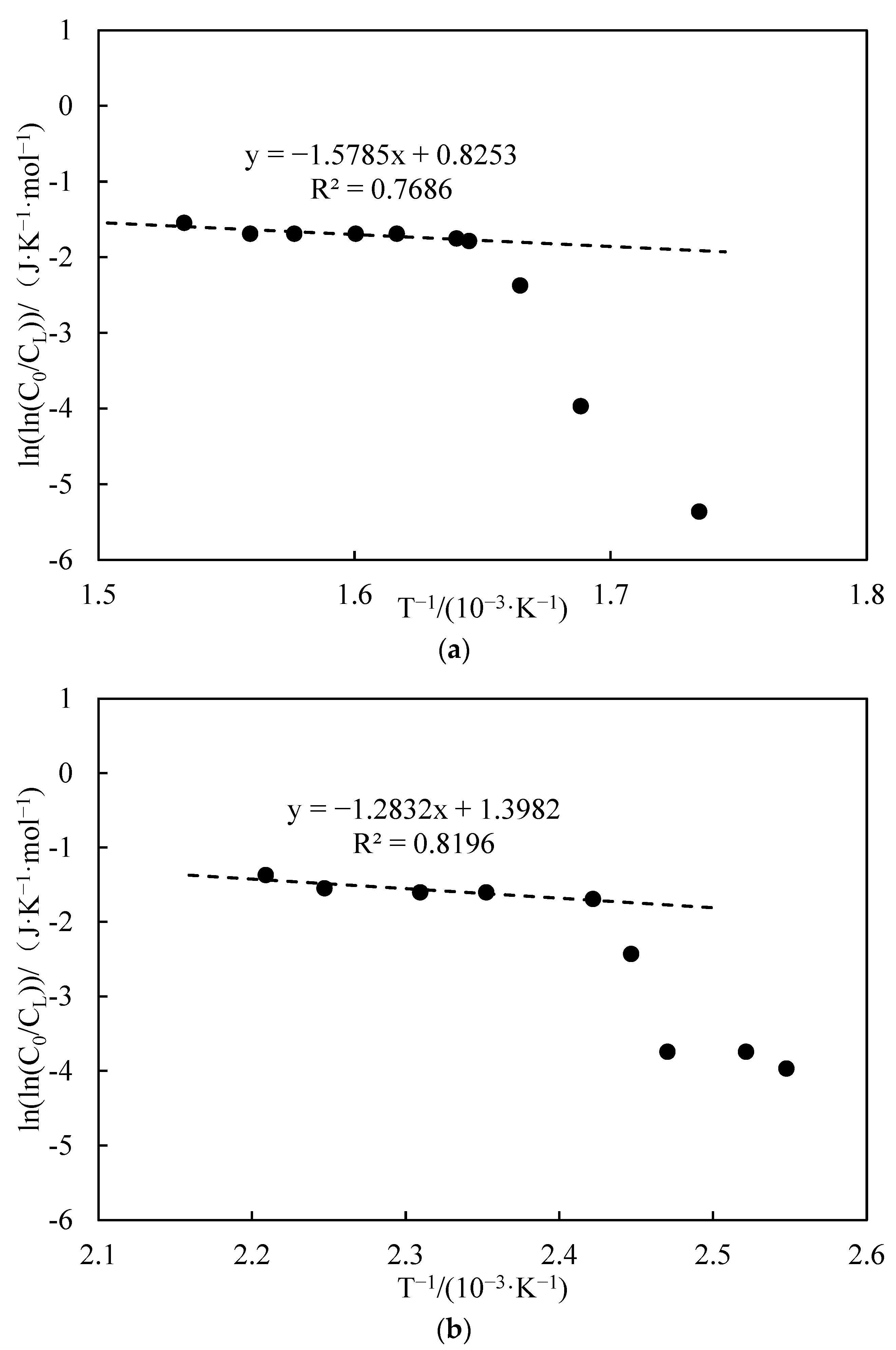
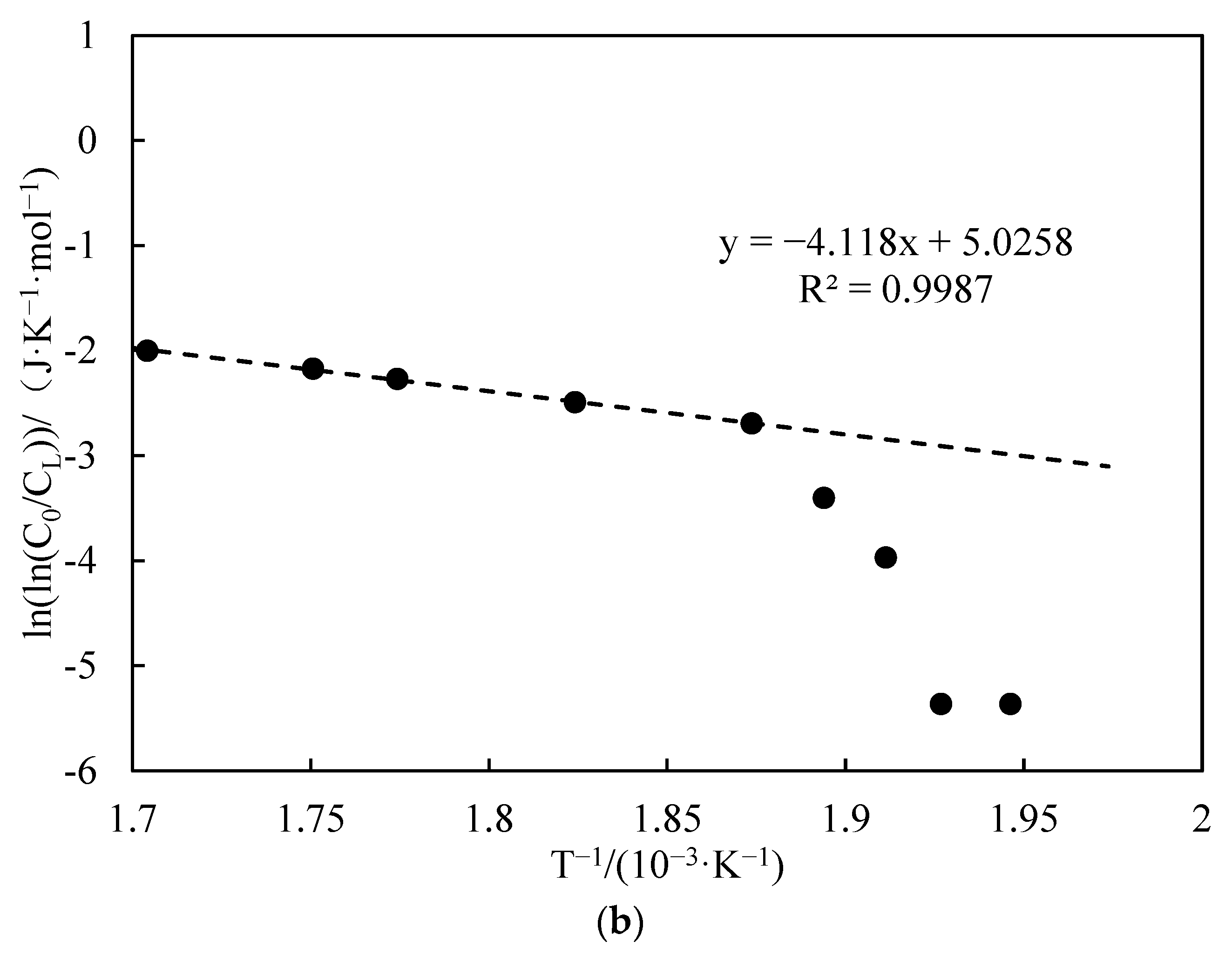
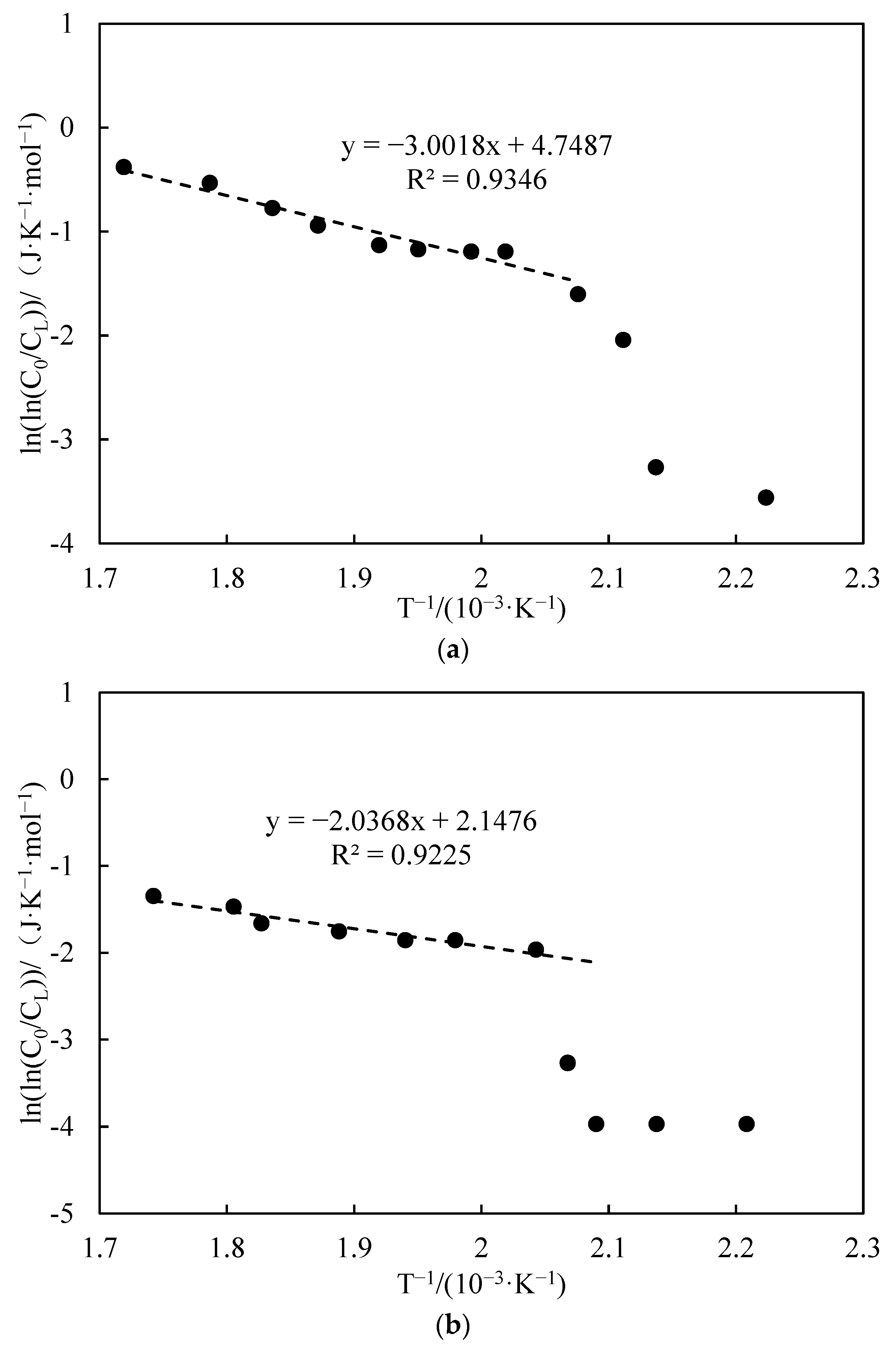
3.3.3. Apparent Activation Energy Analysis
4. Conclusions
- 7.
- The experimental system can achieve real-time monitoring of crude oil combustion in situ. The combination of fuzzy theory and PID control enables thermal tracking compensation, achieving a temperature control accuracy of ±0.1 °C. This device is designed to meet the experimental requirements of high-pressure, rapid temperature changes and complex gas production components during crude oil combustion. It has a pressure resistance of 10 MPa, a sampling resolution of 0.1 °C, and the capability to monitor and record the content of seven tail gas components in real time.
- 8.
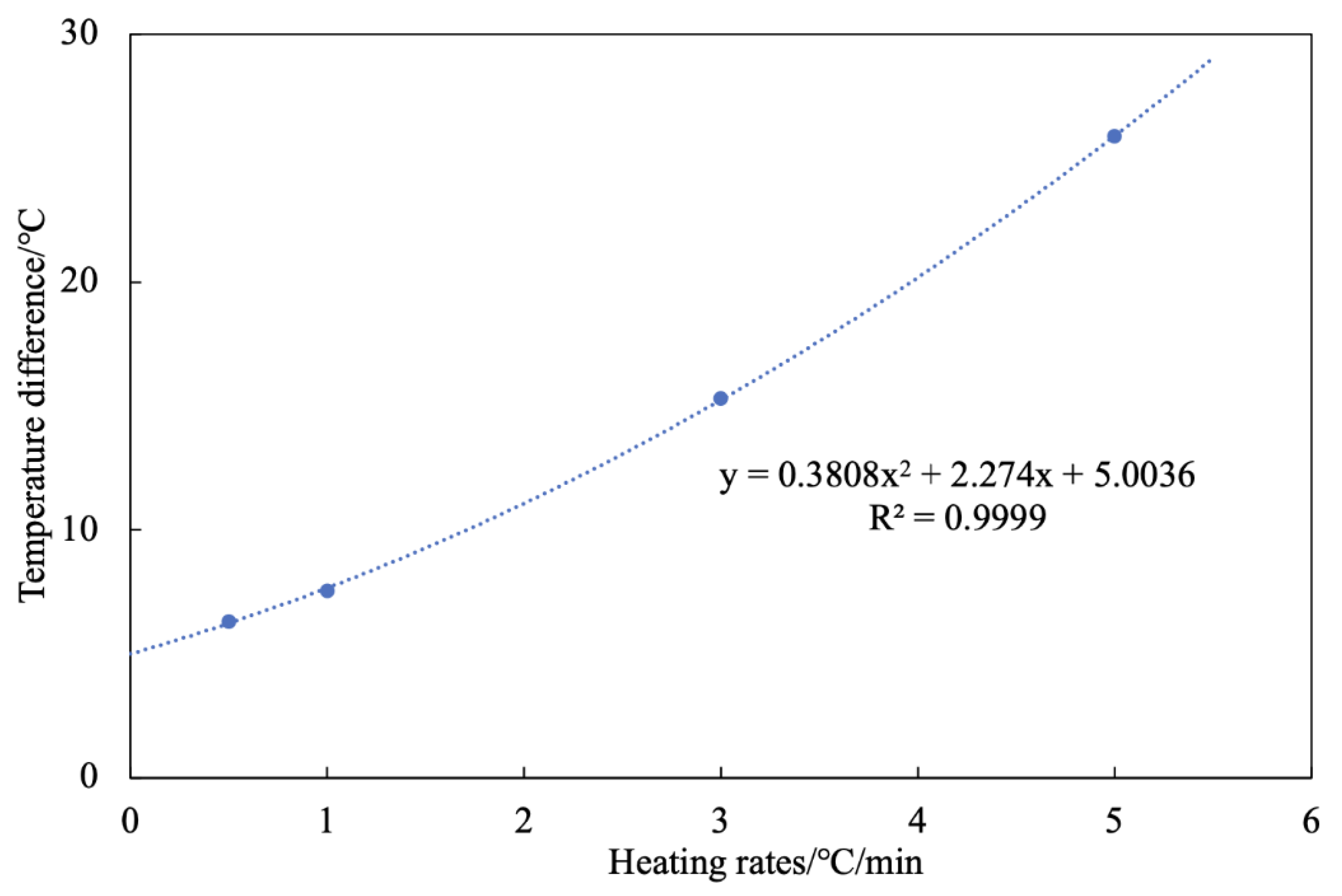
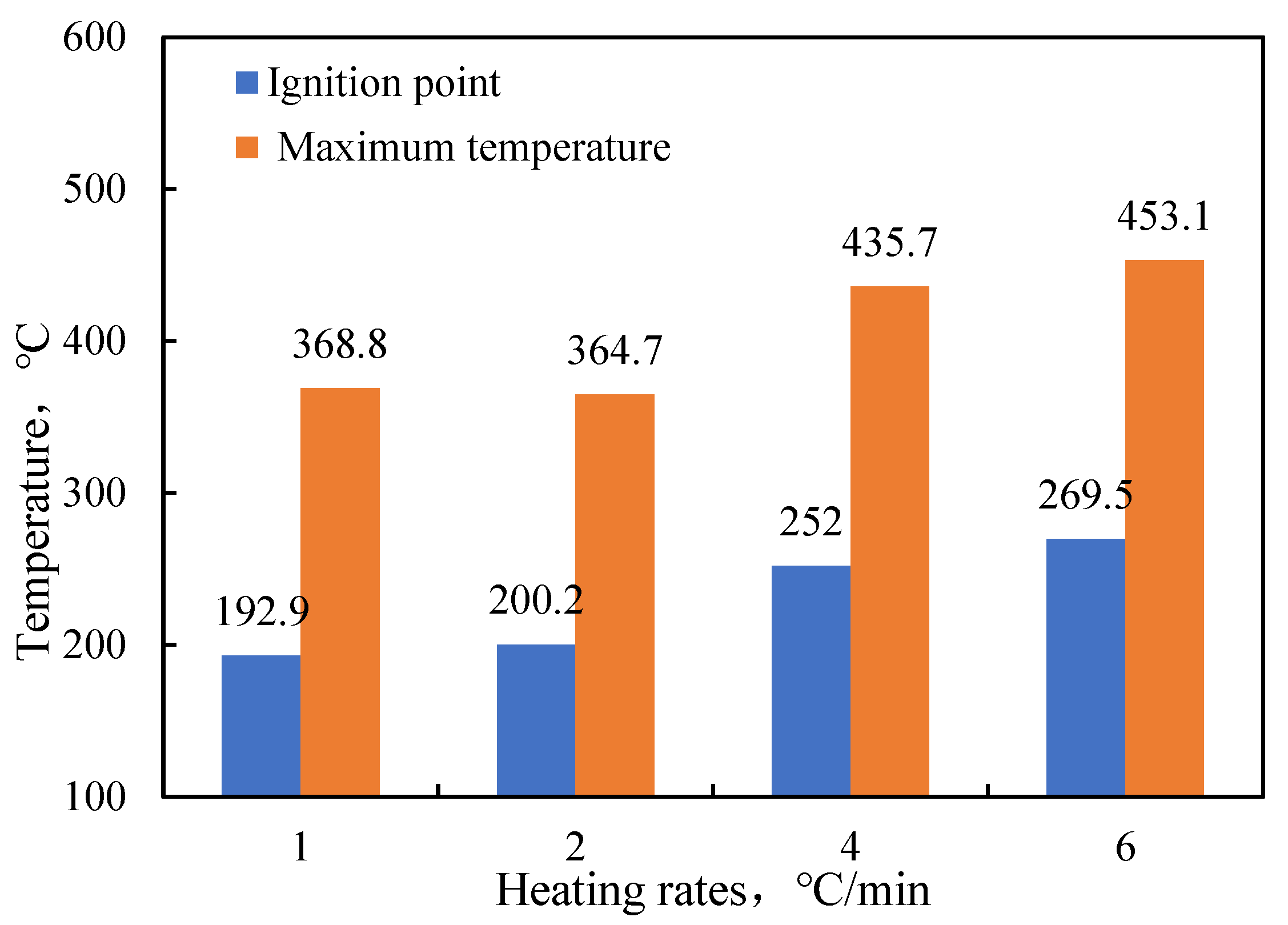
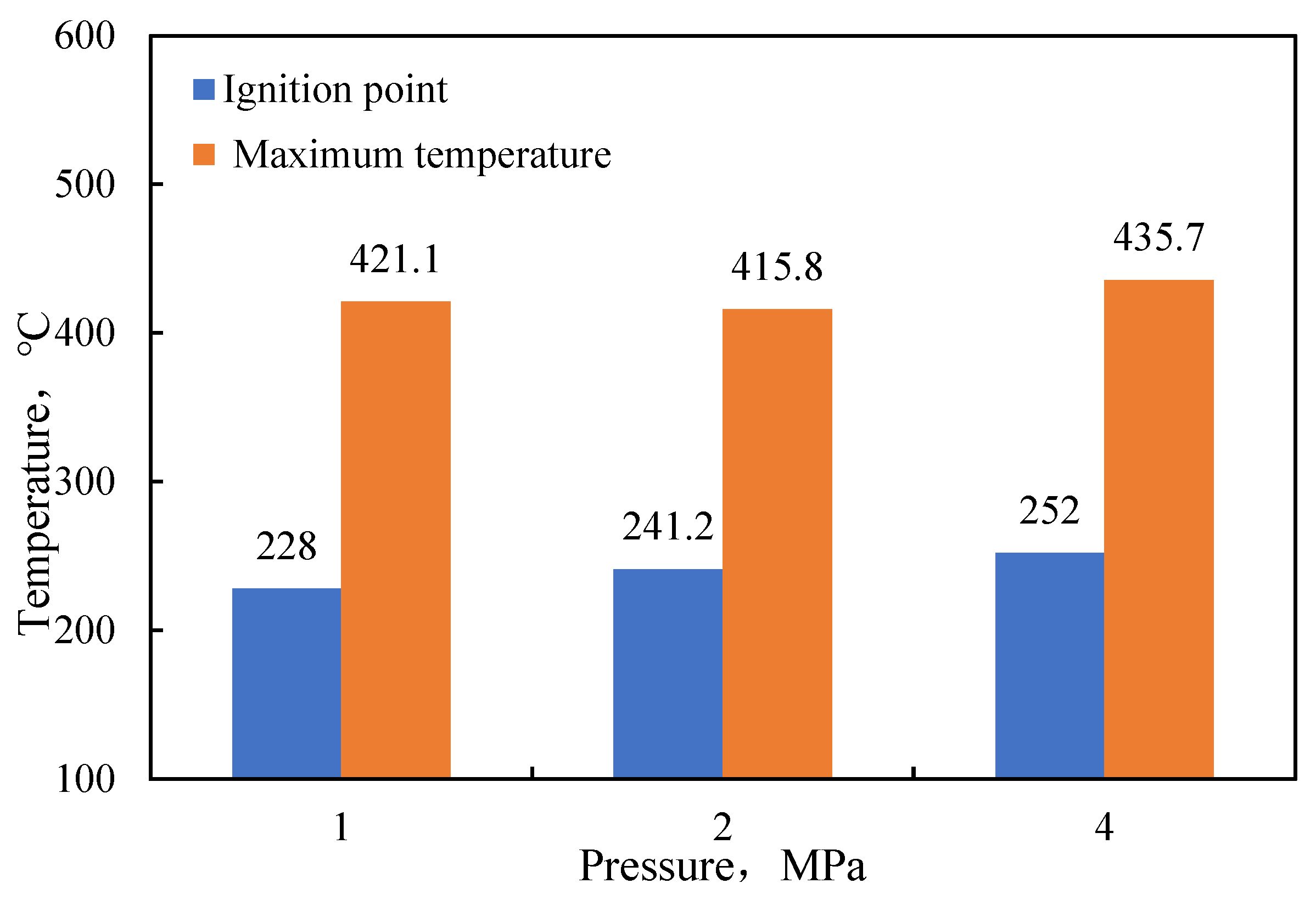
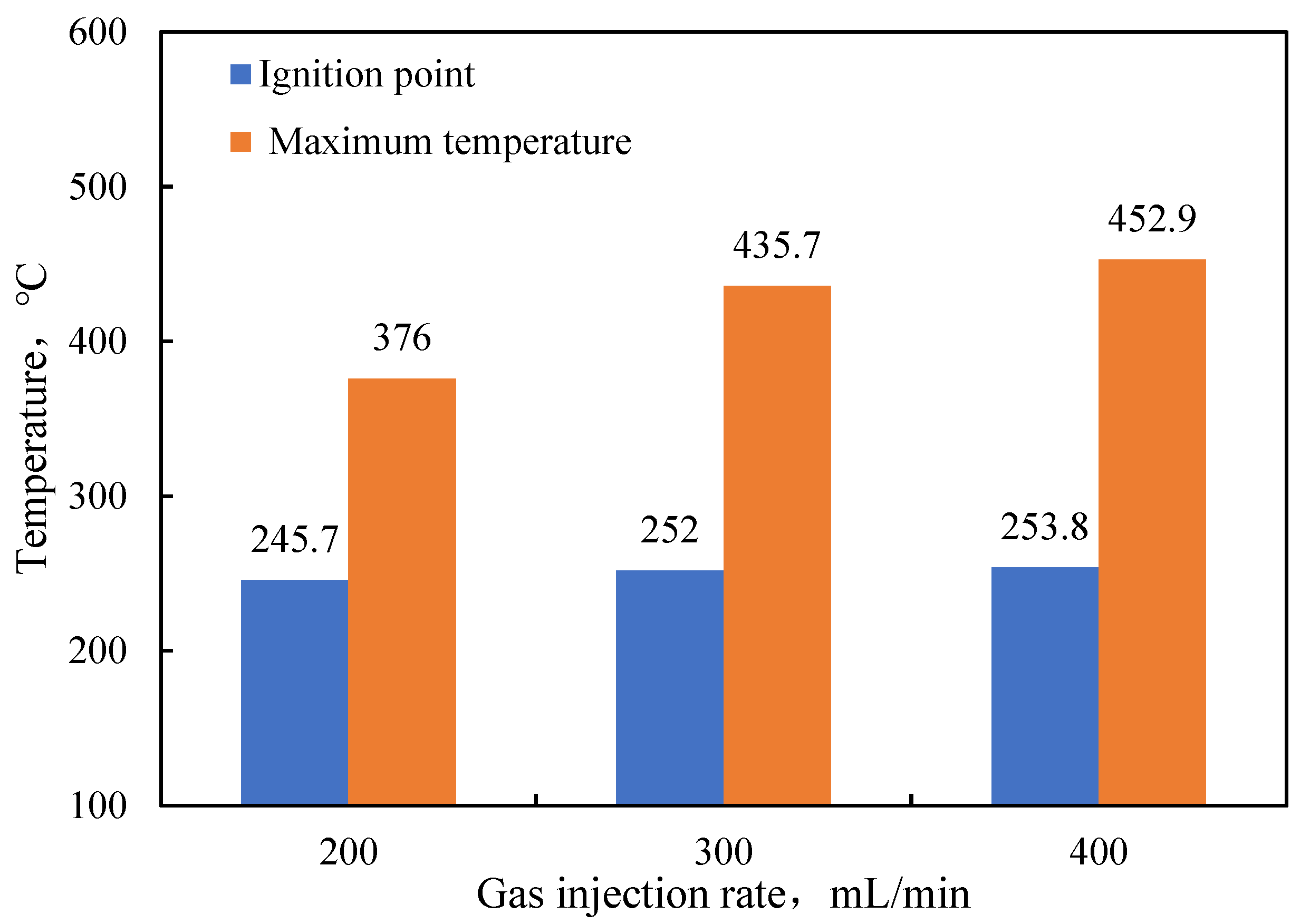
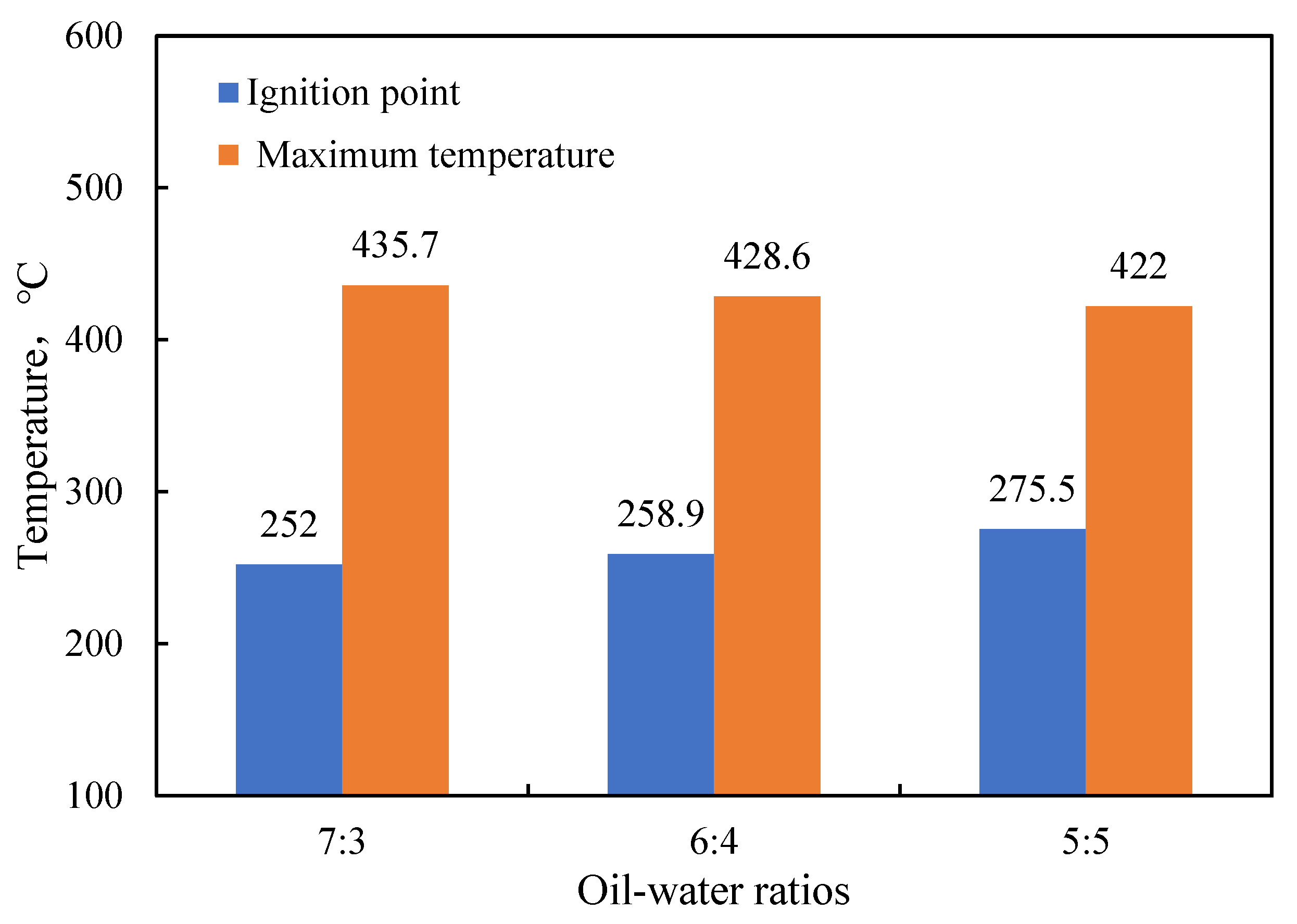
- Functional experiments showed that the variation of with during the experimental process exhibited a distinct stage bounded by an inflection point. The linearity of the curves improved only when the temperature exceeded a specific inflection point temperature. The apparent activation energies and finger front factors were calculated for the HTO stage under different pressure conditions. The results showed that, as the pressure increased, the auto-ignition point, inflection point temperature, and apparent activation energy of the crude oil decreased. This indicates that increasing the pressure can promote the HTO of the crude oil. The spontaneous ignition point of the crude oil exhibited an upward trend as the heating rate increased. Additionally, the maximum temperature during the combustion process generally rose with the increase in heating rate, reaching a maximum temperature of 453.1 °C.
- 9.
- Using the experimental data obtained from the high-temperature and high-pressure dynamic ignition simulation system, it is possible to calculate the kinetic parameters of crude oil oxidation. This calculation provides essential data support for the implementation of in situ combustion and numerical simulation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shah, A.; Fishwick, R.; Wood, J.; Leeke, G.; Rigby, S.; Greaves, M. A review of novel techniques for heavy oil and bitumen extraction and upgrading. Energy Environ. Sci. 2010, 3, 700–714. [Google Scholar] [CrossRef]
- Changfeng, X.; Wenlong, G.; Jihong, H. Research and Application of Fire-Flooding Technologies in Post-Steam Injected Heavy Oil Reservoir. In Proceedings of the International Petroleum Technology Conference, Beijing, China, 26–28 March 2013. [Google Scholar]
- Hamdy, M.; Mohamed, A.A.; Curran, H. Combination Effect of in-Situ Combustion and Exhaust Gases Recirculation on 1D Combustion Tube: Numerical Approach. Combust. Sci. Technol. 2023, 195, 3296–3309. [Google Scholar] [CrossRef]
- Farahani, M.D.; Abdrabou, M.; Zhang, H.; Zhu, J.; Wang, F.; Lee, K.; Zheng, Y. In situ burning of crude oils using iron oxide nanoparticles as additives. Fuel 2022, 330, 125568. [Google Scholar] [CrossRef]
- Yang, L.F.; Sheng, J.J. Pyrite effects on the oxidation of in situ crude oil. J. Pet. Sci. Eng. 2022, 209, 109812. [Google Scholar] [CrossRef]
- Kok, M.V.; Iscan, A.G. Catalytic effects of metallic additives on the combustion properties of crude oils by thermal analysis techniques. J. Therm. Anal. Calorim. 2001, 64, 1311–1318. [Google Scholar] [CrossRef]
- Li, J.; Mehta, S.A.; Moore, R.G.; Ursenbach, M.G. New Insights Into Oxidation Behaviours of Crude Oils. J. Can. Pet. Technol. 2009, 48, 12–15. [Google Scholar] [CrossRef]
- Zhao, J.-Z.; Jia, H.; Pu, W.-F.; Wang, L.-L.; Peng, H. Sensitivity Studies on the Oxidation Behavior of Crude Oil in Porous Media. Energy Fuels 2012, 26, 6815–6823. [Google Scholar] [CrossRef]
- Gundogar, A.S.; Kok, M.V. Thermal characterization, combustion and kinetics of different origin crude oils. Fuel 2014, 123, 59–65. [Google Scholar] [CrossRef]
- Hamedi Shokrlu, Y.; Maham, Y.; Tan, X.; Babadagli, T.; Gray, M. Enhancement of the efficiency of in situ combustion technique for heavy-oil recovery by application of nickel ions. Fuel 2013, 105, 397–407. [Google Scholar] [CrossRef]
- Ni, J.; Jia, H.; Pu, W.; Jiang, H.; Yang, J.; Ren, Q. Thermal kinetics study of light oil oxidation using TG/DTG techniques. J. Therm. Anal. Calorim. 2014, 117, 1349–1355. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Sitnov, S.A.; Vakhin, A.V. Impact of Iron Tallate on the Kinetic Behavior of the Oxidation Process of Heavy Oils. Energy Fuels 2019, 33, 7678–7683. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, J.-Z.; Pu, W.-F.; Liao, R.; Wang, L.-L. The Influence of Clay Minerals Types on the Oxidation Thermokinetics of Crude Oil. Energy Sources Part A Recovery Util. Environ. Eff. 2012, 34, 877–886. [Google Scholar] [CrossRef]
- Li, J.; Mehta, S.A.; Moore, R.G.; Ursenbach, M.G.; Zalewski, E.; Ferguson, H.; Okazawa, N.E. Oxidation and ignition behaviour of saturated hydrocarbon samples with crude oils using TG/DTG and DTA thermal analysis techniques. J. Can. Pet. Technol. 2004, 43, 45–51. [Google Scholar] [CrossRef]
- Li, J.; Mehta, S.A.; Moore, R.G.; Zalewski, E.; Ursenbach, M.G.; Van Fraassen, K. Investigation of the oxidation behaviour of pure hydrocarbon components and crude oils utilizing PDSC thermal technique. J. Can. Pet. Technol. 2006, 45, 48–53. [Google Scholar] [CrossRef]
- Jiao, A.; Huang, P.; Qian, X.; Feng, C.; Liu, Z. A chemically assisted ignition mathematical model based on the theory of thermal ignition. Energy Rep. 2022, 8, 1358–1369. [Google Scholar] [CrossRef]
- Yuan, C.D.; Sadikov, K.; Varfolomeev, M.; Khaliullin, R.; Pu, W.; Al-Muntaser, A.; Mehrabi-Kalajahi, S.S. Low-temperature combustion behavior of crude oils in porous media under air flow condition for in-situ combustion (ISC) process. Fuel 2020, 259, 116293. [Google Scholar] [CrossRef]
- Ren, S.R.; Greaves, M.; Rathbone, R.R. Air injection LTO process: An IOR technique for light-oil reservoirs. SPE J. 2002, 7, 90–99. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Y.X.; Huang, R.P.; Castanier, L.M.; Kovscek, A.R. An experimental investigation of the in-situ combustion behavior of Karamay crude oil. J. Pet. Sci. Eng. 2015, 127, 82–92. [Google Scholar] [CrossRef]
- Pu, W.F.; Wang, L.-L.; Peng, X.-Q.; Li, N.; Zhao, S. Effects of aromatics, resins, and asphaltenes on oxidation behavior and kinetics of heavy crude oil. Pet. Sci. Technol. 2020, 38, 815–822. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.S.; Lu, N.; Chai, M.J.; Wang, S.; Feng, Q.H.; Chen, Z.X. Integration of ramped temperature oxidation and combustion tube tests for kinetic modeling of heavy oil in-Situ combustion. Energy 2023, 274, 127435. [Google Scholar] [CrossRef]
- Abu, I.I.; Moore, R.G.; Mehta, S.A.; Ursenbach, M.G.; Mallory, D.G.; Almao, P.P.; Ortega, L.C. Upgrading of Athabasca Bitumen Using Supported Catalyst in Conjunction With In-Situ Combustion. J. Can. Pet. Technol. 2015, 54, 220–232. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.; Varfolomeev, M.A.; Yuan, C.; Mehrabi-Kalajahi, S.S.; Saifullin, E.R.; Sadikov, K.; Talipov, S. Low-temperature combustion characteristics of heavy oils by a self-designed porous medium thermo-effect cell. J. Pet. Sci. Eng. 2020, 195, 107863. [Google Scholar] [CrossRef]
- Shouman, A.R. A review of one aspect of the thermal-explosion theory. J. Eng. Math. 2006, 56, 179–184. [Google Scholar] [CrossRef]
- Niu, B.L.; Ren, S.; Liu, Y.; Wang, D.; Tang, L.; Chen, B. Low-Temperature Oxidation of Oil Components in an Air Injection Process for Improved Oil Recovery. Energy Fuels 2011, 25, 4299–4304. [Google Scholar] [CrossRef]
- Ambalae, A.; Mahinpey, N.; Freitag, N. Thermogravimetric studies on pyrolysis and combustion behavior of a heavy oil and its asphaltenes. Energy Fuels 2006, 20, 560–565. [Google Scholar] [CrossRef]
- Fadaei, H.; Castanier, L.; Camp, A.M.M.; Debenest, G.; Quintard, M.; Renard, G. Experimental and Numerical Analysis of In-Situ Combustion in a Fractured Core. SPE J. 2011, 16, 358–373. [Google Scholar] [CrossRef]
- Kuppe, G.J.M.; Mehta, S.A.; Moore, R.G.; Ursenbach, M.G.; Zalewski, E. Heats of combustion of selected crude oils and their SARA fractions. J. Can. Pet. Technol. 2008, 47, 38–42. [Google Scholar] [CrossRef]
- Clara, C.; Durandeau, M.; Quenault, G.; Nguyen, T.-H. Laboratory studies for light-oil air injection projects: Potential application in Handil field. SPE Reserv. Eval. Eng. 2000, 3, 239–248. [Google Scholar] [CrossRef]



| Gases | Measuring Range | Resolution |
|---|---|---|
| CH4 | (0–100)% LEL | 1% LEL |
| O2 | (0–30.0)% LEL | 0.1% LEL |
| H2S | (0–100) PPM | 1 PPM |
| CO | (0–1000) PPM | 1 PPM |
| CO2 | (0–30.0)% LEL | 0.1% LEL |
| H2 | (0–100)% LEL | 1% LEL |
| N2 | (0–100)% LEL | 1%LEL |
| Oil Properties | Value | |
|---|---|---|
| API gravity (°) | 41.1 | |
| Viscosity (mPa⋅s) 50 °C | 0.74 | |
| SARA fractions (%) | Saturates | 84.35 |
| Aromatics | 11.31 | |
| Resins | 4.09 | |
| Asphaltenes | 0.25 | |
| Serial Number | Experimental Sample | Oil–Water Ratio | Sample Quality g | Heating Rates °C/min | Gas Injection Rate mL/min | Gas Injection Pressure MPa |
|---|---|---|---|---|---|---|
| 1 | light crude oil | 7:3 | 5.8 | 4 | 300 | 1 |
| 2 | 2 | |||||
| 3 | 4 | |||||
| 4 | light crude oil | 7:3 | 5.8 | 4 | 200 | 4 |
| 5 | 400 | |||||
| 6 | light crude oil | 6:4 | 5 | 4 | 300 | 4 |
| 7 | 5:5 | 4.1 | ||||
| 8 | light crude oil | 7:3 | 5.8 | 1 | 300 | 4 |
| 9 | 2 | |||||
| 10 | 6 |
| Number | Fitted Linear Slope | Fitted Linear Intercept | Correlation Coefficient | Inflection Temperature (°C) | Apparent Activation Energy (kJ·mol−1) | Prefactor (s−1) | Pressures (MPa) |
|---|---|---|---|---|---|---|---|
| 1 | −3.212 | 3.587 | 0.981 | 267 | 26.70 | 8.41 | 1 |
| 2 | −2.643 | 4.290 | 0.953 | 253 | 21.97 | 16.99 | 2 |
| 3 | −0.963 | 0.009 | 0.937 | 234 | 8.01 | 0.23 | 4 |
| Number | Fitted Linear Slope | Fitted Linear Intercept | Correlation Coefficient | Inflection Temperature (°C) | Apparent Activation Energy (kJ·mol−1) | Prefactor (s−1) | Gas Injection Rate (mL/min) |
|---|---|---|---|---|---|---|---|
| 4 | −1.578 | 0.825 | 0.768 | 333 | 13.12 | 0.53 | 200 |
| 3 | −0.963 | 0.009 | 0.937 | 234 | 8.01 | 0.23 | 300 |
| 5 | −1.283 | 1.398 | 0.819 | 140 | 10.67 | 0.94 | 400 |
| Number | Fitted Linear Slope | Fitted Linear Intercept | Correlation Coefficient | Inflection Temperature (°C) | Apparent Activation Energy (kJ·mol−1) | Prefactor (s−1) | Oil–Water Ratio |
|---|---|---|---|---|---|---|---|
| 6 | −2.438 | 2.294 | 0.985 | 253 | 20.27 | 2.31 | 6:4 |
| 7 | −4.118 | 5.025 | 0.998 | 260 | 34.24 | 35.47 | 5:5 |
| 3 | −0.963 | 0.009 | 0.937 | 234 | 8.01 | 0.23 | 7:3 |
| Number | Fitted Linear Slope | Fitted Linear Intercept | Correlation Coefficient | Inflection Temperature (°C) | Apparent Activation Energy (kJ·mol−1) | Prefactor (s−1) | Heating Rates |
|---|---|---|---|---|---|---|---|
| 8 | −3.001 | 4.748 | 0.934 | 209 | 24.96 | 26.88 | 1 |
| 9 | −2.036 | 2.147 | 0.922 | 217 | 16.93 | 1.99 | 2 |
| 10 | −1.137 | 0.144 | 0.716 | 258 | 9.45 | 0.39 | 6 |
| 3 | −0.963 | 0.009 | 0.937 | 234 | 8.01 | 0.23 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Chen, X.; Yu, X.; Liu, D.; Chen, C.; Zhou, X.; Li, X.; Zhang, L.; Kan, C. Experimental Tests on In Situ Combustion Using Dynamic Ignition Simulation System in High-Temperature and High-Pressure Conditions. Processes 2024, 12, 52. https://doi.org/10.3390/pr12010052
Yin Y, Chen X, Yu X, Liu D, Chen C, Zhou X, Li X, Zhang L, Kan C. Experimental Tests on In Situ Combustion Using Dynamic Ignition Simulation System in High-Temperature and High-Pressure Conditions. Processes. 2024; 12(1):52. https://doi.org/10.3390/pr12010052
Chicago/Turabian StyleYin, Yuchuan, Xinyuan Chen, Xiaocong Yu, Deji Liu, Chao Chen, Xiaosong Zhou, Xiaohui Li, Lidong Zhang, and Changbin Kan. 2024. "Experimental Tests on In Situ Combustion Using Dynamic Ignition Simulation System in High-Temperature and High-Pressure Conditions" Processes 12, no. 1: 52. https://doi.org/10.3390/pr12010052
APA StyleYin, Y., Chen, X., Yu, X., Liu, D., Chen, C., Zhou, X., Li, X., Zhang, L., & Kan, C. (2024). Experimental Tests on In Situ Combustion Using Dynamic Ignition Simulation System in High-Temperature and High-Pressure Conditions. Processes, 12(1), 52. https://doi.org/10.3390/pr12010052






