Abstract
During growth, plants produce bioactive compounds—secondary metabolites. Their concentration can be stimulated by the presence of a stressful factor—an elicitor. Since chlorine dioxide is commonly used in water plants to disinfect drinking water, its application as a plant elicitor seems to be very attractive. The aim of this work was to investigate the influence of a new elicitor, ClO2, on the quality of seeds and bioactive compounds of sprouts. Elicitation of radish and broccoli seeds using ClO2 solutions did not significantly reduce their germination percentage (GP remained over 90%). Radish sprouts sprouted from seeds elicited in chlorine dioxide solutions with concentrations up to 800 ppm did not differ statistically significantly in terms of polyphenol content. Sprouts which were grown in the presence of ClO2 contained significantly fewer polyphenolic compounds. Elicitation of broccoli seeds in 800–1000 ppm ClO2 solutions causes an increase in total phenolic content and concentration of ascorbic acid in sprouts. Elicitation in chlorine dioxide solutions not only increased concentrations of selected bioactive compounds but also improved the microbiological quality of sprouts.
1. Introduction
Plants are the source of many biologically active compounds, which can be divided into nutrients such as proteins, carbohydrates, or fats, and other components like phytochemicals, vitamins, minerals, alkaloids, etc. [1]. The second group is called secondary metabolites [2]. These compounds are specific to plant species so different plants will have individual chemical compositions. Secondary metabolites are chemical compounds different from those needed to meet basic nutritional needs and are not involved in the processes of general metabolism, but are essential to plants’ adaptation to changing environmental conditions and reacting to pathogen invasion [3]. Unlike animals, plants do not have specialised defensive cells to defend against microbial invasion. Their defence systems have evolved in such a way that each cell has acquired the capability to respond to attempted infection and to build up a defensive response. This mechanism is manifested in the activation of secondary metabolite pathways [4,5]. Secondary metabolites are able to protect plants from pathogens and insects. These compounds are also capable of absorbing UV radiation [6,7]. Secondary metabolites present in plants play a significant role as health-promoting compounds and are important in the prevention of metabolic disorders, infectious diseases, and so-called lifestyle diseases such as diabetes, heart disease, stroke, or cancers [8]. Consumption of food rich in bioactive compounds affects the functioning of the human body and can strengthen, weaken, or modify the physiological and metabolic functions of the body.
Due to the growing consumer awareness of the nutritional value and quality of food, one of the directions of development of the food industry is towards functional and fortified food. Products with health-promoting properties are sought after by consumers. In addition, this type of food, apart from meeting the basic needs of the organism, i.e., nutrition, also has a scientifically proven impact on improving health and well-being and/or reducing the risk of diseases. The beneficial effect of functional food on organism functions is caused by the presence of specific bioactive compounds. Therefore, increasing the concentration of the bioactive compounds in food products can increase health-promoting effects and also increase the economic benefits of the industry due to the increased interest in purchasing.
One method of enriching food products is adding bioactive compounds during food processing. Valuable secondary metabolites can be obtained using biotechnological methods of plant cell or organ cultures, which seems to be an attractive alternative to the extraction of whole plant material [9]. Since plants are raw materials for food production, methods of enhancing bioactive compound concentration are more appropriate. To improve the nutritional and nutraceutical quality of plants and crops, many agricultural practices such as mineral nutrition, biofortification, the manipulation of the environment, and the use of biochemical and microbial elicitors are used [10].
Elicitation is a specific response of the plants to a stressful factor (the elicitor), which is similar to a defence mechanism triggered by the presence of pathogens or other environmental factors [1,9,11]. The induction of such a mechanism increases the synthesis of phytochemical compounds. During plant response, specific biochemical pathways are activated which leads to the synthesis of secondary metabolites. Elicitors may be physicochemical factors, chemical substances, and phytohormones acting as mediators (abiotic factors: jasmonic acid, gibberellins, cytokinins, ethanol, acetic acid, and inorganic salts such as mercury (II) chloride, copper (II) sulphate) and microorganisms and fungal or bacterial extracts (biotic factors: cellulose, chitin, poly- and oligosaccharides, and yeast cells) [6,9]. From a scientific point of view, it is important to understand the impact of stress factors on plants and the direction of their action, especially the link between the factor and the induced metabolic pathways. Typically, elicitation is used to obtain plant raw materials with an increased content of bioactive ingredients for further processing or to obtain enriched unprocessed vegetables and fruits.
Recently, there has been a growing research interest in chlorine and its use as an activator for stimulating plant defences [12]. Scientists have made an effort to describe the mechanism that activates plant defence by the presence of different elicitors [13,14,15]. Among chemical elicitors, chlorine compounds, and chlorine dioxide are of particular interest. Chlorine dioxide is a greenish-yellow gas with a characteristic odour similar to ozone [16]. Due to the odd number of chlorine atoms and the presence of an unpaired electron, the chlorine dioxide molecule is considered a free radical [17]. This property makes chlorine dioxide very reactive [18]. It dissolves well in water (up to 3 g/L) and does not hydrolyse. Chlorine dioxide dissolved in water is relatively stable in refrigerated conditions in the absence of light [17,18]. The great advantage of chlorine dioxide over other chlorine compounds is that it generates halogenated organic compounds only in trace amounts. Additionally, chlorine dioxide does not react with ammonium ions, so no chloramines are formed as a result of its presence [19]. Using aqueous solutions of chlorine dioxide for sprout treatment did not change the organoleptic properties of the product [20].
Chlorine dioxide is commonly used to disinfect drinking water and sewage [21]. The first use of chlorine dioxide for drinking water treatment was recorded in 1944 in the USA, and nowadays more and more water treatment plants choose ClO2 over chlorine gas and ozone [22,23,24]. The reason for disinfecting drinking water with chlorine dioxide is that a smaller amount of harmful by-products are generated in the process compared to the commonly used disinfectants [25]. Additionally, chlorine dioxide has a lower effective concentration compared to chlorine gas [26]. It has been proven that in the case of human exposure to ClO2, this compound and its metabolites are eliminated from the body more rapidly than chlorine, and they do not appear to increase trihalomethane concentrations at low dosages [27]. The disinfecting effect of chlorine dioxide solutions has been used in the agri-food industry [21,28,29]. Chlorine dioxide solutions are used to clean food contact surfaces, greenhouses, and animal rooms [30]. Chlorine dioxide is also found in stationary CIP (clean-in-place) cleaning devices, as well as in bottle washers used in breweries and beverage production [31]. In some cases, ClO2 can be used in the processing of fruits and vegetables for washing, or to wash and disinfect fish and seafood [19,30,32]. Due to a low effective concentration, chlorine dioxide use should not cause surface corrosion [19].
Since chlorine dioxide is commonly used to disinfect drinking water, its application as a plant elicitor, especially to sprouts, seems to be very attractive. Sprouts are one of the plant food products that have gained popularity in recent years and can be easily subjected to elicitation [20,33,34,35]. These young plants provide essential nutrients and also biologically active compounds [36,37]. Depending on the plant origin, the type and the concentration of bioactive compounds are different. Some sprouts additionally exhibit antimicrobial, anticancer, anti-inflammatory as well as anti-obesity properties [38,39], especially in the case of broccoli sprouts, which contain high levels of glucoraphanin which is transformed by myrosinase into sulphoraphane with proven anti-cancer properties. Strong antioxidant properties are also shown by other cruciferous sprouts (e.g., radish) which contain higher concentrations of bioactive compounds than their mature equivalents [39]. The great advantage of sprouts over fruit or vegetables is their year-round availability, short growth period, and the simple methods of obtaining them, which do not involve large financial outlays. In the case of sprouts, elicitation can be carried out at the stage of initiating plant growth by soaking seeds in elicitors, fumigation (introducing elicitors in gas form to the sprouting environment), as well as during growth by spraying/watering with elicitor solution or providing an eliciting factor in a growing medium/soil. Due to the elicitation, sprouts can constitute functional food. Sprouts subjected to treatments that activate secondary metabolites pathways can also be a source of bioactive compounds dedicated to enriching other food products, used as pharmaceuticals, or used in other industries, e.g., in polymer processing as a source of compounds improving the thermal stability of plastics or as a compound in active or intelligent packaging [13,14,39,40,41,42]. Nowadays, scientists are interested in improving polymer properties with essential oils, which, when incorporated into a polymer matrix or applied to a polymer film surface, can give new properties like antimicrobial or antioxidant features to the polymer [43,44]. Thermal stability can be enhanced by adding, e.g., lignin, curcuminoids, and eugenol [45,46,47].
The aim of this work was to investigate the influence of a new elicitor, chlorine dioxide, on the quality of radish and broccoli seeds and sprouts, especially bioactive components present in sprouts, to verify if sprouts submitted to the proposed treatment meet requirements for functional food. In addition, the influence of ClO2 on the microbiological quality and organoleptic characteristics was also verified.
2. Materials and Methods
2.1. Materials
Seeds and Sprouts
Seeds of broccoli Brassica olreacea var. italica cv. Calabrese Natalino and radish Raphanus sativus var. sativus cv. Opolanka were purchased from TORSEED S.A. Garden Seed and Nursery Stock Company in Toruń (Toruń, Poland). Sprouts for comparing the properties between elicited sprouts and sprouts available on the market were purchased from a local store in Bydgoszcz, Poland.
2.2. Methods
2.2.1. Sprouting
To obtain sprouts, a tank method that ensures continuous access to water, air, and light during the germination process was used (Figure 1). Sprouting was conducted in a glass reactor with a volume of 1 L. The reactor consists of two parts—a conical bottom with a stub pipe to provide compressed air (flow 2 L/min) and an upper cylindrical part. The germination reactor was poured with a growing medium, either distilled water or ClO2 solution up to 90% of tank volume, and seeds which constituted a 5% suspension. The growing medium was changed every 24 h. Sprouting of radish and broccoli seeds lasted, respectively, 2 and 3 days. When sprouts reached the appropriate age, they were separated from the growing medium on a sieve, where excess water was drained. Next, the remaining water was gently removed with the use of blotting paper.
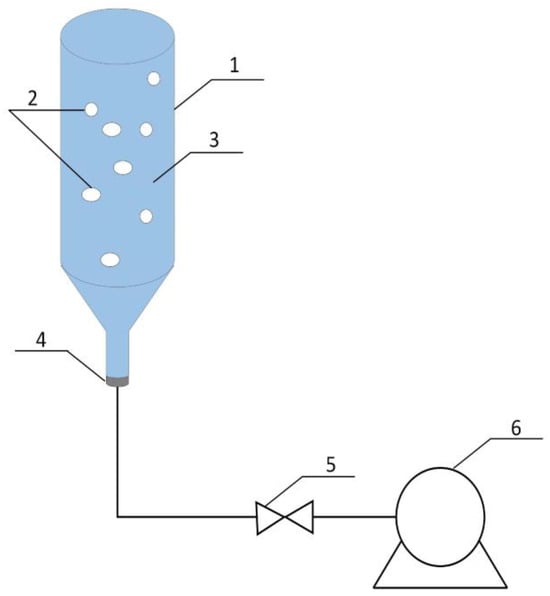
Figure 1.
Scheme of a sprouting stand (1—a glass reactor, 2—air bubbles, 3—growing medium, 4—a plug with a stub pipe, 5—a valve, 6—an air pump).
Preparing ClO2 Solutions
Chlorine dioxide (ClO2) used in the research was prepared from commercially available compounds—a stabilised solution of chlorine dioxide Armex 5 (Mexeo, Poland) and its activator, an aqueous solution of citric acid Mexacid D (Mexeo, Poland). Both liquids were mixed with distilled water in a ratio of 1 mL:1 mL:23 mL (stabilised chlorine dioxide: citric acid: water), thus obtaining a solution of chlorine dioxide with a content of approximately 2000 ppm. The initial solution was diluted with distilled water into solutions of concentration from 10 to 1000 ppm ClO2.
Elicitation
Elicitation was performed by immersing seeds in chlorine dioxide solution with concentrations of 100, 200, 400, 600, 800, and 1000 ppm. Seed elicitation time was 60 min and seeds to ClO2 solution ratio was 1:9. After the treatment, seeds were immersed in distilled water while maintaining the same 1:9 ratio as during elicitation. Seed washing time was 15 min and the procedure was performed twice. Then, the seeds were dried in a convection dryer with forced air circulation (SLW 115 STD, POL-EKO, Wodzisław Śląski, Poland) at an air temperature of 40 °C for 12 h. Seeds of one of the elicitation variants (immered in 800 ppm ClO2 solution) were also elicited with chlorine dioxide solutions (concertation from 10 to 50 ppm) during growth (germination). The growing medium (ClO2 solution) was changed every 24 h.
2.2.2. Effect of Chlorine Dioxide Solutions on Seed Germination
Elicited seeds were tested only in the case of their viability. Seed quality was determined according to the guidelines of the International Seed Testing Association (ISTA) [48]. The number of sprouted seeds was counted on the 4th and 6th days of germination in the case of radish seeds, and on the 4th and 10th days in the case of broccoli seeds.
2.2.3. Determination of Bioactive Compounds
Determination of Concentration of Total Phenolic Compounds in Sprouts
To determine the total phenolic content in sprout extracts, the Folin–Ciocalteu method was used [49]. The extract was obtained by grinding 2 g of whole sprouts (first leaves, a stem, and a root) in a mortar, which next was transferred to a test tube with 40 mL of 1% acetic acid in methanol and subjected to sonification in a cold water bath for 8 min (PS-10A Ultrasonic Waterbath Adverti, Łódź, Poland). During extraction, the samples were protected from light. After extraction, samples were centrifuged at 3500 rpm for 5 min (Rotina 380 Hettich, Kirchlengern, Germany). A total of 1 mL of supernatant was transferred to a test tube and mixed with 6 mL of distilled water and 0.5 mL of Folin–Ciocalteu reagent (Chempur, Piekary Śląskie, Poland). After 2 min of incubation, 1.5 mL of saturated disodium carbonite solution (Chempur, Poland) and 1.9 mL of distilled water were added. Next, the sample was vortexed (LLG-uniTEXER 1 LLG-Labware, Meckenheim, Germany) and incubated for 30 min in a water bath (37 °C) (WNB 22, Memmert, Schwabach, Germany). Finally, the absorbance of the sample was measured at 765 nm (HP/Agilent 8453 UV/Vis Spectrophotometer, Santa Clara, CA, USA). The content of total phenolic compounds was expressed as mg of gallic acid equivalent (mg GAE) per g of fresh weight, based on the standard curve.
Determination of Ascorbic Acid Content in Sprouts
To determine the ascorbic acid content of the sprout extracts, the spectrophotometric method with Tillmans (2,6-dichlorophenolindophenol, POCH, Gliwice, Poland) reagent solution was used [50]. Extracts were obtained by homogenizing 5 g of whole sprouts (first leaves, a stem and a root) with 30 mL 2% oxalic acid solution (POCH, Poland) at 15,000 rpm for 2 min (H 500 POL-EKO, Wodzisław Śląski, Poland). Homogenate was then diluted to 50 mL with 2% oxalic acid solution and centrifuged at 3500 rpm for 10 min (Rotina 380 Hettich, Kirchlengern, Germany). Next, 5 mL of supernatant was transferred to the test tube with 5 mL 2% oxalic acid solution and 1 mL 40% formaldehyde solution (POCH, Poland). The sample was mixed and incubated for 24 h at 4 °C. Then, 2 mL Tillmans reagent solution and 10 mL xylene (POCH, Poland) were added. After vortexing (LLG-uniTEXER 1 LLG-Labware, Meckenheim, Germany) for 10 s, the phase separation top layer was taken for measuring absorbance at 500 nm (HP/Agilent 8453 UV/ViS Spectrophotometer, Santa Clara, CA USA). Total ascorbic acid content was expressed as mg per 100 g of fresh weight, based on the standard curve.
Determination of Chlorophyll Content in Sprouts
To determine chlorophyll content in sprouts, a spectrophotometric method was used [51]. Extracts were obtained by grinding 2 g of whole sprouts (first leaves, a stem and a root) with 20 mL 80% aqueous acetone solution (POCH, Gliwice, Poland) in a mortar. Homogenate was then transferred to the test tube and diluted to 50 mL with 80% aqueous acetone solution. The sample was then centrifuged at 3500 rpm for 10 min. Finally, the absorbance of the supernatant was measured at 646 and 663 nm (HP/Agilent 8453 UV/ViS Spectrophotometer, Santa Clara, CA, USA). Based on the absorbance and formulas (1–2), the contents of chlorophyll a and chlorophyll b were calculated. The results were expressed as μg per 100 g of fresh weight.
where:
Chla = 12.25·A663 − 2.55·A646,
Chlb = 20.31·A646 − 4.91·A663,
Chla—content of chlorophyll a in sample (μg/100 g FW),
Chlb—content of chlorophyll b in sample (μg/100 g FW),
AXXX—absorbance at chosen wavelength.
2.2.4. Colourimetric Measurements
The colour of whole sprouts was assessed according to the guidelines of the Commission Internationale de l’Eclairage (CIE) by measuring the L*a*b* colour coordinates. A sample of sprouts weighing approximately 10 g was placed in a measurement dish. L* (brightness), a* (red-green), and b* (yellow-blue) were recorded using a colourimetric spectrophotometer (Chroma Meter CR-410, Tokyo, Japan) where an illuminant and a reference angle were D65 and 10°, respectively. The total colour difference (ΔE) was calculated as follows [52]:
ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2
2.2.5. Microbial Analysis
For microbial population analysis of the total number of bacteria, yeast, mould, and Enterobacteriaceae, 10 g of whole sprouts (first leaves, a stem, and a root) were homogenised in a Stomacher Lab Blender (model 400 Circulator, Seward Laboratory) with 90 mL sterilised normal saline, and then serially diluted. Then, 1 mL of the selected dilution was transferred onto a plate and a liquid medium was added (pour-plate method). Plates with cultures were incubated under conditions depending on the type of microorganisms:
- Total number of aerobic mesophilic and psychrophilic bacteria according to PN-EN ISO 4833-1:2013-12 [53]. Duplicate plates with nutrient broth (BIOCORP, Warsaw, Poland) were counted after incubation at 37 ± 1 °C for 24–48 h for mesophilic, and 15 ± 1 °C for 72–96 h for psychrophilic.
- Total number of aerobic mesophilic and psychrophilic yeasts and moulds according to PN-EN ISO 21527-1:2009 [54]. Duplicate plates with medium containing antibiotic Chloramphenicol (BIOCORP, Warsaw, Poland) were counted after incubation at 30 ± 1 °C for 72 h for mesophilic, and 15 ± 1 °C for 5–7 days for psychrophilic.
- Enterobacteriaceae enumeration according to PN-EN ISO 21528-2:2017-08 [55]. Duplicate plates with Violet Red Bile Lactose Agar selective medium (OXOID, Hampshire, UK) were counted after incubation at 37 ± 1 °C for 24 h.
The colonies were counted as the number of viable microorganisms in cfu/g of fresh weight.
2.2.6. Organoleptic Tests
The organoleptic tests were carried out by 25 participants (13 women and 12 men) trained for this purpose. Panellists were selected, trained, and monitored according to PN-EN ISO 8586:2014-03 [56]. The methodology and testing sheets of organoleptic tests were based on PN-ISO 4121:1998 [57]. Tests were carried out by using the five-point method. Quality features such as appearance, flavour, aroma, texture, and colour were evaluated. The tested properties were components of the overall assessment, and the impact of individual features on the overall quality of sprouts was determined using weighting factors (Supplementary Materials).
2.2.7. Statistical Analysis
The presented results of chemical and physical properties are the average of three repetitions. Averages are presented as values with standard deviation (average ± SD). A one-way variance analysis ANOVA was used to evaluate significant statistical differences in parameters. The comparison of averages was performed using Tukey’s method. Statistical calculations were performed in Microsoft Excel 2016. Significance was defined as p < 0.05.
3. Results and Discussion
3.1. Effect of Chlorine Dioxide Solutions on Seed Germination
Radish seeds that were exposed to the eliciting solution of ClO2 with a concentration of up to 800 ppm were characterised by a percentage of sprouted seeds above 90%, similar to untreated control samples (Table 1). Similar observations were made in the case of broccoli seeds. Due to the high viability and germination rate (number of counted seedlings on the 4th day of germination) of both species, there were no statistically significant differences observed between 4 and 6 days of sprouting for radish seeds and between 4 and 10 days of sprouting for broccoli seeds. Elicitation with ClO2 solution of concentrations up to 400 ppm did not statistically differ in the percentage of sprouted radish and broccoli seeds in comparison to untreated seeds, which amounted to about 94–95%. Increasing the concentration of the elicitor from 400 to 800 ppm decreased the percentage of both species’ sprouted seeds to about 90%. These results corroborate with the ones reported by Singh et al. [58], who studied chlorine dioxide and its effect on alfalfa seeds. Alfalfa seeds were immersed in ClO2 solutions with concentrations ranging from 10 to 50 ppm and time of exposition from 3 to 10 min. This treatment did not influence the germination percentage of alfalfa, which was approximately 90%. Hassan et al. [15] also observed the impact of elicitor on the percentage of sprouted seeds. Application of ultrasound treatment on sorghum seeds caused changes, both increases and decreases, in the number of sprouted seeds depending on the amplitude and exposure time. The application of different organic acids and acids with chitosan mixtures had an impact on the sprouting of kidney bean seeds [59]. The authors showed that ascorbic acid caused the biggest increase in the germination rate in the first 4 days of sprouting, with no significant changes in sprouting between 6 and 8 days. On the other hand, folic acid caused the biggest decrease in the germination rate in the first 4 days, and the biggest increase from 6 to 8 days. Therefore, it can be concluded that the percentage of sprouted seeds depends on the type of applied elicitor, its concentration, and sprouting time.

Table 1.
Effect of ClO2 elicitation on percentage of germination of broccoli and radish seeds.
3.2. Determination of Bioactive Compounds
Elicited broccoli sprouts with ClO2 concentrations ranging from 0 to 600 ppm did not differ in the concentration of polyphenols (Table 2). As the elicitor concentration used for seed treatment increased in the range of 600–1000 ppm, the total phenolic compounds (TPCs) also increased in comparison to the control sample. Application of 800 and 1000 ppm ClO2 solution for broccoli seed elicitation caused an increase in total phenolic compounds concentration from 109.8 mg GAE/100 g FW for the control sample to 197.3 and 236.3 mg GAE/100 g FW, respectively. The presence of phenolic acids, such as gallic, protocatechuic, caffeic, p-coumaric, ferulic, chlorogenic, and sinapic, can be expected [60]. When the elicitor was present during sprouting, the content of polyphenols decreased, but sprouts grown in ClO2 solutions were characterised with higher polyphenols content than sprouts from raw seeds (Table 2). The concentration of polyphenols in radish sprouts decreased with increased concentration of the elicitor (Table 2). TPCs were from 6 to 15% lower after elicitation in comparison with the control radish sprouts; however, changes in TPCs in sprouts elicited with ClO2 solutions of concentration between 100 and 800 ppm were not statistically significant and amounted to approximately 630 mg GAE/100 g FW. Among these compounds, the presence of phenolic acids can be expected, including gallic, protocatechuic, caffeic, p-coumaric, ferulic, and sinapic [60]. The presence of the elicitor during germination decreased total phenolic compound concentration in radish sprouts, even by fivefold (Table 2). Since broccoli and radish sprouts are among the most popular sprouts available on the market [36,38], those species are the subject of research by scientists. Depending on the variety, broccoli sprouts contain from 200 to 412 mg GAE/100 g FW, while radish sprouts contain from 75 to 292 mg GAE/100 g FW [51,60,61,62,63,64,65]. Przybysz et al. [66] supplemented radish sprouts during growth in magnesium in the form of MgSO4·H2O. The presence of Mg caused an increase in phenolic compound concentration from 13.9 to 21.7% in comparison to the control. The elicitation of radishes with saline changed the total phenolic contents of 3- and 5-day-old sprouts, and TPCs were significantly increased by 25–50% after treatment with 100 mM of NaCl [65]. In the case of elicitation treatment, more research was conducted with broccoli sprouts since broccoli is a great source of sulforaphane possessing potent anti-cancer activity, and [67,68,69] studied the influence of hydrogen peroxide on broccoli sprouts. Elicitation by 0–1000 mM H2O2 solutions did not cause a significant change in total phenolic compounds concentration in the fresh matter of sprouts, and it was approximately 160 mg GAE/100 g FW. However, on the dry weight basis, the highest H2O2 concentration caused a small but significant reduction of 17% in the phenolics content as compared to the control. Elicitation of broccoli sprouts studied by Pérez-Balibrea et al. [70] resulted in an increase in total phenolic concentration when exogenous elicitors chitosan, salicylic acid, and methyl jasmonate were sprayed at low concentrations during germination. The presence of light also influences the chemical composition of sprouts. Paśko et al. [71] compared phenolic content in amaranthus sprouts which were sprouted with and without access to light. Sprouts germinated in the presence of light had 1/3 more polyphenols than sprouts with limited light, and TPCs was 2.9 and 2.0 mg GAE/g DM, respectively [71].

Table 2.
Effect of ClO2 elicitation on content of selected bioactive compounds in sprouts.
Broccoli sprouts contain from 6.8 to 51.7 mg/100 g FW ascorbic acid [51,61,72]. Radish sprouts are a greater source of this bioactive compound, containing from 11.6 to 71.9 mg/100 g FW [51,61,73]. In the present research, untreated broccoli and radish sprouts contained 8.3 and 18.9 mg/100 g FW, respectively (Table 2). Elicitation in ClO2 solutions changed ascorbic acid content in sprouts. In the case of broccoli sprouts, a positive correlation can be observed (Table 2). When broccoli sprouts were exposed to chlorine dioxide solutions during growth, ascorbic acid content decreased almost twice, up to 16.4 mg/100 g FW in comparison to the control sample (Table 2). Elicitation with ClO2 solutions of radish seeds and sprouts resulted in a decrease in vitamin C content. Even if the concentration of ascorbic acid in radish sprouts was 50% lower than in untreated sprouts, in comparison to sprouts purchased from the local market elicited radish sprouts had three times more vitamin C (Table 2). Elicitation of broccoli sprouts with methionine and tryptophan caused a decrease in vitamin C, while the presence of methyl jasmonate did not change vitamin C concentration when sprouts were older than 5 days. The presence of salicylic acid and chitosan positively affected ascorbic acid concentration in 5- and 7-day-old sprouts [70]. Salicylic acid is a well-known inducer of plant systemic acquired resistance (SAR) and has diverse effects on tolerance to abiotic stress, so it was expected to influence the increase in secondary metabolic pathways and biosynthesis of vitamins [74]. In the case of broccoli sprouts, chlorine dioxide can also be considered as a stimulator of the synthesis of bioactive compounds, like vitamins and phenolic compounds [65,75,76].
Depending on the plant species, sprouts can range in colour from white through yellow to vivid green or red-violet. The pink, red, and purple colours are caused by the presence of anthocyanins. The green colour of sprouts is related to the presence of chlorophylls, and the concentration of this compound is associated with access to light during growth. In Asia, legume sprouts are usually grown in climatic cabinets in the dark, hence their colour is rather white and yellow [77]. Chlorophylls are desirable not only because of the appearance of the sprouts and their attractiveness but also because of the biological activity of chlorophylls, manifested, among other ways, in their antioxidant properties [73]. There are very limited data in the literature regarding the content of chlorophylls in broccoli and radish sprouts. The concentration of chlorophylls is related to both the plant variety and the growing conditions. Michalczyk and Macura [51] in their research on the impact of storage conditions on the quality of minimally processed vegetable products determined chlorophylls in radish sprouts at the level of 5.5 mg/100 g FW, while Gałązka-Czarnecka and Krala [73] in similar research used radish sprouts, which contained 102.1 mg/100 g DM. In the case of broccoli sprouts, the literature states that their chlorophyll content ranges from 0.83 to approximately 3.0 mg/100 g DM [62]. Untreated radish and broccoli sprouts contained 15.39 and 7.30 mg/100 g DM chlorophylls, respectively. Elicitation in ClO2 solutions caused a decrease in chlorophyll concentration in radish and broccoli sprouts (Table 2). Additionally, the biggest decrease was observed in sprouts germinated in 50 ppm ClO2 solution as the growing medium.
3.3. Colourimetric Measurements
Colour parameters L* and b* of elicited radish and broccoli sprouts did not differ significantly (Table 3). In the case of broccoli sprouts, the highest value of L* parameter was observed for the control sample (52.9) and the lowest for sprouts elicited for 1 h in 800 ppm ClO2 solution, then sprouted in 10 ppm ClO2 solution (47.9). Parameter b* was between 9.7 and 13.0. Significant differences were observed for a* parameter (green to red). Immersing broccoli seeds in ClO2 solutions changed a* parameter from −2.9 to −1.9 for control sprouts and elicited with 1000 ppm ClO2 solution, respectively. Modification of the growing medium by adding chlorine dioxide had the biggest impact on the green colour of sprouts. The presence of ClO2 solutions during sprouting changed a* parameter to −0.6 and −0.2 when the growing medium was 10 and 25 ppm ClO2 solutions, respectively, and even caused the appearance of the red colour (3.0 of a* parameter) when the growing medium was 50 ppm ClO2 solution. In the case of radish sprouts, the L* parameter increased after using ClO2 solution as the elicitor (Table 3). The lowest value of L* was observed for the control sample (46.0) and the highest value for sprouts elicited with 100 ppm ClO2 solution (51.3). Elicitation caused a decrease in the b* parameter; however, changes were not statistically significant. Elicitation with ClO2 solutions significantly changed the value of the a* parameter from −6.9 for the control sample to 3.4 for the sample elicited for 1 h with 800 ppm ClO2 solution, then sprouted with 50 ppm ClO2 solution. A significantly lower share of green colour was also observed for sprouts elicited in 1000 ppm ClO2 solution. The colour parameters of sprouts correspond with chlorophyll concentration. The presence of chlorophylls affects the appearance and colour of the sprouts. These features of sprouts impact consumers’ decisions when choosing food products. The application of ClO2 solution as the elicitor caused a decrease in the chlorophyll concentration, which caused changes in the colour of the sprouts from greenish to reddish. Such a change may, to some extent, confuse the consumer, who will usually expect the sprouts to have a vivid green colour or other characteristic appearance. Colour can have a significant impact on consumer perception and decision-making [78,79]. In the case of elicited sprouts, their colour changes unfavourably, but broccoli sprouts are characterised by a higher content of bioactive compounds and this may offset this disadvantage for consumers.

Table 3.
Effect of ClO2 elicitation on the colour of sprouts in CIELab colour space and Adobe RGB space.
3.4. Microbial Analysis
Sprouts are valued for their health-promoting and nutritional values. Much recent research confirms that sprouts may be important in cancer prevention and in the prevention and treatment of civilization diseases (diseases that affect an increasing number of people every year connected with their lifestyle and the environment), but sprouts may carry risks as they are a source of pathogens. Sprouts present a unique risk to consumers because they require humidity and warmth to grow. These same conditions are ideal for pathogens. The presence of pathogenic microflora on sprouts may result from its presence on the seeds. Sprouts may also become infected during germination as a result of failure to provide hygienic conditions [80,81,82]. Kordušienė [61] determined the total number of bacteria on radish, alfalfa, amaranth and broccoli seeds and sprouts. Seeds were characterised with levels of contamination ranging from 1.3 × 103 to 4.5 × 103 cfu/g. However, as a result of germination, this number increased and ranged from 3.1 × 105 to 5.4 × 106 cfu/g. Martinez-Villaluenga et al. [72] assessed the microbiological quality of broccoli and radish seeds and sprouts. The total number of mesophilic and psychrophilic bacteria in the seeds was 106–107 cfu/g. Sprouting caused in both broccoli and radish sprouts an increase in contamination, and the number of microbes was 100 times higher. Michalczyk and Macura [51] examined sprouts commercially available on the Polish market. The total number of bacteria and the total number of yeasts in sunflower, alfalfa and radish sprouts were 108–109 cfu/g and 105–106 cfu/g, respectively. Other studies have shown that the contamination of sprouts and their mix available on the Polish market with bacteria ranges from 108 to 1010 cfu/g [83]. Similar studies carried out on sprouts available on the Spanish market showed that microbial contamination amounted to 107–108 cfu/g [84]. This data proves the high risk connected to sprout consumption.
For the assessment of microbiological and organoleptic quality, the number of elicitation variants was limited. In the case of the aerobic mesophilic, psychrophilic bacteria, aerobic mesophilic moulds, and aerobic psychrophilic yeast and moulds, radish sprouts had higher levels of contamination than broccoli sprouts (Table 4). Furthermore, changes in the chemical composition of sprouts elicited in ClO2 solutions caused an additional important effect by improving microbiological quality. For both broccoli and radish sprouts, immersing seeds for 1 h in 800 ppm ClO2 solution and modifying the growing medium by the addition of ClO2 solution caused a decrease in the total number of all analysed bacteria, yeast, and moulds. Additionally, increasing the concentration of ClO2 in the growing medium to 25 and 50 ppm reduces the number of microbials below the limit of detection (10 cfu/g) (Table 4). Chlorine dioxide solutions have been used to disinfect fresh sprouts. It has been proven that the short-term impact of ClO2 can eliminate pathogens present on the surface of sprouts. When mung bean sprouts were soaked in a 100 ppm chlorine dioxide solution for 5 min, the number of L. monocytogenes and S. typhimurium present on the surface was reduced by 1.5 log and 3 log cfu/g, respectively [85]. Broccoli sprouts were washed in a 50 ppm chlorine dioxide solution for 5 min. This variant resulted in a reduction in the population of bacteria present on the surface in the range of 101 to 102 cfu/g [86]. Similar observations were made when ozone was applied as an elicitor. Ozonation can be introduced at the stage of sowing seeds, planting, or during growth. Plant ozone defence responses depend on the interconnectedness between many complex signalling pathways and metabolic signals [3]. Sharma et al. [87] soaked alfalfa seeds and sprouts and sprayed them with ozonated water with an ozone concentration of 21 ppm for 64 min. There was a 2.2 log reduction in the number of E. coli when ozone was applied by spraying for 64 min and a 0.85 log reduction when ozone was applied by soaking, observed by [87]. Wade et al. [88] conducted a similar experiment in which, after 20 min of treatment of alfalfa seeds in ozonated water with a concentration of 21.8 ppm of ozone, they achieved a 50% reduction in the population L. monocytogenes (from 2.99 to 1.51 log).

Table 4.
Effect of ClO2 elicitation on microbiological quality of sprouts.
3.5. Organoleptic Tests
Organoleptic quality assessment was carried out for three sprout variants: the control, elicited by immersing for 1 h in 800 ppm ClO2 solution then sprouted in 25 ppm ClO2 solution, and commercially available sprouts purchased from the local market (Table 5). In the case of appearance, texture, and overall quality of broccoli and radish sprouts, the highest note was given to the control sample, in contrast to sprouts purchased from the market, which were given the lowest note. Both the control samples of radish and broccoli sprouts were rated the highest note in terms of colour. They were evaluated with higher than 4.0 notes, which means that radish sprouts were characterised by green leaves and white to white-red roots, and broccoli sprouts had green leaves and white to white-yellow roots (Figure 2). The leaves of the remaining compared sprouts were yellow-green. The aroma of sprouts germinated in the presence of ClO2 was moderately noticeable, free from unusual and foreign odours, but still characteristic and proper for sprouts. In the case of the commercially available sprouts and the control samples, the evaluation team detected foreign odours. Some evaluators described the smell of the control sprouts as musty and unpleasant. All sprouts had an intense and typical taste. The texture of radish sprouts did not differ statistically significantly, and the evaluators found the radish sprouts firm and crispy. Commercially available broccoli sprouts obtained the lowest score in the case of the texture (3.3), which corresponds to average crispiness. In the organoleptic evaluation, the best notes were given to the control samples. The overall quality of radish and broccoli sprouts germinated in ClO2 solution was lower than that of the control samples, but the difference was not statistically significant, so they can be classified with the same quality in the organoleptic evaluation. Commercially available sprouts were rated the worst in the overall assessment.

Table 5.
Effect of ClO2 elicitation on organoleptic quality of sprouts.

Figure 2.
Appearance of radish and broccoli sprouts: (a) the control sample of radish sprouts; (b) radish sprouts grown from seeds disinfected for an hour in a chlorine dioxide solution at a concentration of 800 ppm and then sprouted in the presence of 25 ppm chlorine dioxide; (c) radish sprouts purchased from the market; (d) the control sample of broccoli sprouts grown from untreated seeds; (e) broccoli sprouts grown from seeds subjected to an hour of disinfection in a chlorine dioxide solution at a concentration of 800 ppm and then sprouted in the presence of 25 ppm chlorine dioxide; (f) broccoli sprouts purchased from the market.
During the examination of elicitation by ozone on alfalfa sprouts, Wade et al. [88] also analysed the sensory quality of sprouts. On the first day of storage, sprouts soaked in water with ozone did not differ significantly in terms of appearance, colour and aroma from sprouts soaked in water. After 7 days of storage, differences in organoleptic assessment between sprouts were significant and sprouts treated with ozone were considered the worst. Taormina and Beuchat [89] have used sodium hypochlorite, calcium hypochlorite, chlorine dioxide, hydrogen peroxide, and sodium phosphate (V) for the elicitation of alfalfa sprouts. Within a certain range of concentrations of chemical compounds used, the organoleptic properties of the sprouts did not deteriorate and were even better than the control sprouts with which no elicitation was used. The application of vitamin C, folic acid, and chitosan in different concentrations changed some of the sensory properties of elicited sprouts [90]. The most significant changes were observed especially in the case of the texture and overall acceptability which increased after eliciting with chitosan in both concentrations (1000 and 1500 ppm).
4. Conclusions
Generally, due to the high concentration of bioactive compounds, sprouts can be classified as an example of a functional food. The concentration of selected bioactive compounds can be increased by stimulating metabolic pathways. Elicitation in chlorine dioxide solutions can be considered as a feasible tool to obtain sprouts with enhanced levels of health-promoting compounds, such as vitamin C or phenolic compounds. However, because of the growing conditions, storage with relatively high humidity and concentration of nutrients such as saccharides and proteins, sprouts may be a source of pathogens. Hence, the huge advantage of chlorine dioxide as the elicitor is its antimicrobial properties. Elicitation in chlorine dioxide solutions not only increased concentrations of selected bioactive compounds but also improved the microbiological quality of sprouts. The reduction in microbiological contamination obtained in this study while improving the phytochemical value by increasing the concentration of health-promoting compounds may contribute to increasing the multi-aspect quality of sprouts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr12010174/s1, Table S1. Definitions of score notes in the radish sprouts evaluation survey. Table S2. Definitions of score notes in the broccoli sprout evaluation survey.
Author Contributions
Conceptualization, J.S. and K.C.; methodology, J.S., K.C., A.D. and G.G.; software, J.S.; validation, J.S., K.C. and A.D.; formal analysis, J.S., B.B. and G.G.; investigation, J.S. and A.D.; resources, K.C., J.S. and G.G.; data curation, J.S. and B.B.; writing—original draft preparation, J.S. and B.B.; writing—review and editing, B.B. and J.S.; visualization, J.S. and B.B.; supervision, K.C. and G.G.; project administration, J.S. and K.C.; funding acquisition, K.C. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education and Science.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article.
Acknowledgments
The paper was prepared using the apparatus purchased within the framework of the “Implementation of the second stage of the Regional Innovation Center” project, co-financed from funds of the European Regional Development Fund for Operational Program of the Kuyavian-Pomeranian Voivodeship for years 2007–2013.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Delgoda, R.; Murray, J.E. Chapter 7—Evolutionary Perspectives on the Role of Plant Secondary Metabolites. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 93–100. ISBN 978-0-12-802104-0. [Google Scholar]
- Sachadyn-Król, M.; Agriopoulou, S. Ozonation as a Method of Abiotic Elicitation Improving the Health-Promoting Properties of Plant Products—A Review. Molecules 2020, 25, 2416. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Deepa, P.R.; Sharma, P.K. Silent Metabolism and Not-so-Silent Biological Activity: Possible Molecular Mechanisms of Stress Response in Edible Desert Legumes. J. Plant Biochem. Biotechnol. 2021, 30, 640–645. [Google Scholar] [CrossRef]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A Tool for Enriching the Bioactive Composition of Foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Mandal, S.K.; Puri, S.; Asati, V.; Deepa, P.R.; Sharma, P.K. Investigating the Antioxidant Activity Enhancer Effect of Cyamopsis Tetragonoloba Seed Extract on Phenolic Phytochemicals. Front. Plant Sci. 2023, 14, 1131173. [Google Scholar] [CrossRef]
- Singla, R.K. Secondary Metabolites as Treatment of Choice for Metabolic Disorders and Infectious Diseases and Their Metabolic Profiling: Part 1. Curr. Drug Metab. 2020, 21, 480–481. [Google Scholar] [CrossRef] [PubMed]
- Namdeo, A.G. Plant Cell Elicitation for Production of Secondary Metabolites: A Review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar]
- Cabrera-De la Fuente, M.; González-Morales, S.; Juárez-Maldonado, A.; Leija-Martínez, P.; Benavides-Mendoza, A. Chapter 4—Plant Nutrition and Agronomic Management to Obtain Crops with Better Nutritional and Nutraceutical Quality. In Therapeutic Foods; Holban, A.M., Grumezescu, A.M., Eds.; Handbook of Food Bioengineering; Academic Press: Cambridge, MA, USA, 2018; pp. 99–140. ISBN 978-0-12-811517-6. [Google Scholar]
- Dal Bosco Ducatti, R. Plant Elicitation: The Generation of Misleading and Biased Information. J. Plant Growth Regul. 2023, 42, 3785–3788. [Google Scholar] [CrossRef]
- Ramsey, C.; Sandoval, V.M.; Freebury, P.C.; Newman, D.H.; Dooley, G.; Cseke, L.J.; Newman, S.E. Priming Bean Seedlings to Boost Natural Plant Defenses Against Common Bacterial Wilt: Leaf Architecture, Leaf Area, Foliage Water Content, and Plant Biomass Results (Part 3). Glob. J. Agric. Innov. Res. Dev. 2023, 10, 52–79. [Google Scholar] [CrossRef]
- Gómez-Velázquez, H.D.J.; Aparicio-Fernández, X.; Mora, O.; González Davalos, M.L.; de los Ríos, E.A.; Reynoso-Camacho, R. Chia Seeds and Chemical-Elicited Sprouts Supplementation Ameliorates Insulin Resistance, Dyslipidemia, and Hepatic Steatosis in Obese Rats. J. Food Biochem. 2022, 46, e14136. [Google Scholar] [CrossRef]
- Laila, O.; Murtaza, I.; Muzamil, S.; Imtiyaz Ali, S.; Abid Ali, S.; Ahamad Paray, B.; Gulnaz, A.; Vladulescu, C.; Mansoor, S. Enhancement of Nutraceutical and Anti-Diabetic Potential of Fenugreek (Trigonella foenum-graecum). Sprouts with Natural Elicitors. Saudi Pharm. J. 2023, 31, 1–13. [Google Scholar] [CrossRef]
- Hassan, S.; Imran, M.; Ahmad, M.H.; Khan, M.I.; XU, C.; Khan, M.K.; Muhammad, N. Phytochemical Characterization of Ultrasound-Processed Sorghum Sprouts for the Use in Functional Foods. Int. J. Food Prop. 2020, 23, 853–863. [Google Scholar] [CrossRef]
- Vandekinderen, I.; Devlieghere, F.; Van Camp, J.; Kerkaert, B.; Cucu, T.; Ragaert, P.; De Bruyne, J.; De Meulenaer, B. Effects of Food Composition on the Inactivation of Foodborne Microorganisms by Chlorine Dioxide. Int. J. Food Microbiol. 2009, 131, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Dobson, S.; Cary, R.; World Health Organization; International Labour Organisation. Chlorine Dioxide (Gas); WHO: Geneva, Switzerland, 2002; ISBN 978-92-4-153037-8. [Google Scholar]
- Bergmann, H.; Koparal, S. The Formation of Chlorine Dioxide in the Electrochemical Treatment of Drinking Water for Disinfection. Electrochim. Acta 2005, 50, 5218–5228. [Google Scholar] [CrossRef]
- Humphries, E.G.; Fleming, H.P. Chlorine Dioxide Use in Pickling Cucumber Hydrocooler Operations. Appl. Eng. Agric. 1996, 12, 715–7200. [Google Scholar] [CrossRef]
- Kaniewska, J.; Domoradzki, M.; Poćwiardowski, W. Preparation of seeds for the production of edible sprouts. Acta Agroph. 2010, 16, 315–325. [Google Scholar]
- Jin, D.-S.; Deshwal, B.-R.; Park, Y.-S.; Lee, H.-K. Simultaneous Removal of SO2 and NO by Wet Scrubbing Using Aqueous Chlorine Dioxide Solution. J. Hazard. Mater. 2006, 135, 412–417. [Google Scholar] [CrossRef]
- Aieta, E.M.; Berg, J.D. A Review of Chlorine Dioxide in Drinking Water Treatment. J.-Am. Water Work. Assoc. 1986, 78, 62–72. [Google Scholar] [CrossRef]
- Van Der Hoek, J.P.; Bertelkamp, C.; Verliefde, A.R.D.; Singhal, N. Drinking Water Treatment Technologies in Europe: State of the Art—Challenges—Research Needs. J. Water Supply Res. Technol.—Aqua 2014, 63, 124–130. [Google Scholar] [CrossRef]
- Zbieć, E.; Dojlido, J. Uboczne Produkty Dezynfekcji Wody. Ochr. Sr. 1999, 3, 37–44. [Google Scholar]
- Hua, G.; Reckhow, D.A. Comparison of Disinfection Byproduct Formation from Chlorine and Alternative Disinfectants. Water Res. 2007, 41, 1667–1678. [Google Scholar] [CrossRef]
- Maćkiewicz, J.; Dziubek, A.M.; Czarniecka, J. Zapotrzebowanie Na Dwutlenek Chloru w Uzdatniania Wód Infiltracyjnych. Ochr. Sr. 2003, 25, 9–12. [Google Scholar]
- Abdel-Rahman, M.S.; Couri, D.; Bull, R.J. Metabolism and Pharmacokinetics of Alternate Drinking Water Disinfectants. Environ. Health Perspect. 1982, 46, 19–23. [Google Scholar] [CrossRef]
- Deshwal, B.R.; Jin, D.S.; Lee, S.H.; Moon, S.H.; Jung, J.H.; Lee, H.K. Removal of NO from Flue Gas by Aqueous Chlorine-Dioxide Scrubbing Solution in a Lab-Scale Bubbling Reactor. J. Hazard. Mater. 2008, 150, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.-S.; Huxsoll, C.C.; Robertson, G. Prevention of Potato Spoilage During Storage by Chlorine Dioxide. J. Food Saf. 2001, 66, 472–477. [Google Scholar] [CrossRef]
- Sanders, F.T. Reregistration Eligibility Decision (RED) for Chlorine Dioxide and Sodium Chlorite (Case 4023); U.S. Environmental Protection Agency, Prevention, Pesticides and Toxic Substances: Washington, DC, USA, 2006.
- Mielczarek, M. Dezynfekcja Wody Dwutlenkiem Chloru. Ochr. Sr. 1995, 4, 45–48. [Google Scholar]
- Andrews, L.S.; Key, A.M.; Martin, R.L.; Grodner, R.; Park, D.L. Chlorine Dioxide Wash of Shrimp and Crawfish an Alternative to Aqueous Chlorine. Food Microbiol. 2002, 19, 261–267. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Sugier, D. Improvement of nutraceutical value of broccoli sprouts by natural elicitors. Acta Sci. Pol. Hortorum Cultus 2013, 12, 129–140. [Google Scholar]
- Szulc, J.; Czaczyk, K.; Gozdecka, G. Metody otrzymywania kiełków—Od upraw domowych do produkcji przemysłowej. Żywność Nauka Technol. Jakość 2017, 112, 27–40. [Google Scholar] [CrossRef]
- Lewicki, P.P. Kiełki nasion jako źródło cennych składników odżywczych. Żywność Nauka Technol. Jakość 2010, 17, 18–33. [Google Scholar]
- Weiss, A.; Hammes, W. Efficacy of Heat Treatment in the Reduction of Salmonella and Escherichia coli O157:H- on Alfalfa, Mung Bean, and Radish Seeds Used for Sprout Production. Eur. Food Res. Technol. 2005, 221, 187–191. [Google Scholar] [CrossRef]
- Pasko, P.; Gdula-Argasinska, J.; Podporska-Carroll, J.; Quilty, B.; Wietecha-Posluszny, R.; Tyszka-Czochara, M.; Zagrodzki, P. Influence of Selenium Supplementation on Fatty Acids Profile and Biological Activity of Four Edible Amaranth Sprouts as New Kind of Functional Food. J. Food Sci. Technol. 2015, 52, 4724–4736. [Google Scholar] [CrossRef]
- Bokić, J.; Škrobot, D.; Tomić, J.; Šeregelj, V.; Abellán-Victorio, Á.; Moreno, D.A.; Ilić, N. Broccoli Sprouts as a Novel Food Ingredient: Nutritional, Functional and Sensory Aspects of Sprouts Enriched Pasta. LWT 2022, 172, 114203. [Google Scholar] [CrossRef]
- Waliat, S.; Arshad, M.S.; Hanif, H.; Ejaz, A.; Khalid, W.; Kauser, S.; Al-Farga, A. A Review on Bioactive Compounds in Sprouts: Extraction Techniques, Food Application and Health Functionality. Int. J. Food Prop. 2023, 26, 647–665. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Hamed, Y.S.; Kamel, R.M.; Shawir, S.M.S.; Sakr, H.; Ali, M.; Ammar, A.; Saleh, M.N.; Fadly, E.E.; Salama, M.A.; et al. Enhanced Physical Properties, Antioxidant and Antibacterial Activity of Bio-Composite Films Composed from Carboxymethyl Cellulose and Polyvinyl Alcohol Incorporated with Broccoli Sprout Seed Extract for Butter Packaging. Int. J. Biol. Macromol. 2024, 255, 128346. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Islam, N.; Choudhuri, C.; Mandal, P. Elicitation of therapeutic potential and oxidative stress assessment of fenugreek sprouts under uv irradiation. Int. J. Pharm. Pharm. Sci. 2017, 9, 91–99. [Google Scholar] [CrossRef][Green Version]
- Rico, D.; Peñas, E.; García, M.D.C.; Martínez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martín-Diana, A.B. Sprouted Barley Flour as a Nutritious and Functional Ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef]
- Skórczewska, K.; Szulc, J.; Lewandowski, K.; Ligocka, A.; Wilczewski, S. Modification of Poly(Vinyl Chloride) with Bio-Based Cassia Oil to Improve Thermo-Mechanical and Antimicrobial Properties. Materials 2023, 16, 2698. [Google Scholar] [CrossRef]
- Szulc, J.; Lewandowski, K.; Skórczewska, K.; Sadkiewicz, J. Release of Thymol from Plasticized Poly(Vinyl Chloride). Polimery 2018, 63, 825–829. [Google Scholar] [CrossRef]
- Matykiewicz, D.; Skórczewska, K. Characteristics and Application of Eugenol in the Production of Epoxy and Thermosetting Resin Composites: A Review. Materials 2022, 15, 4824. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, J.; Wieczorek, M.; Skórczewska, K.; Klapiszewska, I.; Lewandowski, K.; Klapiszewski, Ł. Preparation, Characterization and Tailoring Properties of Poly(Vinyl Chloride) Composites with the Addition of Functional Halloysite–Lignin Hybrid Materials. Materials 2022, 15, 8102. [Google Scholar] [CrossRef] [PubMed]
- Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Osial, M.; Dąbrowska, A.; Nikiforow, K.; Jenczyk, P.; Grzywacz, H. Graphene Modification by Curcuminoids as an Effective Method to Improve the Dispersion and Stability of PVC/Graphene Nanocomposites. Molecules 2023, 28, 3383. [Google Scholar] [CrossRef] [PubMed]
- Małuszyńska, E.; Boros, L.; Kolasińska, K.; Osińska, A. International Seed Testing Association (ISTA) Rules for Seed Testing. Międzynarodowe Przepisy Oceny Nasion; Polska Wersja Wydania; Instytut Hodowli i Aklimatyzacji Roślin, Zakład Nasiennictwa i Nasionoznawstwa: Radzików, Poland, 2004. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- PN-A-04019:1998; Food Products—Determination of Vitamin C Content. Polski Komitet Normalizacyjny: Warsaw, Poland, 1998.
- Michalczyk, M.; Macura, R. Wpływ warunków przechowywania na jakość wybranych, dostępnych w obrocie handlowym, mało przetworzonych produktów warzywnych. Żywność Nauka Technol. Jakość 2008, 15, 96–107. [Google Scholar]
- Hejna, A.; Marć, M.; Skórczewska, K.; Szulc, J.; Korol, J.; Formela, K. Insights into Modification of Lignocellulosic Fillers with Isophorone Diisocyanate: Structure, Thermal Stability and Volatile Organic Compounds Emission Assessment. Eur. J. Wood Prod. 2021, 79, 75–90. [Google Scholar] [CrossRef]
- PN-EN ISO 4833-1:2013-12; Microbiology of the Food Chain Horizontal Method for the Enumeration of Microorganisms Part 1: Colony Count at 30 °C by the Pour Plate Technique. Polski Komitet Normalizacyjny: Warsaw, Poland, 2013.
- PN-ISO 21527-1:2009; Microbiology of Food and Animal Feeding Stuffs Horizontal Method for the Enumeration of Yeasts and Moulds Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. Polski Komitet Normalizacyjny: Warsaw, Poland, 2009.
- PN-EN ISO 21528-2:2017-08; Microbiology of the Food Chain Horizontal Method for the Detection and Enumeration of Enterobacteriaceae Part 2: Colony-Count Technique. Polski Komitet Normalizacyjny: Warsaw, Poland, 2017.
- PN-EN ISO 8586:2014-03; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Sensory Evaluation Experts. Polski Komitet Normalizacyjny: Warsaw, Poland, 2014.
- PN-ISO 4121:1998; Sensory Analysis—Methodology—Evaluation of Food Products Using Scaling Methods. Polski Komitet Normalizacyjny: Warsaw, Poland, 1998.
- Singh, N.; Singh, R.K.; Bhunia, A.K. Sequential Disinfection of Escherichia coli O157:H7 Inoculated Alfalfa Seeds before and during Sprouting Using Aqueous Chlorine Dioxide, Ozonated Water, and Thyme Essential Oil. LWT—Food Sci. Technol. 2003, 36, 235–243. [Google Scholar] [CrossRef]
- Limón, R.I.; Peñas, E.; Martínez-Villaluenga, C.; Frias, J. Role of Elicitation on the Health-Promoting Properties of Kidney Bean Sprouts. LWT—Food Sci. Technol. 2014, 56, 328–334. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic Profile and Antioxidant Activity in Selected Seeds and Sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Kordušienė, S. Maistui Daigintų Sėklų Džioviniomo Ir Šaldymo Būdai Bei Mikrobiologinės Taršos Mažinimas. 2010. Available online: https://talpykla.elaba.lt/elaba-fedora/objects/elaba:1843849/datastreams/MAIN/content (accessed on 20 December 2023).
- De Nicola, G.R.; Bagatta, M.; Pagnotta, E.; Angelino, D.; Gennari, L.; Ninfali, P.; Rollin, P.; Iori, R. Comparison of Bioactive Phytochemical Content and Release of Isothiocyanates in Selected Brassica Sprouts. Food Chem. 2013, 141, 297–303. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Jeżyna, M.; Świeca, M.; Dziki, D.; Baraniak, B.; Czyż, J. Effect of Bioaccessibility of Phenolic Compounds on In Vitro Anticancer Activity of Broccoli Sprouts. Food Res. Int. 2012, 49, 469–476. [Google Scholar] [CrossRef]
- Michalczyk, M. Wpływ naparów herbaty czarnej i zielonej na zanieczyszczenie mikrobiologiczne oraz wzrost kiełków rzodkiewki. Żywność Nauka Technol. Jakość 2012, 19, 175–186. [Google Scholar]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of Salt Stress on Phenolic Compounds, Glucosinolates, Myrosinase and Antioxidant Activity in Radish Sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Przybysz, A.; Wrochna, M.; Małecka-Przybysz, M.; Gawrońska, H.; Gawroński, S.W. The Effects of Mg Enrichment of Vegetable Sprouts on Mg Concentration, Yield and ROS Generation. J. Sci. Food Agric. 2016, 96, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kensler, T.W.; Cho, C.G.; Posner, G.H.; Talalay, P. Anticarcinogenic Activities of Sulforaphane and Structurally Related Synthetic Norbornyl Isothiocyanates. Proc. Natl. Acad. Sci. USA 1994, 91, 3147–3150. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Sorting out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health. Nutrients 2019, 11, 429. [Google Scholar] [CrossRef]
- Vanegas Torres, A.; Tish, N.; Rodov, V. Enhancement of Glucosinolate Formation in Broccoli Sprouts by Hydrogen Peroxide Treatment. Foods 2022, 11, 655. [Google Scholar] [CrossRef]
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Improving the Phytochemical Composition of Broccoli Sprouts by Elicitation. Food Chem. 2011, 129, 35–44. [Google Scholar] [CrossRef]
- Paśko, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, Total Polyphenols and Antioxidant Activity in Amaranth and Quinoa Seeds and Sprouts during Their Growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Peñas, E.; Ciska, E.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C.; Frias, J. Time Dependence of Bioactive Compounds and Antioxidant Capacity during Germination of Different Cultivars of Broccoli and Radish Seeds. Food Chem. 2010, 120, 710–716. [Google Scholar] [CrossRef]
- Gałązka-Czarnecka, I.; Krala, L. Zmiany jakości kiełków rzodkiewki Raphanus sativus L. podczas chłodniczego przechowywania w modyfikowanej atmosferze i w powietrzu. Chłodnictwo Organ Nacz. Organ. Tech. 2009, 44, 56–59. [Google Scholar]
- Smetanska, I.; Krumbein, A.; Schreiner, M.; Knorr, M. Influence of Salicylic Acid and Methyl Jasmonate on Glucosinolate Levels in Turnip. J. Hortic. Sci. Biotechnol. 2007, 82, 690–694. [Google Scholar] [CrossRef]
- Baenas, N.; Ferreres, F.; García-Viguera, C.; Moreno, D.A. Radish Sprouts—Characterization and Elicitation of Novel Varieties Rich in Anthocyanins. Food Res. Int. 2015, 69, 305–312. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Effect of NaCl Treatments on Glucosinolate Metabolism in Broccoli Sprouts. J. Zhejiang Univ. Sci. B 2013, 14, 124–131. [Google Scholar] [CrossRef]
- Lee, J.-D.; Shannon, J.G.; Jeong, Y.-S.; Lee, J.-M.; Hwang, Y.-H. A Simple Method for Evaluation of Sprout Characters in Soybean. Euphytica 2007, 153, 171–180. [Google Scholar] [CrossRef]
- Ren, L.; Chen, Y. Influence of Color Perception on Consumer Behavior. In Proceedings of the HCI in Business, Government, and Organizations; Nah, F.F.-H., Xiao, B.S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 413–421. [Google Scholar]
- Rathee, R.; Rajain, P. Role Colour Plays in Influencing Consumer Behaviour. Int. Res. J. Bus. Stud. 2019, 12, 209–222. [Google Scholar] [CrossRef]
- Taormina, P.J.; Beuchat, L.R.; Slutsker, L. Infections Associated with Eating Seed Sprouts: An International Concern. Emerg. Infect. Dis. 1999, 5, 626–634. [Google Scholar] [CrossRef]
- Mohle-Boetani, J.C.; Farrar, J.; Bradley, P.; Barak, J.D.; Miller, M.; Mandrell, R.; Mead, P.; Keene, W.E.; Cummings, K.; Abbott, S.; et al. Salmonella Infections Associated with Mung Bean Sprouts: Epidemiological and Environmental Investigations. Epidemiol. Infect. 2009, 137, 357–366. [Google Scholar] [CrossRef]
- Smith, M.A. Sprout-Associated Outbreaks and Development of Preventive Controls. In Global Safety of Fresh Produce; Elsevier: Amsterdam, The Netherlands, 2014; pp. 327–339. ISBN 978-1-78242-018-7. [Google Scholar]
- Michalczyk, M.; Kowalińska, J. Zanieczyszczenie mikrobiologiczne kiełkowanych nasion dostępnych w handlu. Żywność Nauka Technol. Jakość 2009, 16, 32–39. [Google Scholar]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological Quality of Fresh, Minimally-Processed Fruit and Vegetables, and Sprouts from Retail Establishments. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef]
- Jin, H.-H.; Lee, S.-Y. Combined Effect of Aqueous Chlorine Dioxide and Modified Atmosphere Packaging on Inhibiting Salmonella typhimurium and Listeria monocytogenes in Mungbean Sprouts. J. Food Sci. 2007, 72, M441–M445. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, M.H.; Song, K.B. Efficacy of Aqueous Chlorine Dioxide and Fumaric Acid for Inactivating Pre-Existing Microorganisms and Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes on Broccoli Sprouts. Food Control 2009, 20, 1002–1005. [Google Scholar] [CrossRef]
- Sharma, R.R.; Demirci, A.; Beuchat, L.R.; Fett, W.F. Application of Ozone for Inactivation of Escherichia coli 0157:H7 on Inoculated Alfalfa Sprouts1. J. Food Process. Preserv. 2003, 27, 51–64. [Google Scholar] [CrossRef]
- Wade, W.N.; Scouten, A.J.; Mcwatters, K.H.; Wick, R.L.; Demirci, A.; Fett, W.F.; Beuchat, L.R. Efficacy of Ozone in Killing Listeria monocytogenes on Alfalfa Seeds and Sprouts and Effects on Sensory Quality of Sprouts. J. Food Prot. 2003, 66, 44–51. [Google Scholar] [CrossRef]
- Taormina, P.J.; Beuchat, L.R. Comparison of Chemical Treatments to Eliminate Enterohemorrhagic Escherichia coli O157:H7 on Alfalfa Seeds. J. Food Prot. 1999, 62, 318–324. [Google Scholar] [CrossRef]
- Hussain, A.; Murtaza, I. Effect of natural elicitors on physical and sensory qualities of fenugreek (Trigonella foenum-graecum L.) sprouts. Int. J. Microbiol. Res. 2018, 10, 975–5276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).