Abstract
Thermo-rechargeable batteries, or tertiary batteries, are prospective energy-harvesting devices that are charged by changes in the battery temperature. Previous studies on tertiary batteries have utilized an electrolyte solution, yet the volume of this electrolyte solution could be a disadvantage in terms of the heat capacity given to the tertiary batteries. To overcome this drawback, the performance of an electrolyte-free tertiary battery consisting of physically joined Na1.60Co[Fe(CN)6]0.902.9H2O (NCF90) and Na0.72Ni[Fe(CN)6]0.685.1H2O (NNF68) thin films was investigated for the first time. During thermal cycling between 5 °C and 15 °C, the thermal voltage (VTB) was observed to be 8.4 mV. This result is comparable to the VTB of conventional tertiary batteries that use electrolyte solutions made of NCF90 and NNF68 thin films.
1. Introduction
Energy-harvesting devices that can efficiently utilize low-temperature environmental heat below 100 °C are attracting attention as an essential technology for achieving carbon neutrality. Among these devices, thermo-rechargeable batteries [1,2,3,4,5,6,7,8,9,10,11,12,13] are promising because they can be charged by changes in the battery’s surrounding temperature (T) due to a difference in the temperature coefficient (α = dE/dT) of the redox potential (E) between their cathode (α+) and anode (α−) materials. Henceforth, we refer to thermo-rechargeable batteries consisting of solid active materials as tertiary batteries. Tertiary batteries generate electricity by utilizing the temperature changes between the low (TL) and high (TH) temperatures during thermal cycling, rather than the temperature difference between the electrodes. This means that a tertiary battery is able to harvest thermal energy close to the room temperature and transduce this energy into electricity. The working mechanism of a tertiary battery is as follows. For simplicity, the initial output voltage (Vcell0) of the tertiary battery is defined as 0 V. In a tertiary battery composed of a cathode and anode with different α values, when the temperature of the tertiary battery is increased from TL to TH in an open-circuit state, a thermal voltage (VTB) is generated for the temperature change ΔT (=TH − TL) in the tertiary battery, and the output voltage (Vcell) becomes VTB (≥0) from 0 V. This can be considered the charge of the tertiary battery due to the temperature change. In addition, by discharging the tertiary battery at TH, a current can be extracted to an external circuit only by the discharge capacity (QTB) corresponding to VTB. Similarly, when the circuit is returned to the open-circuit state at TH and the temperature is decreased from TH to TL, Vcell becomes −VTB from 0 V. Then, by discharging at TL, the current can be extracted to an external circuit only by the QTB corresponding to −VTB. In other words, a tertiary battery behaves like a heat engine in response to the thermal cycle. In a tertiary battery, VTB and QTB are significant performance parameters. The thermal voltage (VTB) is expressed as VTB = (α+ − α−)ΔT [5,6]. In general, the capacity of a battery is determined by the amount of charge for which the electromotive force is equal to the voltage drop. Since the electromotive force is small (tens of mV) in a tertiary battery, the capacity coefficient ( ≡ −d/d; q represents the capacity per unit weight of the active material) of E can be considered a constant. The discharge capacity (QTB) per unit weight of total active material is expressed as , where β+ (β−) and r are the β of the cathode (anode) and the weight ratio (, where m+ (m−) is the weight of active material in the cathode (anode)), respectively [10]. This equation indicates that optimizing α+ (α−), β+ (β−), and r can lead to maximizing QTB. Actually, it has been reported that optimizing α, β, and r can increase the QTB and VTB of a tertiary battery [10]. Thus, there have been many reports of performance improvements in tertiary batteries [7,8,9,10]. However, these previous studies of tertiary batteries used an electrolyte solution, and the volume of this electrolyte solution has the potential to suppress the thermal response. This is because many liquids have a higher specific heat capacity than solids [14], and the volume of the electrolyte solution may be a drawback in terms of the heat capacity given to the tertiary battery, since the volume of liquids tends to be larger than the volume of solids. To overcome this drawback, it is important to develop all-solid-state tertiary batteries that use solid electrolytes or remove the electrolyte layer to reduce the heat capacity of the tertiary battery. Nevertheless, an all-solid-state tertiary battery has not yet been demonstrated.
Prussian blue analogs (M-PBAs: AxM[Fe(CN)6]y, where A and M represent an alkaline metal and a transition metal, respectively) exhibit a crystal structure consisting of a jungle-gym-type 3D framework of transition metals with cyano bridges. In general, M-PBAs in the reduced state have trigonal (Rm; Z = 3) or face-centered cubic (fcc) (Fmm; Z = 4) structures [15,16,17]. Nano spaces within the framework enable the reversible storage of guest species (Na+ and H2O) [15,16]. This property of M-PBAs results in various functionalities, such as lithium/sodium/potassium ion secondary batteries [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45], tertiary batteries [5,6,7,8,9,10], electrochromism [46,47,48,49,50,51,52,53], and so on. Shibata et al. [48] reported that an all-solid-state ion transfer device, in which only Na+ ions but not electrons can pass through the interface, for electrochromic devices can be fabricated with the physical junction of two PBA thin films. The electron barrier at the physically formed interface was attributed to the naturally formed water sheet [48], which acts as a pseudo electrolyte layer. In fact, it has been found that when all-solid-state devices that consist of physically joined NCF90 and NNF68 thin films are fabricated in a glove box and the water sheet is removed, there is no ion transfer [48]. In other words, all-solid-state ion transfer devices can be regarded as electrolyte-free all-solid-state ion transfer devices. However, no current research has been carried out on the application of these all-solid-state devices to an ion secondary battery and/or a tertiary battery. Next, let us consider M-PBAs as an active material for the electrodes of tertiary batteries. The range of values of α for M-PBAs varies from positive to negative. Therefore, a tertiary battery composed of M-PBAs can include materials with both positive and negative α. According to one study [54], the values of α are positive in Fe-PBA and Co-PBA and negative in Mn-PBA, Ni-PBA, Cu-PBA, and Cd-PBA. Among M-PBAs, Co-PBA and Ni-PBA are a typical combination for the cathode and anode active materials in tertiary batteries [8]. This is because the α values of Co-PBA and Ni-PBA are relatively large, resulting in good cycle stabilities of the potential. For example, Takahara et al. [8] reported that a tertiary battery fabricated with Na1.60Co[Fe(CN)6]0.902.9H2O (NCF90) and Na0.72Ni[Fe(CN)6]0.685.1H2O (NNF68) thin films using an electrolyte solution of 17 mol/kg NaClO4 exhibited a VTB of 40 mV at ΔT = 40 K. The battery also exhibited stable operation for at least 10 cycles without any degradation of the discharge capacity. Therefore, if tertiary battery operation can be demonstrated with an all-solid-state ion transfer device consisting of physically joined Co-PBA and Ni-PBA thin films, it is possible to develop a tertiary battery that can utilize the thermal energy more effectively than a conventional tertiary battery using an electrolyte solution.
In this study, the battery performance between TL (=5 °C) and TH (=15 °C) in an electrolyte-free tertiary battery consisting of physically joined NCF90 and NNF68 thin films is investigated as a first report. It was confirmed that the performance of the electrolyte-free NCF90/NNF68 tertiary battery demonstrated comparable performance to a conventional tertiary battery composed of NCF90 and NNF68 during thermal cycling between 5 °C and 15 °C.
2. Materials and Methods
2.1. Materials
NaNO3, Co(NO3)26H2O, Ni(NO3)26H2O, K3[Fe(CN)6], NaClO4, and a 0.1 mol/L HNO3 solution were purchased from FUJIFILM Wako Pure Chemical Co. (Osaka, Japan). An indium tin oxide (ITO) transparent electrode coated on glass (with sheet resistivity of 100 Ω/sq.) was purchased from GEOMATEC Co., Ltd. (Yokohama, Japan).
2.2. Sample Preparation and Characterization
Thin films of Na1.60Co[Fe(CN)6]0.902.9H2O (NCF90) and Na0.72Ni[Fe(CN)6]0.685.1H2O (NNF68) were synthesized on a transparent ITO glass electrode using electrochemical deposition techniques described in previous studies [8,9]. Prior to the deposition of the thin film, the ITO glass electrode surface underwent purification through electrolysis with 0.1 mol/L HNO3 with 2.5 V applied for one minute. The NCF90 thin film was deposited on an ITO glass electrode utilizing an aqueous solution consisting of 0.8 mmol/L K3[Fe(CN)6], 0.5 mmol/L Co(NO3)26H2O, and 5 mol/L NaNO3. The deposition process was carried out under potentiostatic conditions at −0.45 V vs. an Ag/AgCl standard electrode (saturated KCl). Sawtooth wave modulation (±0.35 V, 36 Hz) was also applied to the potential. The obtained thin film was a transparent green color. The NNF68 thin film was deposited on an ITO glass electrode utilizing an aqueous solution consisting of 0.5 mmol/L K3[Fe(CN)6], 0.5 mmol/L Ni(NO3)26H2O, and 1 mol/L NaNO3. The deposition process was carried out under potentiostatic conditions at −0.45 V vs. an Ag/AgCl standard electrode (saturated KCl). Sawtooth wave modulation (±0.35 V, 36 Hz) was also applied to the potential. The obtained thin film was transparent and colorless. The chemical composition was cited from the studies in [8,9]. X-ray diffraction patterns were examined using a MiniFlex 600 X-ray diffractometer (Rigaku; Tokyo, Japan) equipped with a CuKα line (λ = 1.54 Å) as the X-ray source at room temperature. Scanning electron microscope (SEM) images of the surface of the as-grown NCF90 and NNF68 thin films were observed with a Hitachi S4000 SEM (Hitachi High-Tech Co.; Tokyo, Japan) at an acceleration voltage of 5 kV.
The electrochemical properties of the NCF90 and NNF68 thin films were investigated using a potentiostat (HJ1001SD8; MEIDEN HOKUTO; Tokyo, Japan) with a three-electrode beaker cell. The working, reference, and counter electrodes were the NCF90 (NNF68) thin film, Ag/AgCl standard electrode (saturated KCl), and Pt electrode, respectively. The aqueous electrolyte solution was 17 mol/kg NaClO4. The rate of charge and discharge was approximately 0.5 C. The cut-off potentials of the NCF90 and NNF68 thin films were in the ranges of 0.20 V to 1.2 V vs. Ag/AgCl, and 0.20 V to 0.80 V vs. an Ag/AgCl standard electrode (saturated KCl), respectively. The weight of each thin film was determined by measuring its thickness, area, and volume density. The thickness (d) of the thin films was evaluated with a stylus profilometer. The NCF90 and NNF68 thin films had actual volume densities of 0.58 and 0.68 of the ideal density, respectively. The actual volume densities were obtained by carefully removing the thin films and evaluating their weight, which was measured using micro-analytical balances.
The values of α were evaluated with a two-electrode beaker cell whose cathode and anode were the same PBA thin films linked by a salt bridge. The aqueous electrolyte solution was 17 mol/kg NaClO4. The temperature of one electrode was regulated utilizing a thermostatic bath (BB301; Yamato Scientific Co., Ltd.; Tokyo, Japan), while the other electrode’s temperature was kept at room temperature. The potential difference (ΔV) was carefully measured against the temperature difference (ΔT) between the cathode and anode in the open-circuit condition. For each ΔT, we waited 20 min for the potential to stabilize, and then measured the ΔV.
2.3. Electrolyte-Free Tertiary Battery Assembly
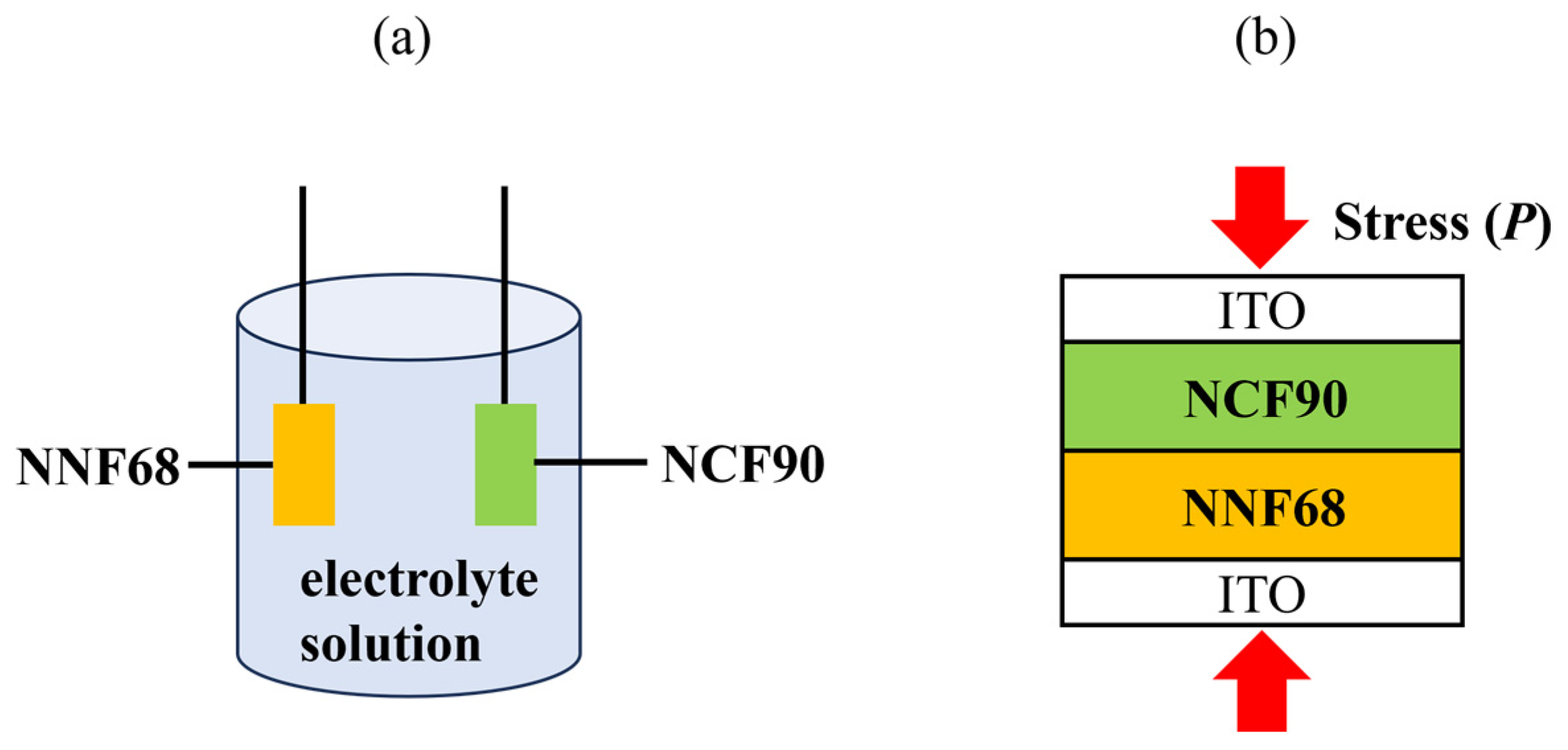
At first, the NCF90 and NNF68 thin films were pre-oxidized. This step is required for tertiary batteries because it adjusts the potential of both electrodes and sets β to a small value. A conventional tertiary battery is composed of the cathode, anode, and electrolyte solution, as shown in Figure 1a, while an electrolyte-free tertiary battery with a contact area of 0.04 cm2 was prepared by joining the surfaces of the NCF90 thin film and NNF68 thin film. This junction was achieved by applying stress (P) from the outside, as shown in Figure 1b. The P was investigated to finely regulate the electrical contact of the films. As P increases, the resistivity between the NCF90 and NNF68 thin films gradually reduces and becomes almost insensitive to P [48]. Consequently, measurements were carried out in the latter P-range. The initial potential E0 was set to be 0.50 V at 5 °C, where β+ (β−) of the NCF90 (NNF68) thin film was small. The parameters for both the NCF90 and NNF68 thin films are summarized in Table 1.
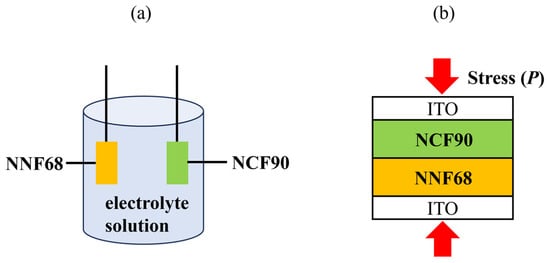
Figure 1.
Schematic illustrations of (a) conventional and (b) electrolyte-free tertiary batteries: (a) The conventional tertiary battery is composed of NCF90, NNF68 thin films, and 17 mol/kg NaClO4 aqueous electrolyte solution. (b) The electrolyte-free tertiary battery is composed of NCF90 and NNF68 thin films. The junction between NCF90 and NNF68 thin films is achieved by applying stress (P) from outside the ITO glass electrodes.

Table 1.
The parameters of NCF90 and NNF68 thin films used in the tertiary battery. α(=dE/dT), β(=dE/dq), d, S, m, and E0 are the temperature coefficient of the redox potential E, the capacity coefficient of E, the thickness of the thin film, the active area of the thin film, the weight of the thin film in the active area of the tertiary battery, and the initial potential (vs. Ag/AgCl standard electrode (saturated KCl)), respectively. The α of conventional tertiary battery’s electrodes were evaluated with a three-electrode beaker cell, whose working, reference, and counter electrodes were the NCF90 (NNF68) thin film, Ag/AgCl standard electrode (saturated KCl), and Pt electrode, respectively [8]. Therefore, the α of Ag/AgCl standard electrode is also included in this α.
2.4. Thermal Cycle Measurement
The thermal cycle properties of the tertiary batteries were measured using a potentiostat (ECstat-301; EC Frontier Co., Ltd.; Kyoto, Japan) between TL(=5 °C) and TH(=15 °C). The cell temperature (Tcell) was controlled by a desktop atmospheric thermostatic chamber (AC200; EC Frontier Co., Ltd.; Kyoto, Japan). The Tcell was assumed to be the temperature of the thermostatic chamber since it was held there for a sufficient period of time. The thermal cycle is composed of four processes: (i) heating and waiting, (ii) discharging at TH, (iii) cooling and waiting, and (iv) discharging at TL. During the (i) heating process, T was gradually increased from TL to TH, and then held at TH for 15 min to stabilize the potential in the open-circuit condition. During the (ii) discharging process, a constant current (=0.50 μA/cm2) was applied until the output voltage (Vcell) reached 0 V. During the (iii) cooling process, T was gradually decreased from TH to TL, and then held at TL for 15 min to stabilize the potential in the open-circuit condition. During the (iv) discharging process, a constant current (=0.50 μA/cm2) was applied until Vcell reached 0 V. The total weight of the active materials in the cathode and anode was defined as the unit weight.
3. Results and Discussion
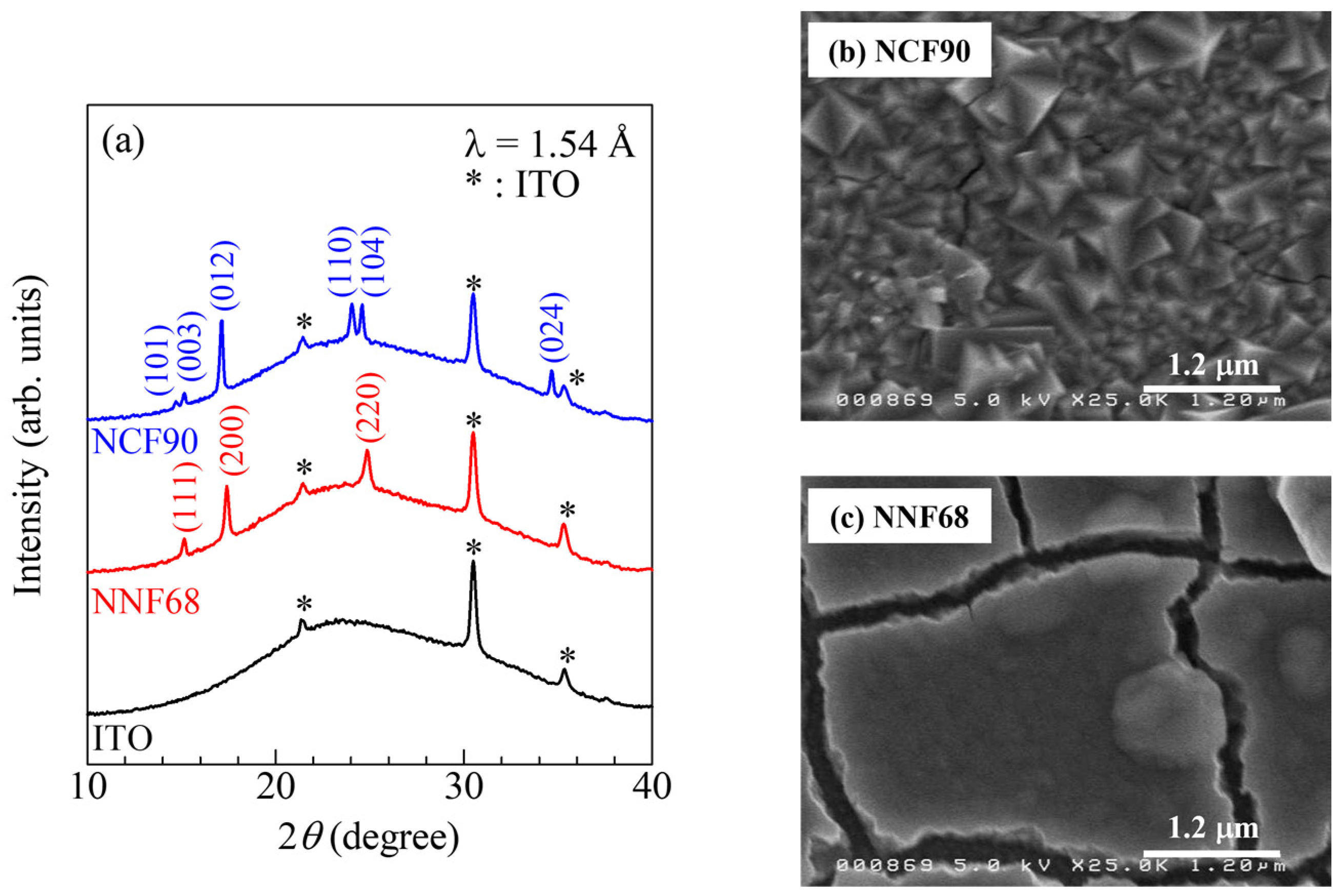
Figure 2a exhibits the X-ray diffraction (XRD) patterns of the as-grown NCF90 and NNF68 films. The asterisks (*) denote the diffraction peaks of the ITO transparent glass electrode. In the NCF90 thin film, the observed diffraction peaks can be assigned an index in the trigonal (Rm; Z = 3) structure. Lattice constants were evaluated using the Rietveld method (Rietan-FP program [55]): a = 7.403 (5) and c = 17.542 (15) . In the NNF68 thin film, the observed diffraction peaks could be assigned an index in the face-centered cubic (fcc) (Fmm; Z = 4) structure. Lattice constant a was 10.172 (2) . These lattice constants of the as-grown NCF90 and NNF68 thin films were consistent with those in the studies in [8,9]. Figure 2b,c are scanning electron microscope (SEM) images of the surfaces of the NCF90 and NNF68 thin films, respectively. The surface of the NCF90 thin film is composed of large crystals. The grain size was roughly evaluated to be 200–500 nm. On the other hand, the surface of the NNF68 thin film seems to be composed of granular particles with low crystallinity. The grain size was roughly evaluated to be 150–300 nm.
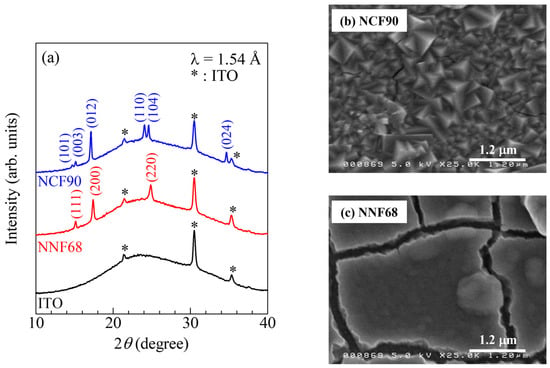
Figure 2.
X-ray diffraction (XRD) patterns and scanning electron microscope (SEM) images of the surfaces of the NCF90 and NNF68 thin films: (a) XRD patterns of NCF90, NNF68, and ITO transparent glass electrodes. The values of NCF90 and NNF68 in the brackets are the indexes in the trigonal and face-centered cubic structures, respectively. SEM images of the surfaces of the (b) NCF90 and (c) NNF68 thin films.
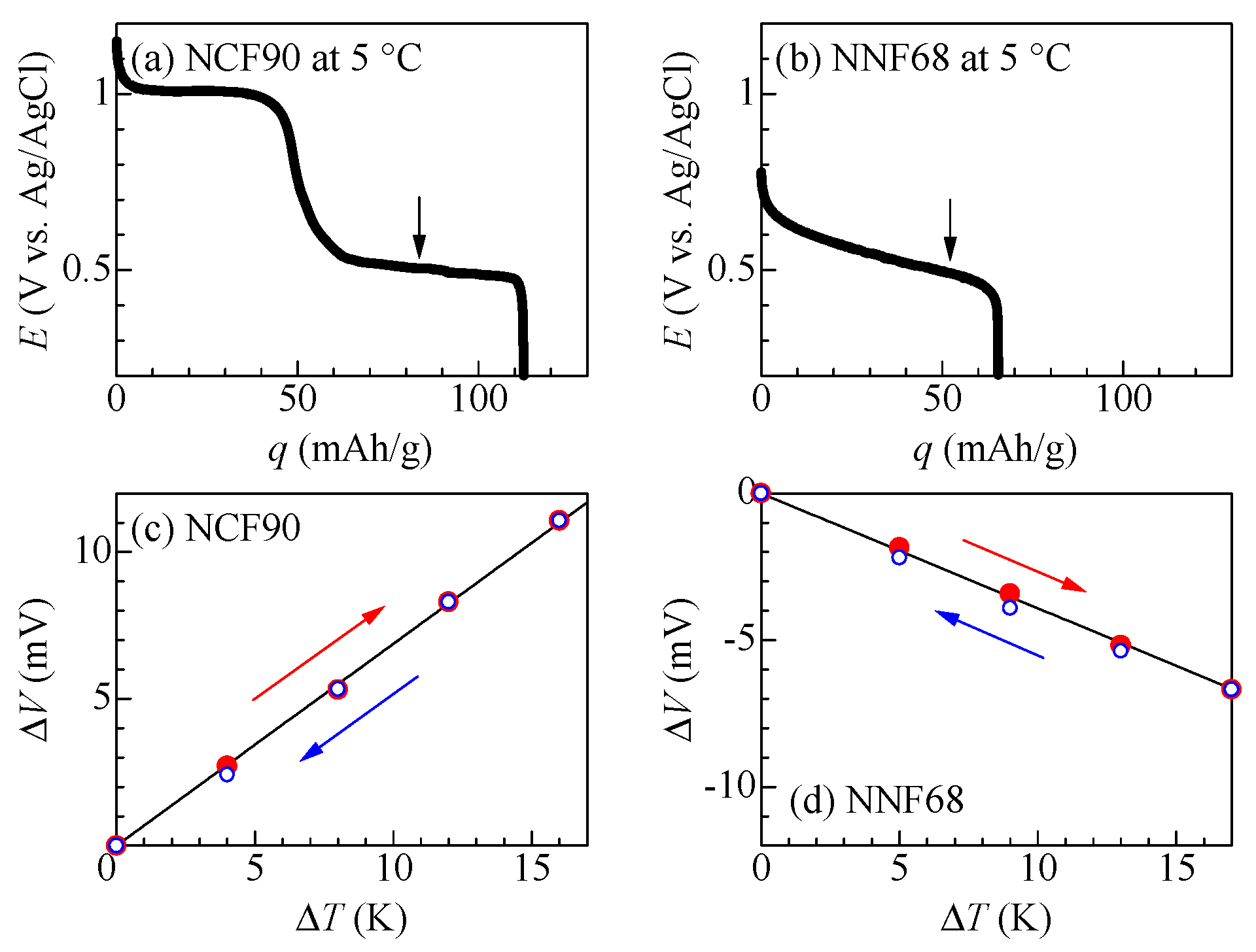
Figure 3a,b exhibit the discharge curves of the NCF90 and NNF68 thin films at 5 °C, respectively. In the NCF90 thin film (Figure 3a), the discharge curve exhibits two plateaus at around 1.0 V and 0.5 V vs. the Ag/AgCl standard electrode. This feature is consistent with that observed in propylene carbonate (PC) with 1mol/L NaClO4 [28]. Therefore, the redox site at the lower (upper) plateau is Co3+/Co2+ ([Fe(CN)6]3−/[Fe(CN)6]4−). The observed capacity (=112 mAh/g) is close to the ideal value (=125 mAh/g). In the NNF68 thin film (Figure 3b), the discharge curve exhibits a single plateau at around 0.55 V vs. the Ag/AgCl standard electrode. The redox site of this plateau is [Fe(CN)6]3−/[Fe(CN)6]4− [54]. The observed capacity (=65 mAh/g) is close to the ideal value (=65 mAh/g). Figure 3c,d show the potential difference (ΔV) between the two-electrode beaker cell, whose cathode and anode were the same PBA thin films linked by a salt bridge, against the temperature difference (ΔT) between TL and TH during the heating (filled red circles) and cooling processes (open blue circles) at the potentials at the arrow positions in Figure 3a,b, respectively. The open and filled circles correspond to the ΔT-increasing and ΔT-decreasing experimental runs, respectively. These results indicate that the NCF90 and NNF68 thin films had no hysteresis between TH and TL. The α values of the NCF90 and NNF68 thin films determined using least squares fitting of each dataset were +0.69 mV/K and −0.39 mV/K, respectively. Here, based on the discussion of Moritomo et al. [54], the value of α is considered for the NCF90 and NNF68 thin films. From a thermodynamical viewpoint, α is expressed as (Sred − Soxi)/e. Here, Sred and Soxi represent the entropy of the system in the reduction and oxidation states, respectively, and e represents the elementary charge (>0). The difference in redox sites is responsible for the difference in the sign of α. In the lower plateau of NCF90, the redox site is the Co site. This reduction process alters the valence (qn) in Co and causes a modification of the Na+ configuration entropy (SNa) around the redox site. The large |qn| strongly constrains the Na+ configuration and decreases the SNa. At the lower plateau of the NCF90, the |qn| of the reduction state (Co2+) is 2, which is smaller than the oxidation state (Co3+) where |qn| is 3. In other words, SNa in the reduction state is expected to be larger than SNa in the oxidation state. Under these conditions, Sred becomes larger than Soxi. This results in the positive value of α, as observed. At the single plateau of NNF68, the redox site is the [Fe(CN)6] site. During this reduction process, the |qn| of the reduction state ([Fe(CN)6]4−) is 4, which is larger than the oxidation state ([Fe(CN)6]3−), where |qn| is 3. In other words, SNa in the reduction state is expected to be smaller than SNa in the oxidation state. Under these conditions, Sred becomes smaller than Soxi. This results in the negative value of α, as observed. Furthermore, these values are consistent with the literature values obtained for electrodes made from Co-PBA and Ni-PBA powder samples [54]. The parameters of NCF90 and NNF68 thin films used in the electrolyte-free tertiary battery are summarized in Table 1, along with the parameters of conventional tertiary batteries reported in a previous study [8]. The difference in the α of the electrode active materials in the electrolyte-free tertiary battery and the conventional tertiary battery reflects the difference in the evaluation method. In addition, the difference in β is assumed to be due to sample dependence.

Figure 3.
First discharge curves of (a) NCF90 and (b) NNF68 thin films at 0.5 C and 5 °C against the capacity (q) per unit weight of the active material. Downward arrows indicate the initial potential of the pre-oxidized (a) NCF90 and (b) NNF68 for a tertiary battery, respectively. Potential difference (ΔV) between the two-electrode beaker cell, whose cathode and anode were the same PBA thin films connected by a salt bridge, against the temperature difference (ΔT) between TL and TH in the heating process (filled red circles) and cooling process (open blue circles) runs in (c) NCF90 and (d) NNF68. Red (blue) arrows indicate the direction of ΔV change in the heating (cooling) process. Solid straight lines represent the results of the least squares fitting.
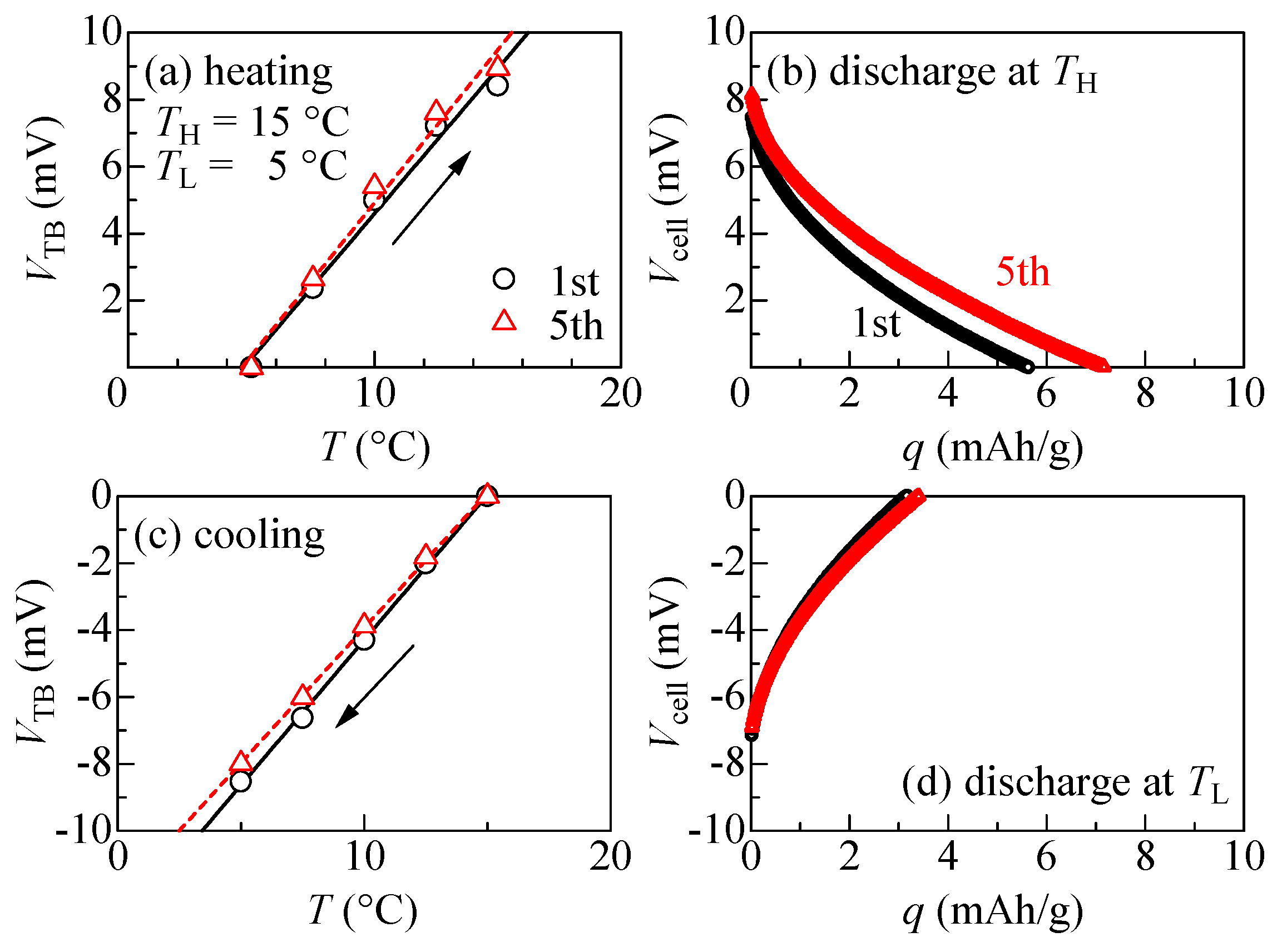
Figure 4 exhibits the thermal cycle behaviors of the electrolyte-free NCF90/NNF68 tertiary battery between TL = 5 °C and TL = 15 °C. During the first cycle (open black circle) of the heating process, as shown in Figure 4a, VTB linearly increases with an increase in T at a rate of 0.9 mV/K. This rate is close to αcell (=1.1 mV/K), which represents αNCF90–αNNF68. At TH, the thermal voltage (VTB) becomes 8.4 mV. During the discharging process at TH, as shown in Figure 4b, the output voltage (Vcell) gradually decreases to 0 V with an increase in capacity (q). The discharge capacity (QTB) per unit weight of the total active materials is 5.6 mAh/g. Here, we compared this observed value and the calculated value (QTBcalc). QTBcalc is expressed as , where β+ (β−) and r are the β of the cathode (anode) and the weight ratio (, where m+ (m−) is the weight of active material in the cathode (anode)), respectively. With the parameters in Table 1, it is estimated that QTBcalc is 5 mAh/g at ΔT = 10 K. Therefore, the observed value is consistent with the calculated value. As shown in Figure 4c, during the cooling process, VTB linearly decreases with an increase in T at a rate of 0.9 mV/K. At TL, VTB becomes −8.5 mV. During the discharging process at TL, as shown in Figure 4d, QTB is 3.2 mAh/g. The thermal cycle properties observed during the fifth cycle were essentially similar to those observed during the first cycle. Furthermore, the observed αcell of the electrolyte-free tertiary battery was consistent with the αcell (=1.1 mV/K) of a conventional NCF90/NNF68 tertiary battery using electrolyte solution in a previous report [8].
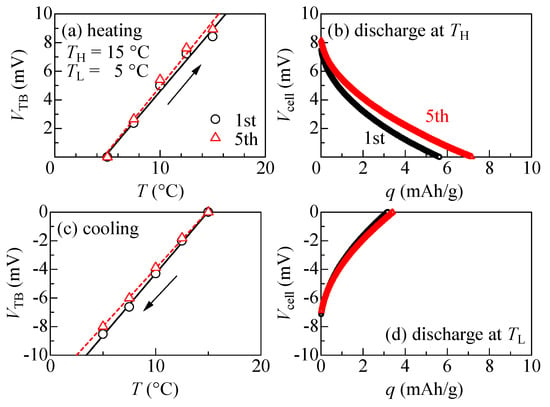
Figure 4.
Thermal cycles of the electrolyte-free tertiary battery composed of NCF90 and NNF68 thin films: (a) Thermal voltage (VTB) of the electrolyte-free tertiary battery against temperature (T) measured in the heating run under the open-circuit condition at the first and fifth cycles; the solid and broken straight lines represent the results of the least squares fitting, respectively. The arrow indicates the direction of VTB change. (b) Discharge curves at TH (=15 °C) against capacity (q) per unit weight of the total active materials at the first and fifth cycles. (c) VTB against T was measured in the cooling run under the open-circuit condition at the first and fifth cycles; the solid and broken straight lines represent the results of the least squares fitting, respectively. The arrow indicates the direction of VTB change. (d) Discharge curves at TL (=5 °C) against q per unit weight of the total active materials at the first and fifth cycles.
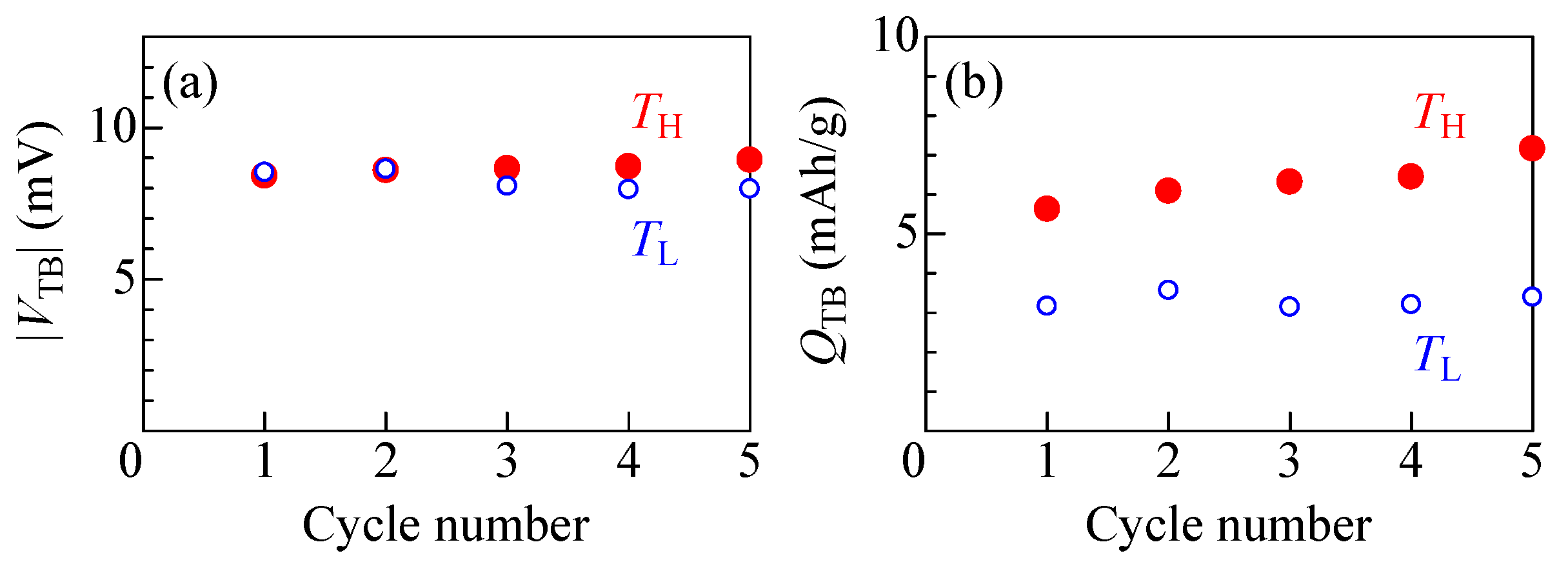
Figure 5a,b exhibit the thermal cycle property of the electrolyte-free NCF90/NNF68 tertiary battery. The open and filled circles represent the data obtained at TL and TH, respectively. In the electrolyte-free NCF90/NNF68 tertiary battery, there was almost no change in either |VTB| or QTB up to the fifth cycle. However, a significant difference exists between the QTB values of TH and TL. The difference in QTB between TH and TL is considered to be due to higher resistance in a physical-junction-type all-solid-state ion-transfer device made of PBA films at lower temperatures, resulting in a lower number of ions that can pass through this interface [48].
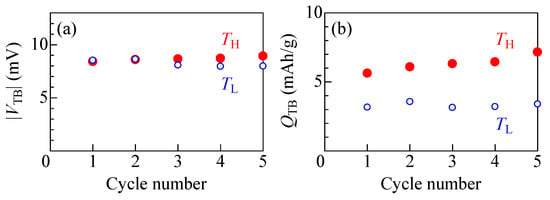
Figure 5.
(a) Thermal voltage (VTB) and (b) discharge capacity (QTB) per unit weight of the total active material against cycle number. Open and filled circles represent the data obtained at TL and TH, respectively.
Here, the thermal efficiency (η) of the first cycle of the electrolyte-free NCF90/NNF68 tertiary battery is roughly evaluated. Details of the evaluation are described in the study in [7]. As shown in Figure 5, the electrolyte-free NCF90/NNF68 tertiary battery exhibited a VTB of 8.4 mV and QTB of 5.6 mAh/g at TH = 15 °C, and a VTB of 8.5 mV and QTB of 3.2 mAh/g at TL = 5 °C. The η is defined as (WH + WL)/Q, where WH (WL) and Q are the electric work at TH (TL) and the input thermal energy, respectively. WH (WL) was estimated to be 1.3 meV/NCF90 (0.75 meV/NCF90), whose value was roughly estimated as q′VTB/2 at TH (TL). Here, q′ is the discharge capacity (q) per unit weight of the total active material of the tertiary battery at TH (TL), converted to the final extraction charge per NCF90. Q was estimated as (C+ + C−)ΔT, where C+ (C−) represents the specific heat of the cathode (anode) material, respectively. Based on the Dulong–Petit law, C+ (C−) was approximated by the specific heat of the ideal Na2Co[Fe(CN)6] (Na2Ni[Fe(CN)6]) in the high temperature limit; C+ = 4.16 meV/K, C− = 15.23 meV/K. As a result, η = 1.1% was obtained, which corresponds to 31% of the Carnot efficiency (ηcarnot = 3.5%).
Finally, the performance of the electrolyte-free tertiary battery is compared with the conventional tertiary battery reported in a previous study [8]. Table 2 summarizes the VTB and QTB values of the electrolyte-free and conventional tertiary batteries at TH. For convenience of explanation, the normalized values, VTB* and QTB*, at ΔT = 10 K are also listed in Table 2. Note that this normalization is easy to perform because the QTB of a tertiary battery is proportional to VTB, and VTB is proportional to ΔT. The electrolyte-free tertiary battery exhibits a VTB* of 8.4 mV and a QTB* of 5.6 mAh/g at ΔT = 10 K. On the other hand, the conventional tertiary battery exhibits a VTB* of 10 mV and a QTB* of 1.3 mAh/g at ΔT = 10 K. The VTB* was found to be similar for both tertiary batteries, while the QTB* was about four times greater for the electrolyte-free tertiary battery. The difference in QTB* between the electrolyte-free tertiary battery and the conventional tertiary battery can be interpreted as due to the difference in the charge coefficient β (see Table 1). Thus, it was found that the electrolyte-free tertiary batteries exhibited high performance. Note that the thermal response properties of electrolyte-free tertiary batteries could not be evaluated due to problems in the experimental environment. This is because the thermostatic chamber used to measure the electrolyte-free tertiary battery could determine the temperature inside the chamber, but could not measure the temperature of the device in real time. It is imperative to evaluate the thermal response of all-solid-state tertiary batteries, as they are expected to have better performance compared to conventional tertiary batteries. This evaluation will be performed in the future.

Table 2.
Performance of the electrolyte-free and conventional tertiary batteries. VTB and QTB are the thermal voltage and discharge capacity per unit weight of the total active materials contained in the cathode and anode at TH. VTB* and QTB* are normalized values at ΔT = 10 K.
4. Conclusions
The operation of an electrolyte-free tertiary battery consisting of physically joined NCF90 and NNF68 thin films is demonstrated for the first time. The results indicate that the performance of an electrolyte-free tertiary battery is comparable to that of a conventional tertiary battery using an electrolyte solution. It is believed that all-solid-state tertiary batteries, including electrolyte-free all-solid-state tertiary batteries and all-solid-state tertiary batteries with solid electrolytes, will be a new direction for future tertiary battery studies.
Author Contributions
Conceptualization, methodology, funding acquisition, and writing: T.S.; investigation: H.M.; data curation: I.N.; supervision: H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by JSPS KAKENHI (Grant No. JP21H01822), an Amano Institute of Technology Research Grant, and the Murata Science Foundation.
Data Availability Statement
The data presented in this study are available upon request to the corresponding author.
Acknowledgments
The SEM observations were performed at the Open Facility of the Tokyo University of Marine Science and Technology, and Y. Tsukada supported the observations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lee, S.W.; Yang, Y.; Lee, H.-W.; Ghasemi, H.; Kraemer, D.; Chen, G.; Cui, Y. An electrochemical system for efficiently harvesting low-grade heat energy. Nat. Commun. 2014, 5, 3942. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, S.W.; Ghasemi, H.; Loomis, J.; Li, X.; Kraemer, D.; Zheng, G.; Cui, Y.; Chen, G. Charging-free electrochemical system for harvesting low-grade thermal energy. Proc. Natl. Acad. Sci. USA 2014, 111, 17011–17016. [Google Scholar] [CrossRef]
- Wang, J.; Feng, S.-P.; Yang, Y.; Hau, N.Y.; Munro, M.; Ferreira-Yang, E.; Chen, G. “Thermal Charging” Phenomenon in Electrical Double Layer Capacitors. Nano Lett. 2015, 15, 5784–5790. [Google Scholar] [CrossRef]
- Gao, C.; Lee, S.W.; Yang, Y. Thermally Regenerative Electrochemical Cycle for Low-Grade Heat Harvesting. ACS Energy Lett. 2017, 2, 2326–2334. [Google Scholar] [CrossRef]
- Shibata, T.; Fukuzumi, Y.; Kobayashi, W.; Moritomo, Y. Thermal power generation during heat cycle near room temperature. Appl. Phys. Express 2018, 11, 017101. [Google Scholar] [CrossRef]
- Fukuzumi, Y.; Amaha, K.; Kobayashi, W.; Niwa, H.; Moritomo, Y. Prussian Blue Analogues as Promising Thermal Power Generation Materials. Energy Technol. 2018, 6, 1865–1870. [Google Scholar] [CrossRef]
- Shibata, T.; Fukuzumi, Y.; Moritomo, Y. Thermal efficiency of a thermocell made of Prussian blue analogues. Sci. Rep. 2018, 8, 14784. [Google Scholar] [CrossRef]
- Takahara, I.; Shibata, T.; Fukuzumi, Y.; Moritomo, Y. Improved Thermal Cyclability of Tertiary Battery Made of Prussian Blue Analogues. ChemistrySelect 2019, 4, 8558–8563. [Google Scholar] [CrossRef]
- Shibata, T.; Iwaizumi, H.; Fukuzumi, Y.; Moritomo, Y. Energy harvesting thermocell with use of phase transition. Sci. Rep. 2020, 10, 1813. [Google Scholar] [CrossRef]
- Shibata, T.; Nakamura, K.; Nozaki, S.; Iwaizumi, H.; Ohnuki, H.; Moritomo, Y. Optimization of electrode parameters of NaxCo[Fe(CN)6]0.88/NaxCd[Fe(CN)6]0.99 tertiary battery. Sustain. Mater. Technol. 2022, 33, e00483. [Google Scholar]
- Li, X.; Li, J.; Yun, J.; Wu, A.; Gao, C.; Lee, S.W. Continuous thermally regenerative electrochemical systems for directly converting low-grade heat to electricity. Nano Energy 2022, 101, 107547. [Google Scholar] [CrossRef]
- Wu, A.; Li, X.; Lee, D.; Li, J.; Yun, J.; Jiang, C.; Li, Z.; Lee, S.W. Thermoresponsive ionic liquid for electrochemical low-grade heat harvesting. Nano Energy 2023, 105, 108022. [Google Scholar] [CrossRef]
- Choi, A.; Song, Y.-Y.; Kim, J.; Kim, D.; Kim, M.-H.; Lee, S.W.; Seo, D.-H.; Lee, H.-W. Enhancing Efficiency of Low-Grade Heat Harvesting by Structural Vibration Entropy in Thermally Regenerative Electrochemical Cycles. Adv. Mater. 2023, 35, 2303199. [Google Scholar] [CrossRef]
- Atkins, P.; De Paula, J. Elements of Physical Chemistry, 7th ed.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Buser, H.J.; Schwarzenbach, D.; Petter, W.; Ludi, A. The crystal structure of Prussian Blue: Fe4[Fe(CN)6]3∙xH2O. Inorg. Chem. 1977, 16, 2704–2710. [Google Scholar] [CrossRef]
- Herren, F.; Fischer, P.; Ludi, A.; Haelg, W. Neutron diffraction study of Prussian Blue, Fe4[Fe(CN)6]3∙xH2O. Location of water molecules and long-range magnetic order. Inorg. Chem. 1980, 19, 956–959. [Google Scholar] [CrossRef]
- Niwa, H.; Kobayashi, W.; Shibata, T.; Nitani, H.; Moritomo, Y. Invariant nature of substituted element in metal-hexacyanoferrate. Sci. Rep. 2017, 7, 13225. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, N.; Morikawa, T.; Kondo, J.; Takeda, Y.; Yamamoto, O.; Kinugasa, N.; Yamagishi, T. Lithium intercalation behavior into iron cyanide complex as positive electrode of lithium secondary battery. J. Power Sources 1999, 79, 215–219. [Google Scholar] [CrossRef]
- Imanishi, N.; Morikawa, T.; Kondo, J.; Yamane, R.; Takeda, Y.; Yamamoto, O.; Sakaebe, H.; Tabuchi, M. Lithium intercalation behavior of iron cyanometallates. J. Power Sources 1999, 81–82, 530–534. [Google Scholar] [CrossRef]
- Okubo, M.; Asakura, D.; Mizuno, Y.; Kim, J.-D.; Mizokawa, T.; Kudo, T.; Honma, I. Switching Redox-Active Sites by Valence Tautomerism in Prussian Blue Analogues AxMny[Fe(CN)6]·nH2O (A: K, Rb): Robust Frameworks for Reversible Li Storage. J. Phys. Chem. Lett. 2010, 1, 2063–2071. [Google Scholar] [CrossRef]
- Matsuda, T.; Moritomo, Y. Thin Film Electrode of Prussian Blue Analogue for Li-ion Battery. Appl. Phys. Express 2011, 4, 047101. [Google Scholar] [CrossRef]
- Takachi, M.; Matsuda, T.; Moritomo, Y. Structural, Electronic, and Electrochemical Properties of LixCo[Fe(CN)6]0.902.9H2O. Jpn. J. Appl. Phys. 2013, 52, 044301. [Google Scholar] [CrossRef]
- Shen, L.; Wang, Z.; Chen, L. Prussian Blues as a Cathode Material for Lithium Ion Batteries. Chem. Eur. J. 2014, 20, 12559–12562. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.-R.; Fu, J.; Shadike, Z.; Cao, M.-H.; Wang, W.-W.; Fu, Z.-W. All-Solid-State Rechargeable Lithium Metal Battery with a Prussian Blue Cathode Prepared by a Nonvacuum Coating Technology. ACS Omega 2018, 3, 7648–7654. [Google Scholar] [CrossRef]
- Zhang, Z.; Avdeev, M.; Chen, H.; Yin, W.; Kan, W.H.; He, G. Lithiated Prussian blue analogues as positive electrode active materials for stable non-aqueous lithium-ion batteries. Nat. Commun. 2022, 13, 7790. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hu, J.; Chen, H.; Zhang, C.; Xu, M.; Zhuang, L.; Ai, X.; Qian, J. Chemical lithiation methodology enabled Prussian blue as a Li-rich cathode material for secondary Li-ion batteries. Energy Storage Mater. 2023, 60, 102803. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Takachi, M.; Moritomo, Y. A sodium manganese ferrocyanide thin film for Na-ion batteries. Chem. Commun. 2013, 49, 2750–2752. [Google Scholar] [CrossRef]
- Takachi, M.; Matsuda, T.; Moritomo, Y. Redox Reactions in Prussian Blue Analogue Films with Fast Na+ Intercalation. Jpn. J. Appl. Phys. 2013, 52, 090202. [Google Scholar] [CrossRef]
- Yang, D.; Xu, J.; Liao, X.-Z.; He, Y.-S.; Liu, H.; Ma, Z.-F. Structure optimization of Prussian blue analogue cathode materials for advanced sodium ion batteries. Chem. Commun. 2014, 50, 13377–13380. [Google Scholar] [CrossRef]
- Lee, H.W.; Wang, R.Y.; Pasta, M.; Lee, S.W.; Liu, N.; Cui, Y. Manganese hexacyanomanganate open framework as a high-capacity positive electrode material for sodium-ion batteries. Nat. Commun. 2014, 5, 5280. [Google Scholar] [CrossRef]
- Wang, L.; Song, J.; Qiao, R.Q.; Wray, L.A.; Hossain, M.A.; Chung, Y.-D.; Yang, W.; Lu, Y.; Evans, D.; Lee, J.-J.; et al. Rhombohedral Prussian White as Cathode for Rechargeable Sodium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 2548–2554. [Google Scholar] [CrossRef]
- Yu, S.; Li, Y.; Lu, Y.; Xu, B.; Wang, Q.; Yan, M.; Jiang, Y. A promising cathode material of sodium iron–nickel hexacyanoferrate for sodium ion batteries. J. Power Sources 2015, 275, 45–49. [Google Scholar] [CrossRef]
- Pasta, M.; Wang, R.Y.; Ruffo, R.; Qiao, R.; Lee, H.-W.; Shyam, B.; Guo, M.; Wang, Y.; Wray, L.A.; Yang, W.; et al. Manganese–cobalt hexacyanoferrate cathodes for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 4211–4223. [Google Scholar] [CrossRef]
- Nakamoto, K.; Sakamoto, R.; Ito, M.; Ktajou, A.; Okada, S. Effect of Concentrated Electrolyte on Aqueous Sodium-ion Battery with Sodium Manganese Hexacyanoferrate Cathode. Electrochemistry 2017, 85, 179–185. [Google Scholar] [CrossRef]
- Rehman, R.; Peng, J.; Yi, H.; Shen, Y.; Yin, J.; Li, C.; Fang, C.; Li, Q.; Han, J. Highly crystalline nickel hexacyanoferrate as a long-life cathode material for sodium-ion batteries. RSC Adv. 2020, 10, 27033–27041. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Y.; Xie, J.; Nie, Y.; Xu, X.; Tu, J.; Shen, C.; Jin, Y.; Li, Y.; Lu, Y.; et al. High-performance Ni/Fe-codoped manganese hexacyanoferrate by scale-up synthesis for practical Na-ion batteries. Mater. Today Sustain. 2022, 18, 100113. [Google Scholar] [CrossRef]
- Eftekhari, A. Potassium secondary cell based on Prussian blue cathode. J. Power Sources 2004, 126, 221–228. [Google Scholar] [CrossRef]
- Bie, X.; Kubota, K.; Hosaka, T.; Chihara, K.; Komaba, S. A novel K-ion battery: Hexacyanoferrate (II)/graphite cell. J. Mater. Chem. A 2017, 5, 4325–4330. [Google Scholar] [CrossRef]
- Wu, X.; Jian, Z.; Li, Z.; Ji, X. Prussian white analogues as promising cathode for non-aqueous potassium-ion batteries. Electrochem. Commun. 2017, 77, 54–57. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, Y.; Zhao, C.; Liu, L.; Zhang, J.; Zhang, Q.; Shen, X.; Zhao, J.; Yu, X.; Li, H.; et al. Building aqueous K-ion batteries for energy storage. Nat. Energy 2019, 4, 495–503. [Google Scholar] [CrossRef]
- Chong, S.; Yang, J.; Sun, L.; Guo, S.; Liu, Y.; Liu, H.K. Potassium nickel iron hexacyanoferrate as ultra-long-life cathode material for potassium-ion batteries with high energy density. ACS Nano 2020, 14, 9807–9818. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.; Lu, Y.; Wang, C.; Zhang, Q.; Zhao, S.; Peng, J.; Zhang, K.; Chou, S.-L.; Chen, J. A Low-Strain Potassium-Rich Prussian Blue Analogue Cathode for High Power Potassium-Ion Batteries. Angew. Chem. 2021, 133, 13160–13166. [Google Scholar] [CrossRef]
- Ge, J.; Fan, L.; Rao, A.M.; Zhou, J.; Lu, B. Surface-substituted Prussian blue analogue cathode for sustainable potassium-ion batteries. Nat. Sustain. 2022, 5, 225–234. [Google Scholar] [CrossRef]
- Le Pham, P.N.; Wernert, R.; Cahu, M.; Sougrati, M.T.; Aquilanti, G.; Johansson, P.; Monconduit, L.; Stievano, L. Prussian blue analogues for potassium-ion batteries: Insights into the electrochemical mechanisms. J. Mater. Chem. A 2023, 11, 3091–3104. [Google Scholar] [CrossRef]
- Gotoh, A.; Uchida, H.; Ishizaki, M.; Satoh, T.; Kaga, S.; Okamoto, S.; Ohta, M.; Sakamoto, M.; Kawamoto, T.; Tanaka, H. Simple synthesis of three primary colour nanoparticle inks of Prussian blue and its analogues. Nanotechnology 2007, 18, 345609. [Google Scholar] [CrossRef]
- Hara, S.; Shiozaki, H.; Omura, A.; Tanaka, H.; Kawamoto, T.; Tokumoto, M.; Yamada, M.; Gotoh, A.; Kurihara, M.; Sakamoto, M. Color-Switchable Glass and Display Devices Fabricated by Liquid Processes with Electrochromic Nanoparticle “Ink”. Appl. Phys. Express 2008, 1, 104002. [Google Scholar] [CrossRef]
- Shibata, T.; Kamioka, H.; Moritomo, Y. Simultaneous Measurement of Electron and Ion Transfer in All-Solid Ion-Transfer Device Made of Transition Metal Cyanide Films. Jpn. J. Appl. Phys. 2011, 50, 124101. [Google Scholar] [CrossRef]
- Ishizaki, M.; Kanaizuka, K.; Abe, M.; Hoshi, Y.; Sakamoto, M.; Kawamoto, T.; Tanaka, H.; Kurihara, M. Preparation of electrochromic Prussian blue nanoparticles dispersible into various solvents for realisation of printed electronics. Green Chem. 2012, 14, 1537–1544. [Google Scholar] [CrossRef]
- Lee, K.-M.; Tanaka, H.; Takahashi, A.; Kim, K.H.; Kawamura, M.; Abe, Y.; Kawamoto, T. Accelerated coloration of electrochromic device with the counter electrode of nanoparticulate Prussian blue-type complexes. Electrochim. Acta 2015, 163, 288–295. [Google Scholar] [CrossRef]
- Assis, L.M.N.; Leones, R.; Kanicki, J.; Pawlicka, A.; Silva, M.M. Prussian blue for electrochromic devices. J. Electroanal. Chem. 2016, 777, 33–39. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Y.; Zhou, K.; Wang, H.; Yan, H. Improving Electrochromic Cycle Life of Prussian Blue by Acid Addition to the Electrolyte. Materials 2019, 12, 28. [Google Scholar] [CrossRef]
- Tang, D.; Wang, J.; Liu, X.-A.; Tong, Z.; Ji, H.; Qu, H.-Y. Low-Spin Fe Redox-Based Prussian Blue with excellent selective dual-band electrochromic modulation and energy-saving applications. J. Colloid Interface Sci. 2023, 636, 351–362. [Google Scholar] [CrossRef]
- Moritomo, Y.; Yoshida, Y.; Inoue, D.; Iwaizumi, H.; Kobayashi, S.; Kawaguchi, S.; Shibata, T. Origin of the Material Dependence of the Temperature Coefficient of the Redox Potential in Coordination Polymers. J. Phys. Soc. Jpn. 2021, 90, 063801. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).