The Application of Dextran Sodium Sulfate to the Efficient Separation of Ilmenite and Forsterite, as a Flotation Depressant
Abstract
1. Introduction
2. Experimental Section
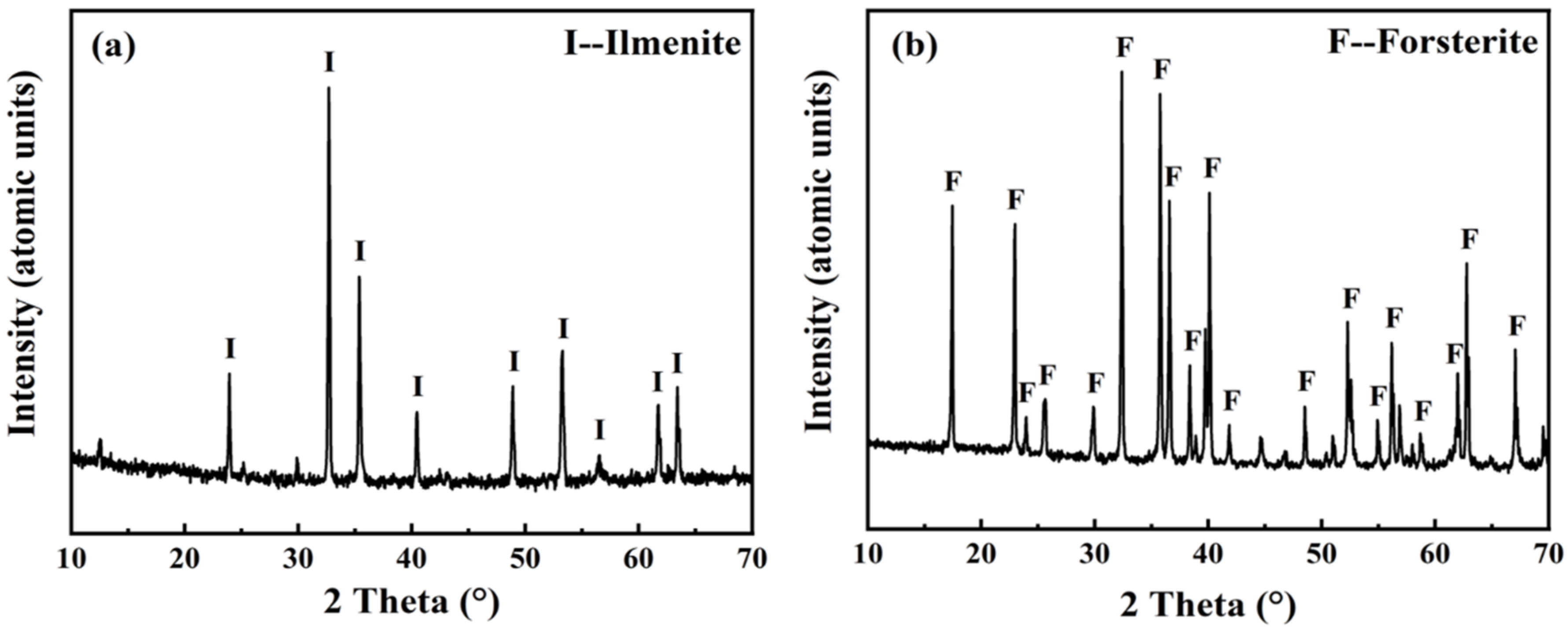
2.1. Materials
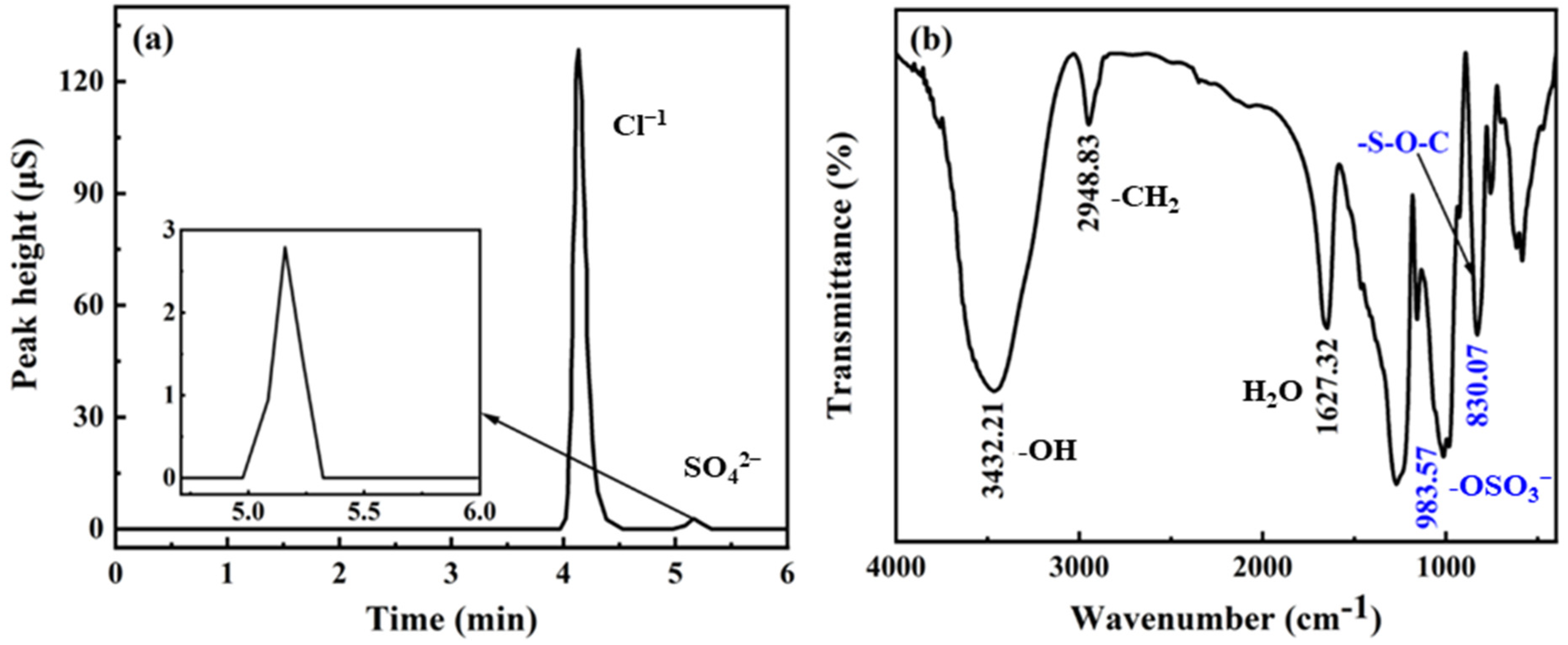
2.2. Characterization of DSS
2.3. Flotation Tests
2.4. Interaction Mechanisms
2.5. Molecular Dynamics Simulation
2.5.1. DSS Molecule and Mineral Crystal Structure
2.5.2. Dynamics Simulation Details
3. Results and Discussion
3.1. Characterization of DSS
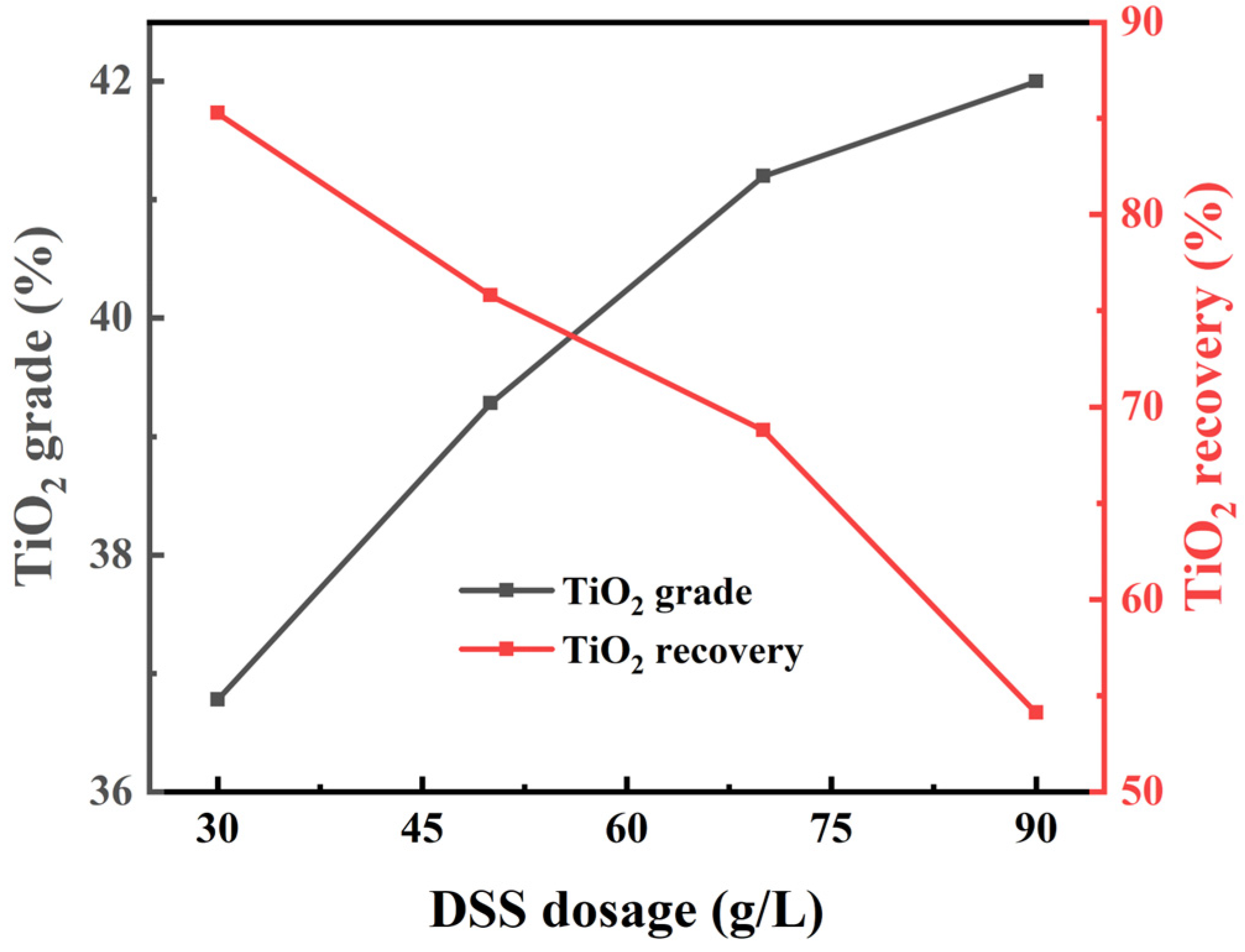
3.2. Flotation Experiments
3.2.1. Micro-Flotation of Single Minerals
3.2.2. Laboratory Flotation of Ilmenite Ore
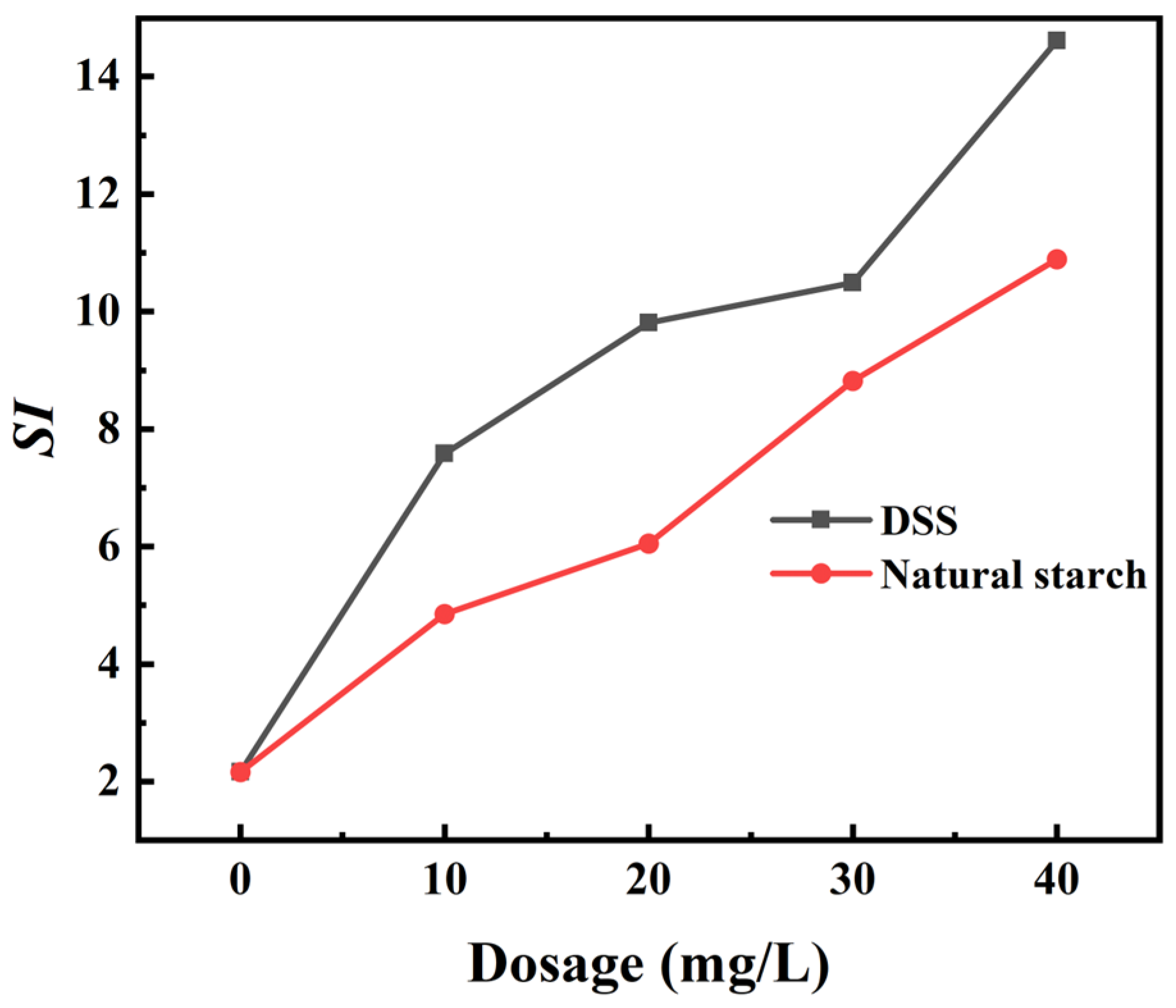
3.3. Interaction Mechanisms of the DSS on Ilmenite/Forsterite Surface
3.3.1. Electrostatic Interaction Analysis
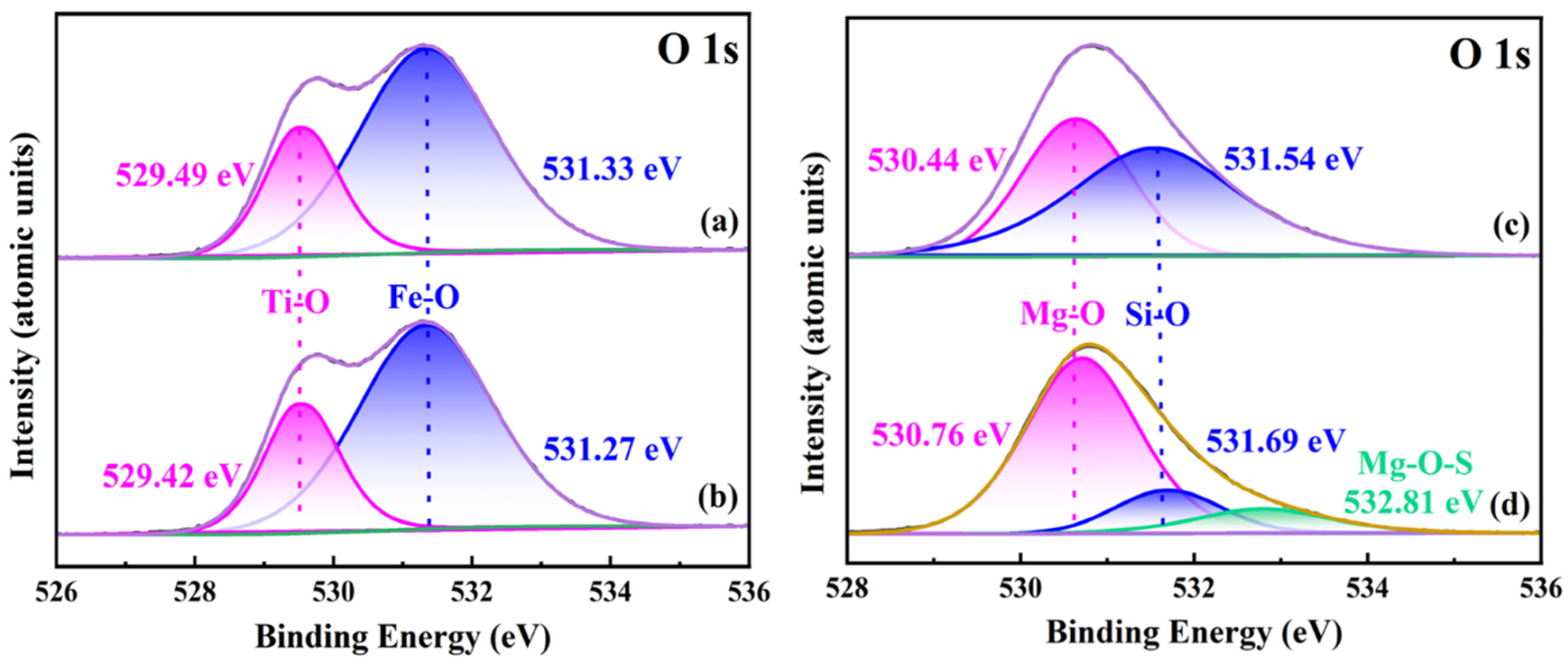
3.3.2. Changes in Mineral-Surface Functional Groups before and after DSS Treatment
3.3.3. Surface Morphology Analysis
3.3.4. Changes in the Binding Energy of Minerals with/without DSS Treatment
3.3.5. Dynamic Adsorption Behavior of DSS on the Ilmenite/Forsterite Surface
3.3.6. Adsorption Configuration of DSS on Ilmenite and Forsterite Surfaces
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Desheng, C.; Longsheng, Z.; Tao, Q.; Guoping, H. Desilication from titanium–vanadium slag by alkaline leaching. Trans. Nonferrous Met. Soc. China 2013, 23, 3076–3082. [Google Scholar] [CrossRef]
- Samal, S.; Mohapatra, B.K.; Mukherjee, P.S.; Chatterjee, S.K. Integrated XRD, EPMA and XRF study of ilmenite and titania slag used in pigment production. J. Alloys Compd. 2009, 474, 484–489. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, J.; Chang, X.; Guo, S. Effect of microwave irradiation on selective heating behavior and magnetic separation characteristics of Panzhihua ilmenite. Appl. Surf. Sci. 2014, 300, 171–177. [Google Scholar] [CrossRef]
- Deng, R.; Yang, X.; Hu, Y.; Ku, J. Effect of Fe(II) as assistant depressant on flotation separation of scheelite from calcite. Miner. Eng. 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, C.; Wang, T.; Deng, J.; Cao, Y.; Chang, L.; Zhou, G.; Wu, Y.; Li, P. New insight into surface adsorption thermodynamic, kinetic properties and adsorption mechanisms of sodium oleate on ilmenite and titanaugite. Adv. Powder Technol. 2020, 31, 3628–3639. [Google Scholar] [CrossRef]
- Fan, X.; Rowson, N.A. The effect of Pb(NO3)2 on the ilmenite flotation. Miner. Eng. 2000, 13, 205–215. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Yu, L.; Xu, Y.; Du, Y. Study on the activation mechanism of lead ions in the flotation of ilmenite using benzyl hydroxamic acid as collector. J. Ind. Eng. Chem. 2018, 62, 209–216. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, C.; Wang, H.; Xu, L.; Li, P.; Li, J.; Li, G.; Peng, W.; Zhang, F.; Fan, G.; et al. A novel sodium trans-2-nonene hydroxamate for the flotation separation of ilmenite and forsterite: Superior collecting and selectivity. Sep. Purif. Technol. 2024, 333, 125830. [Google Scholar] [CrossRef]
- Liu, X.; Huang, G.Y.; Li, C.X.; Cheng, R.J. Depressive effect of oxalic acid on titanaugite during ilmenite flotation. Miner. Eng. 2015, 79, 62–67. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, Q.Y.; Yuan, Z.T.; Zhang, Y.; Li, L. Effect of sodium silicate on the magnetic separation of ilmenite from titanaugite by magnetite selective coating. Powder Technol. 2019, 344, 233–241. [Google Scholar] [CrossRef]
- Yang, Y.H.; Xu, L.H.; Tian, J.; Liu, Y.C.; Han, Y.X. Selective flotation of ilmenite from olivine using the acidified water glass as depressant. Int. J. Miner. Process. 2016, 157, 73–79. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, Z.; Cao, J. New depression mechanism of polymeric depressant on titanaugite in ilmenite flotation. Sep. Purif. Technol. 2021, 264, 118468. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Y.; Liu, C.; Huang, L.; Huang, Z.; Li, H. The anionic flotation of fluorite from barite using gelatinized starch as the depressant. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124794. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L. Measurement of froth zone and collection zone recoveries with various starch depressants in anionic flotation of hematite and quartz. Miner. Eng. 2019, 138, 31–42. [Google Scholar] [CrossRef]
- Ludovici, F.; Hartmann, R.; Rudolph, M.; Liimatainen, H. Thiol-Silylated Cellulose Nanocrystals as Selective Biodepressants in Froth Flotation. ACS Sustain. Chem. Eng. 2023, 11, 16176–16184. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, H.; Wu, Q.; Zheng, Y.; Cui, Y.; Yan, W.; Deng, J.; Peng, T. Comparative study on adsorption and depressant effects of carboxymethyl cellulose and sodium silicate in flotation. J. Mol. Liq. 2018, 268, 140–148. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, X.; Meng, Q.; Zhang, Y.; Xu, Y.; Li, L. Adsorption mode of sodium citrate for achieving effective flotation separation of ilmenite from titanaugite. Miner. Eng. 2021, 171, 107086. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Yu, L.; Xu, Y.; Du, Y.; Zhang, C. Selective depression of titanaugite in the ilmenite flotation with carboxymethyl starch. Appl. Surf. Sci. 2018, 440, 955–962. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, R.; Wang, L. Adsorption of polystyrenesulfonate on titanaugite surface: Experiments and quantum chemical calculations. J. Mol. Liq. 2020, 319, 114167. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, Z.; Sun, H.; Yin, W.; Hong, J.; Cao, S.; Tang, Y.; Zhao, C.; Yao, J. Improving flotation separation of apatite from dolomite using PAMS as a novel eco-friendly depressant. Miner. Eng. 2020, 156, 106492. [Google Scholar] [CrossRef]
- Cao, Z.; Cao, Y.; Qu, Q.; Zhang, J.; Mu, Y. Separation of bastnäsite from fluorite using ethylenediamine tetraacetic acid as depressant. Miner. Eng. 2019, 134, 134–141. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, Z.; Meng, Q.; Zhao, X.; Du, Y. Study on the flotation behavior and interaction mechanism of ilmenite with mixed BHA/NaOL collector. Miner. Eng. 2021, 170, 107034. [Google Scholar] [CrossRef]
- Du, Y.; Meng, Q.; Yuan, Z.; Xu, Y.; Li, L. New cognitions into three new classified titanaugite in mineralogical characteristics and fundamental performances. Miner. Eng. 2021, 169, 106962. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M.; Rezai, B. Effect of crystal chemistry and surface properties on ilmenite flotation behavior. Int. J. Miner. Process. 2015, 137, 71–81. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Li, L.; Lu, J.; Yang, J. Modification mechanism of lead ions and its response to wolframite flotation using salicylhydroxamic acid. Powder Technol. 2020, 366, 477–487. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, X.; Meng, Q.; Zhang, Y. Investigation of selective adsorption of sodium oleate during separation of ilmenite from titanaugite via surface magnetization. Miner. Eng. 2021, 171, 107128. [Google Scholar] [CrossRef]
- Zhang, C.; Li, P.; Cao, Y.; Hao, H. Synthesis of sodium oleate hydroxamate and its application as a novel flotation collector on the ilmenite-forsterite separation. Sep. Purif. Technol. 2022, 284, 120283. [Google Scholar] [CrossRef]
- Tazikeh, S.; Kondori, J.; Zendehboudi, S.; Amin, J.S. Molecular dynamics simulation to investigate the effect of polythiophene-coated Fe3O4 nanoparticles on asphaltene precipitation. Chem. Eng. Sci. 2021, 237, 116417. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Dong, F.; Gao, Z. Anisotropic adsorption of oleate on diaspore and kaolinite crystals: Implications for their flotation separation. Appl. Surf. Sci. 2014, 321, 331–338. [Google Scholar] [CrossRef]
- Hao, H.; Li, L.; Yuan, Z.; Liu, J. Molecular arrangement of starch, Ca2+ and oleate ions in the siderite-hematite-quartz flotation system. J. Mol. Liq. 2018, 254, 349–356. [Google Scholar] [CrossRef]
- Hao, Z.; Jiankun, W.; Wenjing, L.; Fengyan, L. Microwave-assisted synthesis, characterization, and textile sizing property of carboxymethyl corn starch. Fibers Polym. 2015, 16, 2308–2317. [Google Scholar] [CrossRef]
- Saboktakin, M.R.; Tabatabaie, R.M.; Maharramov, A.; Ramazanov, M.A. Synthesis and characterization of new electrorheological fluids by carboxymethyl starch nanocomposites. Carbohydr. Polym. 2010, 79, 1113–1116. [Google Scholar] [CrossRef]
- Yuan, Z.; Du, Y.; Meng, Q.; Zhang, C.; Xu, Y.; Zhao, X. Adsorption differences of carboxymethyl cellulose depressant on ilmenite and titanaugite. Miner. Eng. 2021, 166, 106887. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q. The flotation separation of scheelite from calcite and fluorite using dextran sulfate sodium as depressant. Int. J. Ofmineral Process. 2017, 169, 53–59. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Wang, W.; Deng, J.; Chen, B.; Yan, W.; Xiong, S.; Huang, Y.; Liu, J. Flotation behaviors of ilmenite, titanaugite, and forsterite using sodium oleate as the collector. Miner. Eng. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Rhein, F.; Zhai, O.; Schmid, E.; Nirschl, H. Multidimensional Separation by Magnetic Seeded Filtration: Experimental Studies. Powders 2023, 2, 588–606. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Hailing, Z.; Wenhao, J. New insights into the carboxymethyl cellulose adsorption on scheelite and calcite: Adsorption mechanism, AFM imaging and adsorption model. Appl. Surf. Sci. 2019, 463, 105–114. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Laskowski, J.S. The adsorption of polysaccharides onto mineral surfaces: An acid/base interaction. Int. J. Miner. Process. 2000, 60, 229–245. [Google Scholar] [CrossRef]
- Daou, T.J.; Begin, S.C.; Greneche, J.M.; Thomas, F.; Derory, A.; Bernhardt, P.; Legaré, P.; Pourroy, G. Phosphate adsoption properties of magnetite-base nanoparticles. Chem. Mater. 2007, 19, 4494–4505. [Google Scholar] [CrossRef]
- Ouerd, A.; Dumont, C.A.; Berthome, G.; Normand, B. Reactivity of titanium in physiological medium: I. Electrochemical characterization of the metal/protein interface. J. Electrochem. Soc. 2007, 154, 593–601. [Google Scholar] [CrossRef]
- Sugiyama, S.; Miyamoto, T.; Hayashi, H.; Moffat, J.B. Oxidative coupling of methane on mgo-mgso4 catalysts in the presence and absence of carbon tetrachloride. Bull. Chem. Soc. Jpn. 1996, 69, 235–240. [Google Scholar] [CrossRef]
- Haycock, D.E.; Kasrai, M.; Nicholls, C.J.; Urch, D.S. ChemInform abstract: The electronic structure of magnesium hydroxide (brucite) using X-ray emission, X-ray photoelectron, and auger spectroscopy. Chem. Informationsdienst 1979, 10, 1791–1796. [Google Scholar] [CrossRef]
- Seyama, H.; Soma, M. Bonding-state characterization of the constituent elements of silicate minrals by X-ray photoelectron spectroscopy. Chem. Informationsdienst 1985, 16, 2199–2924. [Google Scholar]
- Seyama, H.; Soma, M. X-ray Photoelectron Spectroscopic Study of Montmorillonite Containing Exchangeable Divalent Cations. J. Am. Chem. Soc. 1984, 80, 237–248. [Google Scholar] [CrossRef]
- Wu, B.; Raghavan, S. Removal of BTA Adsorbed on Cu: A Feasibility Study Using the Quartz Crystal Microbalance with Dissipation (QCMD) Technique. ECS J. Solid State Sci. Technol. 2019, 8, P3114–P3117. [Google Scholar] [CrossRef]
- Gao, F.; Dong, L.; Xue, Z.; Cai, S.; Fan, M.; Hao, B.; Fan, P.; Bao, W.; Wang, J. Dodecylamine enhanced coal gasification fine slag flotation and its molecular dynamics simulation. Miner. Eng. 2023, 203, 108322. [Google Scholar] [CrossRef]
- Tang, Y.; Kelebek, S.; Yin, W. Surface chemistry of magnesite and calcite flotation and molecular dynamics simulation of their cetyl phosphate adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125246. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Zhang, H.; Lyu, W.; Zhu, Y.; Ma, Y.; Li, F. The role of gellan gum in the selective flotation separation of fluorite from calcite: An experimental and molecular dynamics simulation study. Powder Technol. 2024, 432, 119156. [Google Scholar] [CrossRef]
- Huang, H.; Gao, W.; Li, X. Effect of SO42− and PO43− on flotation and surface adsorption of dolomite: Experimental and molecular dynamics simulation studies. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132699. [Google Scholar] [CrossRef]



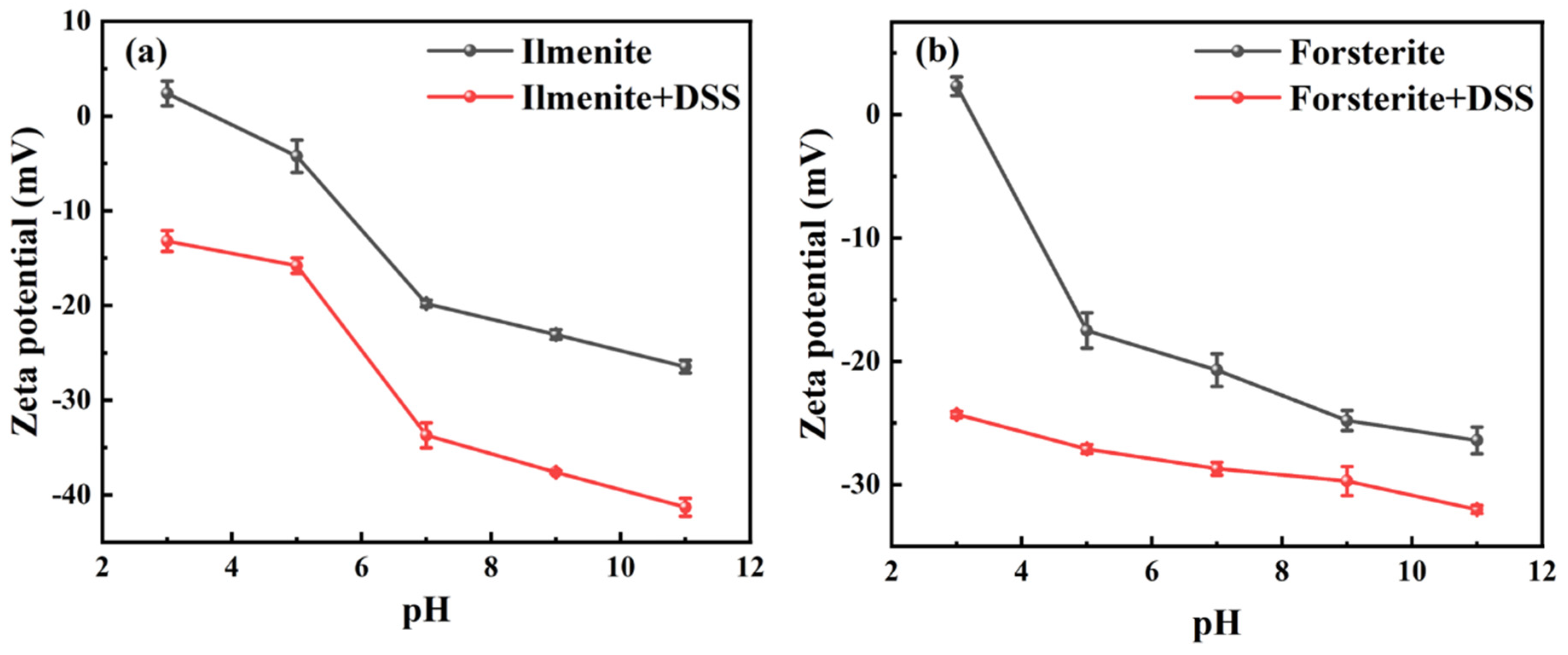






| Sample | ξ2 (mV) | ξ1 (mV) | ∆|ξ| (mV) | τs (mol/L) | ∆G0ads (kJ) |
|---|---|---|---|---|---|
| Ilmenite | −15.80 | −4.24 | 11.56 | K × exp(0.01156) | −1.12 |
| Forsterite | −27.10 | −17.50 | 9.60 | K × exp(0.00960) | −0.93 |
| Samples | Relative Atomic Content of the Elements (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Fe | Si | Ti | Mg | Ca | Al | |
| Ilmenite | 19.09 | 57.42 | 9.74 | 5.35 | 8.40 | — | — | — |
| Ilmenite + DSS | 19.55 | 56.36 | 10.63 | 7.17 | 6.29 | — | — | — |
| Forsterite | 13.52 | 48.04 | 1.83 | 12.74 | — | 14.53 | 6.20 | 3.14 |
| Forsterite + DSS | 17.02 | 46.18 | 1.88 | 13.56 | — | 12.26 | 5.95 | 3.15 |
| Sample | D (×10−6) | F (Hz) | Thickness (nm) | Mass (ng/cm2) |
|---|---|---|---|---|
| Ilmenite | 3.66 | −21.51 | 25.78 | 2578.38 |
| Forsterite | 16.48 | −45.68 | 82.05 | 8204.62 |
| Sample | Interaction Energy (kJ/mol) |
|---|---|
| Ilmenite | −497.17 |
| Forsterite | −805.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, G.; Zhang, H.; Tian, F.; Wang, H.; Xu, L.; Cao, Y.; Xu, H.; Zhang, F.; He, J.; Li, G. The Application of Dextran Sodium Sulfate to the Efficient Separation of Ilmenite and Forsterite, as a Flotation Depressant. Processes 2024, 12, 134. https://doi.org/10.3390/pr12010134
Fan G, Zhang H, Tian F, Wang H, Xu L, Cao Y, Xu H, Zhang F, He J, Li G. The Application of Dextran Sodium Sulfate to the Efficient Separation of Ilmenite and Forsterite, as a Flotation Depressant. Processes. 2024; 12(1):134. https://doi.org/10.3390/pr12010134
Chicago/Turabian StyleFan, Guixia, Huaiyao Zhang, Fuqiang Tian, Hongbin Wang, Longhua Xu, Yijun Cao, Hongxiang Xu, Fanfan Zhang, Jianyong He, and Guosheng Li. 2024. "The Application of Dextran Sodium Sulfate to the Efficient Separation of Ilmenite and Forsterite, as a Flotation Depressant" Processes 12, no. 1: 134. https://doi.org/10.3390/pr12010134
APA StyleFan, G., Zhang, H., Tian, F., Wang, H., Xu, L., Cao, Y., Xu, H., Zhang, F., He, J., & Li, G. (2024). The Application of Dextran Sodium Sulfate to the Efficient Separation of Ilmenite and Forsterite, as a Flotation Depressant. Processes, 12(1), 134. https://doi.org/10.3390/pr12010134











