Abstract
Melanoidins, as macromolecular heterogeneous organic polymers, are produced from the Maillard reaction between amino and carbonyl groups during the thermal hydrolysis pretreatment (THP) of sludge. The brown color and recalcitrance of melanoidins pose a serious threat to wastewater treatment systems, such as invalidating UV disinfection and decreasing the efficiency of anaerobic digestion; thus, they have gradually received much concern in recent years. However, currently the study on THP-origin melanoidins is limited by a lack of reliable extraction and quantification methods. This paper presents a comprehensive review of the physical, chemical, and biological properties of melanoidins from different sources to fill the research gap on THP-origin melanoidins. The adverse effects of melanoidins on the management of wastewater and sludge are discussed, and for the first time, special attention is paid to the potential environmental hazards of THP-origin melanoidins to natural ecosystems. The removal technologies of melanoidins are summarized and compared as well. Finally, the suggested areas that future studies should focus on are provided. This review is dedicated to providing guidance on melanoidin research and management for the better development of the THP industry.
1. Introduction
Environmental costs rapidly increase hand-in-hand with industrialization and urbanization. Following the worldwide crisis of organic solid waste (OSW) generation, in recent years, it has attracted attention on the continuous uptrend of energy consumption for OSW management [1,2]. The sewage sludge produced in wastewater treatment plants (WWTPs) is one of the most concerning OSWs due to its huge quantity, serious pollution, difficulty in disposal, as well as great potential for resource recovery [3,4,5]. The cost of sludge disposal is up to half of the total running costs in WWTPs [6,7]. Anaerobic digestion (AD) is a widespread effective technology for sludge management, with low energy requirements, high volume reduction, and renewable energy generation [8,9,10]. However, as hydrolysis is the rate-limiting step of AD, thermal hydrolysis pretreatment (THP) has come into being to strengthen the disintegration and solubilization of particulate organics under high temperatures and pressure by breaking microbial cells and disrupting the floc structure of sludge [5,11]. In addition to improving the biogas production rate of the AD process, THP also has some other remarkable advantages, such as enhancing sludge dewaterability, providing pathogen-free biosolids, eliminating scum and the foaming of sludge, and so on [8,12]. Therefore, THP combined with AD has been commercialized and applied globally in full scale [13,14]. As an example, one of the most mature commercial THP technologies is Cambi® (Cambi ASA, Asker, Norway), operated at 165–180 °C and 6–8 bar for 30–60 min [15,16,17], which up to now has served as sludge disposal in 88 full-scale facilities in 27 countries (data collected from www.cambi.com (accessed on 1 January 2024)).
However, along with the benefits, the drawbacks induced by THP should not be ignored, namely the formation of refractory by-products [18]. Most of these refractory substances belong to Maillard reaction products (MRPs) called melanoidins [19,20]. The Maillard reaction (MR) is a non-enzymatic browning reaction, which occurs between the carbonyl of reducing sugar and the amino group of the protein, amino acid, or peptide under heating conditions, including a series of sequential and parallel reaction pathways and depending strongly on the reaction conditions [21,22]. Melanoidins, as late-stage MRPs, are dark-colored macromolecular heterogeneous polymers, which mainly involve the structures of heterocyclic amines, furans, aldehydes, ketones, etc. [23,24]. Traditionally, the focus of melanoidins has been mainly on the food field, and melanoidins are considered to be present in many foods, like coffee, roasted malt, vinegar, bread, and beverages (such as sweet wine and dark beer), and have obvious effects on the texture, flavor, storage, and nutrition of food [25,26]. In the environmental field, the typical conditions of sludge THP overlap with those of MRs, namely, sludge providing sufficient reactants (polysaccharide and protein) and high temperatures that provide reaction occasions, thus inevitably resulting in the formation of melanoidins [18]. With the characteristics of a dark color, strong ultraviolet (UV)-quenching ability, and extremely poor biodegradability, the adverse effect of melanoidins on wastewater treatment and sludge disposal has become a difficult issue to be solved, and the existence of melanoidins usually leads to non-compliance with the environmental standards for discharged wastewater [18,22]. Moreover, THP-origin melanoidins with dark colors and high organic nitrogen have strong potential to contaminate natural water and soil [27,28].
As shown in Figure 1, in the last two decades, the studies on melanoidins have mainly been divided into three aspects: (1) melanoidins from model MR systems (blue pattern), (2) melanoidins in food (red pattern), and (3) melanoidins associated with wastewater (yellow pattern). An in-depth literature investigation revealed that melanoidins are coming into ever-sharper focus in the environmental field, for example, in food-engineering wastewater (particularly characteristics of melanoidins, the dose effect, and the application of effective decolorization treatments). However, the research on THP-origin melanoidins in sludge is still in the early stages; the published research and review articles have mainly focused on their formation, characterization, or effects on subsequent AD process [29,30]. However, to date, not only there is a lack of systematic knowledge on the targeted qualitative and quantitative analyses, but it also appears that fully comprehending the properties of melanoidins to solve their effects and regulation is largely overlooked by the relevant literature.
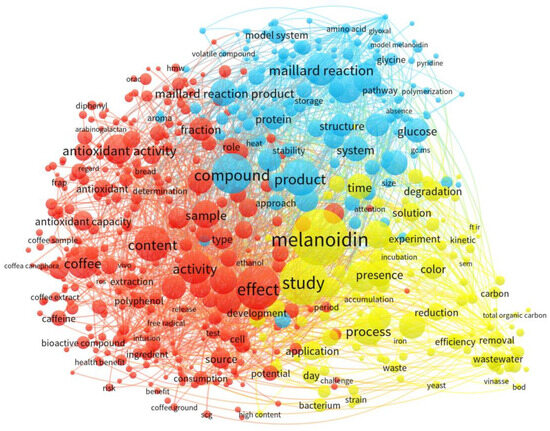
Figure 1.
Bibliometric map of studies on melanoidins as visualized by keyword network from 1122 articles published from 2000 to 2023, retrieved from Web of Science database.
Therefore, the main objective of this article was to comprehensively summarize the properties of melanoidins formed during the THP of sludge and emphasize their environmental hazards and removal technologies. This review can provide guidance on melanoidin management and improve the development of the THP industry. It is worthwhile to note that due to the limited research on melanoidins in the field of sludge, many references in this review come from the THP of other OSWs, food processing wastewater, and even the food industry to pave the way for future research on THP-origin melanoidins in sludge.
2. Foundation of the Research on THP-Origin Melanoidins in Sludge
2.1. Methods for Melanoidin Extraction
Developing extraction methods for melanoidins is critical to the study of precise characterization. Through extraction, the adverse impacts of melanoidins on the environmental field might also be mitigated [11]. As for food-origin melanoidins, the extraction methods are divided into physical methods (such as dialysis, ultrafiltration, gel filtration chromatography, and macroporous adsorption resin) [31] and chemical methods (such as organic solvent extraction and acid precipitation) [26]. During physical extraction, especially membrane separation (such as dialysis and ultrafiltration), establishing a universal method that applies to all kinds of melanoidins is the main obstacle. The molecular weight (MW) of melanoidins serves as the basis of purification, distinguishing them from most of the other dissolved matters in the substrate [32,33]. However, the MWs of melanoidins are variable with the differences in reactants [18]. To date, the frequently used membrane cut-off is 5 kDa or 10 kDa according to the type of matrix. For example, the MW cut-off is set as 5 kDa for extracting the melanoidins from cocoa beans and dark beer, whereas it is 10 kDa for extracting melanoidins from coffee and vinegar [34,35]. It is worth conducting pre-experiment analysis (e.g., size exclusion chromatography) to determine the MW contribution of the matrix, so as to provide a reference for melanoidin extraction [33,36].
Among the chemical methods, organic solvent extraction is the most adopted [31]. For example, an isopropanol extraction method was developed for melanoidins from sugarcane molasses [37], while ethyl acetate extraction was reported to be effective for melanoidins from distillery wastewater [38,39]. However, the main drawbacks of organic solvent extraction include the low recovery rate, low selectivity, large amount of solvent consumption, high overall cost, and potential toxicity [40,41]. Moreover, it is hard to maintain the structural integrity of melanoidins after extraction by organic solvent, which becomes a limitation of the applicability of this method [26]. To improve the efficiency of melanoidin extraction by organic solvents, the selection of the solvent is an important factor which mainly depends on the chemical structure of melanoidins, especially the polarity [42].
That is why macroporous resin adsorption has gradually gained acceptance as a powerful method for melanoidin extraction, since it is capable of yielding a relatively complete melanoidin profile [43,44]. For instance, Zhang et al. [26] compared acetone precipitation and macroporous resin adsorption in melanoidin extraction from dark beer, and concluded that resin adsorption was more effective at maintaining the accurate structure and profitable antioxidant activity of the extracted melanoidins. Generally, macroporous resin adsorption is a sustainable and eco-friendly method along with resin regeneration and reuse [45]. Our group, for the first time, established the protocol of a macroporous resin method to extract melanoidins from thermal hydrolyzed sludge (THS) and determined the optimal operating conditions [19,46]. This laid the foundation for further study on THP-origin melanoidins.
It is noteworthy that new developments gradually appear in melanoidin extraction with some auxiliary techniques, and multi-step purification has been established with higher efficiency [31]. For example, high hydrostatic pressure was successfully adopted to release melanoidins from black garlic to assist extraction [47]. Nevertheless, there is no consensus on which extraction method works best, since this should be determined according to the specific melanoidins. Unfortunately, at present, there have been no articles on the comparison of melanoidin extraction methods targeting the differences in their structures and properties, and thus, related investigation is needed to fill this gap.
On the other hand, in recent years, water-insoluble melanoidins have attracted much attention [26,48]. To solve the insolubility problem, some pretreatments can be adopted [49]. For instance, Rodriguez et al. [50] and Celik et al. [51] used enzymatic hydrolysis to release the insoluble protein structures in melanoidins from dairy products, coffee, and bread crusts. Alves and Perrone [52] and Oracz et al. [53] obtained water-insoluble melanoidins from bread and cocoa beans by acid and alkali hydrolysis, respectively, peeling off the bound phenolic compounds in melanoidins to make them soluble. However, much is still unknown about the existence of water-insoluble melanoidins in THS, thus failing to distinguish the similarities and differences between soluble and insoluble melanoidins, and this can be a future research direction.
2.2. Methods for Melanoidin Quantification
Quantification is as necessary and fundamental as extraction in the study of melanoidins. The content of melanoidins is closely associated with their effects on the environment and ecology [54,55], thus serving as a basis to evaluate their environmental risks. Due to the hazard rating of THP-origin melanoidins still being uncertain, accurate quantification of melanoidins is of vital importance for regulating their in situ formation and THP conditions [56]. To date, the major hinderance to developing a straightforward way to calculate the content of melanoidins is that the composition and structure are both variable without a fixed form [18]. Most previous researchers were limited by using some indirect indicators to represent the melanoidin content in solution, such as COD, color, or browning index [57,58]; however, due to being short of targeted representativeness, the above indicators are not convincing enough. For example, some melanoidins are dark brown and others are light yellow at the same concentration, so using 475 nm to represent brown is not a reliable method [32].
Indeed, there are some relative quantification methods for melanoidins [42]. One of the common approaches is excitation–emission matrix fluorescence (EEM) semi-quantification [59,60], employing fluorescence regional integration and parallel factor analysis to indicate the relative content of melanoidins in all dissolved organic matters [60]. Hyphenated techniques with steric exclusion chromatography, photo diode array detectors, or fluorescence systems have also been adopted in melanoidin semi-quantification, which are usually used to measure the main characteristic indices of refractory dissolved organic matters for each chromatograph peak [42], but these hyphenated methods are expensive and cannot cover all the components in melanoidins [18].
Even if the relative quantification methods seem to be mature, knowing the absolute concentration of melanoidins is necessary but unsettled. One of the most common methods is gravimetric estimation, in which the melanoidin content can be expressed as the weight of the melanoidins extracted after freeze-drying [31,61]. However, the disadvantage of this method is that the lyophilized product perhaps contains other substances in the matrix besides melanoidins. Further, earlier research by Martins and van Boekel [62] absolutely quantified melanoidins through measuring the concentration of 14C-labeled sugars incorporated into melanoidins; however, this method has not yet been widely recognized. Comparatively, colorimetric quantification with a model MR system as the standard is a promising method to obtain the absolute concentration of melanoidins, due to its rapid and simple operation and high reproducibility. Meanwhile, it is little affected by the complex composition of melanoidins [63,64]. For example, the melanoidins in THS [46] and distilled spent grain [65] have been absolutely quantified by colorimetry as 445.78 mg/L and 268.60 mg/g, respectively. Kaspchak et al. [66], Yang et al. [67], and Yang et al. [68] also used colorimetry to determine the molar concentration of melanoidins in several types of environmental samples. However, refinement of colorimetric quantification is needed, specifically the selection of standard model melanoidins, the determination of proper wavelengths, and the validation of the methodology [42]. Therefore, to our knowledge, there is still room for the development and application of melanoidin quantification methods, especially in complex matrices.
3. Properties of Melanoidins
3.1. Physical Properties
In the food field, melanoidins have gained significant attention due to their close association with the color, aroma, flavor, taste, and viscosity of various solid and liquid foods such as coffee, beer, cocoa, honey, bakery products, and malt [21]. The dark brown color is the most typical physical property of melanoidins [69]. Carrying chromophore groups in high MW (HMW) final MRPs is the main reason for the color appearance [70,71]. The color of melanoidins has become a concern in wastewater treatment which can seriously interfere with UV disinfection systems and ultimately result in the darkening of the effluent [20]. On the other hand, the color of melanoidins provides a basis for their approximate quantification (usually at 420 or 475 nm) in complex matrices such as distillery and molasses wastewater [72].
Another major physical property of melanoidins is the surface property. Extensive studies have used microscopic imaging to visualize the surface structure of melanoidins from different sources. For example, glucose/L-asparagine model melanoidins were observed through Transmission Electron Microscopy (TEM), and results showed that the number and size of the amorphous aggregates increased over time, reaching lengths of several micrometers [73]. Scanning electron microscopy (SEM) was employed to illustrate the irregular polygonal block and granular morphological structures of ginseng melanoidins [74]. Additionally, Atomic Force Microscopy (AFM) was applied for black garlic melanoidins for surface feature analysis [47]. In general, the surface morphology of melanoidins is irregular and rough. This rough surface of melanoidins can induce molecular aggregation and then reduce the liquid viscosity [47], which may affect the treatment efficiency of melanoidin-containing wastewater. In addition to roughness, melanoidins also have an amphiphilic nature and chargeability on the surface, which are attributed to the presence of hydrophilic–hydrophobic components and the negative charge carried, respectively [46,75]. The amphiphilic properties of melanoidins make them available as a good emulsifier [76]. For example, Feng et al. [75] used confocal laser scanning microscopy (CLSM) to indicate the stabilized emulsions of 0.25 wt% coffee melanoidins; it was also observed that the foaming of coffee melanoidins was related to their amphiphilic property [77]. Additionally, the negative charge of melanoidins is also a unique property, which is the foundation of their adsorption behavior. For example, coffee melanoidins were determined to expose negative charges through Anion Exchange Chromatography elution, and the charge distribution was found to be heterogeneous with a polyanionic feature [46].
As for THS melanoidins, Wang et al. [78] observed the surface features through Atomic Force Microscopy and compared them to glucose–glycine model melanoidins, and it was found that THS melanoidins showed larger agglomerates on the surface than model melanoidins [78]. This means that THS melanoidins may hold more sludge components on the surface gaps, probably affecting the flocculation of sludge. However, to date, little is known about hydrophobicity and chargeability of THS melanoidins, and the effect of the surface properties of melanoidins on sludge rheology and dewaterability needs to be well explored as well.
3.2. Chemical Properties
Melanoidins are formed through the cyclization, dehydration, retro aldolization, rearrangement, isomerization, and condensation of MR intermediates [79]. Due to the complexity of the products, the chemical structure of melanoidins still remains relatively unknown [77]. Melanoidins’ chemical properties are determined by their chemical structure and exhibit distinct characteristics in various fields. The discussion here mainly focuses on metal chelation ability, antioxidant activity, and chemical stability, as these chemical properties are closely correlated with melanoidin management in the environmental field.
The anionic hydrophilic nature of melanoidins allows them to form stable complexes with metal cations [21]. The ketone and hydroxyl groups of pyranone or pyridone residues act as the main donor in melanoidins chelating with metals [80]. Melanoidins have different affinities for different types of metals. For example, lactose–glycine model melanoidins mainly had the ability to chelate Cu2+, Fe2+, and Zn2+ [81], whereas molasses melanoidins could form complexes with more kinds of metals, including Pb2+, Zn2+, Ni2+, Cu2+, Fe2+, Cd2+, and Co2+ [82]. Added to that, there are differences in the chelating properties of melanoidins from different sources. For example, the melanoidins from traditional balsamic vinegar showed higher Fe2+-chelating capacity but lower heme-binding ability compared with the melanoidins from barley coffee and dark beer [34]. According to Morales [83], the composition of carbohydrates was considered a crucial factor in melanoidins–metal chelation, with glucose demonstrating higher efficiency than lactose. In addition, melanoidins can form large complex molecules with various heavy metals in an acidic medium, resulting in precipitation [80], so they have the potential to be developed into repair agents to solve metal contamination in soil [84]. Finally, it should be emphasized that the chelating properties of melanoidins will also affect their antioxidant and antibacterial activities [34,35].
According to existing research, the antioxidant activity of melanoidins is attributed to multiple mechanisms, such as trapping positively charged electrophilic species, radical scavenging, metal chelation, or the termination of radical chain reactions [25]. The reducing activity is ascribed to certain structures with the ability to offer electrons, for instance, the hydroxyl group, pyrrole and other N-heterocyclic aromatic structures, and exposed amino acid groups (e.g., tryptophan, tyrosine, and methionine), while the free radical scavenging capacity is usually due to the aromatic and phenolic structures [77]. The antioxidation behavior of melanoidins can be measured through different methods, such as being indicated by ferric ion reducing antioxidant power (FRAP), 2,20-azino-bis-(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), free radical 2,2-diphenyl-1-picrylhydrazy (DPPH), and hydroxyl or superoxide anion radical scavenging activity [26,33]. Although the antioxidant activity of food melanoidins is regarded as a profitable property to help reduce the risk of disease [85], for THS melanoidins, antioxidation will perhaps make a negative impact, since melanoidins as antioxidants may be involved in hydrogen transfer and/or electron donations within the sludge, then interfere with biological treatment processes, which deserves more attention and in-depth research.
The chemical stabilities of melanoidins are reflected in many aspects, such as being exposed to heating, strong oxidizing or reducing conditions, and UV irradiation [47]. The thermal stability of melanoidins is typically evaluated by observing the variation in mass with increasing temperatures using synchronous thermal analysis technology [26,47]. The oxidizing/reducing and UV stabilities can be evaluated by exposing melanoidins to H2O2/Na2SO3 solutions and UV light, respectively, to calculate the degradation ratio of melanoidins [47]. It was showed that black garlic melanoidins were unstable under strong oxidizing conditions, but could maintain a relatively stable state under strong reducing conditions, and similar results were observed in beer melanoidins [26,47]. Generally, the chemical stabilities of melanoidins are influenced by several conditions, such as the pressure and heating temperature of the MR, the metal ions incorporated into the melanoidins, and the aggregation degree of melanoidin aggregates [86,87]. Due to their high chemical stabilities, melanoidins may accumulate in the environment and have a persistent impact on the natural ecosystem.
3.3. Biological Properties
The biological properties of melanoidins mainly include anti-inflammatory and anti-microbial capacities, anti-cancer abilities, anti-photoaging abilities, phytotoxicity, cytotoxicity, genotoxicity and so on [21,69,88], among which some functions deserve special concern in the environmental field [89]. The antibacterial property has been extensively reported for different melanoidins [90,91,92,93]. The melanoidins from coffee, beer, and dessert wine all have significantly stronger antibacterial activity over Gram-positive bacteria than Gram-negative bacteria who have a lipopolysaccharide component in their outer membrane [35,93,94]. The antimicrobial activity can be mediated by the interaction of melanoidins chelating with Mg2+ in the cell membrane via membrane-damage mechanisms [35,91]. In the environmental field, the melanoidins from molasses distillery wastewater were reported to be the main contributor of antimicrobial components in wastewater, compared with hexose, polyphenol, and caramel [95]. Moreover, the HMW melanoidins in the >100 kDa fraction of molasses distillery wastewater showed higher antioxidant and antimicrobial characteristics than the low MW (LMW) melanoidins [72]. In terms of THS melanoidins, their adverse effects on methane production during AD [29,94] and side-stream nitrogen removal [12] indicate, from the side, that melanoidins should have potential in antimicrobial function, but thorough direct research is still lacking.
Recently, the phytotoxicity induced by wastewater-origin melanoidins has been experimentally confirmed. Seed germination and plant growth bioassays showed that the root and shoot lengths decreased significantly when the seeds of Allium cepa and Cicer arietinum were treated with increasing concentrations of melanoidin-containing distillery effluent, implying the concentration dependence of melanoidin phytotoxicity [96,97]. In an earlier study, Yuan et al. [98] tested the fertilizer potential of the liquid product from the hydrothermal treatment of swine manure that contained melanoidins, and results showed that the higher temperature led to more dangerous behavior in seed germination. This circumstantial evidence suggests that targeted study on phytotoxicity induced by THS melanoidins is worthwhile.
At the cellular and genetic levels, there has been little agreement on whether melanoidins are capable of cytotoxicity and genotoxicity or not. For example, Glosl et al. [99] reported that glucose–glycine model melanoidins exhibited modest but significant genotoxic effects on human lymphocytes; in particular, the LMW fraction showed the most reactive effect on Caco-2 cells. However, Diaz-Morales et al. [88] found that both the raw and digested melanoidins from three different bakery products displayed no cytotoxicity towards Caco-2 and HUVEC cells, which totally differed from the former study. For food-origin melanoidins, it is now considered that higher heating temperatures and longer heating times are more likely to produce melanoidins with stronger toxicities [31]. Recently, Chowdhary et al. [96] clearly showed that the melanoidins from distillery wastewater were genotoxic because they could cause chromosomal variation phenomena in a chromosomal aberration study of Allium cepa. Moreover, from the ultra microstructure of Allium cepa observed using TEM, multi-vacuoles were formed in root tip cells under melanoidin treatment, which represented the defense mechanism to melanoidins [100]. Based on these studies, there is a strong possibility that THP-origin melanoidins may have cytotoxicity and genotoxicity, considering that they are generated under more violent heating conditions than those from food or wastewater, but unfortunately, related research is almost non-existent.
3.4. Summary of Methods to Characterize Melanoidins’ Properties
In order to gain comprehensive knowledge of melanoidins’ properties, the common characterization techniques used are summarized and compared in Table 1.

Table 1.
Methods and techniques for determining the characteristics of melanoidins.
4. Effects of Melanoidins in Practical Applications
4.1. Effects of Melanoidins on Wastewater Treatment and Sludge Disposal
THS melanoidins will be retained in the sludge digestion effluent, due to hardly biodegrading during AD, and returned to the main stream of the WWTP to affect the entire wastewater treatment system [20,109]. Dwyer et al. [20] continuously monitored Oxley Creek water reclamation plant (65 mL/d, Brisbane, Australia) for a year and a half after implementing THP, and found that the plant effluent experienced increased color, decreased UV transmission, and increased dissolved organic nitrogen. Devos et al. [110] also documented that the total nitrogen concentration in the sludge digestion return liquor increased with the increase in THP temperature. Additionally, melanoidins can seriously interfere with UV disinfection systems for wastewater due to their UV-quenching property. Some reported that the melanoidin quenching was mainly attributed to the LMW fraction (<10 kDa) [111], whereas others reported that the cause laid in the HMW fraction (>10 kDa) [20,112]. Although the mechanism of melanoidins’ UV-quenching activity is still controversial, it can be concluded that there is a correlation between UV quenching and MW distribution. As for biological effects, the above-mentioned toxic properties of melanoidins contribute to the inhibition of side-stream nitrogen removal when treating digestion return liquor, as melanoidins inhibit the microbial community and nitrogen-associated metabolic pathway [113].
THS melanoidins can also affect the chemical composition and properties of sludge. The formation of melanoidins needs to consume reducing sugars and hydrolyzed proteins, thus leading to a drop in the contents of carbohydrates and proteins in sludge [114,115]. Instead, the aromatic and cyclic structures and humic and polymeric substances all increase [11,116]. Moreover, it was experimentally demonstrated that melanoidins would deteriorate sludge dewaterability by trapping massive water molecules and carrying negative charges, and that the water holding capacity of a sludge cake with 480 mg melanoidins/g TS increased by 20% compared to that without melanoidins [117]. It was reported that the hydrophobicity of THS was improved by 17% with the THP temperature increasing from 120 to 210 °C, since the protein structure was destroyed at higher THP temperatures and more hydrophobic groups (e.g., peptide bonds) were exposed [118].
Melanoidin-containing THS will face some challenges in the subsequent AD. Many publications have demonstrated the negative impact of THP temperatures above 175 °C on biogas production [119,120]. For example, Abe et al. [121] reported a decrease in biogas of 33% when the THP temperature changed from 170 to 200 °C. These researchers ascribe the inhibition of biogas to melanoidin formation at extremely high temperatures, although there is no direct evidence. Yin et al. [30] also reported that the production of volatile fatty acids (VFAs) during AD was reduced by 12% when affected by the melanoidins produced from thermal pretreated food waste. The inhibitory effects of melanoidins are closely dependent on their dose, since melanoidins at different concentrations present different bacteriostatic or bactericidal results [21]. As speculated in a previous review [68], the presence of melanoidins does not just cause inhibitory effects, while it may have no effect or even promote AD at low doses. However, the mechanism of the influence of melanoidins on AD is unclear, and in particular, whether melanoidins can promote biogas or VFA production needs to be confirmed in depth.
4.2. Potential Hazards and Risks of Melanoidins for Natural Environment
The melanoidins in original THS liquor were measured to be 455.78 mg/L [46], while their concentrations in the digestion liquor or final effluent of WWTPs have not yet been measured or estimated. However, considering the widespread application of THP and the high refractoriness of THP-origin melanoidins, those existing in the treated effluent ought to be easily accumulated in aquatic and soil environments, and may even interact with other environmental factors to aggravate pollutions. As reported, once the melanoidin-containing distillery wastewater was used for long-term land irrigation without proper treatment, it was harmful to crop growth and biological health [122], and the melanoidins from distillery wastewater in high concentrations have strong mutagenic, carcinogenic, and cytotoxic effects on cells [99]. More importantly, the released melanoidins in the natural environment may interfere with the original biogeochemical fate and dynamics of abiotic components in soils, sediments, and waters. For example, melanoidins could be engaged in electronic competition with anaerobic methanogenic bacteria to affect the natural carbon cycle [29]. Therefore, the polluting path and fate of melanoidins, as well as their effects on the environment, shall not be overlooked.
In addition, toxic substances in ecosystems are often persistent and affect all the biota, including humans, through food and water supplies [123]. Taking into account the high stability and increased release of THP melanoidins into the environment, they may potentially interact with organisms through the food chain or be mediated by environmental factors to finally affect human health [124]. According to statistics, the residents in the distillery wastewater contaminated area faced higher probabilities of health problems such as irritation of the eyes, skin allergies, headache, fever, vomiting sensations, stomach pain, etc. [40]. This is not a unique instance; as documented, people residing in the vicinity of a coffee processing plant who consumed this polluted water suffered from similar symptoms, and even breathing problems as well [125]. In addition, melanoidins in the body were also found to accelerate the progression of various diseases, such as cardiovascular complications, diabetes mellitus, and Alzheimer’s disease [40]. This highlights an urgent need to treat melanoidins in a proper way before WWTP effluents enter the outer environment.
5. Methods of Melanoidin Removal
5.1. Physicochemical Removal
There is a high concentration of melanoidins (2–20 g/L) in several types of agro-industrial wastewater, such as distillery, winery, and brewery wastewater, for which the decolorization and degradation of melanoidins during treatment have attracted much attention [57,126]. Several studies have performed physicochemical methods to remove melanoidins, such as adsorption [127], flocculation [128], ozonation [129], coagulation [27], ultrafiltration [130], UV/H2O2 oxidation [131], electrochemical methods [132], and membrane treatments [133]. Among these, adsorption, especially by activated carbon (AC), is widely used to remove color and specific organic contaminants due to its simplicity, effectiveness, and economy [56]. There is plenty of research applying AC derived from different biomasses, such as bagasse, sawdust, wood, and rice husk [134], and variously modified ACs, such as Cu-impregnated [135], amine-modified [28], and H2O2-modified ACs [136], in adsorption experiments on model melanoidins, and generally, satisfactory results have been obtained with a removal rate higher than 80%.
Another melanoidin removal method of concern is the advanced oxidation process (AOP), and hydroxyl radicals are thought to be involved in all AOPs [136]. Cañizares et al. [137] compared three AOP methods, including conductive-diamond electrochemical oxidation (CDEO), Fenton oxidation, and ozonation to treat melanoidin-containing colored wastewater produced in the fermentation process, and found that CDEO and ozonation had better decolorization results than Fenton oxidation. In addition, some studies have combined two physicochemical treatments to complementally intensify melanoidin removal, such as an electrolytic treatment combined with activated carbon adsorption [138], and ultrasound combined with Fenton oxidation [139]. However, there are few studies on the removal and decolorization of THP-origin melanoidins in actual wastewater.
5.2. Biological Removal
In fact, the costs of different methods to remove melanoidins highly depend on the specific operations and actual wastewater situations, and comparatively, physical or/and chemical methods are not suitable for full-scale treatment [140]. Agarwal et al. [141] reviewed the technologies employed globally for melanoidin removal and concluded that an efficient and cost-effective treatment scheme should comprise a physicochemical treatment followed by a biological treatment. A microbial treatment is generally a good choice that is eco-friendly and economically competitive over a single physicochemical approach [142]. To date, quite a few bacteria and fungi have shown an excellent ability to remove melanoidins from wastewater, such as the bacteria Pseudomonas putida, Bacillus licheniformis, Alcaligenes sp., and Lactobacillus plantarum [40,122,140]; white-rot fungi; and yeasts [143,144]. Comparatively, fungi have the capacity to biodegrade recalcitrant pollutants with a higher tolerance to toxins and more robustness [145]; however, the main constriction for removing melanoidins using fungi is the high demand in food supplements and the necessity of dilution [92]. Microbial melanoidin removal is documented to present a dual mechanism: some adsorb the color, whereas some degrade melanoidins biologically; some may use both adsorbing and degrading mechanisms [146]. However, the investigation concerning the removal pathways during melanoidin degradation and the involved mechanisms is far from sufficient. And this happens to be an important aspect for safety considerations, since it was found that the toxicity of molasses wastewater was increased after treatment with Pleurotus sp. [92].
It is worth mentioning that extracellular enzymes play an important role in melanoidin removal by bacteria and fungi, such as manganese peroxidase and lignin peroxidase [37], so enzymatic treatment is regarded as a promising method for melanoidin removal as well. Zhang et al. [147] improved the melanoidins’ decolorization efficiency through the addition of cutinase from Thermobifida alba, which acted on the conjugated structures in melanoidins. In addition, the exploitation of new microbial species is needed, and in particular, the construction of potential mixed microbial consortia should become a priority to improve the practical adaptability to complex melanoidin-containing wastewater [148]. Unfortunately, although there are many studies on the biological treatment of molasses and distillery wastewater [122,141,147], it seems that the removal of THP-origin melanoidins has not yet entered the sight of researchers. Last but not least, practically combining melanoidin removal with other processes installed in WTTPs is of special consideration, so as to ensure the developed methods are both efficient and economically viable.
6. Conclusions and Outlook
This paper presented a systematic summary of the properties of THP-origin melanoidins and highlighted the potential hazards and removal methods. Melanoidins are macromolecular heterogeneous organic polymers, and they are non-enzymatic browning reaction products of MRs during the THP of sludge. They have a significant impact on wastewater treatment, as well as the natural environment and health of organisms, which means that the formulation of related legal standards for melanoidin discharge is urgent in the future.
So far, reliable extraction and quantification methods for melanoidins are still lacking, which poses a huge challenge to the research on THP-origin melanoidins. Therefore, not only the understanding of the structure of melanoidins is inadequate, but also the physicochemical and biological properties exhibited need to be better elucidated. The technologies for THP-origin melanoidin removal are currently less discussed; however, molasses wastewater treatment has proved that physical, chemical, and/or biological methods are able to function in removing melanoidins, providing an opportunity for WWTPs to imitate the practice and develop their own systems to manage melanoidins.
As a final note, based on the sustainable development conception, it is necessary to seek the potential values of THP-origin melanoidins, which will help reduce the environmental risk, while possibly changing unwanted melanoidins into value-added products. As previously reported, the melanoidins in wastewater after extraction and purification might be applicable in the biomedical industry and they can also be potentially used as nutritional feed additives, antimicrobial agents, and preservatives [46]. In addition, the high stability and interface features of melanoidins make them profitable as potential emulsifiers in the materials field [149]. Dedicated research is needed to realize the promising applicability of THP-origin melanoidins.
Author Contributions
Conceptualization, Y.L. and J.H.; validation, L.W. and Q.Y.; project administration, J.H.; visualization, Y.L. and Q.Z.; funding acquisition, J.H.; writing—original draft preparation, Y.L. and Q.Z.; writing—review and editing, J.H., Y.L., Q.Z. and S.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 42007371] and the Technology Research and Development Program of Beijing Municipal Education Commission, China [grant number KM202110005015].
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, H.N.; Wu, S.B.; Tian, Y.J.; Zhang, J.; Liu, H.T. Application of machine learning methods for the prediction of organic solid waste treatment and recycling processes: A review. Bioresour. Technol. 2021, 319, 124114. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe-Arachchige, S.P.; Abeysiriwardana-Arachchige, I.S.A.; Delanka-Pedige, H.M.K.; Nirmalakhandan, N. Biofertilizer recovery from organic solid wastes via hydrothermal liquefaction. Bioresour. Technol. 2021, 338, 125497. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhuo, Y.; Peng, D.; Yao, Q.; Li, H.; Qu, Q. Influence of thermal hydrolysis pretreatment on organic transformation characteristics of high solid anaerobic digestion. Bioresour. Technol. 2017, 244, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Dai, X.; Song, L.; Dai, L.; Dong, B. Effects of stepwise thermal hydrolysis and solid-liquid separation on three different sludge organic matter solubilization and biodegradability. Bioresour. Technol. 2019, 290, 121753. [Google Scholar] [CrossRef] [PubMed]
- Gahlot, P.; Balasundaram, G.; Tyagi, V.K.; Atabani, A.E.; Suthar, S.; Kazmi, A.A.; Stepanec, L.; Juchelkova, D.; Kumar, A. Principles and potential of thermal hydrolysis of sewage sludge to enhance anaerobic digestion. Environ. Res. 2022, 214, 113856. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Sun, F.; Zhou, Y. Insights into anaerobic transformation of key dissolved organic matters produced by thermal hydrolysis sludge pretreatment. Bioresour. Technol. 2018, 266, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Meshref, M.N.A.; Dhar, B.R. Optimization of thermal hydrolysis process for enhancing anaerobic digestion in a wastewater treatment plant with existing primary sludge fermentation. Bioresour. Technol. 2021, 321, 124498. [Google Scholar] [CrossRef]
- Alfaro, N.; Cano, R.; Fdz-Polanco, F. Effect of thermal hydrolysis and ultrasounds pretreatments on foaming in anaerobic digesters. Bioresour. Technol. 2014, 170, 477–482. [Google Scholar] [CrossRef]
- Choi, J.M.; Han, S.K.; Lee, C.Y. Enhancement of methane production in anaerobic digestion of sewage sludge by thermal hydrolysis pretreatment. Bioresour. Technol. 2018, 259, 207–213. [Google Scholar] [CrossRef]
- McNamara, P.J.; Wilson, C.A.; Wogen, M.T.; Murthy, S.N.; Novak, J.T.; Novak, P.J. The effect of thermal hydrolysis pretreatment on the anaerobic degradation of nonylphenol and short-chain nonylphenol ethoxylates in digested biosolids. Water Res. 2012, 46, 2937–2946. [Google Scholar] [CrossRef]
- Devos, P.; Haddad, M.; Carrère, H. Thermal Hydrolysis of Municipal sludge: Finding the Temperature Sweet Spot: A Review. Waste Biomass Valorization 2021, 12, 2187–2205. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Q.; Wang, H.; Han, B.; Xia, D.; Wang, D.; Zhang, W. Molecular mechanisms of interaction between enzymes and Maillard reaction products formed from thermal hydrolysis pretreatment of waste activated sludge. Water Res. 2021, 206, 117777. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Chang, S.W.; Ngo, H.H.; Guo, W.; Nghiem, L.D.; Banu, J.R.; Jeon, B.H.; Nguyen, D.D. Influence of thermal hydrolysis pretreatment on physicochemical properties and anaerobic biodegradability of waste activated sludge with different solids content. Waste Manag. 2019, 85, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.J.; Beightol, S.; Mandahar, U.; Suzuki, R.; Xiao, S.; Lu, H.W.; Le, T.; Mah, J.; Pathak, B.; DeClippeleir, H.; et al. Pretreatment of a primary and secondary sludge blend at different thermal hydrolysis temperatures: Impacts on anaerobic digestion, dewatering and filtrate characteristics. Water Res. 2017, 122, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Abu-Orf, M.; Goss, T. Comparing Thermal Hydrolysis Processes (CAMBI and EXELYS) for Solids Pretreatmet Prior to Anaerobic Digestion. Proc. Water Environ. Fed. 2012, 2012, 1024–1036. [Google Scholar] [CrossRef]
- Barber, W.P.F. Thermal hydrolysis for sewage treatment: A critical review. Water Res. 2016, 104, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Keymer, P.; Ruffell, I.; Pratt, S.; Lant, P. High pressure thermal hydrolysis as pre-treatment to increase the methane yield during anaerobic digestion of microalgae. Bioresour. Technol. 2013, 131, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Feng, Y.; Huang, H.; Khunjar, W.; Wang, Z.W. Recalcitrant dissolved organic nitrogen formation in thermal hydrolysis pretreatment of municipal sludge. Environ. Int. 2020, 138, 105629. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hao, J.; Yu, X.; Zhang, B.; Sui, J.; Wang, C. Method development for the identification, extraction and characterization of melanoidins in thermal hydrolyzed sludge. Sci. Total Environ. 2023, 864, 161204. [Google Scholar] [CrossRef]
- Dwyer, J.; Starrenburg, D.; Tait, S.; Barr, K.; Batstone, D.J.; Lant, P. Decreasing activated sludge thermal hydrolysis temperature reduces product colour, without decreasing degradability. Water Res. 2008, 42, 4699–4709. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Qian, H.; Yao, W.-R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Mu, D.; Qu, F.; Zhu, Z.; Wu, D.; Qi, H.; Ahmed Mohamed, T.; Liu, Y.; Wei, Z. Effect of Maillard reaction on the formation of humic acid during thermophilic phase of aerobic fermentation. Bioresour. Technol. 2022, 357, 127362. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Kim, L. Storage-induced chemical changes in active components of honey de-regulate its antibacterial activity. Food Chem. 2011, 126, 1155–1163. [Google Scholar] [CrossRef]
- Kitts, D.D.; Chen, X.M.; Jing, H. Demonstration of antioxidant and anti-inflammatory bioactivities from sugar-amino acid maillard reaction products. J. Agric. Food Chem. 2012, 60, 6718–6727. [Google Scholar] [CrossRef] [PubMed]
- Echavarría, A.P.; Pagán, J.; Ibarz, A. Melanoidins Formed by Maillard Reaction in Food and Their Biological Activity. Food Eng. Rev. 2012, 4, 203–223. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, M.; Emilia Coldea, T.; Yang, H.; Zhao, H. Structure, chemical stability and antioxidant activity of melanoidins extracted from dark beer by acetone precipitation and macroporous resin adsorption. Food Res. Int. 2023, 164, 112045. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, Y.; Zhou, Y.; Liu, H. Coagulation removal of melanoidins from biologically treated molasses wastewater using ferric chloride. Chem. Eng. J. 2009, 152, 88–94. [Google Scholar] [CrossRef]
- Verma, R.; Kundu, L.M.; Pandey, L.M. Enhanced melanoidin removal by amine-modified Phyllanthus emblica leaf powder. Bioresour. Technol. 2021, 339, 125572. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Z.Y.; Geng, Z.Q.; Tian, Y.C.; Ji, W.X.; Li, W.T.; Dai, K.; Zeng, R.J.; Zhang, F. Elucidating the production and inhibition of melanoidins products on anaerobic digestion after thermal-alkaline pretreatment. J. Hazard. Mater. 2022, 424, 127377. [Google Scholar] [CrossRef]
- Yin, J.; Liu, J.; Chen, T.; Long, Y.; Shen, D. Influence of melanoidins on acidogenic fermentation of food waste to produce volatility fatty acids. Bioresour. Technol. 2019, 284, 121–127. [Google Scholar] [CrossRef]
- Yang, S.; Fan, W.; Xu, Y. Melanoidins present in traditional fermented foods and beverages. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4164–4188. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.S. Enolization and racemization reactions of glucose and fructose on heating with amino-acid enantiomers and the formation of melanoidins as a result of the Maillard reaction. Amino Acids 2009, 36, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; Correia, E.; Lopes, L.; Guido, L.F. Further insights into the role of melanoidins on the antioxidant potential of barley malt. Food Chem. 2014, 160, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. Effect of Dietary Melanoidins on Lipid Peroxidation during Simulated Gastric Digestion: Their Possible Role in the Prevention of Oxidative Damage. J. Agric. Food Chem. 2010, 58, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Rurián-Henares, J.A.; Morales, F.J. Antimicrobial activity of melanoidins against Escherichia coli is mediated by a membrane-damage mechanism. J. Agric. Food Chem. 2008, 56, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Pirestani, S.; Nasirpour, A.; Keramat, J.; Desobry, S. Preparation of chemically modified canola protein isolate with gum Arabic by means of Maillard reaction under wet-heating conditions. Carbohydr. Polym. 2017, 155, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Kumar, V.; Tripathi, S. Evaluation of molasses-melanoidin decolourisation by potential bacterial consortium discharged in distillery effluent. 3 Biotech 2018, 8, 187. [Google Scholar] [CrossRef]
- Tripathi, S.; Sharma, P.; Purchase, D.; Tiwari, M.; Chakrabarty, D.; Chandra, R. Biodegradation of organo-metallic pollutants in distillery wastewater employing a bioaugmentation process. Environ. Technol. Innov. 2021, 23, 101774. [Google Scholar] [CrossRef]
- Tripathi, S.; Sharma, P.; Singh, K.; Purchase, D.; Chandra, R. Translocation of heavy metals in medicinally important herbal plants growing on complex organometallic sludge of sugarcane molasses-based distillery waste. Environ. Technol. Innov. 2021, 22, 101434. [Google Scholar] [CrossRef]
- Chowdhary, P.; Raj, A.; Bharagava, R.N. Environmental pollution and health hazards from distillery wastewater and treatment approaches to combat the environmental threats: A review. Chemosphere 2018, 194, 229–246. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Faixo, S.; Gehin, N.; Balayssac, S.; Gilard, V.; Mazeghrane, S.; Haddad, M.; Gaval, G.; Paul, E.; Garrigues, J.C. Current trends and advances in analytical techniques for the characterization and quantification of biologically recalcitrant organic species in sludge and wastewater: A review. Anal. Chim. Acta 2021, 1152, 338284. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, J.; Chen, L.; Wang, Z. Adsorption and desorption of chlorogenic acid by macroporous adsorbent resins during extraction of Eucommia ulmoides leaves. Ind. Crops Prod. 2020, 149, 112336. [Google Scholar] [CrossRef]
- Pu, Y.; Ding, T.; Zhang, N.; Jiang, P.; Liu, D. Identification of bitter compounds from dried fruit of Ziziphus jujuba cv. Junzao. Int. J. Food Prop. 2017, 20, S26–S35. [Google Scholar] [CrossRef]
- Qiang, X.; Luo, J.; Guo, S.; Cao, W.; Hang, X.; Liu, J.; Wan, Y. A novel process for molasses utilization by membrane filtration and resin adsorption. J. Clean. Prod. 2019, 207, 432–443. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Hao, J.; Li, Y.; Yu, X.; Zhang, B. Sustainable recovery of melanoidins from thermal hydrolyzed sludge by macroporous resin and properties characterization. J. Environ. Manag. 2023, 331, 117277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ding, Y.; Wang, D.; Deng, Y.; Zhao, Y. Effect of high hydrostatic pressure conditions on the composition, morphology, rheology, thermal behavior, color, and stability of black garlic melanoidins. Food Chem. 2021, 337, 127790. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Pastoriza de la Cueva, S.; Peinado, M.J.; Rufian-Henares, J.A.; Navarro, M.P.; Rubio, L.A. Modifications in bacterial groups and short chain fatty acid production in the gut of healthy adult rats after long-term consumption of dietary Maillard reaction products. Food Res. Int. 2017, 100, 134–142. [Google Scholar] [CrossRef]
- Herzfeld, J.; Rand, D.; Matsuki, Y.; Daviso, E.; Mak-Jurkauskas, M.; Mamajanov, I. Molecular structure of humin and melanoidin via solid state NMR. J. Phys. Chem. B 2011, 115, 5741–5745. [Google Scholar] [CrossRef]
- Rodriguez, A.; Lema, P.; Bessio, M.I.; Moyna, G.; Panizzolo, L.A.; Ferreira, F. Isolation and Characterization of Melanoidins from Dulce de Leche, A Confectionary Dairy Product. Molecules 2019, 24, 4163. [Google Scholar] [CrossRef]
- Çelik, E.E.; Rubio, J.M.A.; Andersen, M.L.; Gökmen, V. Interactions of coffee and bread crust melanoidins with hydroxycinnamic and hydroxybenzoic acids in aqueous radical environment. Food Res. Int. 2018, 108, 286–294. [Google Scholar] [CrossRef]
- Alves, G.; Perrone, D. Breads enriched with guava flour as a tool for studying the incorporation of phenolic compounds in bread melanoidins. Food Chem. 2015, 185, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Identification and quantification of free and bound phenolic compounds contained in the high-molecular weight melanoidin fractions derived from two different types of cocoa beans by UHPLC-DAD-ESI-HR-MSn. Food Res. Int. 2019, 115, 135–149. [Google Scholar] [CrossRef]
- Habinshuti, I.; Chen, X.; Yu, J.; Mukeshimana, O.; Duhoranimana, E.; Karangwa, E.; Muhoza, B.; Zhang, M.; Xia, S.; Zhang, X. Antimicrobial, antioxidant and sensory properties of Maillard reaction products (MRPs) derived from sunflower, soybean and corn meal hydrolysates. LWT 2019, 101, 694–702. [Google Scholar] [CrossRef]
- Hauser, C.; Muller, U.; Sauer, T.; Augner, K.; Pischetsrieder, M. Maillard reaction products as antimicrobial components for packaging films. Food Chem. 2014, 145, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Bharagava, R.N.; Rai, V. Melanoidins as major colourant in sugarcane molasses based distillery effluent and its degradation. Bioresour. Technol. 2008, 99, 4648–4660. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.; Kavanagh, L.; Lant, P. The degradation of dissolved organic nitrogen associated with melanoidin using a UV/H2O2 AOP. Chemosphere 2008, 71, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Arimi, M.M.; Zhang, Y.; Geißen, S.-U. Color removal of melanoidin-rich industrial effluent by natural manganese oxides. Sep. Purif. Technol. 2015, 150, 286–291. [Google Scholar] [CrossRef]
- Cooper, J.; Antony, A.; Luiz, A.; Kavanagh, J.; Razmjou, A.; Chen, V.; Leslie, G. Characterisation of dissolved organic matter in fermentation industry effluents and comparison with model compounds. Chemosphere 2019, 234, 630–639. [Google Scholar] [CrossRef]
- Liu, J.; Yin, J.; He, X.; Chen, T.; Shen, D. Three-Dimensional Excitation and Emission Fluorescence-Based Method for Evaluation of Maillard Reaction Products in Food Waste Treatment. J. Chem. 2018, 2018, 6758794. [Google Scholar] [CrossRef]
- Huang, F.; Liu, H.; Wen, J.; Zhao, C.; Dong, L.; Liu, H. Underestimated humic acids release and influence on anaerobic digestion during sludge thermal hydrolysis. Water Res. 2021, 201, 117310. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.I.F.S.; van Boekel, M.A.J.S. Melanoidins extinction coefficient in the glucose/glycine Maillard reaction. Food Chem. 2003, 83, 135–142. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; Morales, F.J. Functional properties of melanoidins: In vitro antioxidant, antimicrobial and antihypertensive activities. Food Res. Int. 2007, 40, 995–1002. [Google Scholar] [CrossRef]
- Singh, N.; Petrinic, I.; Helix-Nielsen, C.; Basu, S.; Balakrishnan, M. Concentrating molasses distillery wastewater using biomimetic forward osmosis (FO) membranes. Water Res. 2018, 130, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fan, W.; Xu, Y. Melanoidins from Chinese Distilled Spent Grain: Content, Preliminary Structure, Antioxidant, and ACE-Inhibitory Activities In Vitro. Foods 2019, 8, 516. [Google Scholar] [CrossRef] [PubMed]
- Kaspchak, E.; Toci, A.T.; Menezes, L.R.A.; Igarashi-Mafra, L.; Mafra, M.R. Effect of phytic acid, tannic acid and saponin on asparagine-glucose Maillard reaction. Food Chem. 2022, 394, 133518. [Google Scholar] [CrossRef]
- Yang, N.; Yang, S.; Zheng, X. Inhibition of Maillard reaction during alkaline thermal hydrolysis of sludge. Sci. Total Environ. 2022, 814, 152497. [Google Scholar] [CrossRef]
- Yang, N.; Yang, S.; Yang, L.; Song, Q.; Zheng, X. Exploration of browning reactions during alkaline thermal hydrolysis of sludge: Maillard reaction, caramelization and humic acid desorption. Environ. Res. 2022, 217, 114814. [Google Scholar] [CrossRef]
- Langner, E.; Rzeski, W. Biological Properties of Melanoidins: A Review. Int. J. Food Prop. 2013, 17, 344–353. [Google Scholar] [CrossRef]
- Del Pino-García, R.; González-SanJosé, M.L.; Rivero-Pérez, M.D.; Muñiz, P. Influence of the Degree of Roasting on the Antioxidant Capacity and Genoprotective Effect of Instant Coffee: Contribution of the Melanoidin Fraction. J. Agric. Food Chem. 2012, 60, 10530–10539. [Google Scholar] [CrossRef]
- Silván, J.M.; Morales, F.J.; Saura-Calixto, F. Conceptual Study on Maillardized Dietary Fiber in Coffee. J. Agric. Food Chem. 2010, 58, 12244–12249. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Basu, S.; Batra, V.S.; Balakrishnan, M. Fractionation of sugarcane molasses distillery wastewater and evaluation of antioxidant and antimicrobial characteristics. Ind. Crops Prod. 2018, 118, 73–80. [Google Scholar] [CrossRef]
- Pagán, J.; Ibarz, A.; Elvira, L.; Trillo, J.; de Frutos, S.; Echavarría, A.-P. Monitoring the behavior of melanoidin from a glucose/l-asparagine solution. Food Res. Int. 2012, 48, 802–807. [Google Scholar] [CrossRef]
- Liu, R.; Wu, Q.; Xu, J.; Gao, Y.; Zhi, Z.; Wu, T.; Sui, W.; Zhang, M. Isolation of melanoidins from heat-moisture treated ginseng and its inhibitory effect on choline metabolism. J. Funct. Foods 2023, 100, 105370. [Google Scholar] [CrossRef]
- Feng, J.L.; Berton-Carabin, C.C.; Guyot, S.; Gacel, A.; Fogliano, V.; Schroën, K. Coffee melanoidins as emulsion stabilizers. Food Hydrocoll. 2023, 139, 9. [Google Scholar] [CrossRef]
- Bekedam, E.K.; Roos, E.; Schols, H.A.; van Boekel, M.; Smit, G. Low molecular weight melanoidins in coffee brew. J. Agric. Food Chem. 2008, 56, 4060–4067. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Berton-Carabin, C.C.; Fogliano, V.; Schroën, K. Maillard reaction products as functional components in oil-in-water emulsions: A review highlighting interfacial and antioxidant properties. Trends Food Sci. Technol. 2022, 121, 129–141. [Google Scholar] [CrossRef]
- Bekedam, E.K.; De Laat, M.; Schols, H.A.; Van Boekel, M.; Smit, G. Arabinogalactan proteins are incorporated in negatively charged coffee brew melanoidins. J. Agric. Food Chem. 2007, 55, 761–768. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, Y.-S. Effect of reaction pH on enolization and racemization reactions of glucose and fructose on heating with amino acid enantiomers and formation of melanoidins as result of the Maillard reaction. Food Chem. 2008, 108, 582–592. [Google Scholar] [CrossRef]
- Migo, V.P.; Del Rosario, E.J.; Matsumura, M. Flocculation of melanoidins induced by inorganic ions. J. Ferment. Bioeng. 1997, 83, 287–291. [Google Scholar] [CrossRef]
- Ramonaitytė, D.T.; Keršienė, M.; Adams, A.; Tehrani, K.A.; De Kimpe, N. The interaction of metal ions with Maillard reaction products in a lactose–glycine model system. Food Res. Int. 2009, 42, 331–336. [Google Scholar] [CrossRef]
- Hatano, K.-I.; Kanazawa, K.; Tomura, H.; Yamatsu, T.; Tsunoda, K.-I.; Kubota, K. Molasses melanoidin promotes copper uptake for radish sprouts: The potential for an accelerator of phytoextraction. Environ. Sci. Pollut. Res. 2016, 23, 17656–17663. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.J. Assessing the non-specific hydroxyl radical scavenging properties of melanoidins in a Fenton-type reaction system. Anal. Chim. Acta 2005, 534, 171–176. [Google Scholar] [CrossRef]
- Yang, M.M.; Ding, L.; Wang, P.Y.; Wu, Y.F.; Areeprasert, C.; Wang, M.; Chen, X.L.; Wang, F.C.; Yu, G.S. Formation of melanoidins and development of characterization techniques during thermal pretreatment of organic solid waste: A critical review. Fuel 2023, 334, 126790. [Google Scholar] [CrossRef]
- Yang, D.; Gao, X. Research progress on the antioxidant biological activity of beer and strategy for applications. Trends Food Sci. Technol. 2021, 110, 754–764. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Balasubramaniam, V.M.; Niranjan, K.; Knorr, D. Opportunities and Challenges in High Pressure Processing of Foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.; Shorbagi, M.; Lorenzo, J.M.; Farag, M.A. Dissecting dietary melanoidins: Formation mechanisms, gut interactions and functional properties. Crit. Rev. Food Sci. Nutr. 2022, 62, 8954–8971. [Google Scholar] [CrossRef]
- Diaz-Morales, N.; Cavia-Saiz, M.; Salazar, G.; Rivero-Perez, M.D.; Muniz, P. Cytotoxicity study of bakery product melanoidins on intestinal and endothelial cell lines. Food Chem. 2021, 343, 128405. [Google Scholar] [CrossRef]
- Kumar, P.; Chandra, R. Decolourisation and detoxification of synthetic molasses melanoidins by individual and mixed cultures of Bacillus spp. Bioresour. Technol. 2006, 97, 2096–2102. [Google Scholar] [CrossRef]
- Goulas, V.; Nicolaou, D.; Botsaris, G.; Barbouti, A. Straw Wine Melanoidins as Potential Multifunctional Agents: Insight into Antioxidant, Antibacterial, and Angiotensin-I-Converting Enzyme Inhibition Effects. Biomedicines 2018, 6, 83. [Google Scholar] [CrossRef]
- Kukuminato, S.; Koyama, K.; Koseki, S. Antibacterial Properties of Melanoidins Produced from Various Combinations of Maillard Reaction against Pathogenic Bacteria. Microbiol. Spectr. 2021, 9, e01142-21. [Google Scholar] [CrossRef] [PubMed]
- Arimi, M.M.; Zhang, Y.; Götz, G.; Kiriamiti, K.; Geißen, S.-U. Antimicrobial colorants in molasses distillery wastewater and their removal technologies. Int. Biodeterior. Biodegrad. 2014, 87, 34–43. [Google Scholar] [CrossRef]
- Martínez-Tomé, M.; Jiménez-Monreal, A.M.; García-Jiménez, L.; Almela, L.; García-Diz, L.; Mariscal-Arcas, M.; Murcia, M.A. Assessment of antimicrobial activity of coffee brewed in three different ways from different origins. Eur. Food Res. Technol. 2011, 233, 497–505. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; Morales, F.J. Microtiter plate-based assay for screening antimicrobial activity of melanoidins against E. coli and S. aureus. Food Chem. 2008, 111, 1069–1074. [Google Scholar] [CrossRef]
- Hosseini Koupaie, E.; Bazyar Lakeh, A.A.; Azizi, A.; Hafez, H.; Elbeshbishy, E. Integrated two-phase acidogenic-methanogenic treatment of municipal sludge with thermal hydrolysis. Waste Manag. 2022, 144, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, P.; Singh, A.; Chandra, R.; Kumar, P.S.; Raj, A.; Bharagava, R.N. Detection and identification of hazardous organic pollutants from distillery wastewater by GC-MS analysis and its phytotoxicity and genotoxicity evaluation by using Allium cepa and Cicer arietinum L. Chemosphere 2022, 297, 134123. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Agrawal, S.; Shahi, S.K.; Motghare, A.; Singh, S.; Ramamurthy, P.C. Bioremediation potential of newly isolated Bacillus albus strain VKDS9 for decolourization and detoxification of biomethanated distillery effluent and its metabolites characterization for environmental sustainability. Environ. Technol. Innov. 2022, 26, 102260. [Google Scholar] [CrossRef]
- Yuan, T.; Cheng, Y.; Huang, W.; Zhang, Z.; Lei, Z.; Shimizu, K.; Utsumi, M. Fertilizer potential of liquid product from hydrothermal treatment of swine manure. Waste Manag. 2018, 77, 166–171. [Google Scholar] [CrossRef]
- Glosl, S.; Wagner, K.H.; Draxler, A.; Kaniak, M.; Lichtenecker, S.; Sonnleitner, A.; Somoza, V.; Erbersdobler, H.; Elmadfa, I. Genotoxicity and mutagenicity of melanoidins isolated from a roasted glucose-glycine model in human lymphocyte cultures, intestinal Caco-2 cells and in the Salmonella typhimurium strains TA98 and TA102 applying the AMES test. Food Chem. Toxicol. 2004, 42, 1487–1495. [Google Scholar] [CrossRef]
- Kumar, V.; Ameen, F.; Islam, M.A.; Agrawal, S.; Motghare, A.; Dey, A.; Shah, M.P.; Americo-Pinheiro, J.H.P.; Singh, S.; Ramamurthy, P.C. Evaluation of cytotoxicity and genotoxicity effects of refractory pollutants of untreated and biomethanated distillery effluent using Allium cepa. Environ. Pollut. 2022, 300, 118975. [Google Scholar] [CrossRef]
- Hofmann, T.; Czerny, M.; Calligaris, S.; Schieberle, P. Model studies on the influence of coffee melanoidins on flavor volatiles of coffee beverages. J. Agric. Food Chem. 2001, 49, 2382–2386. [Google Scholar] [CrossRef] [PubMed]
- Fay, L.B.; Brevard, H. Contribution of mass spectrometry to the study of the Maillard reaction in food. Mass Spectrom. Rev. 2005, 24, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.J.; Fernandez-Fraguas, C.; Jiménez-Pérez, S. Iron-binding ability of melanoidins from food and model systems. Food Chem. 2005, 90, 821–827. [Google Scholar] [CrossRef]
- Getachew, A.T.; Chun, B.S. Influence of hydrothermal process on bioactive compounds extraction from green coffee bean. Innov. Food Sci. Emerg. Technol. 2016, 38, 24–31. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; Morales, F.J. A new application of a commercial microtiter plate-based assay for assessing the antimicrobial activity of Maillard reaction products. Food Res. Int. 2006, 39, 33–39. [Google Scholar] [CrossRef]
- Lang, A.; Lan, W.; Xie, J. Preparation and antimicrobial mechanism of Maillard reaction products derived from epsilon-polylysine and chitooligosaccharides. Biochem. Biophys. Res. Commun. 2023, 650, 30–38. [Google Scholar] [CrossRef]
- Feng, T.; Zhou, Y.; Wang, X.; Wang, X.; Xia, S. α-Dicarbonyl compounds related to antimicrobial and antioxidant activity of maillard reaction products derived from xylose, cysteine and corn peptide hydrolysate. Food Biosci. 2021, 41, 100951. [Google Scholar] [CrossRef]
- Shu, Q.; Niu, Y.; Zhao, W.; Chen, Q. Antibacterial activity and mannosylerythritol lipids against vegetative cells and spores of Bacillus cereus. Food Control 2019, 106, 106711. [Google Scholar] [CrossRef]
- Phothilangka, P.; Schoen, M.A.; Huber, M.; Luchetta, P.; Winkler, T.; Wett, B. Prediction of thermal hydrolysis pretreatment on anaerobic digestion of waste activated sludge. Water Sci. Technol. 2008, 58, 1467–1473. [Google Scholar] [CrossRef]
- Devos, P.; Filali, A.; Grau, P.; Gillot, S. Sidestream characteristics in water resource recovery facilities: A critical review. Water Res. 2023, 232, 119620. [Google Scholar] [CrossRef]
- Zhang, D.; An, Z.; Strawn, M.; Broderick, T.; Khunjar, W.; Wang, Z.-W. Understanding the formation of recalcitrant dissolved organic nitrogen as a result of thermal hydrolysis and anaerobic digestion of municipal sludge. Environ. Sci. Water Res. Technol. 2021, 7, 335–345. [Google Scholar] [CrossRef]
- Penaud, V.; Delgenès, J.-P.; Moletta, R. Characterization of soluble molecules from thermochemically pretreated sludge. J. Environ. Eng. 2000, 126, 397–402. [Google Scholar] [CrossRef]
- Gu, Z.L.; Li, Y.; Yang, Y.F.; Xia, S.Q.; Hermanowicz, S.W.; Alvarez-Cohen, L. Inhibition of anammox by sludge thermal hydrolysis and metagenomic insights. Bioresour. Technol. 2018, 270, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, I.; Mottet, A.; Carrere, H.; Deleris, S.; Vedrenne, F.; Steyer, J.P. Modified ADM1 disintegration/hydrolysis structures for modeling batch thermophilic anaerobic digestion of thermally pretreated waste activated sludge. Water Res. 2009, 43, 3479–3492. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Li, A.M. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: The dewatering performance and the characteristics of products. Water Res. 2015, 68, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Qian, W.; Wu, L.; Yu, S.H.; Wei, R.; Chen, W.F.; Ni, J.Z. Spectral characteristics of dissolved organic carbon (DOC) derived from biomass pyrolysis: Biochar-derived DOC versus smoke-derived DOC, and their differences from natural DOC. Chemosphere 2022, 302, 134869. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; He, R.; Jia, M. Characterization of melanoidins in thermal hydrolysis sludge and effects on dewatering performance. Environ. Res. 2023, 239, 117226. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Zhang, L.; Li, A.M. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: Influence of operating conditions and the process energetics. Water Res. 2014, 65, 85–97. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Souza, T.S.; Fdz-Polanco, F.; Perez-Elvira, S.I. Thermal steam explosion pretreatment to enhance anaerobic biodegradability of the solid fraction of pig manure. Bioresour. Technol. 2014, 152, 393–398. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Huang, R.; Zhang, W.; He, W.; Deng, Z.; Han, Y.; Xiao, B.; Luo, H.; Qu, W. Effects of temperature and total solid content on biohydrogen production from dark fermentation of rice straw: Performance and microbial community characteristics. Chemosphere 2022, 286, 131655. [Google Scholar] [CrossRef]
- Abe, N.; Tang, Y.Q.; Iwamura, M.; Morimura, S.; Kida, K. Pretreatment followed by anaerobic digestion of secondary sludge for reduction of sewage sludge volume. Water Sci. Technol. 2013, 67, 2527–2533. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, P.; Sammi, S.R.; Pandey, R.; Kaithwas, G.; Raj, A.; Singh, J.; Bharagava, R.N. Bacterial degradation of distillery wastewater pollutants and their metabolites characterization and its toxicity evaluation by using Caenorhabditis elegans as terrestrial test models. Chemosphere 2020, 261, 127689. [Google Scholar] [CrossRef]
- Efthimiou, I.; Georgiou, Y.; Vlastos, D.; Dailianis, S.; Deligiannakis, Y. Assessing the cyto-genotoxic potential of model zinc oxide nanoparticles in the presence of humic-acid-like-polycondensate (HALP) and the leonardite HA (LHA). Sci. Total Environ. 2020, 721, 137625. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Tripathi, S.; Chandra, R. Maillard reaction product and its complexation with environmental pollutants: A comprehensive review of their synthesis and impact. Bioresour. Technol. Rep. 2021, 15, 100779. [Google Scholar] [CrossRef]
- Haddis, A.; Devi, R. Effect of effluent generated from coffee processing plant on the water bodies and human health in its vicinity. J. Hazard. Mater. 2008, 152, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.K.; Johnson, I.; Kumar, M. Melanoidin removal in multi-oxidant supplemented microwave system: Optimization of operating conditions using response surface methodology and cost estimation. J. Water Process Eng. 2020, 33, 101008. [Google Scholar] [CrossRef]
- Goswami, L.; Pakshirajan, K.; Pugazhenthi, G. Biological treatment of biomass gasification wastewater using hydrocarbonoclastic bacterium Rhodococcus opacus in an up-flow packed bed bioreactor with a novel waste-derived nano-biochar based bio-support material. J. Clean. Prod. 2020, 256, 120253. [Google Scholar] [CrossRef]
- Fan, L.; Nguyen, T.; Roddick, F.A. Characterisation of the impact of coagulation and anaerobic bio-treatment on the removal of chromophores from molasses wastewater. Water Res. 2011, 45, 3933–3940. [Google Scholar] [CrossRef]
- Coca, M.; Peña, M.; González, G. Kinetic study of ozonation of molasses fermentation wastewater. J. Hazard. Mater. 2007, 149, 364–370. [Google Scholar] [CrossRef]
- Bernal, M.; Ruiz, M.O.; Geanta, R.M.; Benito, J.M.; Escudero, I. Colour removal from beet molasses by ultrafiltration with activated charcoal. Chem. Eng. J. 2016, 283, 313–322. [Google Scholar] [CrossRef][Green Version]
- Dwyer, J.; Lant, P. Biodegradability of DOC and DON for UV/H2O2 pre-treated melanoidin based wastewater. Biochem. Eng. J. 2008, 42, 47–54. [Google Scholar] [CrossRef]
- Kobya, M.; Delipinar, S. Treatment of the baker’s yeast wastewater by electrocoagulation. J. Hazard. Mater. 2008, 154, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Rafigh, S.M.; Soleymani, A.R. Melanoidin removal from molasses wastewater using graphene oxide nanosheets. Sep. Sci. Technol. 2020, 55, 2281–2293. [Google Scholar] [CrossRef]
- Satyawall, Y.; Balakrishnan, A. Removal of color from biomethanated distillery spentwash by treatment with activated carbons. Bioresour. Technol. 2007, 98, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Singh, A.; Gupta, S.K. A parametric study using Box-Behnken design for melanoidin removal via Cu-impregnated activated carbon prepared from waste leaves biomass. Appl. Water Sci. 2022, 12, 9. [Google Scholar] [CrossRef]
- Jembere, A.L.; Genet, M.B. Comparative adsorptive performance of adsorbents developed from sugar industrial wastes for the removal of melanoidin pigment from molasses distillery spent wash. Water Resour. Ind. 2021, 26, 17. [Google Scholar] [CrossRef]
- Cañizares, P.; Hernández-Ortega, M.; Rodrigo, M.A.; Barrera-Díaz, C.E.; Roa-Morales, G.; Sáez, C. A comparison between Conductive-Diamond Electrochemical Oxidation and other Advanced Oxidation Processes for the treatment of synthetic melanoidins. J. Hazard. Mater. 2009, 164, 120–125. [Google Scholar] [CrossRef]
- Satyawali, Y.; Verstraete, W.; Balakrishnan, M. Integrated Electrolytic Treatment and Adsorption for the Removal of Melanoidins. Clean-Soil Air Water 2010, 38, 409–412. [Google Scholar] [CrossRef]
- Lan, G.H.; Luo, B.; Li, Q.; Jian, L.; Meng, X.X.; Wei, Y. Combined ultrasound and fenton (US-Fenton) process for the treatment of caramel wastewater. Fresenius Environ. Bull. 2018, 27, 2091–2109. [Google Scholar]
- Jiranuntipon, S.; Chareonpornwattana, S.; Damronglerd, S.; Albasi, C.; Delia, M.L. Decolorization of synthetic melanoidins-containing wastewater by a bacterial consortium. J. Ind. Microbiol. Biotechnol. 2008, 35, 1313–1321. [Google Scholar] [CrossRef][Green Version]
- Agarwal, R.; Lata, S.; Gupta, M.; Singh, P. Removal of melanoidin present in distillery effluent as a major colorant: A Review. J. Environ. Biol. 2010, 31, 521–528. [Google Scholar] [PubMed]
- Li, J.; Zhang, W.; Li, X.; Ye, T.; Gan, Y.; Zhang, A.; Chen, H.; Xue, G.; Liu, Y. Production of lactic acid from thermal pretreated food waste through the fermentation of waste activated sludge: Effects of substrate and thermal pretreatment temperature. Bioresour. Technol. 2018, 247, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, J.; Singh, D.; Nigam, P. Decolourisation of synthetic and spentwash melanoidins using the white-rot fungus Phanerochaete chrysosporium JAG-40. Bioresour. Technol. 2001, 78, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, S.; Tsioptsias, C.; Samaras, P. Biodegradation and decolorization of melanoidin solutions by manganese peroxidase yeasts. Water Sci. Technol. 2016, 73, 2436–2445. [Google Scholar] [CrossRef][Green Version]
- Georgiou, R.P.; Tsiakiri, E.P.; Lazaridis, N.K.; Pantazaki, A.A. Decolorization of melanoidins from simulated and industrial molasses effluents by immobilized laccase. J. Environ. Chem. Eng. 2016, 4, 1322–1331. [Google Scholar] [CrossRef]
- Ziaei-Rad, Z.; Nickpour, M.; Adl, M.; Pazouki, M. Bioadsorption and enzymatic biodecolorization of effluents from ethanol production plants. Biocatal. Agric. Biotechnol. 2020, 24, 101555. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Li, D.; Xiao, J.; Wu, L.; Geng, X.; Wu, G.; Zeng, Z.; Hu, J. Decolorization of molasses alcohol wastewater by thermophilic hydrolase with practical application value. Bioresour. Technol. 2021, 323, 124609. [Google Scholar] [CrossRef]
- Kumar, V.; Chandra, R. Characterisation of manganese peroxidase and laccase producing bacteria capable for degradation of sucrose glutamic acid-Maillard reaction products at different nutritional and environmental conditions. World J. Microbiol. Biotechnol. 2018, 34, 32. [Google Scholar] [CrossRef]
- Kan, X.; Chen, G.; Zhou, W.; Zeng, X. Application of protein-polysaccharide Maillard conjugates as emulsifiers: Source, preparation and functional properties. Food Res. Int. 2021, 150, 110740. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).