Combined Application of Juniperus communis Essential Oil and Amikacin, Clarithromycin and Rifampicin against Mycobacterium avium and Mycobacterium intracellulare
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil
2.2. Strains and Growth Media
2.3. Antimicrobial Drugs
2.4. Sterile Tap Water
2.5. Determination of the Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
2.6. Checkerboard Synergy Method
2.7. Determination of Cellular Content Leakage
2.8. Transmission Electron Microscopy
2.9. Statistical Analysis
3. Results
3.1. Susceptibility of Nontuberculous Mycobacteria to Juniperus communis Essential Oil and Selected Antimicrobial Drugs
3.2. Synergistic Effect of J. communis EO and Selected Antimicrobial Drugs on Nontuberculous Mycobacteria
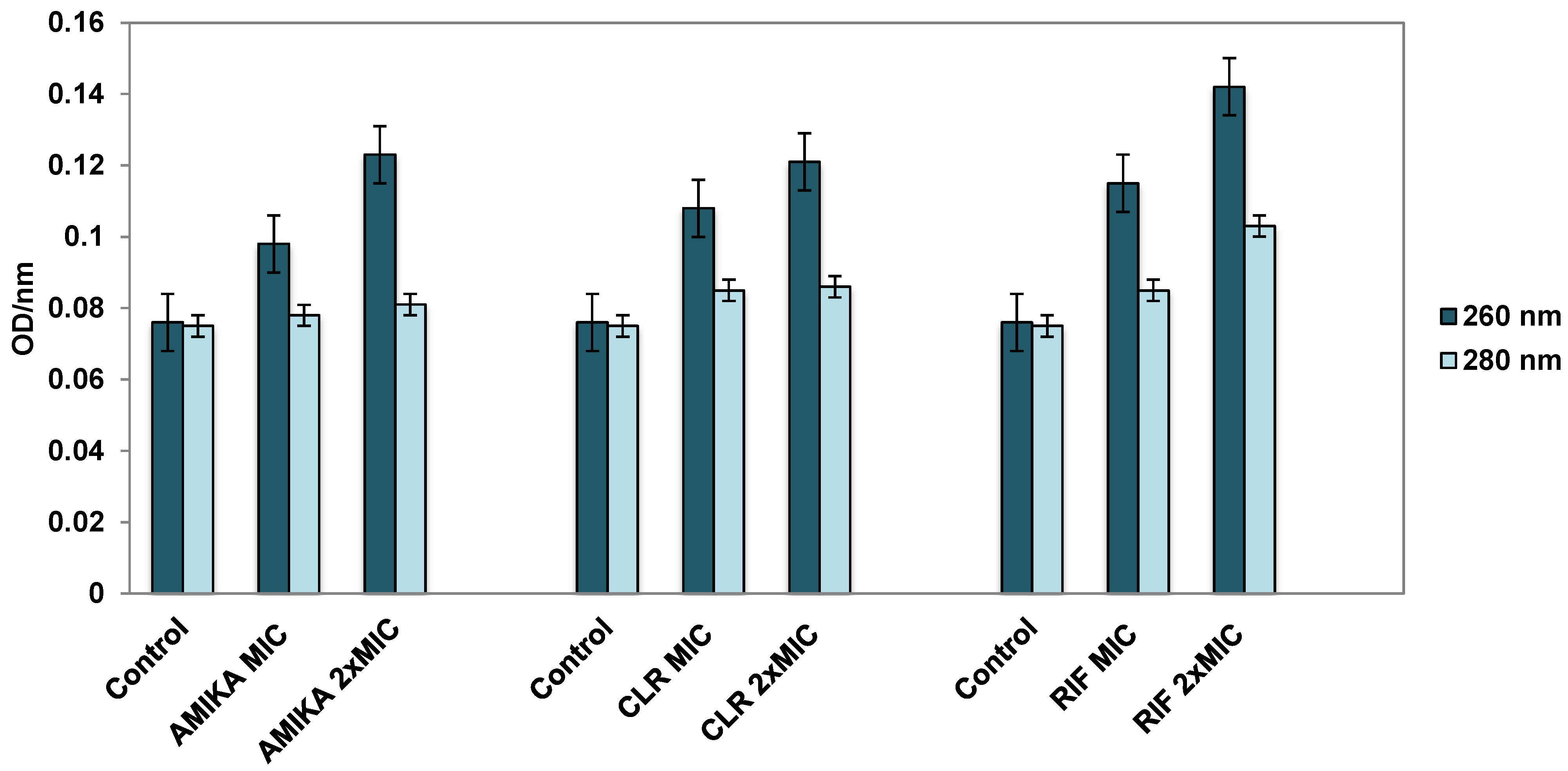
3.3. Leakage of Cellular Contents after Exposure to Selected Antimicrobial Drugs
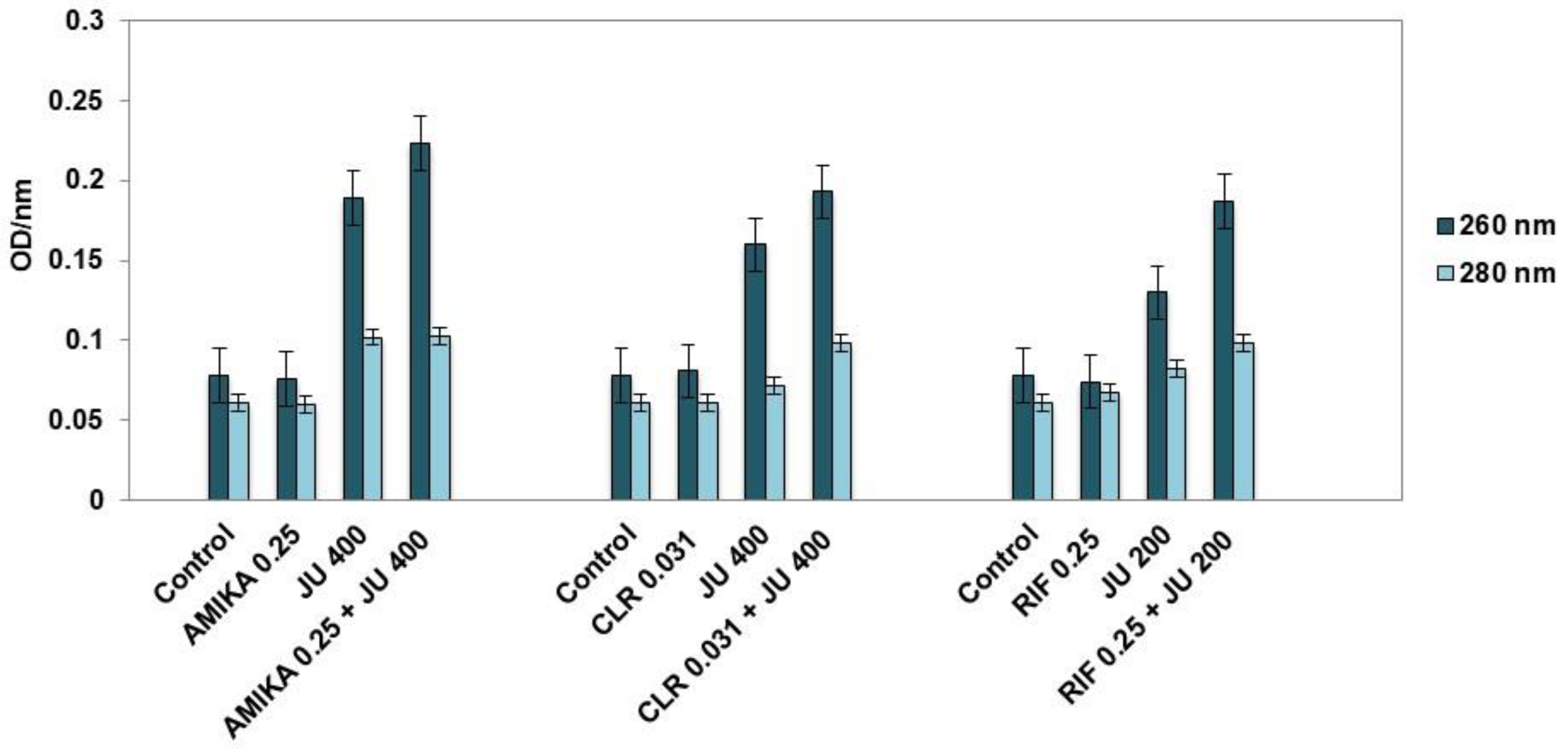
3.4. Leakage of Cellular Contents after Exposure to Synergistic Combinations of J. communis EO and Selected Antimicrobial Drugs
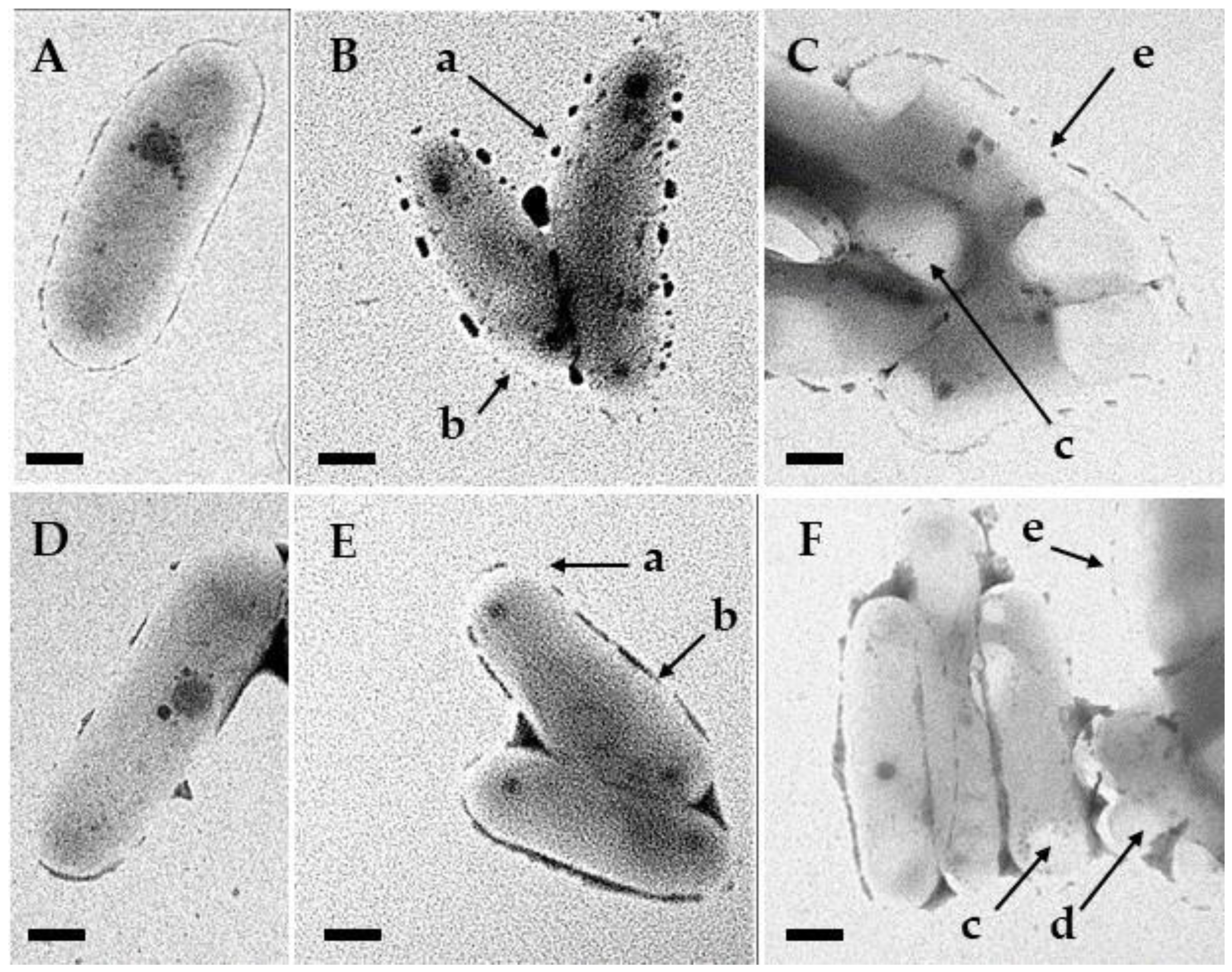
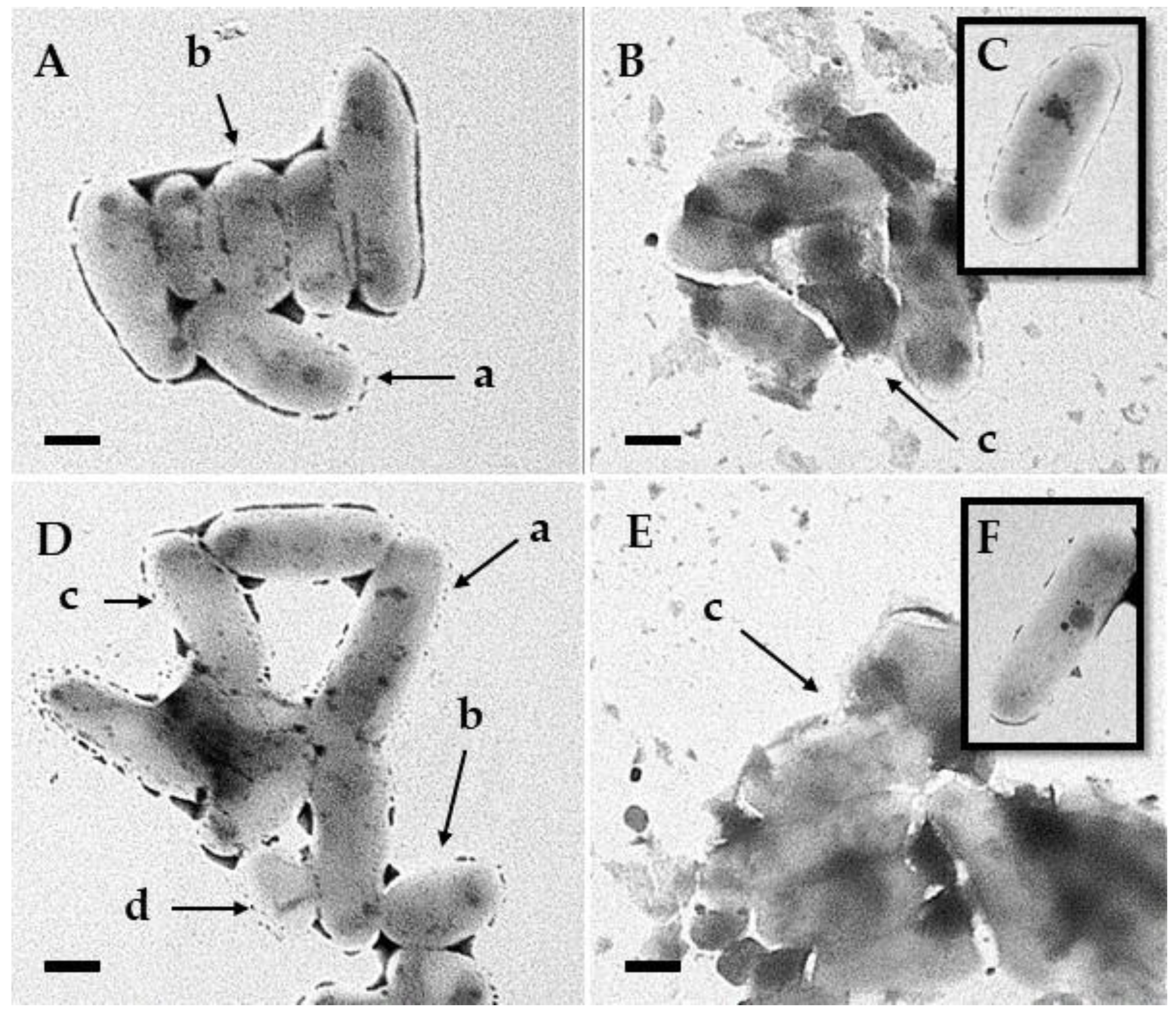
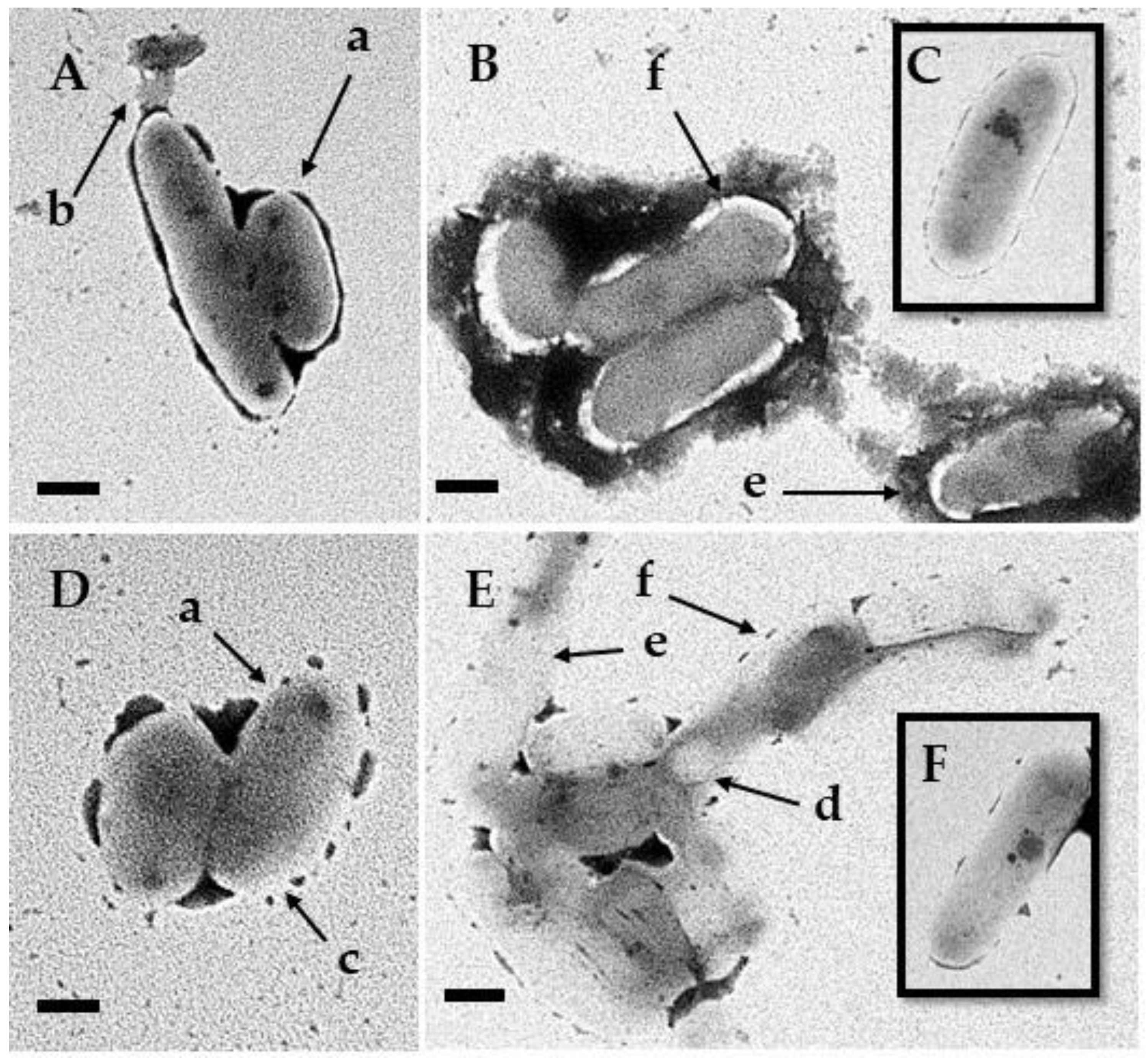
3.5. Transmission Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sousa, S.; Bandeira, M.; Carvalho, P.A.; Duarte, A.; Jordao, L. Nontuberculous Mycobacteria Pathogenesis and Biofilm Assembly. Int. J. Mycobacteriol. 2015, 4, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Buhler, V.B.; Pollak, A. Human Infection with Atypical Acid-Fast Organisms: Report of Two Cases with Pathologic Findings. Am. J. Clin. Pathol. 1953, 23, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Henkle, E.; Winthrop, K.L. Nontuberculous mycobacteria Infections in Immunosuppressed Hosts. Clin. Chest Med. 2015, 36, 91–99. [Google Scholar] [CrossRef]
- Zmak, L.; Obrovac, M.; Katalinic Jankovic, V.; Jankovic, M.; Krajinovic, V.; Viskovic, K.; Sestan Crnek, S.; Haris, V. A Fatal Mycobacterium Chelonae Infection in an Immunosuppressed Patient with Systemic Lupus Erythematosus and Concomitant Fahr’s Syndrome. J. Infect. Chemother. 2011, 17, 264–267. [Google Scholar] [CrossRef]
- Jankovic Makek, M.; Pavlisa, G.; Jakopovic, M.; Redzepi, G.; Zmak, L.; Vukic Dugac, A.; Hecimovic, A.; Mazuranic, I.; Jaksch, P.; Klepetko, W.; et al. Early Onset of Nontuberculous Mycobacterial Pulmonary Disease Contributes to the Lethal Outcome in Lung Transplant Recipients: Report of Two Cases and Review of the Literature. Transpl. Infect. Dis. 2016, 18, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Pollak, A.; Buhler, V.B. The Cultural Characteristics and the Animal Pathogenicity of an Atypical Acid-Fast Organism That Causes Human Disease. Trans. Annu. Meet. Natl. Tuberc. Assoc. 1953, 49, 86–88. [Google Scholar] [PubMed]
- Timpe, A.; Runyon, E.H. The Relationship of Atypical Acid-Fast Bacteria to Human Disease; a Preliminary Report. J. Lab. Clin. Med. 1954, 44, 202–209. [Google Scholar] [CrossRef]
- Varley, C.D.; Ku, J.H.; Henkle, E.; Schafer, S.D.; Winthrop, K.L. Disseminated Nontuberculous Mycobacteria in HIV-Infected Patients, Oregon, USA, 2007–2012. Emerg. Infect. Dis. 2017, 23, 533–535. [Google Scholar] [CrossRef]
- Wood, L.E.; Buhler, V.B.; Pollak, A. Human Infection with the Yellow Acid-Fast Bacillus; a Report of Fifteen Additional Cases. Am. Rev. Tuberc. 1956, 73, 917–929. [Google Scholar] [CrossRef]
- Andréjak, C.; Nielsen, R.; Thomsen, V.Ø.; Duhaut, P.; Sørensen, H.T.; Thomsen, R.W. Chronic Respiratory Disease, Inhaled Corticosteroids and Risk of Non-Tuberculous Mycobacteriosis. Thorax 2013, 68, 256–262. [Google Scholar] [CrossRef]
- Hoefsloot, W.; Van Ingen, J.; Andrejak, C.; Ängeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The Geographic Diversity of Nontuberculous Mycobacteria Isolated from Pulmonary Samples: An NTM-NET Collaborative Study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Forbes, B.A.; Hall, G.S.; Miller, M.B.; Novak, S.M.; Rowlinson, M.-C.; Salfinger, M.; Somoskövi, A.; Warshauer, D.M.; Wilson, M.L. Practical Guidance for Clinical Microbiology Laboratories: Mycobacteria. Clin. Microbiol. Rev. 2018, 31, e00038-17. [Google Scholar] [CrossRef] [PubMed]
- Pfyffer, G.; Brown-Elliot, B.; Wallace, R. Mycobacterium: General Characteristics, Isolation, and Staining Procedures. In Manual of Clinical Microbiology, 8th ed.; Murray, P.R., Ed.; American Society for Microbiology: Washington, DC, USA, 2003; Volume 1, pp. 532–584. [Google Scholar]
- Faria, S.; Joao, I.; Jordao, L. General Overview on Nontuberculous Mycobacteria, Biofilms, and Human Infection. J. Pathog. 2015, 2015, 809014. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.E.; Koh, W.-J.; Yew, W.W. Update on Pulmonary Disease Due to Non-Tuberculous Mycobacteria. Int. J. Infect. Dis. 2016, 45, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An Official ATS/IDSA Statement: Diagnosis, Treatment, and Prevention of Nontuberculous Mycobacterial Diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of Plant Volatiles: Nature’s Diversity and Ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial Agents from Plants: Antibacterial Activity of Plant Volatile Oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Klančnik, A.; Zorko, Š.; Toplak, N.; Kovač, M.; Bucar, F.; Jeršek, B.; Smole Možina, S. Antiadhesion Activity of Juniper (Juniperus communis L.) Preparations against Campylobacter jejuni Evaluated with PCR-Based Methods. Phytother. Res. 2018, 32, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Juniper Oil. European Pharmacopoeia, 8th ed.; Council of Europe: Strasbourg Cedex, France, 2013; Volume 1, pp. 1285–1286. [Google Scholar]
- Angioni, A.; Barra, A.; Russo, M.T.; Coroneo, V.; Dessí, S.; Cabras, P. Chemical Composition of the Essential Oils of Juniperus from Ripe and Unripe Berries and Leaves and Their Antimicrobial Activity. J. Agric. Food Chem. 2003, 51, 3073–3078. [Google Scholar] [CrossRef] [PubMed]
- Mastelić, J.; Miloš, M.; Kuštrak, D.; Radonić, A. Essential Oil and Glycosidically Bound Volatile Compounds from the Needles of Common Juniper (Juniperus communis L.). Croat. Chem. Acta 2000, 73, 585–593. [Google Scholar]
- Rezvani, S.; Ali Rezai, M.; Mahmodi, N. Analysis of essential oils of Juniperus polycarpous and Juniperus communis. Asian J. Chem. 2007, 19, 5019–5022. [Google Scholar]
- Karapandzova, M.; Stefkov, G.; Cvetkovikj, I.; Kulevanova, S.; Sela, F. Chemical Composition and Antimicrobial Activity of Essential Oils of Juniperus Excelsa Bieb. (Cupressaceae) Grown in R. Macedonia. Pharmacogn. Res. 2015, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Peruč, D.; Gobin, I.; Abram, M.; Broznić, D.; Svalina, T.; Štifter, S.; Staver, M.M.; Tićac, B. Antimycobacterial Potential of the Juniper Berry Essential Oil in Tap Water. Arch. Ind. Hyg. Toxicol. 2018, 69, 46–54. [Google Scholar] [CrossRef]
- Peruč, D.; Tićac, B.; Abram, M.; Broznić, D.; Štifter, S.; Staver, M.M.; Gobin, I. Synergistic potential of Juniperus communis and Helichrysum italicum essential oils against nontuberculous mycobacteria. J. Med. Microbiol. 2019, 68, 703–710. [Google Scholar] [CrossRef]
- Peruc, D.; Ticac, B.; Broznic, D.; Maglica, Z.; Sarolic, M.; Gobin, I. Juniperus communis essential oil limit the biofilm formation of Mycobacterium avium and Mycobacterium intracellulare on polystyrene in a temperature-dependent manner. Int. J. Environ. Health Res. 2020, 32, 141–154. [Google Scholar] [CrossRef]
- Peruč, D.; Broznić, D.; Maglica, Ž.; Marijanović, Z.; Karleuša, L.; Gobin, I. Biofilm Degradation of Nontuberculous Mycobacteria Formed on Stainless Steel Following Treatment with Immortelle (Helchrysum italicum) and Common Juniper (Juniperus communis) Essential Oils. Processes 2021, 9, 362. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth, and Its Application in the in Vitro Antibacterial Screening of Phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Andréjak, C.; Almeida, D.V.; Tyagi, S.; Converse, P.J.; Ammerman, N.C.; Grosset, J.H. Characterization of Mouse Models of Mycobacterium avium Complex Infection and Evaluation of Drug Combinations. Antimicrob. Agents Chemother. 2015, 59, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard, and E test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.T. The Diagnosis and Management of Disease Caused by M. avium Complex, M. Kansasii, and Other Mycobacteria. Clin. Chest Med. 1989, 10, 431–443. [Google Scholar]
- Falkinham, J.O., III. Surrounded by Mycobacteria: Nontuberculous Mycobacteria in the Human Environment. J. Appl. Microbiol. 2009, 107, 356–367. [Google Scholar] [CrossRef]
- Brode, S.K.; Daley, C.L.; Marras, T.K. The Epidemiologic Relationship between Tuberculosis and Non-Tuberculous Mycobacterial Disease: A Systematic Review. Int. J. Tuberc. Lung Dis. 2014, 18, 1370–1377. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Farshidpour, M.; Ebrahimi, G.; Aliberti, S.; Falkinham, J.O. Management of Nontuberculous Mycobacterial Infection in the Elderly. Eur. J. Intern. Med. 2014, 25, 356–363. [Google Scholar] [CrossRef]
- Mirsaeidi, M.; Machado, R.F.; Garcia, J.G.N.; Schraufnagel, D.E. Nontuberculous Mycobacterial Disease Mortality in the United States, 1999–2010: A Population-Based Comparative Study. PLoS ONE 2014, 9, e91879. [Google Scholar] [CrossRef]
- van Ingen, J.; Hoefsloot, W.; Dekhuijzen, P.N.R.; Boeree, M.J.; van Soolingen, D. The Changing Pattern of Clinical Mycobacterium Avium Isolation in the Netherlands. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2010, 14, 1176–1180. [Google Scholar]
- Broznić, D.; Ratkaj, I.; Malenica Staver, M.; Kraljević Pavelić, S.; Žurga, P.; Bubalo, D.; Gobin, I. Evaluation of the Antioxidant Capacity, Antimicrobial and Antiproliferative Potential of Fir (Abies Alba Mill.) Honeydew Honey Collected from Gorski Kotar (Croatia). Food Technol. Biotechnol. 2018, 56, 533–545. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Sieniawska, E.; Swatko-Ossor, M.; Sawicki, R.; Ginalska, G. Morphological Changes in the Overall Mycobacterium tuberculosis H37Ra Cell Shape and Cytoplasm Homogeneity Due to Mutellina purpurea L. Essential Oil and Its Main Constituents. Med. Princ. Pract. 2015, 24, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial Activity of Essential Oils and Other Plant Extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of Diffusion and Dilution Methods to Determine the Antibacterial Activity of Plant Extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Peruč, D.; Tićac, B.; Broznić, D.; Gobin, I. Juniper and Immortelle Essential Oils Synergistically Inhibit Adhesion of Nontuberculous Mycobacteria to Acanthamoeba castellanii. Arch. Ind. Hyg. Toxicol. 2020, 71, 223–230. [Google Scholar] [CrossRef]
- Sieniawska, E.; Sawicki, R.; Swatko-Ossor, M.; Napiorkowska, A.; Przekora, A.; Ginalska, G.; Swatko-Ossor, M.; Augustynowicz-Kopec, E. The Effect of Combining Natural Terpenes and Antituberculous Agents against Reference and Clinical Mycobacterium tuberculosis Strains. Molecules 2018, 23, 176. [Google Scholar] [CrossRef]
- Sawicki, R.; Golus, J.; Przekora, A.; Ludwiczuk, A.; Sieniawska, E.; Ginalska, G. Antimycobacterial Activity of Cinnamaldehyde in a Mycobacterium tuberculosis (H37Ra) Model. Molecules 2018, 23, 2381. [Google Scholar] [CrossRef]
- Rodríguez–Beltrán, É.; López, G.D.; Anzola, J.M.; Rodríguez–Castillo, J.G.; Carazzone, C.; Murcia, M.I. Heterogeneous fitness landscape cues, pknG high expression, and phthiocerol dimycocerosate low production of Mycobacterium tuberculosis ATCC25618 rpoB S450L in enriched broth. Tuberculosis 2022, 132, 102156. [Google Scholar] [CrossRef]
- Rastogi, N.; Goh, K.S.; Horgen, L.; Barrow, W.W. Synergistic Activities of Antituberculous Drugs with Cerulenin and Trans -Cinnamic Acid against Mycobacterium tuberculosis. FEMS Immunol. Med. Microbiol. 1998, 21, 149–157. [Google Scholar] [CrossRef]
- Rastogi, N.; Goh, K.S.; Wright, E.L.; Barrow, W.W. Potential Drug Targets for Mycobacterium avium Defined by Radiometric Drug-Inhibitor Combination Techniques. Antimicrob. Agents Chemother. 1994, 38, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between Essential Oil Components and Antibiotics: A Review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Sherry, E.; Warnke, P.H. Successful Use of an Inhalational Phytochemical to Treat Pulmonary Tuberculosis: A Case Report. Phytomedicine 2004, 11, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Brown-Elliott, B.A.; Nash, K.A.; Wallace, R.J. Antimicrobial Susceptibility Testing, Drug Resistance Mechanisms, and Therapy of Infections with Nontuberculous mycobacteria. Clin. Microbiol. Rev. 2012, 25, 545–582. [Google Scholar] [CrossRef] [PubMed]
- Chiaradia, L.; Lefebvre, C.; Parra, J.; Marcoux, J.; Burlet-Schiltz, O.; Etienne, G.; Tropis, M.; Daffe, M. Dissecting the mycobacterial cell envelope and defining the composition of the native mycomembrane. Sci. Rep. 2017, 7, 12807. [Google Scholar] [CrossRef]
- Bakker-Woudenberg, I.A.J.M.; Van Vianen, W.; Van Soolingen, D.; Verbrugh, H.A.; Van Agtmael, M.A. Antimycobacterial Agents Differ with Respect to Their Bacteriostatic versus Bactericidal Activities in Relation to Time of Exposure, Mycobacterial Growth Phase, and Their Use in Combination. Antimicrob. Agents Chemother. 2005, 49, 2387–2398. [Google Scholar] [CrossRef]
- Reisner, B.S.; Woods, G.L.; Popov, V.L. Electron Microscopic Analysis of Mycobacterium avium Complex Isolates Exposed to Ciprofloxacin, Rifabutin, Ethambutol and Clarithromycin. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 1997, 1, 270–275. [Google Scholar]


| MIC/MBC (μg mL−1) | ||
|---|---|---|
| M. avium | M. intracellulare | |
| J. communis EO | 1600/1600 | 1600/1600 |
| Amikacin | 2/4 | 1/4 |
| Clarithromycin | 0.5/2 | 0.062/0.5 |
| Rifampicin | 2/8 | 1/2 |
| J. communis EO * | MIC = 1600 | 400 | 400 | 400 | 800 | 800 | 800 |
| Amikacin * | MIC = 2 | 0.25 | 0.5 | 1 | 0.25 | 0.5 | 1 |
| FICJU | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 | |
| FICAmika | 0.125 | 0.25 | 0.50 | 0.125 | 0.125 | 0.5 | |
| FICi | 0.375 | 0.5 | 0.75 | 0.625 | 0.75 | 1 | |
| Interaction | Si | Si | Ad | Ad | Ad | Ad | |
| J. communis EU * | MIC = 1600 | 200 | 200 | 200 | 400 | 400 | 400 |
| Clarithromycin * | MIC= 0.5 | 0.062 | 0.125 | 0.25 | 0.031 | 0.062 | 0.125 |
| FICJU | 0.125 | 0.125 | 0.125 | 0.25 | 0.25 | 0.25 | |
| FICCLR | 0.124 | 0.25 | 0.5 | 0.062 | 0.124 | 0.25 | |
| FICi | 0.249 | 0.375 | 0.625 | 0.312 | 0.374 | 0.5 | |
| Interaction | Si | Si | Ad | Si | Si | Si | |
| J. communis EU * | MIC = 1600 | 400 | 800 | 800 | 800 | 800 | |
| Clarithromycin * | MIC = 0.5 | 0.25 | 0.031 | 0.062 | 0.125 | 0.25 | |
| FICJU | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 | ||
| FICCLR | 0.5 | 0.062 | 0.124 | 0.25 | 0.5 | ||
| FICi | 0.75 | 0.562 | 0.624 | 0.75 | 1 | ||
| Interaction | Ad | Ad | Ad | Ad | Ad | ||
| J. communis EO * | MIC = 1600 | 25 | 50 | 100 | 200 | 200 | 200 |
| Rifampicin * | MIC = 2 | 1 | 1 | 1 | 0.25 | 0.5 | 1 |
| FICJU | 0.0156 | 0.03125 | 0.0625 | 0.125 | 0.125 | 0.125 | |
| FICRIF | 0.5 | 0.5 | 0.5 | 0.125 | 0.25 | 0.5 | |
| FICi | 0.516 | 0.531 | 0.562 | 0.250 | 0.375 | 0.625 | |
| Interaction | Ad | Ad | Ad | Si | Si | Ad | |
| J. communis EO * | MIC = 1600 | 400 | 400 | 400 | 800 | 800 | 800 |
| Rifampicin * | MIC = 2 | 0.25 | 0.5 | 1 | 0.25 | 0.5 | 1 |
| FICJU | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 | |
| FICRIF | 0.125 | 0.25 | 0.50 | 0.125 | 0.125 | 0.5 | |
| FICi | 0.375 | 0.5 | 0.75 | 0.625 | 0.75 | 1 | |
| Interaction | Si | Si | Ad | Ad | Ad | Ad | |
| J. communis EO * | MIC = 1600 | 400 | 400 | 400 | 800 | 800 | 800 | ||
| Amikacin * | MIC = 1 | 0.25 | 0.5 | 1 | 25 | 0.5 | 1 | ||
| FICJU | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 | |||
| FICAmika | 0.25 | 0.5 | 1 | 0.25 | 0.5 | 1 | |||
| FICi | 0.5 | 0.75 | 1.25 | 0.75 | 1 | 1.5 | |||
| Interaction | Si | Ad | In | Ad | Ad | In | |||
| J. communis EO * | MIC = 1600 | 800 | 800 | 800 | 800 | 1600 | 1600 | 1600 | 1600 |
| Clarithromycin * | MIC = 0.062 | 0.003875 | 0.00775 | 0.0155 | 0.031 | 0.003875 | 0.00775 | 0.0155 | 0.031 |
| FICJU | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | |
| FICCLR | 0.063 | 0.125 | 0.25 | 0.5 | 0.063 | 0.125 | 0.25 | 0.5 | |
| FICi | 0.563 | 0.625 | 0.75 | 1 | 1.063 | 1.125 | 1.25 | 1.5 | |
| Interaction | Ad | Ad | Ad | Ad | In | In | In | In | |
| J. communis EO * | MIC = 1600 | 400 | 400 | 400 | 400 | 400 | 400 | ||
| Rifampicin * | MIC = 1 | 0.015 | 0.031 | 0.062 | 0.125 | 0.25 | 0.5 | ||
| FICJU | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | |||
| FICRIF | 0.015 | 0.031 | 0.062 | 0.125 | 0.25 | 0.5 | |||
| FICi | 0.265 | 0.281 | 0.312 | 0.375 | 0.5 | 0.75 | |||
| Interaction | Si | Si | Si | Si | Si | Ad | |||
| J. communis EU * | MIC = 1600 | 800 | 800 | 800 | 800 | 800 | 800 | ||
| Rifampicin * | MIC = 1 | 0.015 | 0.031 | 0.062 | 0.125 | 0.25 | 0.5 | ||
| FICJU | 0.015 | 0.031 | 0.062 | 0.125 | 0.25 | 0.5 | |||
| FICRIF | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |||
| FICi | 0.515 | 0.531 | 0.562 | 0.625 | 0.75 | 1 | |||
| Interaction | Ad | Ad | Ad | Ad | Ad | Ad | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peruč, D.; Štifter-Vretenar, S.; Planinić, A.; Gobin, I. Combined Application of Juniperus communis Essential Oil and Amikacin, Clarithromycin and Rifampicin against Mycobacterium avium and Mycobacterium intracellulare. Processes 2024, 12, 111. https://doi.org/10.3390/pr12010111
Peruč D, Štifter-Vretenar S, Planinić A, Gobin I. Combined Application of Juniperus communis Essential Oil and Amikacin, Clarithromycin and Rifampicin against Mycobacterium avium and Mycobacterium intracellulare. Processes. 2024; 12(1):111. https://doi.org/10.3390/pr12010111
Chicago/Turabian StylePeruč, Dolores, Sanja Štifter-Vretenar, Ana Planinić, and Ivana Gobin. 2024. "Combined Application of Juniperus communis Essential Oil and Amikacin, Clarithromycin and Rifampicin against Mycobacterium avium and Mycobacterium intracellulare" Processes 12, no. 1: 111. https://doi.org/10.3390/pr12010111
APA StylePeruč, D., Štifter-Vretenar, S., Planinić, A., & Gobin, I. (2024). Combined Application of Juniperus communis Essential Oil and Amikacin, Clarithromycin and Rifampicin against Mycobacterium avium and Mycobacterium intracellulare. Processes, 12(1), 111. https://doi.org/10.3390/pr12010111







