Photodynamic Inactivation of Opportunistic Premise Plumbing Pathogens and Their Biofilms
Abstract
:1. Introduction
Current Treatments in Water Disinfection and Eradication of OPPPs
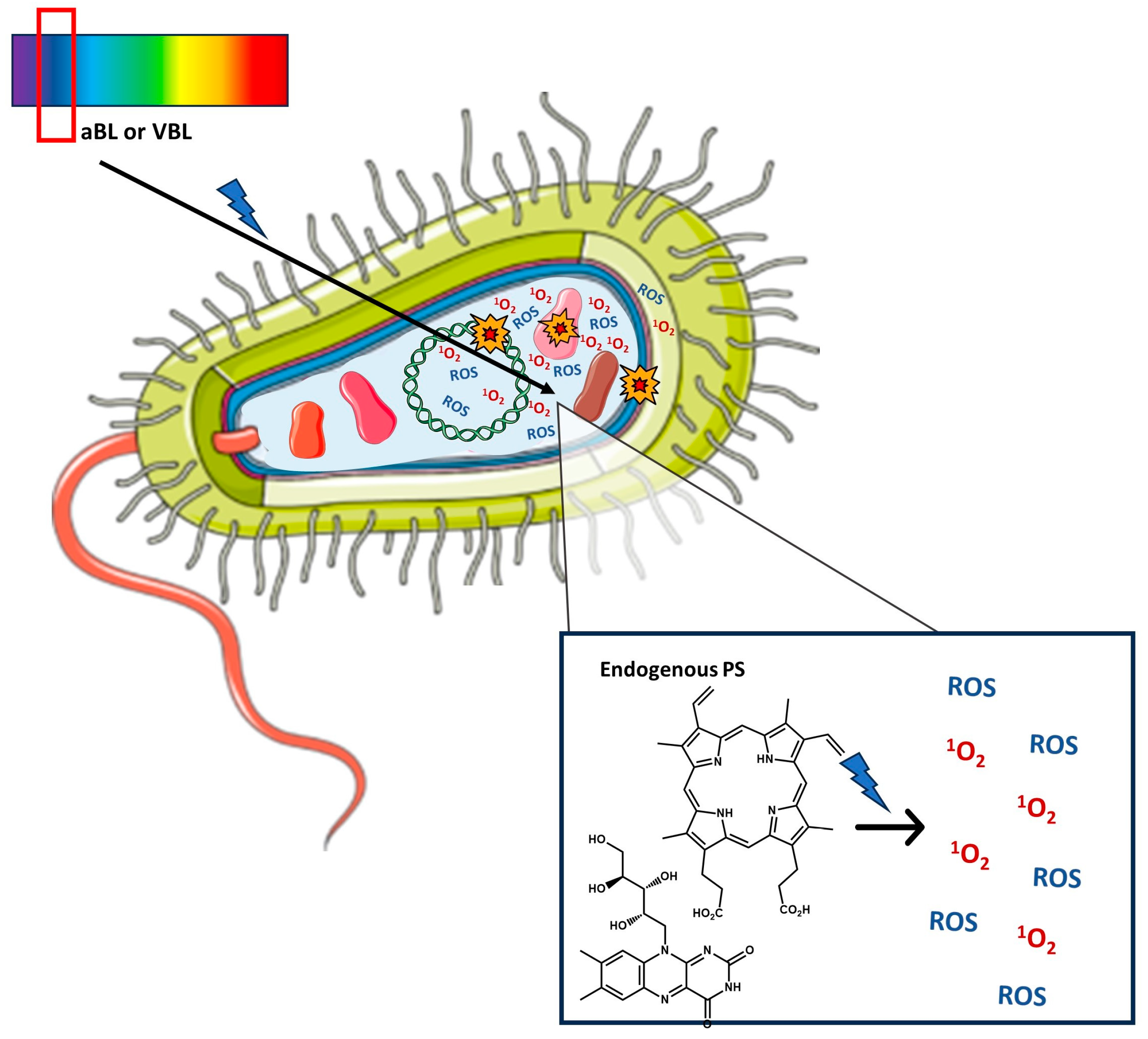
2. Antimicrobial Photoinactivation
2.1. Photodynamic Inactivation (PDI)—Mechanism, Exogenous Photosensitizers, Advantages, Limitations
3. Photodynamic Inactivation against OPPPs
3.1. Pseudomonas aeruginosa
3.2. Legionella pneumophila
3.3. Mycobacteria
3.4. Acinetobacter baumannii
3.5. Aeromonas hydrophila
| Bacterium | Photosensitizer/ Concentration | Irradiation Conditions | Antimicrobial Activity/CFU Reduction | Ref. |
|---|---|---|---|---|
| A. baumannii, clinical isolate II-a | riboflavin/0.011 mM | 440 nm; 25 mW/cm2; light dose 45 J/cm2 | 5-log reduction after 24 h | [164] |
| A. baumannii, MDR strain | riboflavin derivative (FLASH-07a)/50 μM | 380–600 nm; fluence rate 50 mW/cm2; light dose 4.5 J/cm2 | 6.7-log reduction | [124] |
| A. baumannii, MDR strain | 5,10,15,20-tetra(N-methylpyridinium-4-yl)porphyrin (TMPyP4)/ 5 μM | 380–700 nm; irradiance 40 W/m2; light dose 64.8 J/cm2 | >5-log reduction after 24 h | [162] |
| A. baumannii, MDR strain | methylene blue (MB)/ 0.1 mg/mL | 660 nm; irradiance 42.8 mW/cm2; light dose 30 J/cm2 | >7-log reduction after 24 h | [168] |
| A. baumannii, carbapenem-resistant | 2,7-dibromo-9-mesityl-10-methylacridinium perchlorate (YM-3)/170 μM | blue light; irradiance 15 W/m2; light dose 10.8 J/cm2 | complete photoinactivation | [170] |
| A. hydrophila, MDR strain | Zn(II) phthalocyanine (ZnPcOPyHe)/3 μM | 635 nm; fluence rate 100 mW/cm2; light dose 30 J/cm2 | complete photoinactivation after 24 h | [174] |
| A. hydrophila, MDR strain | Zn(II) phthalocyanine (ZnPcMe)/5 μM | 665 nm; fluence rate 100 mW/cm2; light dose 50 J/cm2 | complete photoinactivation after 48 h | [175] |
| L. pneumophila serogroup 1, strain 130b | 5-(4-octadecanamidophenyl)-10,15,20-tris(N-methylpyridinium-3-yl)porphyrin (TMPyP3-C17H35)/0.024 μM | 394 nm; fluence rate; 20 mW/cm2; light dose 12 J/cm2 | complete photoinactivation after 3–5 days | [143] |
| L. pneumophila serogroup 1, strain 130b | 5,10,15,20-tetra(N-methylpyridinium-3-yl)porphyrin (TMPyP3)/0.39 μM | 394 nm; fluence rate; 20 mW/cm2; light dose 36 J/cm2 | complete photoinactivation after 3–5 days | [144] |
| M. abscessus subsp. Abscessus | Pt(II) porphyrin (3-PtTPyP)/0.73 μg/mL | 400–800 nm; fluence rate 50 mW/cm2; light dose 270 J/cm2 | complete photoinactivation within 24 h | [155] |
| M. fortuitum | 5,10,15,20-tetra(N-methylpyridinium-4-yl)porphyrin (TMPyP4)/1.562 μM | 370–800 nm; fluence rate 50 mW/cm2; light dose 45 J/cm2 | complete photoinacti-vation within 48 h | [152] |
| P. aeruginosa, KCTC 2004 | methylene blue (MB)/ 750 μM | 660 nm; laser power 300 mW; light dose 30 J/cm2 | 5.5-log reduction after 24 h | [127] |
| P. aeruginosa, environmental strain | 5,10,15,20-tetra(N-methylpyridinium-4-yl)porphyrin (TMPyP4)/20 μM | 380–700 nm; irradiance 4 mW/cm2; light dose 43.2 J/cm2 | complete photoinactivation after 24 h | [135] |
| P. aeruginosa, MDR strain | pentacationic chlorin (derivative of TPPF20)/ 10 μM | 400–800 nm or 530–800 nm; 150 mW/cm2; light dose 270 J/cm2 | ~7-log reduction after 18 h | [138] |
| P. aeruginosa, MDR strain | riboflavin derivative (FLASH-01a)/50 μM | 380–600 nm; fluence rate 50 mW/cm2; light dose 1.5 J/cm2 | 6.8-log reduction | [124] |
| Stenotrophomonas maltophilia, clinical isolate SM3 | chlorophyllin/0.015 mM | 402 nm; 42 mW/cm2; light dose 50.4 J/cm2 | 4.2-log reduction after 24 h | [164] |
| Bacterium | Photosensitizer/ Concentration | Irradiation Conditions | Antimicrobial Activity/CFU Reduction | Ref. |
|---|---|---|---|---|
| A. baumannii, clinical isolate II-a | chlorophyllin/0.15 mM | 402 nm; 42 mW/cm2; light dose 151.2 J/cm2 | >4-log reduction after 17–20 h | [164] |
| A. baumannii, MDR strain | methylene blue (MB)/ 0.2 mg/mL | 660 nm; irradiance 42.8 mW/cm2; light dose 30 J/cm2 | 3.9-log reduction after 24 h | [168] |
| L. pneumophila serogroup 1, strain Philadelphia ST1 | 5-(4-acetamidophenyl)-10,15,20-tris(N-methylpyridinium-3-yl)porphyrin (TMPyP3-CH3)/3.125 μM | 394 nm; fluence rate; 20 mW/cm2; light dose 12 J/cm2 | complete biofilm destruction after 3–5 days | [145] |
| P. aeruginosa, KCTC 2004 | methylene blue (MB)/ 750 μM | 660 nm; laser power 300 mW; light dose 30 J/cm2 | 3-log reduction after 24 h | [127] |
| P. aeruginosa, environmental strain | 5,10,15,20-tetra(N-methylpyridinium-4-yl)porphyrin (TMPyP4)/20 μM | 380–700 nm; irradiance 4 mW/cm2; light dose 64.6 J/cm2 | 2.8-log reduction of viable cells and 81% reduction of polysaccharide content in the matrix after 24 h | [135] |
| P. aeruginosa, PAO1 wild type | 5,10,15,20-tetra(N-methylpyridinium-4-yl)porphyrin (TMPyP4)/225 μM | 400–600 nm (mercury lamp); 220–240 J/cm2 | >4-log reduction and detachment of the biofilm after 24 h | [133] |
| P. aeruginosa, PAO1 | dicationic diaryl porphyrin/30 μM | 410 nm; 100 mW/cm2; light dose 30 J/cm2 | 2-log reduction of adherent and planktonic cells in 24 h-old biofilm | [137] |
| S. maltophilia, clinical isolate SM3 | chlorophyllin/0.15 mM | 402 nm; 42 mW/cm2; light dose 151.2 J/cm2 | 5.3-log reduction after 17–20 h | [164] |
4. Major Challenges and Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leslie, E.; Hinds, J.; Hai, F.I. Causes, Factors, and Control Measures of Opportunistic Premise Plumbing Pathogens—A Critical Review. Appl. Sci. 2021, 11, 4474. [Google Scholar] [CrossRef]
- Falkinham, J. Common Features of Opportunistic Premise Plumbing Pathogens. Int. J. Environ. Res. Public Health 2015, 12, 4533–4545. [Google Scholar] [CrossRef]
- Wainwright, M.; Ali, T.A.; Barakah, F. A Review of the Role of Oligotrophic Micro-Organisms in Biodeterioration. Int. Biodeterior. Biodegrad. 1993, 31, 1–13. [Google Scholar] [CrossRef]
- Falkinham, J.; Pruden, A.; Edwards, M. Opportunistic Premise Plumbing Pathogens: Increasingly Important Pathogens in Drinking Water. Pathogens 2015, 4, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Falkinham, J.O.; Hilborn, E.D.; Arduino, M.J.; Pruden, A.; Edwards, M.A. Epidemiology and Ecology of Opportunistic Premise Plumbing Pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ. Health Perspect. 2015, 123, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B. Acinetobacter spp. as Nosocomial Pathogens: Epidemiology and Resistance Features. Saudi J. Biol. Sci. 2018, 25, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bravo, A.; Figueras, M.J. An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef]
- Ryan, M.P.; Pembroke, J.T. Brevundimonas spp.: Emerging Global Opportunistic Pathogens. Virulence 2018, 9, 480–493. [Google Scholar] [CrossRef]
- Booth, S.J. Chryseobacterium Related Genera Infections. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Wisplinghoff, H. Pseudomonas spp., Acinetobacter spp. and Miscellaneous Gram-Negative Bacilli. In Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1579–1599.e2. [Google Scholar]
- Owen, R.J. Helicobacter—Species Classification and Identification. Br. Med. Bull. 1998, 54, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Portal, E.; Descours, G.; Ginevra, C.; Mentasti, M.; Afshar, B.; Chand, M.; Day, J.; Echahidi, F.; Franzin, L.; Gaia, V.; et al. Legionella Antibiotic Susceptibility Testing: Is It Time for International Standardization and Evidence-Based Guidance? J. Antimicrob. Chemother. 2021, 76, 1113–1116. [Google Scholar] [CrossRef]
- Mazzotta, M.; Salaris, S.; Pascale, M.R.; Girolamini, L.; Cristino, S. Occurrence of Legionella Spp. in Man-Made Water Sources: Isolates Distribution and Phylogenetic Characterization in the Emilia-Romagna Region. Pathogens 2021, 10, 552. [Google Scholar] [CrossRef]
- Kovaleva, J.; Degener, J.E.; van der Mei, H.C. Methylobacterium and Its Role in Health Care-Associated Infection. J. Clin. Microbiol. 2014, 52, 1317–1321. [Google Scholar] [CrossRef]
- Busatto, C.; Vianna, J.S.; da Silva, L.V.; Ramis, I.B.; da Silva, P.E.A. Mycobacterium Avium: An Overview. Tuberculosis 2019, 114, 127–134. [Google Scholar] [CrossRef]
- Bédard, E.; Prévost, M.; Déziel, E. Pseudomonas aeruginosa in Premise Plumbing of Large Buildings. Microbiologyopen 2016, 5, 937–956. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M. Importance of the Identification of Segniliparus Species from Pulmonary Infection. New Microbes New Infect. 2018, 25, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.P.; Adley, C.C. Sphingomonas paucimobilis: A Persistent Gram-Negative Nosocomial Infectious Organism. J. Hosp. Infect. 2010, 75, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Chatterjee, S.; Mandal, N.C. Stenotrophomonas. In Beneficial Microbes in Agro-Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 427–442. [Google Scholar]
- Guimaraes, A.J.; Gomes, K.X.; Cortines, J.R.; Peralta, J.M.; Peralta, R.H.S. Acanthamoeba spp. as a Universal Host for Pathogenic Microorganisms: One Bridge from Environment to Host Virulence. Microbiol. Res. 2016, 193, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Delafont, V.; Rodier, M.-H.; Maisonneuve, E.; Cateau, E. Vermamoeba vermiformis: A Free-Living Amoeba of Interest. Microb. Ecol. 2018, 76, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Jahangeer, M.; Mahmood, Z.; Munir, N.; Waraich, U.; Tahir, I.M.; Akram, M.; Ali Shah, S.M.; Zulfqar, A.; Zainab, R. Naegleria fowleri: Sources of Infection, Pathophysiology, Diagnosis, and Management; A Review. Clin. Exp. Pharmacol. Physiol. 2020, 47, 199–212. [Google Scholar] [CrossRef]
- Piñero, J.E.; Chávez-Munguía, B.; Omaña-Molina, M.; Lorenzo-Morales, J. Naegleria fowleri . Trends Parasitol. 2019, 35, 848–849. [Google Scholar] [CrossRef]
- Tavares, M.; Kozak, M.; Balola, A.; Sá-Correia, I. Burkholderia cepacia Complex Bacteria: A Feared Contamination Risk in Water-Based Pharmaceutical Products. Clin. Microbiol. Rev. 2020, 33, e00139-19. [Google Scholar] [CrossRef]
- Yung, C.-F.; Maiwald, M.; Loo, L.H.; Soong, H.Y.; Tan, C.B.; Lim, P.K.; Li, L.; Tan, N.W.; Chong, C.-Y.; Tee, N.; et al. Elizabethkingia anophelis and Association with Tap Water and Handwashing, Singapore. Emerg. Infect. Dis. 2018, 24, 1730–1733. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, M.; Jeong, S.J.; Choi, J.Y.; Lee, I.-Y.; Yong, T.-S.; Yong, D.; Jeong, S.H.; Lee, K. Risk Factors for Elizabethkingia Acquisition and Clinical Characteristics of Patients, South Korea. Emerg. Infect. Dis. 2019, 25, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.P.; Sevjahova, L.; Gorman, R.; White, S. The Emergence of the Genus Comamonas as Important Opportunistic Pathogens. Pathogens 2022, 11, 1032. [Google Scholar] [CrossRef]
- Ryan, M.P.; Pembroke, J.T. The Genus Ochrobactrum as Major Opportunistic Pathogens. Microorganisms 2020, 8, 1797. [Google Scholar] [CrossRef] [PubMed]
- Said, M.; van Hougenhouck-Tulleken, W.; Naidoo, R.; Mbelle, N.; Ismail, F. Outbreak of Ralstonia mannitolilytica Bacteraemia in Patients Undergoing Haemodialysis at a Tertiary Hospital in Pretoria, South Africa. Antimicrob. Resist. Infect. Control 2020, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jin, Y.; Bai, F.; Jin, S. Pseudomonas aeruginosa. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 753–767. [Google Scholar]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical Relevance of the ESKAPE Pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Favero, M.S.; Carson, L.A.; Bond, W.W.; Petersen, N.J. Pseudomonas aeruginosa: Growth in Distilled Water from Hospitals. Science 1971, 173, 836–838. [Google Scholar] [CrossRef]
- Gonçalves, I.G.; Simões, L.C.; Simões, M. Legionella pneumophila . Trends Microbiol. 2021, 29, 860–861. [Google Scholar] [CrossRef]
- Hayward, C.; Ross, K.E.; Brown, M.H.; Bentham, R.; Whiley, H. The Presence of Opportunistic Premise Plumbing Pathogens in Residential Buildings: A Literature Review. Water 2022, 14, 1129. [Google Scholar] [CrossRef]
- Dowdell, K.; Haig, S.-J.; Caverly, L.J.; Shen, Y.; LiPuma, J.J.; Raskin, L. Nontuberculous Mycobacteria in Drinking Water Systems—The Challenges of Characterization and Risk Mitigation. Curr. Opin. Biotechnol. 2019, 57, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Szwetkowski, K.J.; Falkinham, J.O. Methylobacterium spp. as Emerging Opportunistic Premise Plumbing Pathogens. Pathogens 2020, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Carvalheira, A.; Silva, J.; Teixeira, P. Acinetobacter Spp. in Food and Drinking Water—A Review. Food Microbiol. 2021, 95, 103675. [Google Scholar] [CrossRef]
- Lee, K.; Yong, D.; Jeong, S.H.; Chong, Y. Multidrug-Resistant Acinetobacter spp.: Increasingly Problematic Nosocomial Pathogens. Yonsei Med. J. 2011, 52, 879. [Google Scholar] [CrossRef]
- Rhoads, W.J.; Pruden, A.; Edwards, M.A. Anticipating Challenges with In-Building Disinfection for Control of Opportunistic Pathogens. Water Environ. Res. 2014, 86, 540–549. [Google Scholar] [CrossRef]
- Wang, H.; Bédard, E.; Prévost, M.; Camper, A.K.; Hill, V.R.; Pruden, A. Methodological Approaches for Monitoring Opportunistic Pathogens in Premise Plumbing: A Review. Water Res. 2017, 117, 68–86. [Google Scholar] [CrossRef]
- Burtscher, M.; Zibuschka, F.; Mach, R.; Lindner, G.; Farnleitner, A. Heterotrophic Plate Count vs. in situ Bacterial 16S RRNA Gene Amplicon Profiles from Drinking Water Reveal Completely Different Communities with Distinct Spatial and Temporal Allocations in a Distribution Net. Water SA 2009, 35, 495–504. [Google Scholar] [CrossRef]
- Ciesielski, C.A.; Blaser, M.J.; Wang, W.L. Role of Stagnation and Obstruction of Water Flow in Isolation of Legionella pneumophila from Hospital Plumbing. Appl. Environ. Microbiol. 1984, 48, 984–987. [Google Scholar] [CrossRef]
- Norton, C.D.; LeChevallier, M.W.; Falkinham, J.O. Survival of Mycobacterium avium in a Model Distribution System. Water Res. 2004, 38, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Grobe, S.; Wingender, J.; Flemming, H.-C. Capability of Mucoid Pseudomonas aeruginosa to Survive in Chlorinated Water. Int. J. Hyg. Environ. Health 2001, 204, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Hessler, C.M.; Panmanee, W.; Hassett, D.J.; Seo, Y. Pseudomonas aeruginosa Inactivation Mechanism Is Affected by Capsular Extracellular Polymeric Substances Reactivity with Chlorine and Monochloramine. FEMS Microbiol. Ecol. 2013, 83, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Ofori, I.; Maddila, S.; Lin, J.; Jonnalagadda, S.B. Chlorine Dioxide Inactivation of Pseudomonas Aeruginosa and Staphylococcus aureus in Water: The Kinetics and Mechanism. J. Water Process Eng. 2018, 26, 46–54. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, C.; Lin, H.; Lv, L.; Yu, X. UV Disinfection Induces a Vbnc State in Escherichia coli and Pseudomonas aeruginosa. Environ. Sci. Technol. 2015, 49, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Bédard, E.; Charron, D.; Lalancette, C.; Déziel, E.; Prévost, M. Recovery of Pseudomonas aeruginosa Culturability Following Copper- and Chlorine-Induced Stress. FEMS Microbiol. Lett. 2014, 356, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.R.; Hanlon, G.W. Resistance of Legionella pneumophila Serotype 1 Biofilms to Chlorine-Based Disinfection. J. Hosp. Infect. 2010, 74, 152–159. [Google Scholar] [CrossRef] [PubMed]
- García, M.T.; Jones, S.; Pelaz, C.; Millar, R.D.; Abu Kwaik, Y. Acanthamoeba Polyphaga Resuscitates Viable Non-Culturable Legionella pneumophila after Disinfection. Environ. Microbiol. 2007, 9, 1267–1277. [Google Scholar] [CrossRef]
- Lin, Y.E.; Vidic, R.D.; Stout, J.E.; McCartney, C.A.; Yu, V.L. Inactivation of Mycobacterium avium by Copper and Silver Ions. Water Res. 1998, 32, 1997–2000. [Google Scholar] [CrossRef]
- Hinenoya, A.; Awasthi, S.P.; Yasuda, N.; Shima, A.; Morino, H.; Koizumi, T.; Fukuda, T.; Miura, T.; Shibata, T.; Yamasaki, S. Chlorine Dioxide Is a Better Disinfectant than Sodium Hypochlorite against Multi-Drug Resistant Staphylococcus aureus, Pseudomonas aeruginosa and Acinetobacter baumannii. Jpn. J. Infect. Dis. 2015, 68, 276–279. [Google Scholar] [CrossRef]
- Cullom, A.; Spencer, M.S.; Williams, M.D.; Falkinham, J.O., III; Pruden, A.; Edwards, M.A. Influence of Pipe Materials on In-Building Disinfection of P. aeruginosa and A. baumannii in Simulated Hot Water Plumbing. Water Res. X 2023, 21, 100189. [Google Scholar] [CrossRef]
- O’Keeffe, J. Climate Change and Opportunistic Pathogens (OPs) in the Built Environment. Environ. Health Rev. 2022, 65, 69–76. [Google Scholar] [CrossRef]
- Pullerits, K.; Ahlinder, J.; Holmer, L.; Salomonsson, E.; Öhrman, C.; Jacobsson, K.; Dryselius, R.; Forsman, M.; Paul, C.J.; Rådström, P. Impact of UV Irradiation at Full Scale on Bacterial Communities in Drinking Water. NPJ Clean Water 2020, 3, 11. [Google Scholar] [CrossRef]
- Gandhi, J.; Prakash, H. Photo-Disinfection Processes for Bacterial Inactivation and Underlying Principles for Water Constituents’ Impact: A Review. Chem. Eng. J. Adv. 2023, 14, 100482. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Singh, H.; Deep, A.; Khatri, M.; Bhaumik, J.; Kim, K.-H.; Bhardwaj, N. UVC-Based Photoinactivation as an Efficient Tool to Control the Transmission of Coronaviruses. Sci. Total Environ. 2021, 792, 148548. [Google Scholar] [CrossRef]
- Yin, R.; Dai, T.; Avci, P.; Jorge, A.E.S.; de Melo, W.C.; Vecchio, D.; Huang, Y.-Y.; Gupta, A.; Hamblin, M.R. Light Based Anti-Infectives: Ultraviolet C Irradiation, Photodynamic Therapy, Blue Light, and Beyond. Curr. Opin. Pharmacol. 2013, 13, 731–762. [Google Scholar] [CrossRef]
- Wang, H.; Hu, H.; Chen, S.; Schwarz, C.; Yin, H.; Hu, C.; Li, G.; Shi, B.; Huang, J. UV Pretreatment Reduced Biofouling of Ultrafiltration and Controlled Opportunistic Pathogens in Secondary Water Supply Systems. Desalination 2023, 548, 116282. [Google Scholar] [CrossRef]
- Halstead, F.D.; Hadis, M.A.; Marley, N.; Brock, K.; Milward, M.R.; Cooper, P.R.; Oppenheim, B.; Palin, W.M. Violet-Blue Light Arrays at 405 Nanometers Exert Enhanced Antimicrobial Activity for Photodisinfection of Monomicrobial Nosocomial Biofilms. Appl. Environ. Microbiol. 2019, 85, e01346-19. [Google Scholar] [CrossRef]
- Amodeo, D.; Lucarelli, V.; De Palma, I.; Puccio, A.; Nante, N.; Cevenini, G.; Messina, G. Efficacy of Violet–Blue Light to Inactive Microbial Growth. Sci. Rep. 2022, 12, 20179. [Google Scholar] [CrossRef]
- Halstead, F.D.; Thwaite, J.E.; Burt, R.; Laws, T.R.; Raguse, M.; Moeller, R.; Webber, M.A.; Oppenheim, B.A. Antibacterial Activity of Blue Light against Nosocomial Wound Pathogens Growing Planktonically and as Mature Biofilms. Appl. Environ. Microbiol. 2016, 82, 4006–4016. [Google Scholar] [CrossRef]
- Hoenes, K.; Bauer, R.; Meurle, T.; Spellerberg, B.; Hessling, M. Inactivation Effect of Violet and Blue Light on ESKAPE Pathogens and Closely Related Non-Pathogenic Bacterial Species—A Promising Tool Against Antibiotic-Sensitive and Antibiotic-Resistant Microorganisms. Front. Microbiol. 2021, 11, 612367. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial Blue Light Inactivation of Pathogenic Microbes: State of the Art. Drug Resist. Updates 2017, 33–35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.; Morici, P.; Ghetti, F.; Sgarbossa, A. Spectroscopic Characterization and Fluorescence Imaging of Helicobacter pylori Endogenous Porphyrins. Biophys. Chem. 2017, 229, 19–24. [Google Scholar] [CrossRef]
- Morici, P.; Battisti, A.; Tortora, G.; Menciassi, A.; Checcucci, G.; Ghetti, F.; Sgarbossa, A. The in Vitro Photoinactivation of Helicobacter pylori by a Novel LED-Based Device. Front. Microbiol. 2020, 11, 283. [Google Scholar] [CrossRef]
- Cossu, M.; Ledda, L.; Cossu, A. Emerging Trends in the Photodynamic Inactivation (PDI) Applied to the Food Decontamination. Food Res. Int. 2021, 144, 110358. [Google Scholar] [CrossRef]
- Murdoch, L.E.; Maclean, M.; Endarko, E.; MacGregor, J.S.; Anderson, G.J. Bactericidal Effects of 405 Nm Light Exposure Demonstrated by Inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium Species in Liquid Suspensions and on Exposed Surfaces. Sci. World J. 2012, 2012, 137805. [Google Scholar] [CrossRef]
- dos Anjos, C.; Sellera, F.P.; de Freitas, L.M.; Gargano, R.G.; Telles, E.O.; Freitas, R.O.; Baptista, M.S.; Ribeiro, M.S.; Lincopan, N.; Pogliani, F.C.; et al. Inactivation of Milk-Borne Pathogens by Blue Light Exposure. J. Dairy Sci. 2020, 103, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Maraccini, P.A.; Wenk, J.; Boehm, A.B. Exogenous Indirect Photoinactivation of Bacterial Pathogens and Indicators in Water with Natural and Synthetic Photosensitizers in Simulated Sunlight with Reduced UVB. J. Appl. Microbiol. 2016, 121, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Luksiene, Z.; Brovko, L. Antibacterial Photosensitization-Based Treatment for Food Safety. Food Eng. Rev. 2013, 5, 185–199. [Google Scholar] [CrossRef]
- Luksiene, Z.; Kokstaite, R.; Katauskis, P.; Skakauskas, V. Novel Approach to Effective and Uniform Inactivation of Gram-Positive Listeria Monocytogenes and Gram-Negative Salmonella enterica by Photosensitization. Food Technol. Biotechnol. 2013, 51, 338–344. [Google Scholar]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal Photodynamic Therapy. Microbiol. Res. 2008, 163, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.A.R.; Ahn, Y.-H. Green Phytoextracts as Natural Photosensitizers in LED-Based Photodynamic Disinfection of Multidrug-Resistant Bacteria in Wastewater Effluent. Chemosphere 2022, 297, 134157. [Google Scholar] [CrossRef]
- Sabbahi, S.; Ben Ayed, L.; Boudabbous, A. Cationic, Anionic and Neutral Dyes: Effects of Photosensitizing Properties and Experimental Conditions on the Photodynamic Inactivation of Pathogenic Bacteria. J. Water Health 2013, 11, 590–599. [Google Scholar] [CrossRef]
- Ndlovu, K.S.; Moloto, M.J.; Sekhosana, K.E.; Nkambule, T.T.I.; Managa, M. Porphyrins Developed for Photoinactivation of Microbes in Wastewater. Environ. Sci. Pollut. Res. 2022, 30, 11210–11225. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Reis, S.; Fontes, M.; Neves, M.; Faustino, M.; Almeida, A. Photodynamic Action against Wastewater Microorganisms and Chemical Pollutants: An Effective Approach with Low Environmental Impact. Water 2017, 9, 630. [Google Scholar] [CrossRef]
- Matafonova, G.; Batoev, V. Dual-Wavelength Light Radiation for Synergistic Water Disinfection. Sci. Total Environ. 2022, 806, 151233. [Google Scholar] [CrossRef] [PubMed]
- Nikinmaa, S.; Alapulli, H.; Auvinen, P.; Vaara, M.; Rantala, J.; Kankuri, E.; Sorsa, T.; Meurman, J.; Pätilä, T. Dual-Light Photodynamic Therapy Administered Daily Provides a Sustained Antibacterial Effect on Biofilm and Prevents Streptococcus mutans Adaptation. PLoS ONE 2020, 15, e0232775. [Google Scholar] [CrossRef] [PubMed]
- Aroso, R.T.; Schaberle, F.A.; Arnaut, L.G.; Pereira, M.M. Photodynamic Disinfection and Its Role in Controlling Infectious Diseases. Photochem. Photobiol. Sci. 2021, 20, 1497–1545. [Google Scholar] [CrossRef]
- Anas, A.; Sobhanan, J.; Sulfiya, K.M.; Jasmin, C.; Sreelakshmi, P.K.; Biju, V. Advances in Photodynamic Antimicrobial Chemotherapy. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100452. [Google Scholar] [CrossRef]
- Bae, I.K.; Shin, J.-Y.; Son, J.-H.; Wang, K.-K.; Han, W.-S. Antibacterial Effect of Singlet Oxygen Depending on Bacteria Surface Charge. Photodiagn. Photodyn. Ther. 2022, 39, 102975. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Magaraggia, M.; Fabris, C.; Soncin, M.; Camerin, M.; Tallandini, L.; Coppellotti, O.; Guidolin, L. Photodynamic Inactivation of Microbial Pathogens: Disinfection of Water and Prevention of Water-Borne Diseases. J. Environ. Pathol. Toxicol. Oncol. 2011, 30, 261–271. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Nadais, H.; Almeida, A. Potential Applications of Porphyrins in Photodynamic Inactivation beyond the Medical Scope. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial Photodynamic Therapy: Overview of a Promising Approach to Fight Antibiotic-Resistant Bacterial Infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar]
- Maisch, T.; Szeimies, R.-M.; Jori, G.; Abels, C. Antibacterial Photodynamic Therapy in Dermatology. Photochem. Photobiol. Sci. 2004, 3, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T. Resistance in Antimicrobial Photodynamic Inactivation of Bacteria. Photochem. Photobiol. Sci. 2015, 14, 1518–1526. [Google Scholar] [CrossRef]
- Spagnul, C.; Turner, L.C.; Boyle, R.W. Immobilized Photosensitizers for Antimicrobial Applications. J. Photochem. Photobiol. B 2015, 150, 11–30. [Google Scholar] [CrossRef]
- Sabino, C.P.; Wainwright, M.; Ribeiro, M.S.; Sellera, F.P.; dos Anjos, C.; Baptista, M.d.S.; Lincopan, N. Global Priority Multidrug-Resistant Pathogens Do Not Resist Photodynamic Therapy. J. Photochem. Photobiol. B 2020, 208, 111893. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Patel, S.K.S.; Lee, J.-K. Bacterial Biofilm Inhibitors: An Overview. Ecotoxicol. Environ. Saf. 2023, 264, 115389. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Cheng, J.; Wang, J.; Li, P.; Lin, J. Treatment of Pseudomonas aeruginosa Infectious Biofilms: Challenges and Strategies. Front. Microbiol. 2022, 13, 3325. [Google Scholar] [CrossRef]
- Songca, S.P.; Adjei, Y. Applications of Antimicrobial Photodynamic Therapy against Bacterial Biofilms. Int. J. Mol. Sci. 2022, 23, 3209. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Rożej, A.; Cydzik-Kwiatkowska, A.; Kowalska, B.; Kowalski, D. Structure and Microbial Diversity of Biofilms on Different Pipe Materials of a Model Drinking Water Distribution Systems. World J. Microbiol. Biotechnol. 2015, 31, 37–47. [Google Scholar] [CrossRef]
- Vaz-Moreira, I.; Egas, C.; Nunes, O.C.; Manaia, C.M. Bacterial Diversity from the Source to the Tap: A Comparative Study Based on 16S RRNA Gene-DGGE and Culture-Dependent Methods. FEMS Microbiol. Ecol. 2013, 83, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-J. Effects of Diverse Water Pipe Materials on Bacterial Communities and Water Quality in the Annular Reactor. J. Microbiol. Biotechnol. 2011, 21, 115–123. [Google Scholar] [CrossRef]
- Morvay, A.A.; Decun, M.; Scurtu, M.; Sala, C.; Morar, A.; Sarandan, M. Biofilm Formation on Materials Commonly Used in Household Drinking Water Systems. Water Supply 2011, 11, 252–257. [Google Scholar] [CrossRef]
- Song, X.; Zhang, G.; Zhou, Y.; Li, W. Behaviors and Mechanisms of Microbially-Induced Corrosion in Metal-Based Water Supply Pipelines: A Review. Sci. Total Environ. 2023, 895, 165034. [Google Scholar] [CrossRef] [PubMed]
- Azimi, S.; Klementiev, A.D.; Whiteley, M.; Diggle, S.P. Bacterial Quorum Sensing During Infection. Annu. Rev. Microbiol. 2020, 74, 201–219. [Google Scholar] [CrossRef]
- Garcez, A.S.; Núñez, S.C.; Azambuja, N.; Fregnani, E.R.; Rodriguez, H.M.H.; Hamblin, M.R.; Suzuki, H.; Ribeiro, M.S. Effects of Photodynamic Therapy on Gram-Positive and Gram-Negative Bacterial Biofilms by Bioluminescence Imaging and Scanning Electron Microscopic Analysis. Photomed. Laser Surg. 2013, 31, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial Photodynamic Therapy for Inactivation of Biofilms Formed by Oral Key Pathogens. Front. Microbiol. 2014, 5, 405. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Späth, A.; Regensburger, J.; Gollmer, A.; Tabenski, L.; Hiller, K.-A.; Bäumler, W.; Maisch, T.; Schmalz, G. Photodynamic Biofilm Inactivation by SAPYR—An Exclusive Singlet Oxygen Photosensitizer. Free Radic. Biol. Med. 2013, 65, 477–487. [Google Scholar] [CrossRef]
- Mohamad, F.; Alzahrani, R.R.; Alsaadi, A.; Alrfaei, B.M.; Yassin, A.E.B.; Alkhulaifi, M.M.; Halwani, M. An Explorative Review on Advanced Approaches to Overcome Bacterial Resistance by Curbing Bacterial Biofilm Formation. Infect. Drug Resist. 2023, 16, 19–49. [Google Scholar] [CrossRef]
- Wainwright, M.; Crossley, K.B. Photosensitising Agents—Circumventing Resistance and Breaking down Biofilms: A Review. Int. Biodeterior. Biodegrad. 2004, 53, 119–126. [Google Scholar] [CrossRef]
- Eckl, D.B.; Landgraf, N.; Hoffmann, A.K.; Schottenhaml, L.; Dirscherl, J.; Weber, N.; Eben, S.S.; Bäßler, P.; Eichner, A.; Huber, H.; et al. Inhibitory Effects of Calcium or Magnesium Ions on PDI. J. Photochem. Photobiol. 2022, 11, 100122. [Google Scholar] [CrossRef]
- Eckl, D.B.; Eben, S.S.; Schottenhaml, L.; Eichner, A.; Vasold, R.; Späth, A.; Bäumler, W.; Huber, H. Interplay of Phosphate and Carbonate Ions with Flavin Photosensitizers in Photodynamic Inactivation of Bacteria. PLoS ONE 2021, 16, e0253212. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu, M.; Monteiro, C.J.P.; Fontes, M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Photodynamic Inactivation of Microorganisms in Different Water Matrices: The Effect of Physicochemical Parameters on the Treatment Outcome. Sci. Total Environ. 2023, 860, 160427. [Google Scholar] [CrossRef] [PubMed]
- Lui, G.Y.; Roser, D.; Corkish, R.; Ashbolt, N.J.; Stuetz, R. Point-of-Use Water Disinfection Using Ultraviolet and Visible Light-Emitting Diodes. Sci. Total Environ. 2016, 553, 626–635. [Google Scholar] [CrossRef]
- Kuznetsova, N.A.; Kaliya, O.L. Photodynamic Water Disinfection. Russ. J. Gen. Chem. 2015, 85, 321–332. [Google Scholar] [CrossRef]
- Camargo, C.; Conceição, V.; Martins, A.; Plepis, A.; Perussi, J.R. Photoinactivation of Gram-Negative Bacteria in Circulating Water Using Chitosan Membranes Containing Porphyrin. Biol. Chem. Res. 2014, 1, 67–75. [Google Scholar]
- Woźniak, A.; Grinholc, M. Combined Antimicrobial Blue Light and Antibiotics as a Tool for Eradication of Multidrug-Resistant Isolates of Pseudomonas aeruginosa and Staphylococcus aureus: In Vitro and In Vivo Studies. Antioxidants 2022, 11, 1660. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, X.; Szewczyk, G.; El-Hussein, A.; Huang, Y.-Y.; Sarna, T.; Hamblin, M.R. Potassium Iodide Potentiates Antimicrobial Photodynamic Inactivation Mediated by Rose Bengal in In Vitro and In Vivo Studies. Antimicrob. Agents Chemother. 2017, 61, e00467-17. [Google Scholar] [CrossRef]
- Rezaie, P.; Pourhajibagher, M.; Chiniforush, N.; Hosseini, N.; Bahador, A. The Effect of Quorum-Sensing and Efflux Pumps Interactions in Pseudomonas aeruginosa Against Photooxidative Stress. J. Lasers Med. Sci. 2018, 9, 161–167. [Google Scholar] [CrossRef]
- Swetha, S.; Santhosh, S.M.; Geetha Balakrishna, R. Enhanced Bactericidal Activity of Modified Titania in Sunlight against Pseudomonas aeruginosa, a Water-Borne Pathogen. Photochem. Photobiol. 2010, 86, 1127–1134. [Google Scholar] [CrossRef]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial Blue Light Inactivation of Pseudomonas aeruginosa by Photo-Excitation of Endogenous Porphyrins: In Vitro and in Vivo Studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef]
- Keshishyan, E.S.; Zaporozhtseva, Z.V.; Zenina, O.M.; Zrodnikov, V.S. Photodynamic Inactivation of Bacteria In Vitro Under the Effect of Blue Light. Bull. Exp. Biol. Med. 2015, 158, 475–477. [Google Scholar] [CrossRef]
- Fila, G.; Krychowiak, M.; Rychlowski, M.; Bielawski, K.P.; Grinholc, M. Antimicrobial Blue Light Photoinactivation of Pseudomonas aeruginosa: Quorum Sensing Signaling Molecules, Biofilm Formation and Pathogenicity. J. Biophotonics 2018, 11, e201800079. [Google Scholar] [CrossRef] [PubMed]
- Fila, G.; Kawiak, A.; Grinholc, M.S. Blue Light Treatment of Pseudomonas aeruginosa: Strong Bactericidal Activity, Synergism with Antibiotics and Inactivation of Virulence Factors. Virulence 2017, 8, 938–958. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, V.T.; Martegani, E.; Bolognese, F. Catalase A Is Involved in the Response to Photooxidative Stress in Pseudomonas aeruginosa. Photodiagn. Photodyn. Ther. 2018, 22, 233–240. [Google Scholar] [CrossRef]
- Bohm, G.C.; Gándara, L.; Di Venosa, G.; Mamone, L.; Buzzola, F.; Casas, A. Photodynamic Inactivation Mediated by 5-Aminolevulinic Acid of Bacteria in Planktonic and Biofilm Forms. Biochem. Pharmacol. 2020, 177, 114016. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-F.; Lee, C.-J.; Chen, C.-T.; Huang, C.-T. δ-Aminolaevulinic Acid Mediated Photodynamic Antimicrobial Chemotherapy on Pseudomonas aeruginosa Planktonic and Biofilm Cultures. J. Photochem. Photobiol. B 2004, 75, 21–25. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Caruso, E.; Banfi, S.; Barbieri, P. Effect of Organic Matter on the In Vitro Photoeradication of Pseudomonas aeruginosa by Means of a Cationic Tetraaryl-Porphyrin. Photochem. Photobiol. 2012, 88, 557–564. [Google Scholar] [CrossRef]
- del Valle, C.A.; Pérez-Laguna, V.; Resta, I.M.; Gavara, R.; Felip-León, C.; Miravet, J.F.; Rezusta, A.; Galindo, F. A Cost-Effective Combination of Rose Bengal and off-the-Shelf Cationic Polystyrene for the Photodynamic Inactivation of Pseudomonas aeruginosa. Mater. Sci. Eng. C 2020, 117, 111302. [Google Scholar] [CrossRef]
- Maisch, T.; Eichner, A.; Späth, A.; Gollmer, A.; König, B.; Regensburger, J.; Bäumler, W. Fast and Effective Photodynamic Inactivation of Multiresistant Bacteria by Cationic Riboflavin Derivatives. PLoS ONE 2014, 9, e111792. [Google Scholar] [CrossRef]
- Hashimoto, M.C.E.; Prates, R.A.; Kato, I.T.; Núñez, S.C.; Courrol, L.C.; Ribeiro, M.S. Antimicrobial Photodynamic Therapy on Drug-Resistant Pseudomonas aeruginosa-Induced Infection. An in Vivo Study. Photochem. Photobiol. 2012, 88, 590–595. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Bolognese, F.; Caruso, E. Searching for Antimicrobial Photosensitizers among a Panel of BODIPYs. Photochem. Photobiol. Sci. 2022, 21, 1233–1248. [Google Scholar] [CrossRef]
- Yang, S.M.; Lee, D.W.; Park, H.J.; Kwak, M.H.; Park, J.M.; Choi, M. Hydrogen Peroxide Enhances the Antibacterial Effect of Methylene Blue-based Photodynamic Therapy on Biofilm-forming Bacteria. Photochem. Photobiol. 2019, 95, 833–838. [Google Scholar] [CrossRef]
- Pereira, A.H.C.; Pinto, J.G.; Freitas, M.A.A.; Fontana, L.C.; Pacheco Soares, C.; Ferreira-Strixino, J. Methylene Blue Internalization and Photodynamic Action against Clinical and ATCC Pseudomonas aeruginosa and Staphyloccocus aureus Strains. Photodiagn. Photodyn. Ther. 2018, 22, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Street, C.N.; Gibbs, A.; Pedigo, L.; Andersen, D.; Loebel, N.G. In Vitro Photodynamic Eradication of Pseudomonas aeruginosa in Planktonic and Biofilm Culture. Photochem. Photobiol. 2009, 85, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Wylie, M.P.; Craig, R.A.; Gorman, S.P.; McCoy, C.P. Development of a High-Level Light-Activated Disinfectant for Hard Surfaces and Medical Devices. Int. J. Antimicrob. Agents 2021, 58, 106360. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Bolognese, F.; Martegani, E.; Cantaluppi, V.; Medana, C.; Barbieri, P. Response to Photo-Oxidative Stress of Pseudomonas aeruginosa PAO1 Mutants Impaired in Different Functions. Microbiology 2017, 163, 1557–1567. [Google Scholar] [CrossRef]
- Chiodaroli, L.; Tolker-Nielsen, T.; Orlandi, V.T.; Bolognese, F.; Barbieri, P. Pigments Influence the Tolerance of Pseudomonas aeruginosa PAO1 to Photodynamically Induced Oxidative Stress. Microbiology 2015, 161, 2298–2309. [Google Scholar] [CrossRef]
- Collins, T.L.; Markus, E.A.; Hassett, D.J.; Robinson, J.B. The Effect of a Cationic Porphyrin on Pseudomonas aeruginosa Biofilms. Curr. Microbiol. 2010, 61, 411–416. [Google Scholar] [CrossRef]
- Urquhart, C.G.; Pinheiro, T.d.R.; da Silva, J.L.G.; Leal, D.B.R.; Burgo, T.A.L.; Iglesias, B.A.; Santos, R.C.V. Antimicrobial Activity of Water-Soluble Tetra-Cationic Porphyrins on Pseudomonas aeruginosa. Photodiagn. Photodyn. Ther. 2023, 42, 103266. [Google Scholar] [CrossRef]
- Beirão, S.; Fernandes, S.; Coelho, J.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Almeida, A.; Cunha, A. Photodynamic Inactivation of Bacterial and Yeast Biofilms with a Cationic Porphyrin. Photochem. Photobiol. 2014, 90, 1387–1396. [Google Scholar] [CrossRef]
- Patel, N.; Swavey, S.; Robinson, J. A Cationic Porphyrin, ZnPor, Disassembles Pseudomonas aeruginosa Biofilm Matrix, Kills Cells Directly, and Enhances Antibiotic Activity of Tobramycin. Antibiotics 2020, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, V.T.; Martegani, E.; Bolognese, F.; Trivellin, N.; Garzotto, F.; Caruso, E. Photoinactivation of Pseudomonas aeruginosa Biofilm by Dicationic Diaryl-Porphyrin. Int. J. Mol. Sci. 2021, 22, 6808. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.S.; Gomes, M.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Cavaleiro, J.A.S.; Almeida, A.; Tomé, J.P.C. Comparative Photodynamic Inactivation of Antibiotic Resistant Bacteria by First and Second Generation Cationic Photosensitizers. Photochem. Photobiol. Sci. 2012, 11, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Meerovich, G.A.; Akhlyustina, E.V.; Tiganova, I.G.; Lukyanets, E.A.; Makarova, E.A.; Tolordava, E.R.; Yuzhakova, O.A.; Romanishkin, I.D.; Philipova, N.I.; Zhizhimova, Y.S.; et al. Photodynamic Inactivation of Pseudomonas aeruginosa Bacterial Biofilms Using New Polycationic Photosensitizers. Laser Phys. Lett. 2019, 16, 115603. [Google Scholar] [CrossRef]
- Schmid, J.; Hoenes, K.; Vatter, P.; Hessling, M. Antimicrobial Effect of Visible Light—Photoinactivation of Legionella rubrilucens by Irradiation at 450, 470, and 620 Nm. Antibiotics 2019, 8, 187. [Google Scholar] [CrossRef]
- Fueda, Y.; Hashimoto, M.; Nobuhara, K.; Yokoi, H.; Komiya, Y.; Shiragami, T.; Matsumoto, J.; Kawano, K.; Suzuki, S.; Yasuda, M. Visible-Light Bactericidal Effect of Silica Gel-Supported Porphyrinatoantimony(V) Catalyst on Legionella Species Occurring in the Living Environmental Fields. Biocontrol Sci. 2005, 10, 55–60. [Google Scholar] [CrossRef]
- Matsumoto, J.; Shiragami, T.; Hirakawa, K.; Yasuda, M. Water-Solubilization of P(V) and Sb(V) Porphyrins and Their Photobiological Application. Int. J. Photoenergy 2015, 2015, 148964. [Google Scholar] [CrossRef]
- Lesar, A.; Begić, G.; Malatesti, N.; Gobin, I. Innovative Approach in Legionella Water Treatment with Photodynamic Cationic Amphiphilic Porphyrin. Water Sci. Technol. Water Supply 2019, 19, 1473–1479. [Google Scholar] [CrossRef]
- Lesar, A.; Mušković, M.; Begić, G.; Lončarić, M.; Tomić Linšak, D.; Malatesti, N.; Gobin, I. Cationic Porphyrins as Effective Agents in Photodynamic Inactivation of Opportunistic Plumbing Pathogen Legionella Pneumophila. Int. J. Mol. Sci. 2020, 21, 5367. [Google Scholar] [CrossRef]
- Mušković, M.; Ćavar, I.; Lesar, A.; Lončarić, M.; Malatesti, N.; Gobin, I. Photodynamic Inactivation of Legionella pneumophila Biofilm Formation by Cationic Tetra- and Tripyridylporphyrins in Waters of Different Hardness. Int. J. Mol. Sci. 2021, 22, 9095. [Google Scholar] [CrossRef]
- Shleeva, M.O.; Savitsky, A.P.; Nikitushkin, V.D.; Solovyev, I.D.; Kazachkina, N.I.; Perevarov, V.V.; Kaprelyants, A.S. Photoinactivation of Dormant Mycobacterium smegmatis Due to Its Endogenous Porphyrins. Appl. Microbiol. Biotechnol. 2019, 103, 9687–9695. [Google Scholar] [CrossRef]
- Choi, S.-R.; Talmon, G.A.; Britigan, B.E.; Narayanasamy, P. Nanoparticulate β-Cyclodextrin with Gallium Tetraphenylporphyrin Demonstrates in Vitro and in Vivo Antimicrobial Efficacy against Mycobacteroides abscessus and Mycobacterium avium. ACS Infect. Dis. 2021, 7, 2299–2309. [Google Scholar] [CrossRef]
- Gong, N.; Tan, Y.; Li, M.; Lu, W.; Lei, X. ALA-PDT Combined with Antibiotics for the Treatment of Multiple Skin Abscesses Caused by Mycobacterium fortuitum. Photodiagn. Photodyn. Ther. 2016, 15, 70–72. [Google Scholar] [CrossRef]
- Wang, X.; Wan, M.; Zhang, L.; Dai, Y.; Hai, Y.; Yue, C.; Xu, J.; Ding, Y.; Wang, M.; Xie, J.; et al. ALA_PDT Promotes Ferroptosis-Like Death of Mycobacterium abscessus and Antibiotic Sterilization via Oxidative Stress. Antioxidants 2022, 11, 546. [Google Scholar] [CrossRef]
- Yue, C.; Wang, L.; Wang, X.; Cen, R.; Chen, J.; Li, L.; Yang, W.; Tan, Y.; Lei, X. In Vitro Study of the Effect of ALA-PDT on Mycobacterium abscessus and Its Antibiotic Susceptibility. Photodiagn. Photodyn. Ther. 2022, 38, 102802. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Feng, Y.; Li, D.; Pang, Z.; Wang, S.; Chen, H.; Jiang, M.; Yan, H.; Li, T.; Fu, H.; et al. 5-Aminolevulinic Acid-Photodynamic Therapy Ameliorates Cutaneous Granuloma by Killing Drug-Resistant Mycobacterium marinum. Photodiagn. Photodyn. Ther. 2022, 38, 102839. [Google Scholar] [CrossRef] [PubMed]
- Guterres, K.B.; Rossi, G.G.; Menezes, L.B.; Anraku de Campos, M.M.; Iglesias, B.A. Preliminary Evaluation of the Positively and Negatively Charge Effects of Tetra-Substituted Porphyrins on Photoinactivation of Rapidly Growing Mycobacteria. Tuberculosis 2019, 117, 45–51. [Google Scholar] [CrossRef]
- Guterres, K.B.; Rossi, G.G.; de Campos, M.M.k.A.; Moreira, K.S.; Burgo, T.A.L.; Iglesias, B.A. Metal Center Ion Effects on Photoinactivating Rapidly Growing Mycobacteria Using Water-Soluble Tetra-Cationic Porphyrins. BioMetals 2020, 33, 269–282. [Google Scholar] [CrossRef]
- Guterres, K.B.; Rossi, G.G.; de Campos, M.M.A.; Moreira, K.S.; Burgo, T.A.L.; Iglesias, B.A. Nanomolar Effective Report of Tetra-Cationic Silver(II) Porphyrins against Non-Tuberculous Mycobacteria in Antimicrobial Photodynamic Approaches. Photodiagn. Photodyn. Ther. 2022, 38, 102770. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.G.; Guterres, K.B.; da Silveira, C.H.; Moreira, K.S.; Burgo, T.A.L.; Iglesias, B.A.; de Campos, M.M.A. Peripheral Tetra-Cationic Pt(II) Porphyrins Photo-Inactivating Rapidly Growing Mycobacteria: First Application in Mycobacteriology. Microb. Pathog. 2020, 148, 104455. [Google Scholar] [CrossRef]
- Shih, M.-H.; Huang, F.-C. Effects of Photodynamic Therapy on Rapidly Growing Nontuberculous Mycobacteria Keratitis. Investig. Opthalmol. Vis. Sci. 2011, 52, 223. [Google Scholar] [CrossRef]
- Miretti, M.; Juri, L.; Peralta, A.; Cosiansi, M.C.; Baumgartner, M.T.; Tempesti, T.C. Photoinactivation of Non-Tuberculous Mycobacteria Using Zn-Phthalocyanine Loaded into Liposomes. Tuberculosis 2022, 136, 102247. [Google Scholar] [CrossRef] [PubMed]
- Leanse, L.G.; Dong, P.-T.; Goh, X.S.; Lu, M.; Cheng, J.-X.; Hooper, D.C.; Dai, T. Quinine Enhances Photo-Inactivation of Gram-Negative Bacteria. J. Infect. Dis. 2020, 221, 618–626. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Chang, K.-C.; Chen, L.-Y.; Wang, P.-C.; Chou, C.-C.; Wu, Z.-B.; Hu, A. Blue Light Irradiation Triggers the Antimicrobial Potential of ZnO Nanoparticles on Drug-Resistant Acinetobacter baumannii. J. Photochem. Photobiol. B 2018, 180, 235–242. [Google Scholar] [CrossRef]
- Boluki, E.; Pourhajibagher, M.; Bahador, A. The Combination of Antimicrobial Photocatalysis and Antimicrobial Photodynamic Therapy to Eradicate the Extensively Drug-Resistant Colistin Resistant Acinetobacter baumannii. Photodiagn. Photodyn. Ther. 2020, 31, 101816. [Google Scholar] [CrossRef] [PubMed]
- da Silva Canielles Caprara, C.; da Silva Freitas, L.; Iglesias, B.A.; Ferreira, L.B.; Ramos, D.F. Charge Effect of Water-Soluble Porphyrin Derivatives as a Prototype to Fight Infections Caused by Acinetobacter baumannii by APDT Approaches. Biofouling 2022, 38, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Costa, L.; Faustino, M.A.F.; Almeida, A. Photodynamic Inactivation of Multidrug-Resistant Bacteria in Hospital Wastewaters: Influence of Residual Antibiotics. Photochem. Photobiol. Sci. 2014, 13, 626–633. [Google Scholar] [CrossRef]
- Stanley, S.; Scholle, F.; Zhu, J.; Lu, Y.; Zhang, X.; Situ, X.; Ghiladi, R. Photosensitizer-Embedded Polyacrylonitrile Nanofibers as Antimicrobial Non-Woven Textile. Nanomaterials 2016, 6, 77. [Google Scholar] [CrossRef]
- Buchovec, I.; Klimkaitė, L.; Sužiedėlienė, E.; Bagdonas, S. Inactivation of Opportunistic Pathogens Acinetobacter baumannii and Stenotrophomonas maltophilia by Antimicrobial Photodynamic Therapy. Microorganisms 2022, 10, 506. [Google Scholar] [CrossRef]
- Ragàs, X.; Dai, T.; Tegos, G.P.; Agut, M.; Nonell, S.; Hamblin, M.R. Photodynamic Inactivation of Acinetobacter baumannii Using Phenothiazinium Dyes: In Vitro and in Vivo Studies. Lasers Surg. Med. 2010, 42, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Marcolan De Mello, M.; De Barros, P.P.; de Cassia Bernardes, R.; Alves, S.R.; Ramanzini, N.P.; Figueiredo-Godoi, L.M.A.; Prado, A.C.C.; Jorge, A.O.C.; Junqueira, J.C. Antimicrobial Photodynamic Therapy against Clinical Isolates of Carbapenem-Susceptible and Carbapenem-Resistant Acinetobacter baumannii. Lasers Med. Sci. 2019, 34, 1755–1761. [Google Scholar] [CrossRef]
- Wanarska, E.; Mielko, K.A.; Maliszewska, I.; Młynarz, P. The Oxidative Stress and Metabolic Response of Acinetobacter baumannii for APDT Multiple Photosensitization. Sci. Rep. 2022, 12, 1913. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-Godoi, L.M.A.; Garcia, M.T.; Pinto, J.G.; Ferreira-Strixino, J.; Faustino, E.G.; Pedroso, L.L.C.; Junqueira, J.C. Antimicrobial Photodynamic Therapy Mediated by Fotenticine and Methylene Blue on Planktonic Growth, Biofilms, and Burn Infections of Acinetobacter baumannii. Antibiotics 2022, 11, 619. [Google Scholar] [CrossRef]
- Huang, L.; Krayer, M.; Roubil, J.G.S.; Huang, Y.-Y.; Holten, D.; Lindsey, J.S.; Hamblin, M.R. Stable Synthetic Mono-Substituted Cationic Bacteriochlorins Mediate Selective Broad-Spectrum Photoinactivation of Drug-Resistant Pathogens at Nanomolar Concentrations. J. Photochem. Photobiol. B 2014, 141, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, M.; Jiang, Y.; Tao, N.; Fu, Y.; Fan, J.; Xu, X.; Shi, H.; Lu, Z.; Shen, C. A New Acridine-Based Photosensitizer with Ultra-Low Light Requirement Efficiently Inactivates Carbapenem-Resistant Acinetobacter baumannii and Methicillin-Resistant Staphylococcus aureus and Degrades Their Antibiotic Resistance Genes. Environ. Int. 2023, 173, 107839. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, T.; Wang, M.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Potentiation of Antimicrobial Photodynamic Inactivation Mediated by a Cationic Fullerene by Added Iodide: In Vitro and in Vivo Studies. Nanomedicine 2015, 10, 603–614. [Google Scholar] [CrossRef]
- Fekrirad, Z.; Darabpour, E.; Kashef, N. Eradication of Acinetobacter baumannii Planktonic and Biofilm Cells Through Erythrosine-Mediated Photodynamic Inactivation Augmented by Acetic Acid and Chitosan. Curr. Microbiol. 2021, 78, 879–886. [Google Scholar] [CrossRef]
- Khan, S.J.; Reed, R.H.; Rasul, M.G. Thin-Film Fixed-Bed Reactor for Solar Photocatalytic Inactivation of Aeromonas hydrophila: Influence of Water Quality. BMC Microbiol. 2012, 12, 285. [Google Scholar] [CrossRef]
- Kussovski, V.; Mantareva, V.; Angelov, I.; Orozova, P.; Wöhrle, D.; Schnurpfeil, G.; Borisova, E.; Avramov, L. Photodynamic Inactivation of Aeromonas hydrophila by Cationic Phthalocyanines with Different Hydrophobicity. FEMS Microbiol. Lett. 2009, 294, 133–140. [Google Scholar] [CrossRef]
- Mantareva, V.N.; Kussovski, V.; Orozova, P.; Dimitrova, L.; Kulu, I.; Angelov, I.; Durmus, M.; Najdenski, H. Photodynamic Inactivation of Antibiotic-Resistant and Sensitive Aeromonas hydrophila with Peripheral Pd(II)- vs. Zn(II)-Phthalocyanines. Biomedicines 2022, 10, 384. [Google Scholar] [CrossRef] [PubMed]

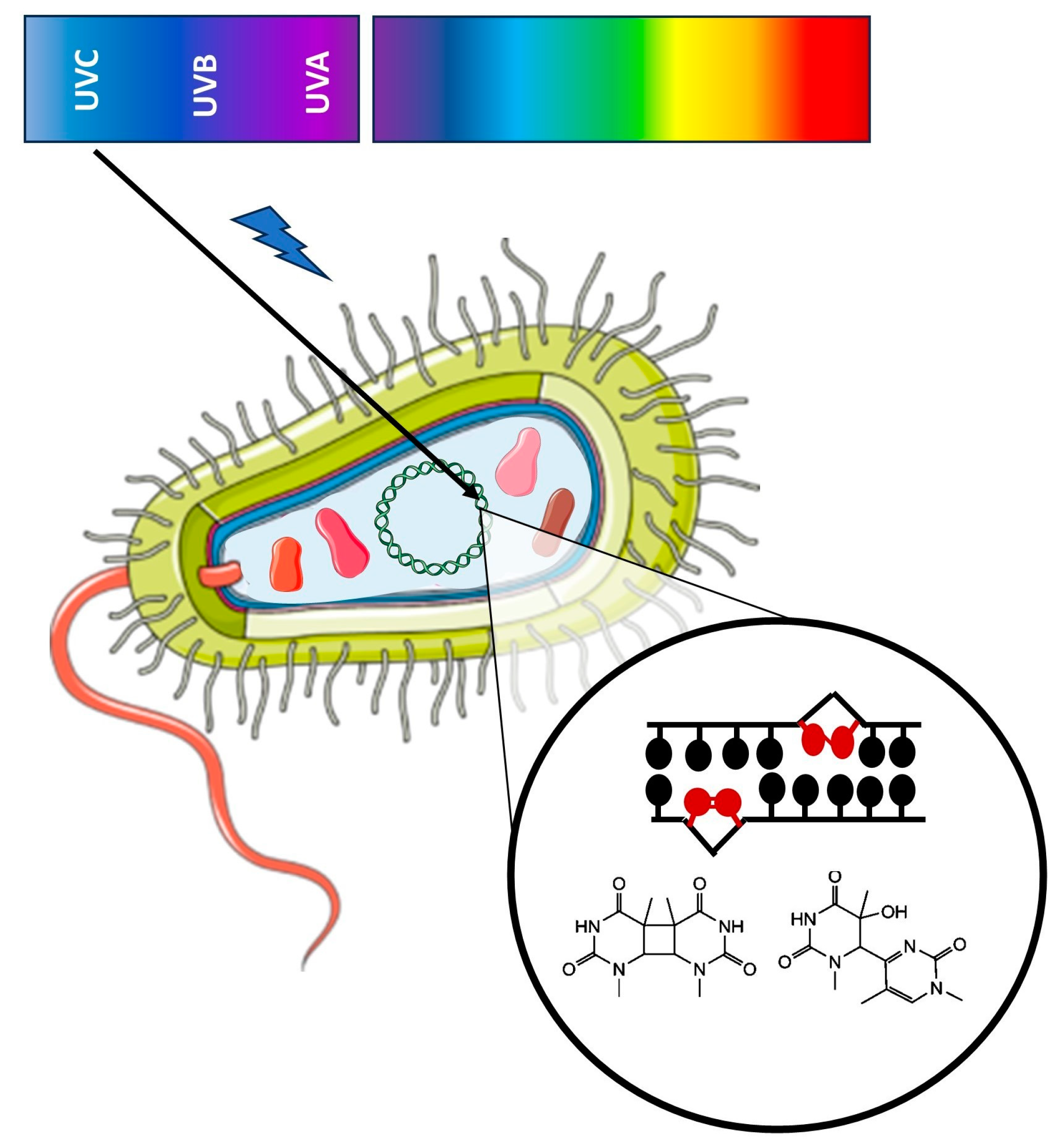


| Bacteria | |||||
|---|---|---|---|---|---|
| Genus | Family | Main Representative(s) | G(+)/ G (−) | Infections and Diseases | Ref. |
| Acinetobacter | Moraxellaceae | A. baumannii | Gram (−) | Hospital and community acquired pneumonia, trauma and wound infections, meningitis, endocarditis, peritonitis, keratitis | [6] |
| Aeromonas | Aeromonadaceae | A. hydrophila | Gram (−) | Gastroenteritis, bacteremia, wound infections | [7] |
| Brevundimonas | Caulobacteraceae | B. diminuta, B. vesicularis | Gram (−) | Bacteremia, septicemia/sepsis, pneumonia/pleuritis, endocarditis, and keratitis | [8] |
| Chryseobacterium | Weeksellaceae | C. meningosepticum, C. indologenes, C. gleum, C. hominis | Gram (−) | Nosocomial infections, pyelonephritis, peritonitis, neonatal meningitis (C. meningosepticum), pneumonia, cystitis, empyema | [9,10] |
| Helicobacter | Helicobacteraceae | H. pylori | Gram (−) | Associated with peptic ulcers, chronic gastritis, and duodenitis | [11] |
| Legionella | Legionellaceae | L. pneumophila, L. rubriculens | Gram (−) | Legionellosis (Pontiac fewer and Legionnaires’ disease)- pneumonia-like diseases | [12,13] |
| Methylobacterium | Methylobacteriae | M. mesophilicum, M. zatmanii, M. extorquens | Gram (−) | Nosocomial infections, bacteremia | [14] |
| Mycobacterium | Mycobacteriaceae | M. avium, M. smegmatis, M. fortuitum, M. abscessus, M. marinum, M. massiliense | Gram (+) | Pulmonary diseases, cystic fibrosis, tuberculosis-like disease, chronic lung infections | [15] |
| Pseudomonas | Pseudomonadaceae | P. aeruginosa | Gram (−) | Pneumonia (cystic fibrosis), bacteremia, urosepsis, and wound infections | [16] |
| Segniliparus | Segniliparaceae | S. rotundus, S. rugosus | Gram (+) | Pulmonary infections, cystic fibrosis, bronchiolitis, pneumonia | [17] |
| Sphingomonas | Sphingomonacedae | S. paucimobilis | Gram (−) | Bacteremia, peritonitis, pneumonia, and urinary tract infections (UTIs) | [18] |
| Stenorophomonas | Xanthomonadaceae | S. maltophilia | Gram (−) | Nosocomial infections, respiratory and urinary tract infections, endocarditis, bacteremia, meningitis, and cellulitis | [10,19] |
| Amoebas | |||||
| Genus | Family | Main representatives | Diseases | Ref. | |
| Achantamoeba | Acanthamoebidae | A. polyphaga, A. castellanii | Associated with diseases in immunocompromised patients (cutaneous, nasopharyngeal, pulmonary, and kidney lesions), keratitis, and granulomatous amoebic encephalitis (GAE) | [20] | |
| Balamuthia | Balamuthiidae | B. mandrillaris | Cutaneous lesions, lung infections, and granulomatous encephalitis connected to immunocompetent infants | [20] | |
| Hartmanella (Vermamoeba) | Hartmannellidae | H. veriformis | Keratitis | [21] | |
| Naegleria | Vahlkampfiidae | N. fowleri | Naegleriasis or primary amoebic meningoencephalitis (PAM) | [22,23] | |
| Possible OPPPs | |||||
| Genus | Family | Main representative(s) | G(+)/ G (−) | Infections and diseases | Ref. |
| Burkholderia | Burkholderiaceae | Burkholderia cepacia complex (Bcc): B. cepacia, B. cenocepacia, B. ambifaria, B. vietnamiensis | Gram (−) | Respiratory infections in patients (cistic fybrosis) | [24] |
| Elizabethkingia | Weeksellaceae | E. anophelis, E meningoseptica | Gram (−) | Nocosomial infections | [25,26] |
| Comamonas | Comamonadaceae | C. testosteroni, C. kerstersii | Gram (−) | Pneumonia, bacteremia, sepsis, and purulent meningitis | [27] |
| Ochrobactrum | Brucellaceae | O. anthropi | Gram (−) | Bacteremia, septicemia/sepsis, pneumonia, endophthalmitis, and keratitis | [28] |
| Ralstonia | Burkholderiaceae | R. mannitolilytica | Gram (−) | Bacteremia | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mušković, M.; Gobin, I.; Malatesti, N. Photodynamic Inactivation of Opportunistic Premise Plumbing Pathogens and Their Biofilms. Processes 2023, 11, 3074. https://doi.org/10.3390/pr11113074
Mušković M, Gobin I, Malatesti N. Photodynamic Inactivation of Opportunistic Premise Plumbing Pathogens and Their Biofilms. Processes. 2023; 11(11):3074. https://doi.org/10.3390/pr11113074
Chicago/Turabian StyleMušković, Martina, Ivana Gobin, and Nela Malatesti. 2023. "Photodynamic Inactivation of Opportunistic Premise Plumbing Pathogens and Their Biofilms" Processes 11, no. 11: 3074. https://doi.org/10.3390/pr11113074
APA StyleMušković, M., Gobin, I., & Malatesti, N. (2023). Photodynamic Inactivation of Opportunistic Premise Plumbing Pathogens and Their Biofilms. Processes, 11(11), 3074. https://doi.org/10.3390/pr11113074








