Experimental Research on the Influence of Ion Channels on the Healing of Skin Wounds in Rats
Abstract
1. Introduction
2. Materials and Methods
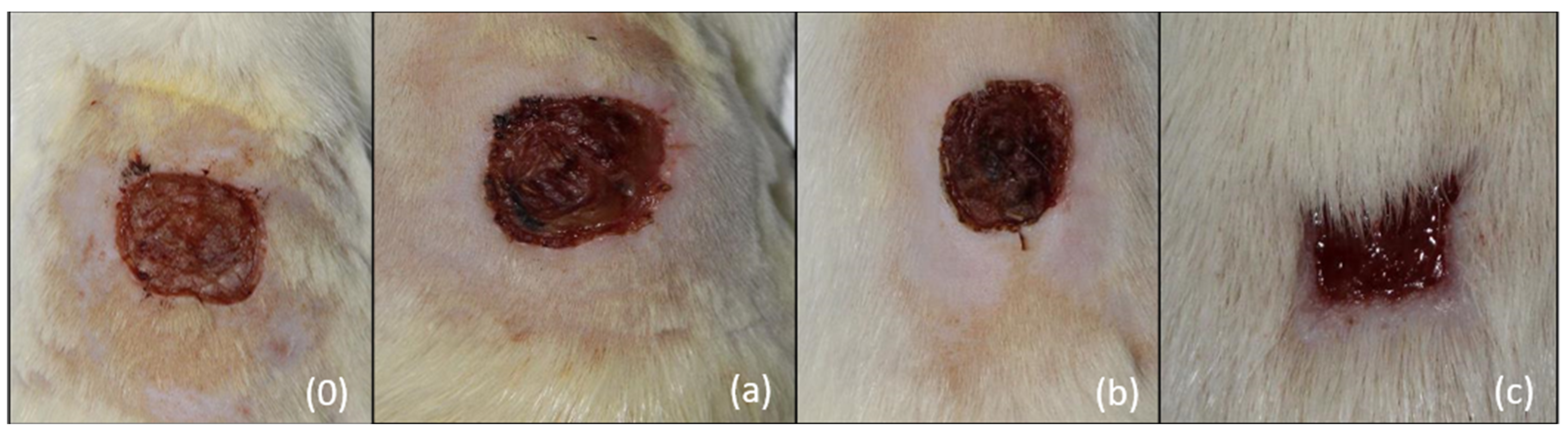
- (a)
- The main parameter—the duration of wound healing measured in days
- (b)
- Secondary parameters
- 1.
- The percentage decrease of the area relative to the value of the initial area, according to the formulawhere S is the percentage decrease in area, St1 is the initial area measured in pixels, and St is the area at the time of measurement in pixels.
- 2.
- The percentage speed decrease of the lesion surface according to the formulawhere V represents the percentage decrease rate per day of the surface, St represents the surface of the lesion at time t, measured in pixels, St+1 represents the surface of the lesion at time t+1, measured in pixels, and t represents the time of surface measurement expressed in days from the beginning of the experiment.
3. Results
3.1. Main Parameter: Average Duration of Wound Healing
Average Duration of Wound Healing
3.2. Secondary Parameters
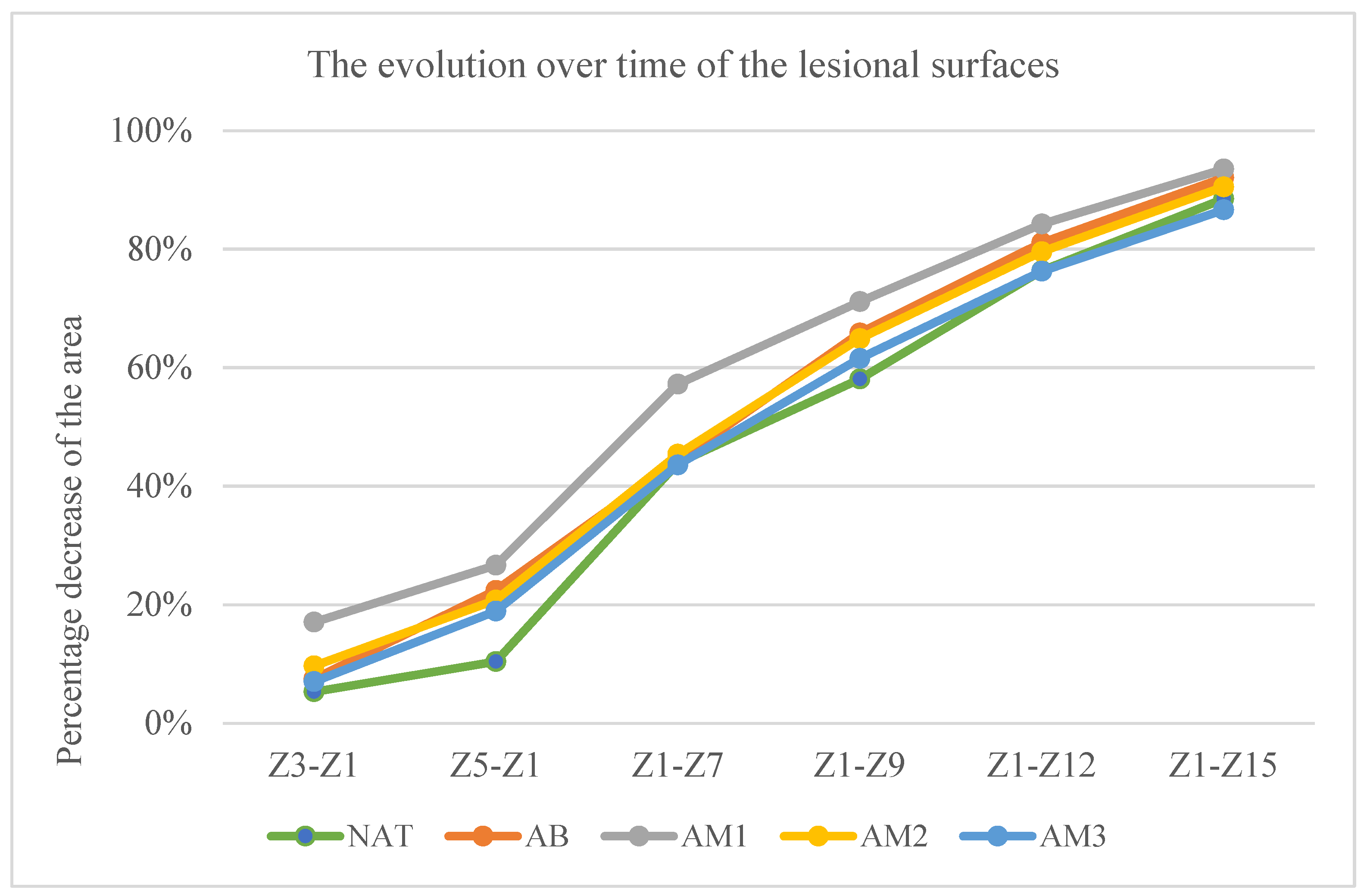
- The percentage decrease of the lesion surfaces compared to the initial surface
- –
- In the untreated batch at time t1—5.33% ± 3.63, t2—10.40% ± 8.09, t3—43.92% ± 15.81, t4—58.08% ± 14.20, t5—76.36% ± 8.20, and t6—88.48% ± 5.92.
- –
- In the batch treated with benzyl alcohol at time t1—7.49% ± 11.09, t2—22.41% ± 13.00, t3—44.35% ± 18.38, t4—65.85% ± 14.13, t5—81.06% ± 4.34, and t6—92.05% ± 4.33.
- –
- In the batch treated with amiodarone in a concentration of 200 nM at time t1—17.03% ± 7.38, t2—26.65% ± 6.20, t3—57.23% ± 11.82, t4—71.13% ± 7.75, t5—84.22% ± 4.18, and t6—93.50% ± 4.46.
- –
- In the batch treated with amiodarone in a concentration of 2000 nM at time t1—9.68% ± 7.57, t2—20.78% ± 10.74, t3—45.39% ± 11.48, t4—64.90% ± 14.69, t5—79.55% ± 6.09, and t6—90.51% ± 5.83.
- –
- In the batch treated with amiodarone in a concentration of 200,000 nM at time t1—7.05% ± 6.98, t2—18.91% ± 6.96, t3—43.59% ± 5.59, t4—61.56% ± 10.35, t5—76.27% and t6—86.63% ± 7.23.
- At time t2 compared to t1: the greatest decrease in surface area was in the batch treated with low concentrations of amiodarone, the difference compared to the untreated batch being statistically significant for a p < 0.001.
- At time t3 compared to t1: the biggest decrease was in the batch treated with low dose amiodarone, the difference compared to the untreated batch being statistically significant for p < 0.0003, but also the group treated with high concentration amiodarone recorded the difference of the untreated batch statistically significant although lower for p < 0.02.
- At time t4 compared to t1, amiodarone in low concentration also registered the greatest decrease in surfaces, but the differences compared to the untreated batch are not statistically significant.
- At times t5 and t6 compared to t1, the only statistically significant difference from the untreated batch was recorded for the low dose of amiodarone (p < 0.02 at time t5, respectively, p < 0.01 at time t6).
- 5
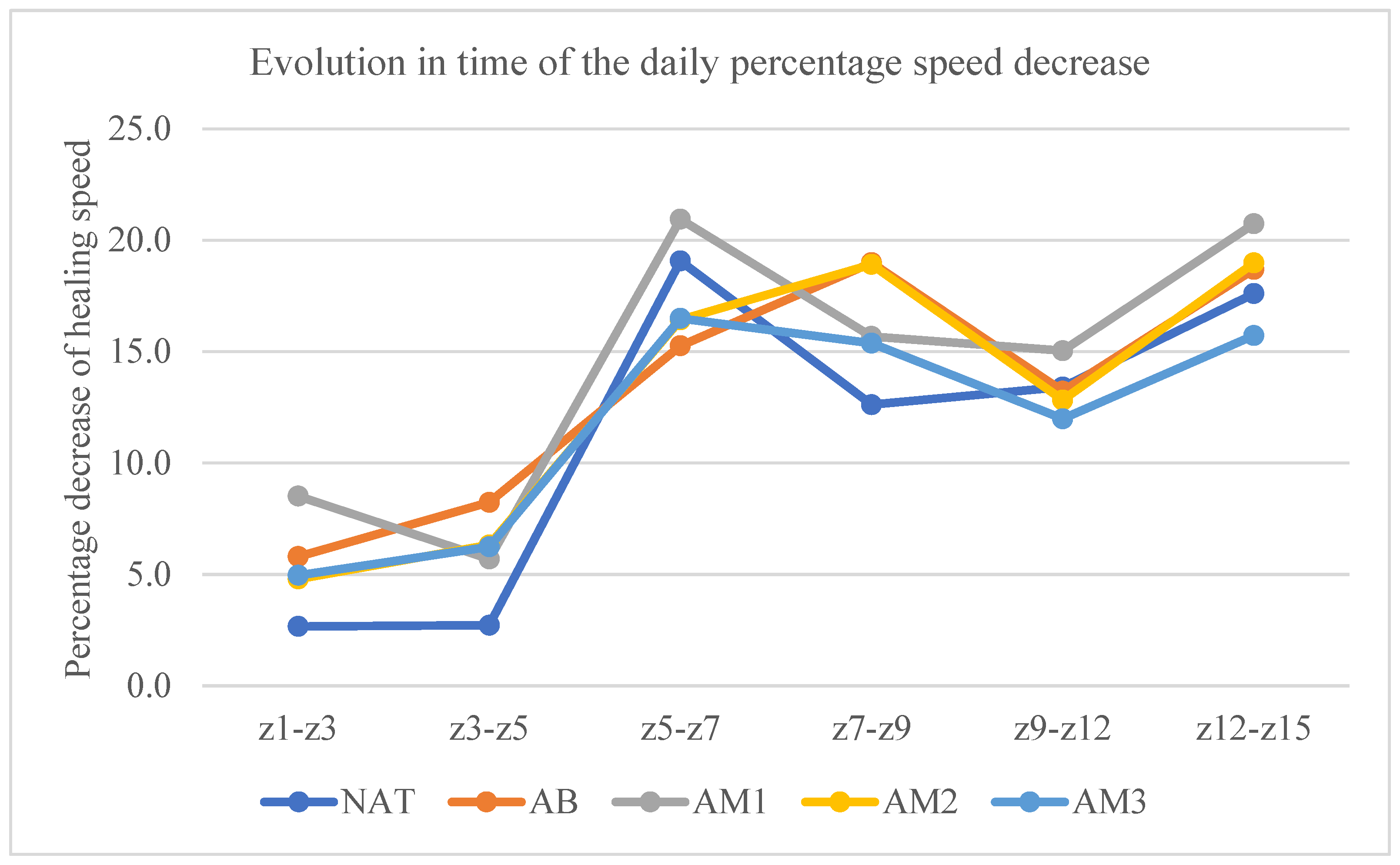
- The rate of daily percentage decrease of the lesion surface
4. Discussion
5. Conclusions
- Low concentration amiodarone promoted wound healing under our experimental conditions, both in terms of duration of healing and speed of healing.
- Blocking potassium channels promotes wound healing.
- Neither medium-dose amiodarone nor high-dose amiodarone had statistically significant effects on wound healing time.
- Given that a potassium current is a depolarizing current, while calcium and sodium currents are repolarizing, it turns out that blocking the potassium current increases the membrane potential; this increase is antagonized by blocking calcium and sodium currents. As a consequence, blocking calcium and/or sodium channels antagonizes the positive effects of blocking potassium channels on wound healing.
- It is possible that the increase in membrane potential produced by blocking potassium channels accelerated the migration of cells into the wound field, which explained the acceleration of healing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, I.K.; Valdearcos, M.; Morioka, K.; Saxena, S.; Feng, X.; Li, R.; Uchida, Y.; Lijun, A.; Li, W.; Pan, J.; et al. Blocking Kv1.3 Potassium Channels Prevents Postoperative Neuroinflammation and Cognitive Decline without Impairing Wound Healing in Mice. Br. J. Anaesth. 2020, 125, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Cai, E.; Xiang, Y.; Zhang, C.; Ge, X.; Wang, J.; Lan, Y.; Xu, H.; Hu, R.; Shen, J. An Immunomodulatory Hydrogel by Hyperthermia-Assisted Self-Cascade Glucose Depletion and ROS Scavenging for Diabetic Foot Ulcer Wound Therapeutics. Adv. Mater. 2023, 35, e2306632. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental Models and Methods for Cutaneous Wound Healing Assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Klinsang, T.; Charoensit, P.; Phimnuan, P.; Luangpraditkun, K.; Ross, G.M.; Viennet, C.; Ross, S.; Viyoch, J. In Vitro Wound Healing Potential of a Fibroin Film Incorporating a Cannabidiol/2-Hydroxypropyl-β-Cyclodextrin Complex. Pharmaceutics 2023, 15, 2682. [Google Scholar] [CrossRef] [PubMed]
- González, C.; Baez-Nieto, D.; Valencia, I.; Oyarzún, I.; Rojas, P.; Naranjo, D.; Latorre, R. K(+) Channels: Function-Structural Overview. Compr. Physiol. 2012, 2, 2087–2149. [Google Scholar] [CrossRef]
- Zhang, W.; Das, P.; Kelangi, S.; Bei, M. Potassium Channels as Potential Drug Targets for Limb Wound Repair and Regeneration. Precis. Clin. Med. 2020, 3, 22–33. [Google Scholar] [CrossRef]
- Pardo, L.A. Voltage-Gated Potassium Channels in Cell Proliferation. Physiology 2004, 19, 285–292. [Google Scholar] [CrossRef]
- Hertel, R.F. Potassium Channel Activation Improves Blood Flow Pattern of Conscious Rats in Cutaneous Microcirculation. Clin. Exp. Pharmacol. Physiol. 1992, 19, 243–248. [Google Scholar] [CrossRef]
- Moulin, V.J.; Dubé, J.; Rochette-Drouin, O.; Lévesque, P.; Gauvin, R.; Roberge, C.J.; Auger, F.A.; Goulet, D.; Bourdages, M.; Plante, M.; et al. Electric Potential Across Epidermis and Its Role During Wound Healing Can Be Studied by Using an In Vitro Reconstructed Human Skin. Adv. Wound Care 2012, 1, 81–87. [Google Scholar] [CrossRef]
- Zhang, W.; Bei, M. Kcnh2 and Kcnj8 Interactively Regulate Skin Wound Healing and Regeneration. Wound Repair. Regen. 2015, 23, 797–806. [Google Scholar] [CrossRef]
- McKeown, L.; Swanton, L.; Robinson, P.; Jones, O.T. Surface Expression and Distribution of Voltage-Gated Potassium Channels in Neurons (Review). Mol. Membr. Biol. 2008, 25, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Florek, J.B.; Lucas, A.; Girzadas, D. Amiodarone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Zhang, Y.; Colenso, C.K.; El Harchi, A.; Cheng, H.; Witchel, H.J.; Dempsey, C.E.; Hancox, J.C. Interactions between Amiodarone and the hERG Potassium Channel Pore Determined with Mutagenesis and in Silico Docking. Biochem. Pharmacol. 2016, 113, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Hancox, J.C. Amiodarone Blocks L-Type Calcium Current in Single Myocytes Isolated from the Rabbit Atrioventricular Node. Gen. Pharmacol. 1997, 29, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Takizawa, T.; Matsumoto, A.; Miki, T.; Seino, S.; Nakaya, H. Inhibition of ATP-Sensitive K+ Channels and L-Type Ca2+ Channels by Amiodarone Elicits Contradictory Effect on Insulin Secretion in MIN6 Cells. J. Pharmacol. Sci. 2011, 116, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Lubic, S.P.; Nguyen, K.P.; Dave, B.; Giacomini, J.C. Antiarrhythmic Agent Amiodarone Possesses Calcium Channel Blocker Properties. J. Cardiovasc. Pharmacol. 1994, 24, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Waldhauser, K.M.; Brecht, K.; Hebeisen, S.; Ha, H.R.; Konrad, D.; Bur, D.; Krähenbühl, S. Interaction with the hERG Channel and Cytotoxicity of Amiodarone and Amiodarone Analogues. Br. J. Pharmacol. 2008, 155, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Soejima, H.; Kawamoto, S.; Akai, J.; Miyoshi, O.; Arai, Y.; Morohka, T.; Matsuo, S.; Niikawa, N.; Kimura, A.; Okubo, K.; et al. Isolation of Novel Heart-Specific Genes Using the BodyMap Database. Genomics 2001, 74, 115–120. [Google Scholar] [CrossRef]
- Yue, Z.; Xie, J.; Yu, A.S.; Stock, J.; Du, J.; Yue, L. Role of TRP Channels in the Cardiovascular System. Am. J. Physiol. Heart. Circ. Physiol. 2015, 308, H157–H182. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Nuccitelli, P.; Ramlatchan, S.; Sanger, R.; Smith, P.J.S. Imaging the Electric Field Associated with Mouse and Human Skin Wounds. Wound Repair. Regen. 2008, 16, 432–441. [Google Scholar] [CrossRef]
- Wang, S.-P.; Wang, J.-A.; Luo, R.-H.; Cui, W.-Y.; Wang, H. Potassium Channel Currents in Rat Mesenchymal Stem Cells and Their Possible Roles in Cell Proliferation. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1077–1084. [Google Scholar] [CrossRef]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-Gated Potassium Channels as Therapeutic Targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.; Nailwal, N.; Bhatia, N.Y.; Doshi, G.; Sathaye, S.; Godad, A.P. An Update On Proficiency of Voltage-Gated Ion Channel Blockers in the Treatment of Inflammation-Associated Diseases. Curr. Drug Targets 2022, 23, 1290–1303. [Google Scholar] [CrossRef] [PubMed]
- Crociani, O.; Guasti, L.; Balzi, M.; Becchetti, A.; Wanke, E.; Olivotto, M.; Wymore, R.S.; Arcangeli, A. Cell Cycle-Dependent Expression of HERG1 and HERG1B Isoforms in Tumor Cells. J. Biol. Chem. 2003, 278, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Choi, T.H.; Han, K.; Son, D.; Kim, J.H.; Kim, S.-H.; Park, J. Regulation of K(+) Channels May Enhance Wound Healing in the Skin. Med. Hypotheses 2008, 71, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Erdem Kış, E.; Tiftik, R.N.; Al Hennawi, K.; Ün, İ. The Role of Potassium Channels in the Proliferation and Migration of Endometrial Adenocarcinoma HEC1-A Cells. Mol. Biol. Rep. 2022, 49, 7447–7454. [Google Scholar] [CrossRef] [PubMed]
- Varró, A.; Biliczki, P.; Iost, N.; Virág, L.; Hála, O.; Kovács, P.; Mátyus, P.; Papp, J.G. Theoretical Possibilities for the Development of Novel Antiarrhythmic Drugs. Curr. Med. Chem. 2004, 11, 1–11. [Google Scholar] [CrossRef]
- Bagheri, M.; Jahromi, B.M.; Mirkhani, H.; Solhjou, Z.; Noorafshan, A.; Zamani, A.; Amirghofran, Z. Azelnidipine, a New Calcium Channel Blocker, Promotes Skin Wound Healing in Diabetic Rats. J. Surg. Res. 2011, 169, e101–e107. [Google Scholar] [CrossRef]
- Ashkani-Esfahani, S.; Hosseinabadi, O.K.; Moezzi, P.; Moafpourian, Y.; Kardeh, S.; Rafiee, S.; Fatheazam, R.; Noorafshan, A.; Nadimi, E.; Mehrvarz, S.; et al. Verapamil, a Calcium-Channel Blocker, Improves the Wound Healing Process in Rats with Excisional Full-Thickness Skin Wounds Based on Stereological Parameters. Adv. Skin Wound Care 2016, 29, 271–274. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, S.H. Skin Barrier and Calcium. Ann. Dermatol. 2018, 30, 265–275. [Google Scholar] [CrossRef]
- Mojiri-Forushani, H. The Role of Calcium Channel Blockers in Wound Healing. Iran J. Basic Med. Sci. 2018, 21, 1198–1199. [Google Scholar] [CrossRef]
- Ma, J.; Kishida, S.; Wang, G.Q.; Meguro, K.; Imuta, H.; Oonuma, H.; Iida, H.; Jo, T.; Takano, H.; Morita, T.; et al. Comparative Effects of Azelnidipine and Other Ca2+-Channel Blockers on the Induction of Inducible Nitric Oxide Synthase in Vascular Smooth Muscle Cells. J. Cardiovasc. Pharmacol. 2006, 47, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Stallmeyer, B.; Kämpfer, H.; Kolb, N.; Pfeilschifter, J. Nitric Oxide Triggers Enhanced Induction of Vascular Endothelial Growth Factor Expression in Cultured Keratinocytes (HaCaT) and during Cutaneous Wound Repair. FASEB J. 1999, 13, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Inami, N.; Kimura, Y.; Omoto, S.; Shouzu, A.; Nishikawa, M.; Iwasaka, T. Effect of Nifedipine on Adiponectin in Hypertensive Patients with Type 2 Diabetes Mellitus. J. Hum. Hypertens. 2007, 21, 38–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Triveni, M.G.; Rudrakshi, C.; Mehta, D.S. Amlodipine-Induced Gingival Overgrowth. J. Indian Soc. Periodontol. 2009, 13, 160–163. [Google Scholar] [CrossRef]
- Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Longaker, M.T.; Gurtner, G.C. Surgical Approaches to Create Murine Models of Human Wound Healing. J. Biomed Biotechnol. 2011, 2011, 969618. [Google Scholar] [CrossRef]
- Golli-Bennour, E.E.; Bouslimi, A.; Zouaoui, O.; Nouira, S.; Achour, A.; Bacha, H. Cytotoxicity Effects of Amiodarone on Cultured Cells. Exp. Toxicol. Pathol. 2012, 64, 425–430. [Google Scholar] [CrossRef]
- Karami, M.Y.; Mansournia, N.; Bagherian, N.; Makarem, A.; MoeinVaziri, N.; Borna, S.; Pourdavood, A.H.; Shamohammadi, I. Effects of Azelnidipine-Carboxymethylcellulose Gel on Healing of Full-Thickness Skin Wounds in Streptozotocin Induced Diabetic Rats. Vet. Med. 2019, 10, 215–222. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kimura, J. Inhibitory Effect of Amiodarone on Na(+)/Ca(2+) Exchange Current in Guinea-Pig Cardiac Myocytes. Br. J. Pharmacol. 2000, 131, 80–84. [Google Scholar] [CrossRef]
- Waite, A.; Gilliver, S.C.; Masterson, G.R.; Hardman, M.J.; Ashcroft, G.S. Clinically Relevant Doses of Lidocaine and Bupivacaine Do Not Impair Cutaneous Wound Healing in Mice. Br. J. Anaesth. 2010, 104, 768–773. [Google Scholar] [CrossRef]


| Batch | Average Scarring Days | Statistical Significance Compared to the Natural Batch |
|---|---|---|
| NAT | 27.17 ± 5.19 days | |
| AB | 21.83 ± 2.71 days | |
| AM1 | 19.57 ± 3.05 days | p = 0.03 |
| AM2 | 22.13 ± 5.17 days | |
| AM3 | 22.25 ± 4.53 days |
| Batch | Z1–Z3 | Z1–Z5 | Z1–Z7 | Z1–Z9 | Z1–Z12 | Z1–Z15 |
|---|---|---|---|---|---|---|
| NAT | 5.33% ± 3.63 | 10.40% ± 8.09 | 43.92% ± 15.81 | 58.08% ± 14.20 | 76.36% ± 8.20 | 88.48% ± 5.92 |
| AB | 7.49% ± 11.09 | 22.41% ± 13.00 (p = 0.02) | 44.35% ± 18.38 | 65.85% ± 14.13 | 81.06% ± 4.34 | 92.05% ± 4.33 |
| AM1 | 17.03% ± 7.38 (p = 0.001) | 26.65% ± 6.20 (p = 0.0003) | 57.23% ± 11.82 (p = 0.04) | 71.13% ± 7.75 (p = 0.02) | 84.22% ± 4.18 (p = 0.01) | 93.50% ± 4.46 (p = 0.04) |
| AM2 | 9.68% ± 7.57 | 20.78% ± 10.74 (p = 0.03) | 45.39% ± 11.48 | 64.90% ± 14.69 | 79.55% ± 6.09 | 90.51% ± 5.83 |
| AM3 | 7.05% ± 6.98 | 18.91% ± 6.96 (p = 0.02) | 43.59% ± 5.59 | 61.56% ± 10.35 | 76.27% ± 6.97 | 86.63% ± 7.23 |
| Batch | Z1–Z3 | Z3–Z5 | Z5–Z7 | Z7–Z9 | Z9–Z12 | Z12–Z15 |
|---|---|---|---|---|---|---|
| NAT | 2.66% ± 1.81 | 2.77% ± 3.168 | 19.06% ± 6.85 | 12.61% ± 5.83 | 13.41% ± 6.02 | 17.60% ± 4.88 |
| AB | 5.80% ± 3.71 (p = 0.03) | 8.24 ± 2.52 (p = 0.001) | 15.26 ± 5.76 | 18.99% ± 8.98 | 13.22% ± 6.12 | 18.69% ± 7.1 |
| AM1 | 8.51 ± 8.51 (p = 0.001) | 5.70% ± 2.89 (p = 0.03) | 20.94% ± 7.11 | 15.68% ± 5.87 | 15.04% ± 2.52 | 20.73% ± 6.74 |
| AM2 | 4.79% ± 3.97 | 6.32% ± 3.08 (p = 0.02) | 16.42% ± 3.66 | 18.91% ± 8.31 | 12.82% ± 4.18 | 18.98% ± 7.46 |
| AM3 | 2.60 ± 5.99 | 6.24% ± 3.93 (p = 0.04) | 16.49% ± 4.33 | 15.37% ± 11.41 | 11.98% ± 6.10 | 15.72% ± 5.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigore, A.; Vatasescu-Balcan, A.; Stoleru, S.; Zugravu, A.; Poenaru, E.; Engi, M.; Coman, O.A.; Fulga, I. Experimental Research on the Influence of Ion Channels on the Healing of Skin Wounds in Rats. Processes 2024, 12, 109. https://doi.org/10.3390/pr12010109
Grigore A, Vatasescu-Balcan A, Stoleru S, Zugravu A, Poenaru E, Engi M, Coman OA, Fulga I. Experimental Research on the Influence of Ion Channels on the Healing of Skin Wounds in Rats. Processes. 2024; 12(1):109. https://doi.org/10.3390/pr12010109
Chicago/Turabian StyleGrigore, Alexandra, Ana Vatasescu-Balcan, Smaranda Stoleru, Aurelian Zugravu, Elena Poenaru, Miruna Engi, Oana Andreia Coman, and Ion Fulga. 2024. "Experimental Research on the Influence of Ion Channels on the Healing of Skin Wounds in Rats" Processes 12, no. 1: 109. https://doi.org/10.3390/pr12010109
APA StyleGrigore, A., Vatasescu-Balcan, A., Stoleru, S., Zugravu, A., Poenaru, E., Engi, M., Coman, O. A., & Fulga, I. (2024). Experimental Research on the Influence of Ion Channels on the Healing of Skin Wounds in Rats. Processes, 12(1), 109. https://doi.org/10.3390/pr12010109







