Study on the Extraction Mechanism of Metal Ions on Small Molecular Phase of Tar-Rich Coal under Ultrasonic Loading
Abstract
1. Introduction
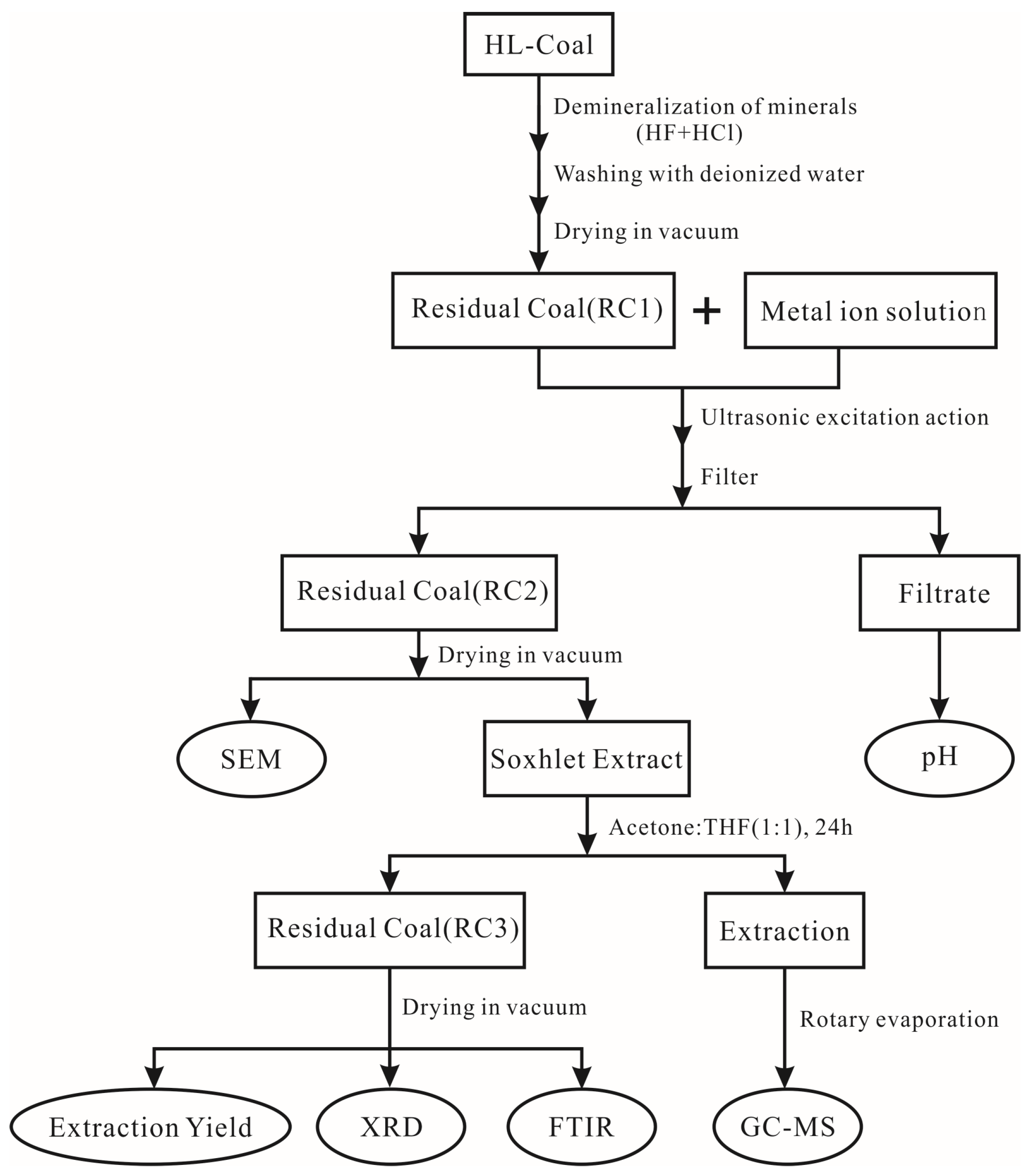
2. Experiment and Methods
2.1. Ultrasonic Loading Experiment of Metal Ions
2.2. Acidity and Alkalinity (pH) Test
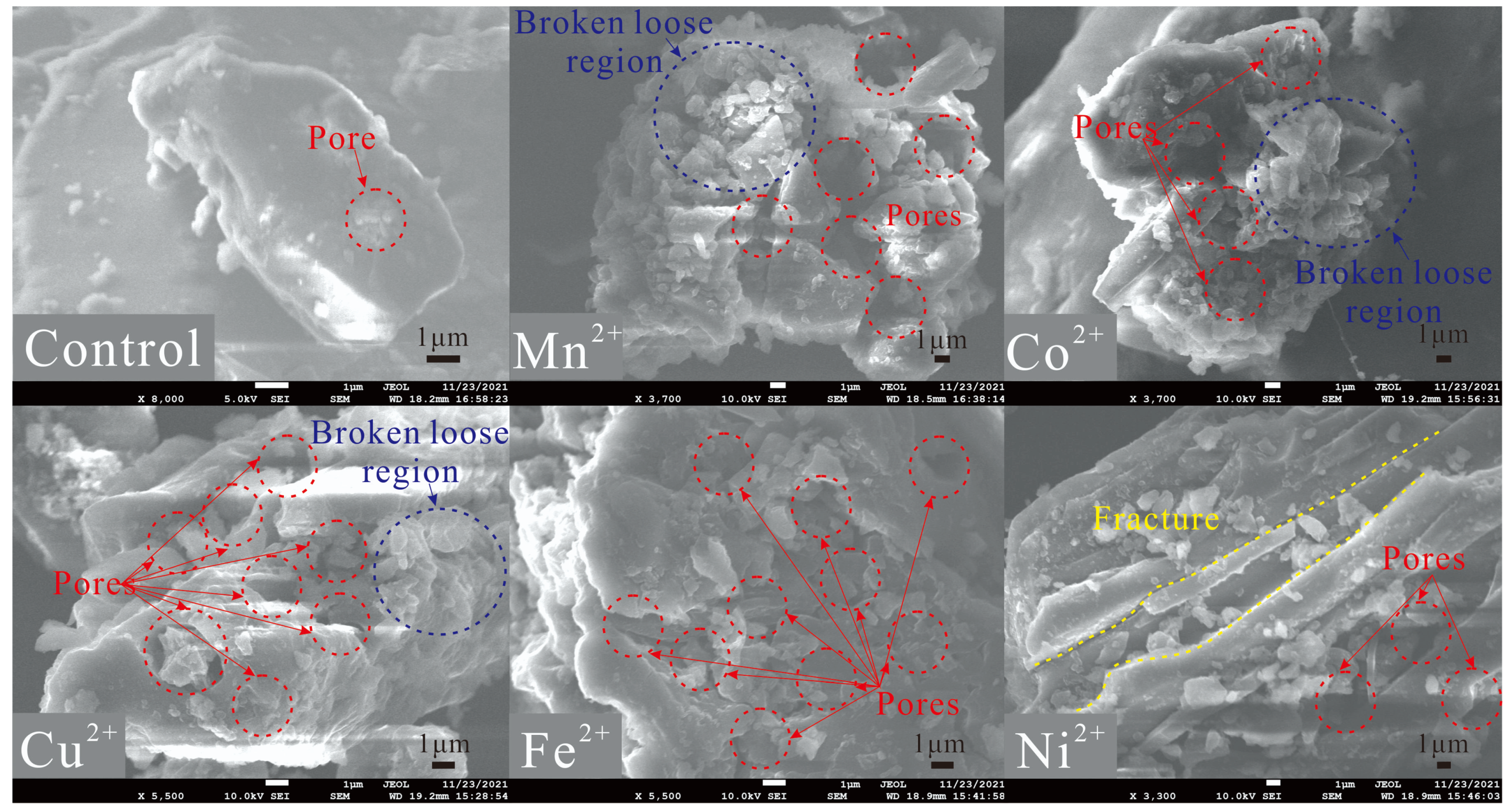
2.3. Scanning Electron Microscopy
2.4. Organic Matter Extraction Experiment
2.5. GC/MS Test Analysis
2.6. Fourier Transform Infrared Spectroscopy
- Approximately 100 mg of potassium bromide, dried in a vacuum oven at 100 °C for 10 h, was weighed and placed into an agate mortar. A small amount of coal sample, which had undergone drying treatment, was thoroughly mixed with the potassium bromide. The mixture was then ground finely.
- The mixture was placed into a mold and subjected to vacuum pressure on a tablet press at 20–25 MPa for 1 min.
- The mold containing a transparent circular thin sheet with a thickness of 0.1–1.0 mm was fixed onto a sample holder.
- Infrared spectroscopy was conducted on the samples using the FTIR spectrometer. The experimental parameters were set as follows: a wavenumber range from 4400 to 400 cm−1 and a resolution of 2 cm−1.
2.7. X-ray Diffraction
3. Results and Discussion
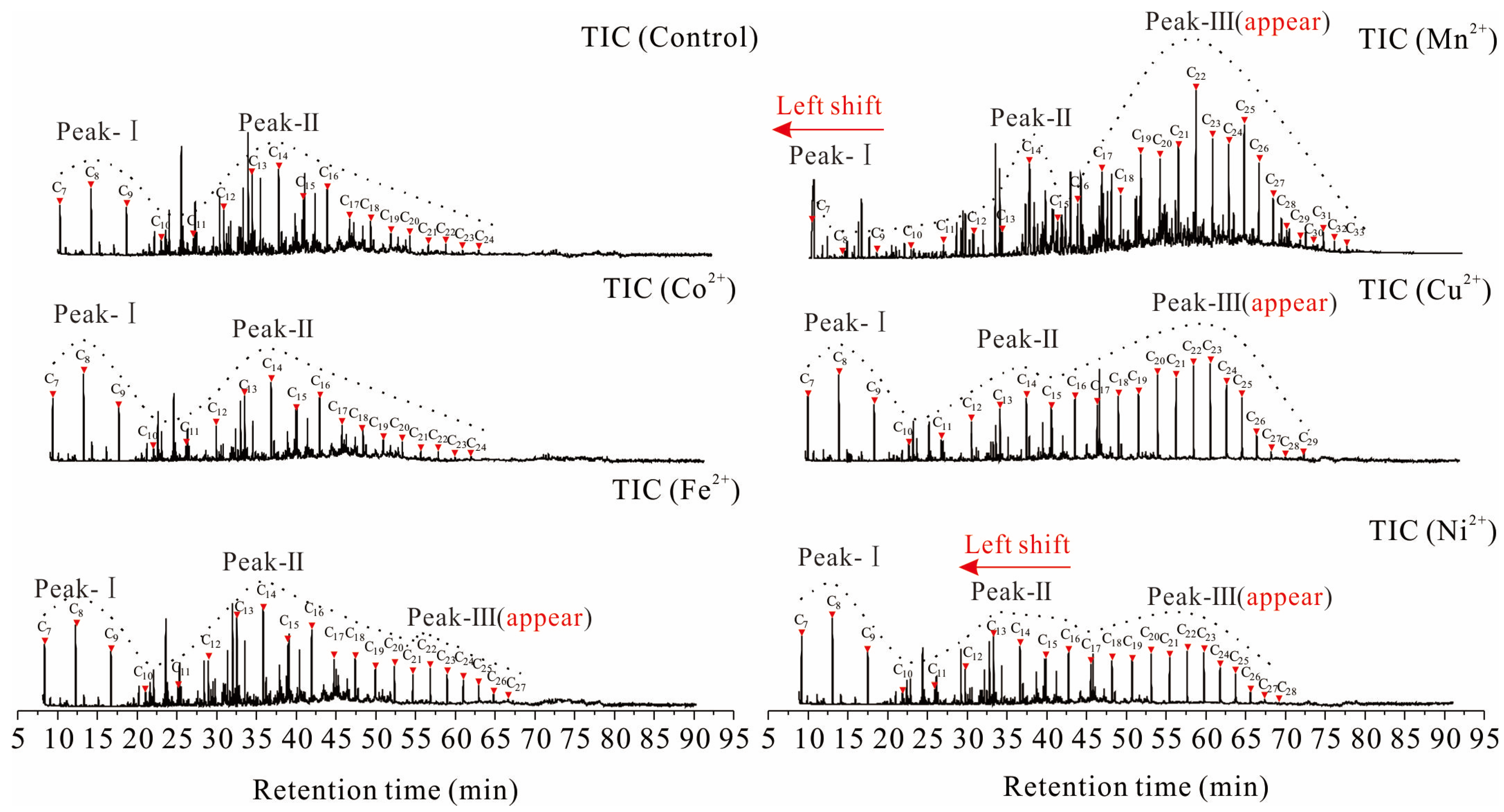
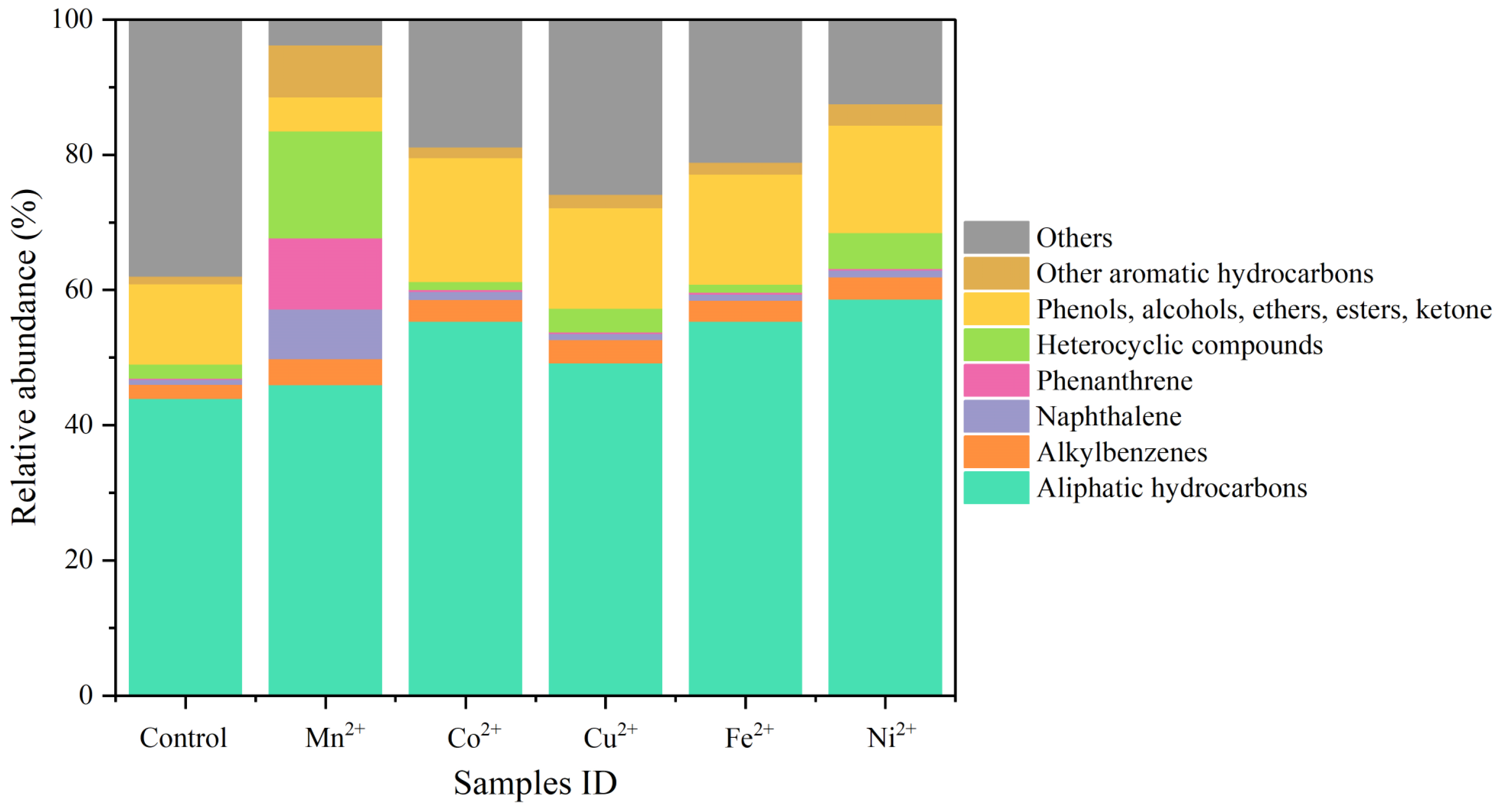
3.1. Extraction Analysis
3.2. Structural Analysis of Raffinate Coal
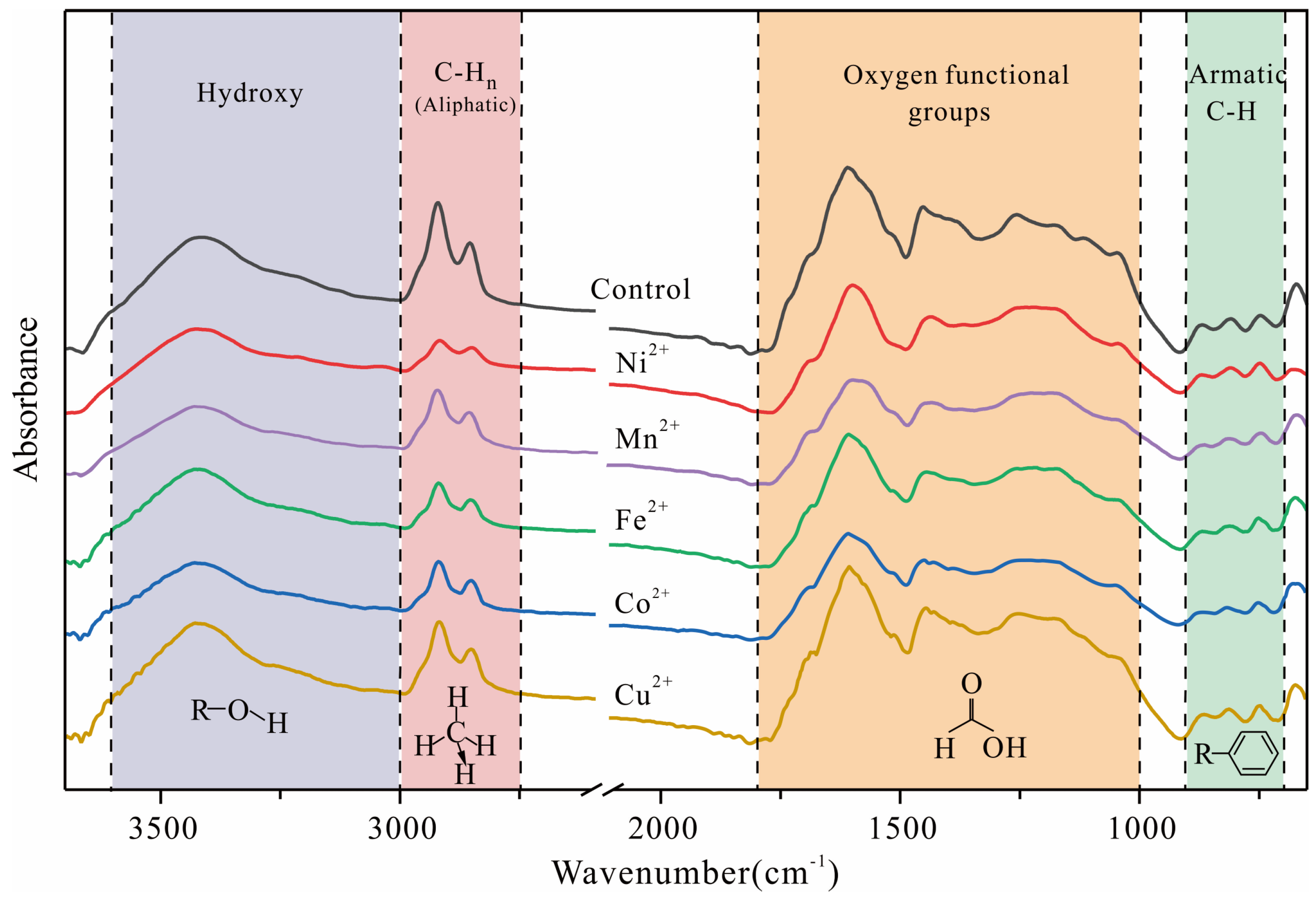
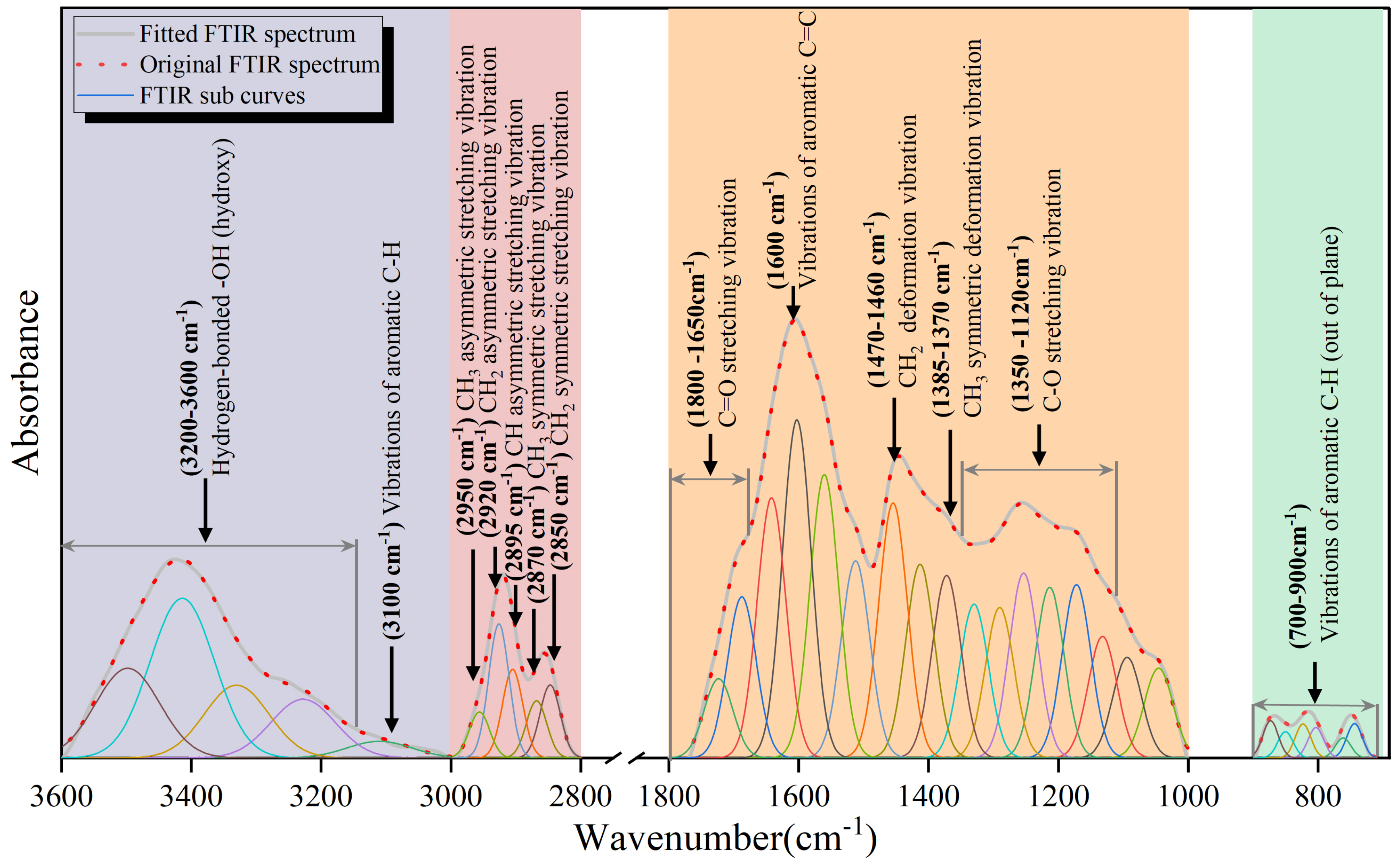
3.2.1. Fourier Transform Infrared Spectroscopy Analysis
- 3600–3000 cm−1: Characteristic absorption peaks of hydroxyl groups.
- 3000–2800 cm−1: Characteristic absorption peaks of aliphatic hydrocarbons.
- 1800–1000 cm−1: Characteristic absorption peaks of oxygen-containing functional groups.
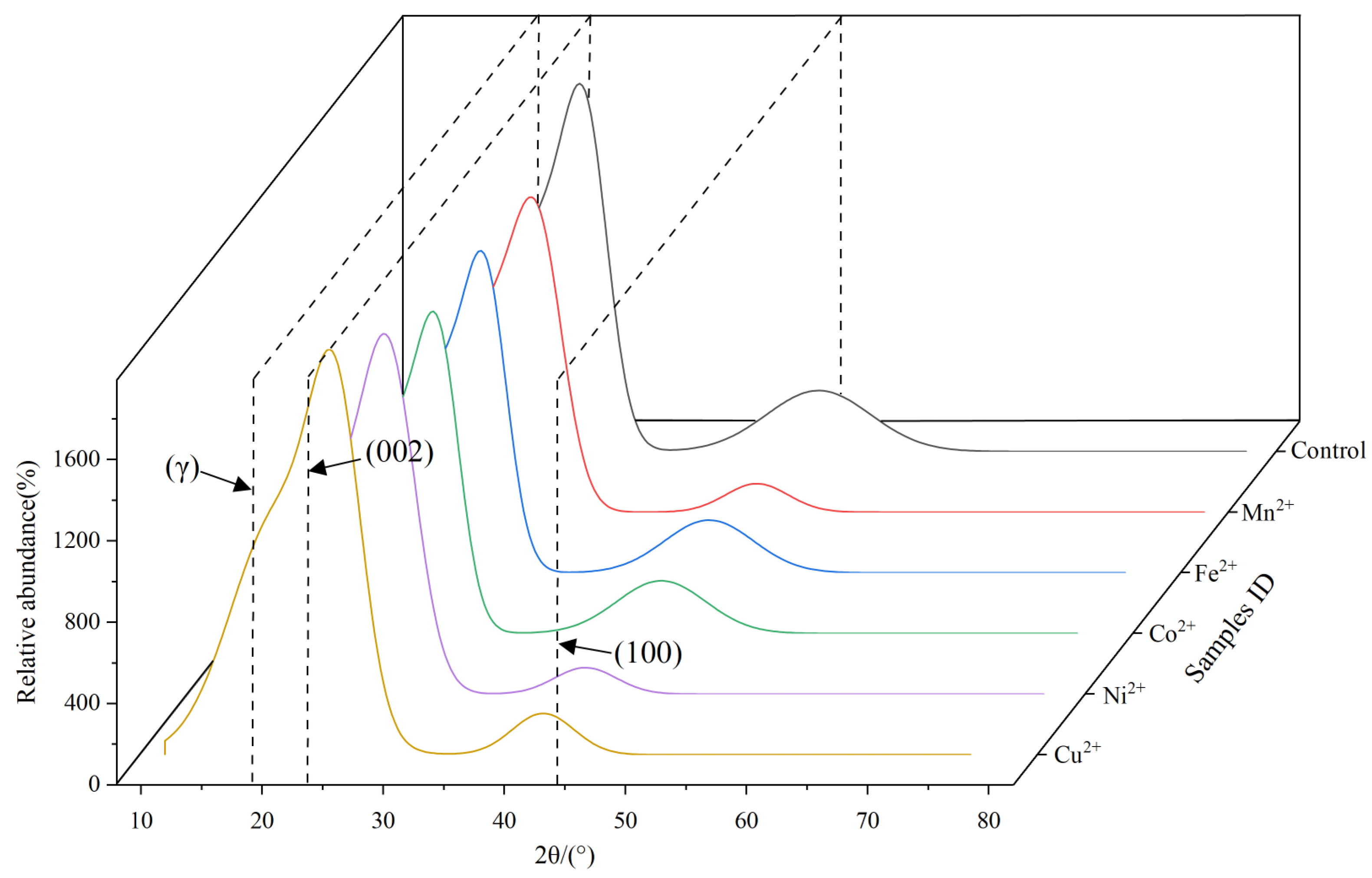
3.2.2. X-ray Diffraction Analysis
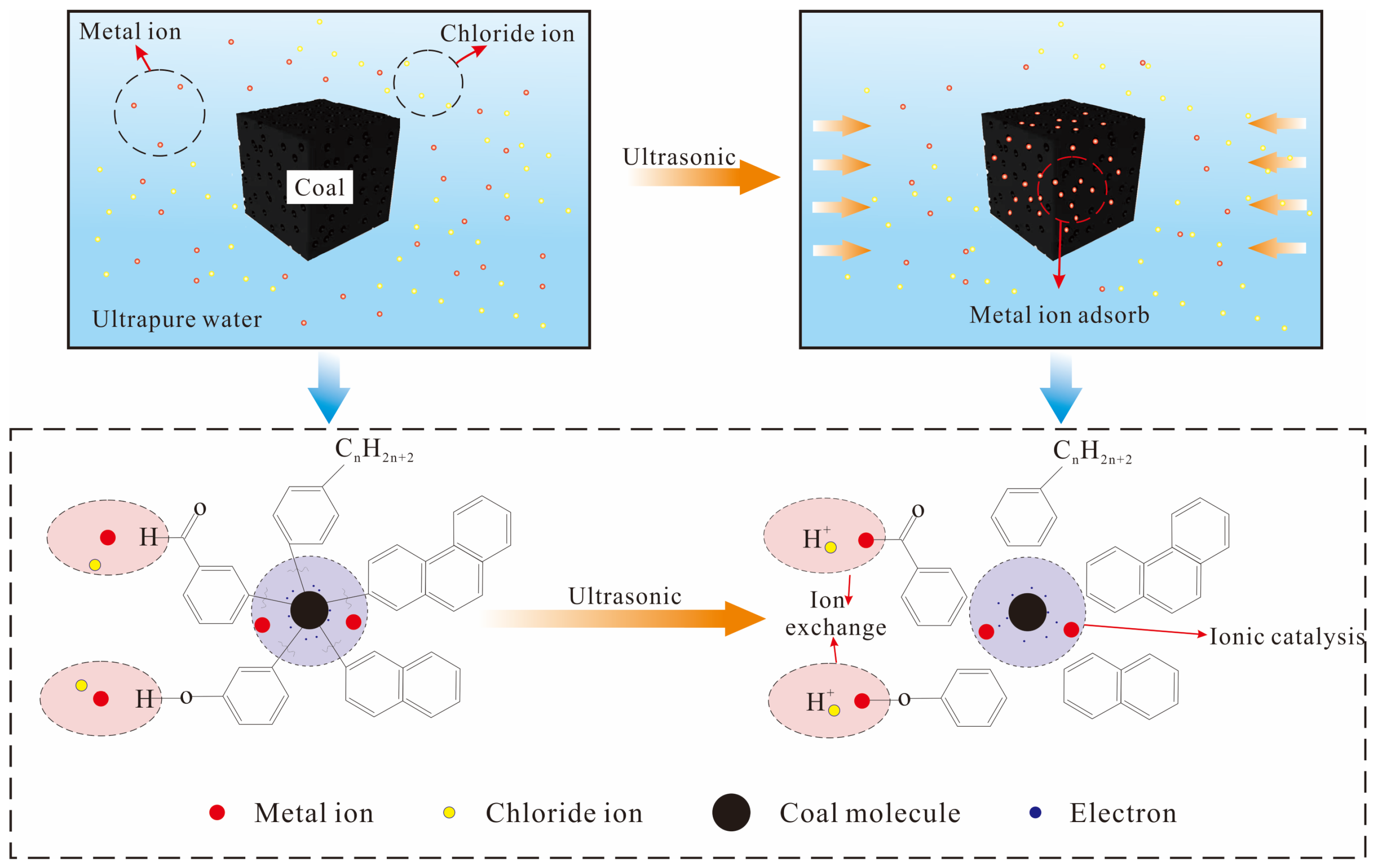
3.3. Mechanism Analysis
4. Conclusions
- Under ultrasonic treatment, Mn2+, Co2+, Cu2+, Fe2+, and Ni2+ metal ions enhanced the extraction efficiency of organic low-molecular-weight fractions. Among these metal ions, Mn2+ exhibited the most significant effect, resulting in a remarkable 212% increase in the extraction rate compared to the blank control group. Following the ultrasonic treatment with different metal ions, there were observable shifts in the peak positions and a number range for normal alkanes present within low-molecular-weight fractions. Additionally, the proportions of aliphatic hydrocarbons, alkylbenzenes, naphthalene, phenanthrene, and other aromatic hydrocarbons increased within this extracted organic fraction obtained from coal samples.
- After subjecting the residual coal samples to ultrasonic loading with various metal ions, consistent trends in the chemical changes were observed. Specifically, there was an increase in the length of aliphatic chains, a decrease in branching (F), a decrease in hydrogen richness (IH), a decrease in aromatic hydrogen content (FARH), an increase in the degree of condensation (DOC), and an increase in aromaticity (AR). Furthermore, the interlayer spacing (d002) within the aromatic structure decreased, while the stacking degree (Lc) increased, and both the expansivity (La) and the number of stacked layers (Nave) decreased.
- After subjecting the coal to ultrasonic loading with various metal ions, the surface of tar-rich coal becomes rougher and exhibits an overall increase in porosity. The metal ions undergo ion exchange with the hydrogen ions present in the coal, thereby altering and influencing the chemical structure of certain functional groups within the coal. Moreover, these metal ions adsorbed onto the coal molecules and effectively formed complexes with unbound electrons within the small molecules of the coal; thus, catalytic effects were facilitated.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, Q.; Li, C.; Wang, S.; Li, D.; Wang, S.; Du, F.; Qiao, J.; Cheng, Q. Effect of the depositional environment on the formation of tar-rich coal: A case study in the northeastern Ordos Basin, China. J. Petrol. Sci. Eng. 2022, 216, 110828. [Google Scholar] [CrossRef]
- Ju, Y.; Zhu, Y.; Zhou, H.; Ge, S.; Xie, H. Microwave pyrolysis and its applications to the in situ recovery and conversion of oil from tar-rich coal: An overview on fundamentals, methods, and challenges. Energy Rep. 2021, 7, 523–536. [Google Scholar] [CrossRef]
- Du, Z.; Li, W. The catalytic effect from alkaline elements on the tar-rich coal pyrolysis. Catalysts 2022, 12, 376. [Google Scholar] [CrossRef]
- Ju, Y.; Zhu, Y.; Zhang, Y.; Zhou, H.; Peng, S.; Ge, S. Effects of high-power microwave irradiation on tar-rich coal for realising in situ pyrolysis, fragmentation, and low-carbon utilisation of tar-rich coal. Int. J. Rock Mech. Min. 2022, 157, 105165. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Shekhawat, D.; Smith, M.W.; Link, D.; Stiegman, A.E. Microwave-assisted pyrolysis of Mississippi coal: A comparative study with conventional pyrolysis. Fuel 2018, 217, 656–667. [Google Scholar] [CrossRef]
- Pan, J.; Lv, M.; Bai, H.; Hou, Q.; Li, M. Effects of metamorphism and deformation on the coal macromolecular structure by laser Raman spectroscopy. Energ. Fuel 2017, 31, 1136–1146. [Google Scholar] [CrossRef]
- Iino, M. Network structure of coals and association behavior of coal-derived materials. Fuel Process. Technol. 2000, 62, 89–101. [Google Scholar] [CrossRef]
- Suzuki, M.; Norinaga, K.; Iino, M. Macroscopic observation of thermal behavior of concentrated solution of coal extracts. Fuel 2004, 83, 2177–2182. [Google Scholar] [CrossRef]
- He, W.; Liu, Z.; Liu, Q.; Shi, L.; Shi, X. Behavior of radicals during solvent extraction of three low rank bituminous coals. Fuel Process. Technol. 2017, 156, 221–227. [Google Scholar] [CrossRef]
- Li, Z.K.; Wei, X.Y.; Yang, Z.S.; Yan, H.L.; Wei, Z.H. Characterization of extracts from geting bituminous coal. Anal. Lett. 2015, 48, 1494–1501. [Google Scholar] [CrossRef]
- Kim, K.; Cho, H.; Lee, S.; Mun, M.; Lee, D. Coal and solvent properties and their correlation with extraction yield under mild conditions. Korean J. Chem. Eng. 2016, 33, 2142–2159. [Google Scholar] [CrossRef]
- Li, F.J.; Wei, X.Y.; Fan, M.H.; Zong, Z.M. Separation and structural characterization of the value-added chemicals from mild degradation of lignites: A review. Appl. Energ. 2016, 170, 415–436. [Google Scholar] [CrossRef]
- Lu, H.Y.; Wei, X.Y.; Yu, R.; Peng, Y.L. Sequential thermal dissolution of Huolinguole lignite in methanol and ethanol. Energ. Fuel 2011, 25, 2741–2745. [Google Scholar] [CrossRef]
- Marzec, A. Towards an understanding of the coal structure: A review. Fuel Process. Technol. 2002, 77–78, 25–32. [Google Scholar] [CrossRef]
- Marta, K. Coal structure studied by means of molecular acoustics methods. Fuel Process. Technol. 2002, 77–78, 33–43. [Google Scholar] [CrossRef]
- Liu, X.; Song, D.; He, X.; Nie, B.; Wang, L. Insight into the macromolecular structural differences between hard coal and deformed soft coal. Fuel 2019, 245, 188–197. [Google Scholar] [CrossRef]
- Bao, Y.; Hu, Y.; Huang, H.; Meng, J.; Zheng, R. Evidence of coal biodegradation from coalbed-produced water—A case study of Dafosi gas field, Ordos Basin, China. ACS Omega 2023, 8, 41885–41896. [Google Scholar] [CrossRef]
- Pajak, J.; Marzec, A.; Severin, D. Compositions of solvent-extracts of a Polish bituminous coal. Fuel 1985, 64, 64–67. [Google Scholar] [CrossRef]
- Ji, H.; Li, Z.; Peng, Y.; Yang, Y.; Tang, Y.; Liu, Z. Pore structures and methane sorption characteristics of coal after extraction with tetrahydrofuran. J. Natural Gas Sci. Eng. 2014, 19, 287–294. [Google Scholar] [CrossRef]
- Vorob’yeva, N.S.; Zemskova, Z.K.; Bodzek, D.; Kiselev, V.I.; Petrov, A.I.A. Hydrocarbons of the soluble part of coal. Petrol. Chem. U.S.S.R. 1983, 23, 232–239. [Google Scholar] [CrossRef]
- Bodzek, D.; Marzec, A. Molecular components of coal and coal structure. Fuel 1981, 60, 47–51. [Google Scholar] [CrossRef]
- Wargadalam, V.J.; Norinaga, K.; Iino, M. Hydrodynamic properties of coal extracts in pyridine. Energ. Fuel 2001, 15, 1123–1128. [Google Scholar] [CrossRef]
- Klotzkin, M.P. Solvent treatment of coals: 1. Effects on microporosity at ambient temperature. Fuel 1985, 64, 1092–1096. [Google Scholar] [CrossRef]
- Klotzkin, M.P. Solvent treatment of coals. 2. Effects on microporosity of extractions in the presence of ultrasonic energy. Fuel 1985, 67, 104–108. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.; Lv, P.; Ren, L.; Kong, J.; Wang, J.; Li, F.; Bai, Y. Effect of n-hexane extraction on the formation of light aromatics from coal pyrolysis and catalytic upgrading. J. Energy Inst. 2020, 93, 1242–1249. [Google Scholar] [CrossRef]
- Hu, R.N.; Wang, Z.C.; Li, L.; Wang, X.; Pan, C.; Kang, S.; Ren, S.; Lei, Z.; Shui, H. Effect of solvent extraction pretreatments on the variation of macromolecular structure of low rank coals. J. Fuel Chem. Technol. 2018, 46, 778–786. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Xue, W.; Yin, Z.; Meng, Z.; Zhou, A. Small molecules from multistep extraction of coal and their effects on coal adsorption of CH4. Catal. Today 2021, 374, 192–199. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, F.; Mo, W.; Wang, Q. Five-stage sequential extraction of Hefeng coal and direct liquefaction performance of the extraction residue. Fuel 2020, 266, 117039. [Google Scholar] [CrossRef]
- Sönmez, Ö.; Yıldız, Ö.; Çakır, M.Ö.; Gözmen, B.; Giray, E.S. Influence of the addition of various ionic liquids on coal extraction with NMP. Fuel 2018, 212, 12–18. [Google Scholar] [CrossRef]
- Yin, J.; Lin, X.; Wang, C.; Dai, J.; Wang, Y.; Xu, Z. Identification of the transformation features of heteroatomic compounds in a low rank coal by combining thermal extraction and various analytical approaches. Fuel 2020, 270, 117480. [Google Scholar] [CrossRef]
- Mahat, R.K.; Rodgers, W.; Basile, F. Microwave Radiation Heating in Pressurized Vessels for the Rapid Extraction of Coal Samples for Broad Spectrum GC–MS Analysis. Energ. Fuel 2014, 28, 6326–6335. [Google Scholar] [CrossRef]
- Yoshida, T.; Takanohashi, T.; Sakanishi, K.; Saito, I.; Fujita, M.; Mashimo, K. The effect of extraction condition on “Hyper Coal” production (1) under room-temperature filtration. Fuel 2002, 81, 1463–1469. [Google Scholar] [CrossRef]
- Liu, L.; Kumar, S.; Wang, Z.; He, Y.; Liu, J.; Cen, K. Catalytic effect of metal chlorides on coal pyrolysis and gasification part I. Combined TG-FTIR study for coal pyrolysis. Thermochim. Acta 2017, 655, 331–336. [Google Scholar] [CrossRef]
- Iino, M.; Takanohashi, T.; Ohsuga, H.; Toda, K. Extraction of coals with CS2-N-methyl-2-pyrrolidinone mixed solvent at room temperature: Effect of coal rank and synergism of the mixed solvent. Fuel 1988, 67, 1639–1647. [Google Scholar] [CrossRef]
- Bao, Y.; Hu, Y.; Wang, W.; Guo, C.; Wang, G. Accumulation model and geochemistry characteristics of oil occurring from Jurassic coal measures in the Huangling mining area of the Ordos Basin, China. Front. Earth Sci.-Prc. 2023, 17, 158–169. [Google Scholar] [CrossRef]
- GB/T 1314-2007; Gray-King Assay of Coal. Standardization Administration of China: Beijing, China, 2007. (In Chinese)
- Bao, Y.; Li, Z.; Meng, J.; Chen, X.; Liu, X. Reformation of coal reservoirs by microorganisms and its significance in CBM exploitation. Fuel 2024, 360, 130642. [Google Scholar] [CrossRef]
- Painter, P.C.; Snyder, R.W.; Starsinic, M.; Coleman, M.M.; Kuehn, D.W.; Davis, A. Concerning the application of FT-IR to the study of coal: A critical assessment of band assignments and the application of spectral analysis programs. Appl. Spectrosc. 1981, 35, 475–485. [Google Scholar] [CrossRef]
- Ibarra, J.; Muñoz, E.; Moliner, R. FTIR study of the evolution of coal structure during the coalification process. Org. Geochem. 1996, 24, 725–735. [Google Scholar] [CrossRef]
- Supaluknari, S.; Larkins, F.P.; Redlich, P.; Jackson, W.R. An FTIR study of Australian coals: Characterization of oxygen functional groups. Fuel Process. Technol. 1988, 19, 123–140. [Google Scholar] [CrossRef]
- Mastalerz, M.; Bustin, R.M. Electron microprobe and micro-FTIR analyses applied to maceral chemistry. Int. J. Coal Geol. 1993, 24, 333–345. [Google Scholar] [CrossRef]
- Thomas, N.; Liliedahl, T.; Krister, S. Metallic iron as a tar breakdown catalyst related to atmospheric, fluidised bed gasification of biomass. Fuel 2006, 85, 689–694. [Google Scholar] [CrossRef]
- Taralas, G.; Kontominas, M.G. Kinetic modelling of VOC catalytic steam pyrolysis for tar abatement phenomena in gasification/pyrolysis technologies. Fuel 2004, 83, 1235–1245. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Nie, B.; Song, D. FTIR and Raman spectroscopy characterization of functional groups in various rank coals. Fuel 2017, 206, 555–563. [Google Scholar] [CrossRef]
- Li, D.; Bao, Y.; Wang, Y.; An, C.; Chang, J. Multiple-experimental investigation on the physicochemical structures alternation during coal biogasification. Fuel 2023, 339, 127433. [Google Scholar] [CrossRef]


| Proximate Analysis (wt%) | Ultimate Analysis (wt%, daf) | Gray-King Assay (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vdaf | FCad | Cdaf | Hdaf | Odaf | Ndaf | St,daf | Moisture content | Tar yield | Semi-coke yield |
| 1.18 | 5.85 | 35.90 | 59.60 | 73.15 | 5.10 | 20.05 | 1.359 | 0.34 | 4.50 | 9.80 | 76.40 |
| Sample ID | F | IH | FARH | DOC | AR |
|---|---|---|---|---|---|
| Control | 2.8007 | 0.1915 | 0.4273 | 0.1307 | 0.2164 |
| Co2+ | 2.8528 | 0.0886 | 0.2583 | 0.2044 | 0.7462 |
| Mn2+ | 3.7552 | 0.1057 | 0.1779 | 0.1578 | 0.5192 |
| Fe2+ | 3.3470 | 0.1299 | 0.3418 | 0.1578 | 0.4276 |
| Ni2+ | 3.6536 | 0.1486 | 0.2308 | 0.1406 | 0.3001 |
| Cu2+ | 2.9102 | 0.1534 | 0.2995 | 0.1590 | 0.3483 |
| Sample ID | d002/nm | La/nm | Lc/nm | Nave (Layers) |
|---|---|---|---|---|
| Control | 0.3593 | 1.7235 | 1.4893 | 4.1450 |
| Co2+ | 0.3538 | 1.8137 | 1.6498 | 4.6631 |
| Fe2+ | 0.3560 | 2.1343 | 1.6348 | 4.5921 |
| Ni2+ | 0.3532 | 2.8315 | 1.8459 | 5.2262 |
| Cu2+ | 0.3573 | 2.7578 | 1.5709 | 4.3966 |
| Mn2+ | 0.3532 | 2.8648 | 1.8990 | 5.3766 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Bao, Y.; Wang, C.; Hu, Y. Study on the Extraction Mechanism of Metal Ions on Small Molecular Phase of Tar-Rich Coal under Ultrasonic Loading. Processes 2024, 12, 104. https://doi.org/10.3390/pr12010104
Wang Z, Bao Y, Wang C, Hu Y. Study on the Extraction Mechanism of Metal Ions on Small Molecular Phase of Tar-Rich Coal under Ultrasonic Loading. Processes. 2024; 12(1):104. https://doi.org/10.3390/pr12010104
Chicago/Turabian StyleWang, Zetang, Yuan Bao, Chaoyong Wang, and Yiliang Hu. 2024. "Study on the Extraction Mechanism of Metal Ions on Small Molecular Phase of Tar-Rich Coal under Ultrasonic Loading" Processes 12, no. 1: 104. https://doi.org/10.3390/pr12010104
APA StyleWang, Z., Bao, Y., Wang, C., & Hu, Y. (2024). Study on the Extraction Mechanism of Metal Ions on Small Molecular Phase of Tar-Rich Coal under Ultrasonic Loading. Processes, 12(1), 104. https://doi.org/10.3390/pr12010104






