Exploitation and Utilization of Generated Oil and Gas by Pyrolysis Simulation Modeling of Shale Source Rocks under the Condition of In Situ Conversion
Abstract
1. Introduction
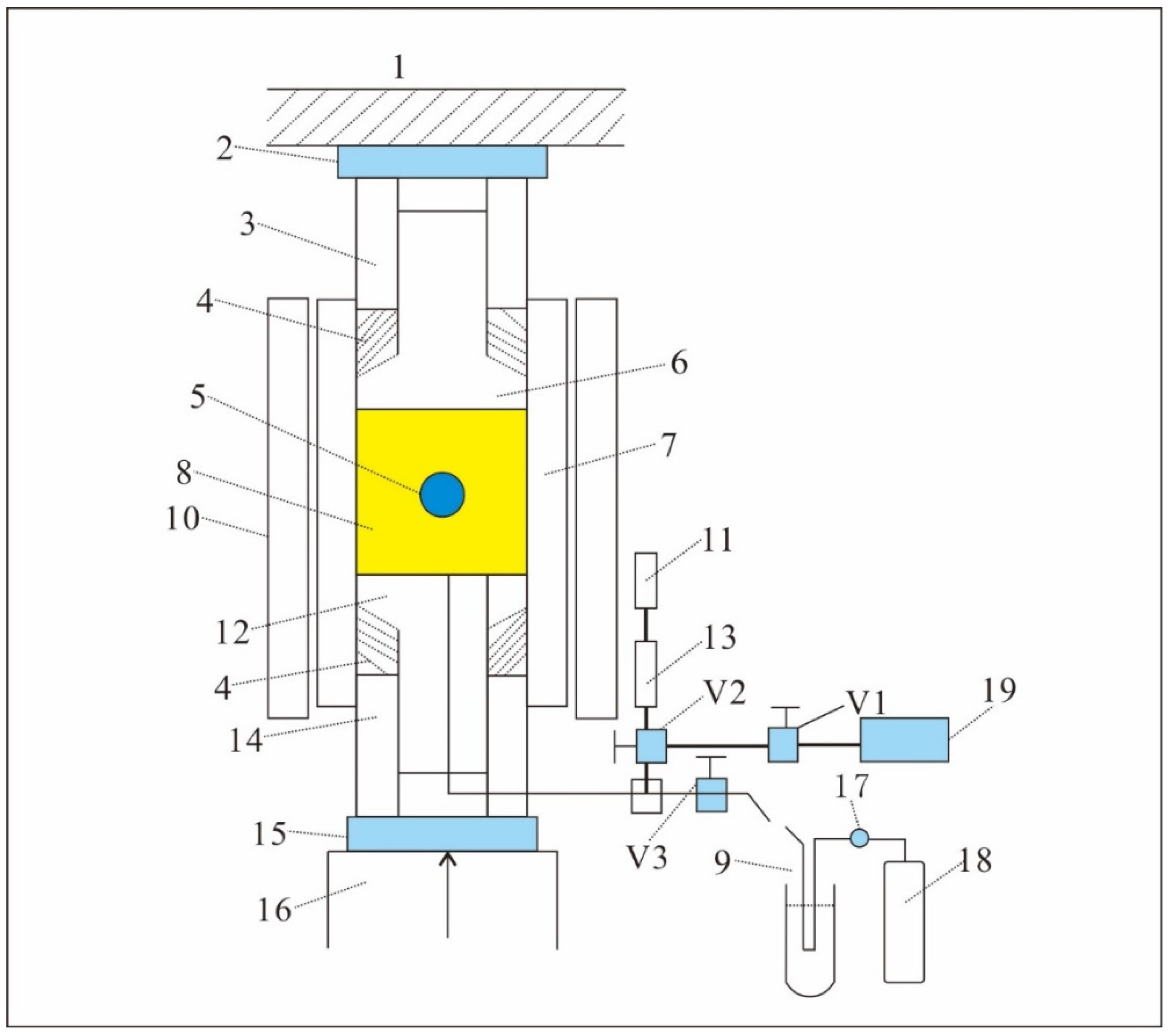
2. Samples and Methods
3. Results and Discussion
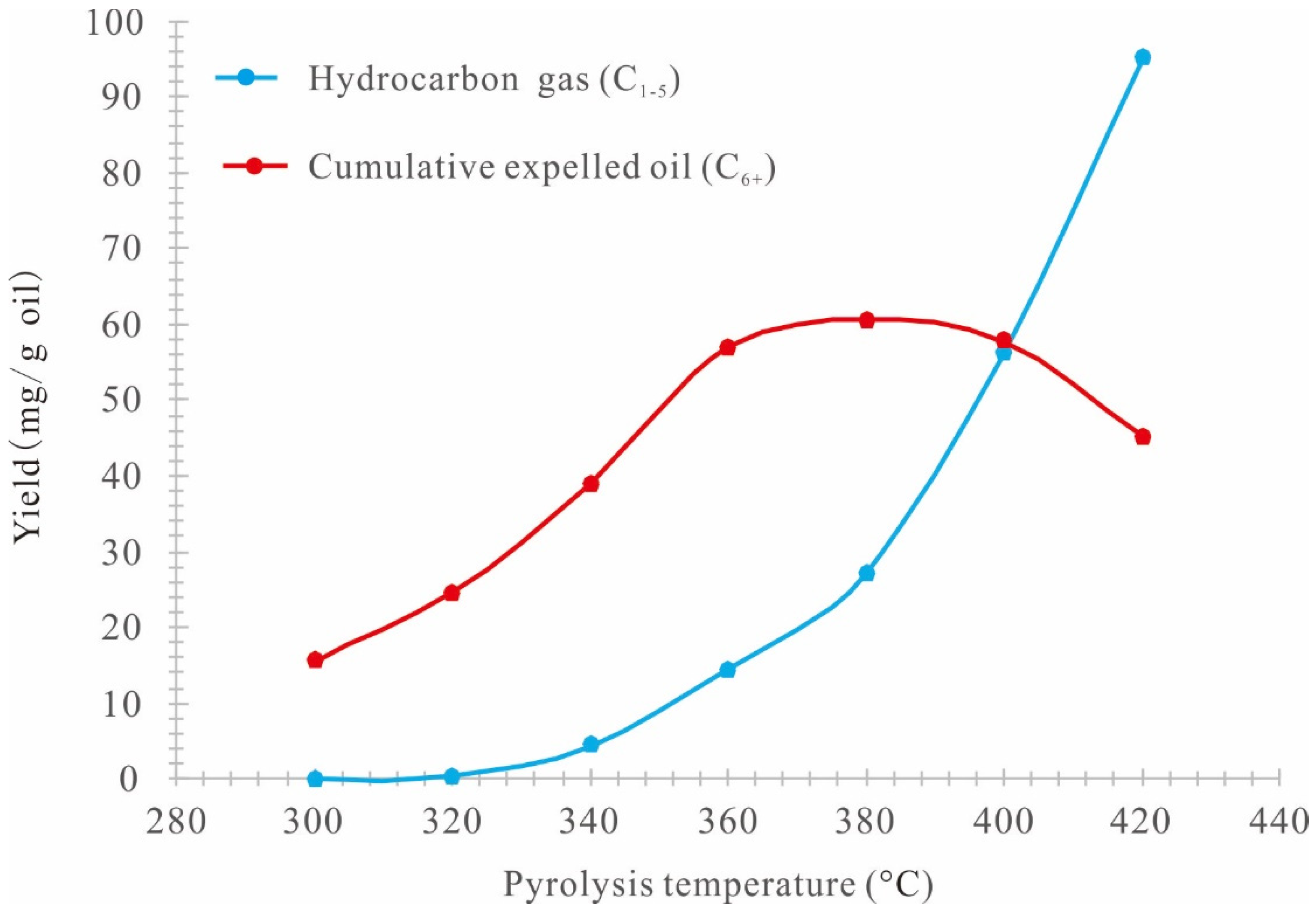
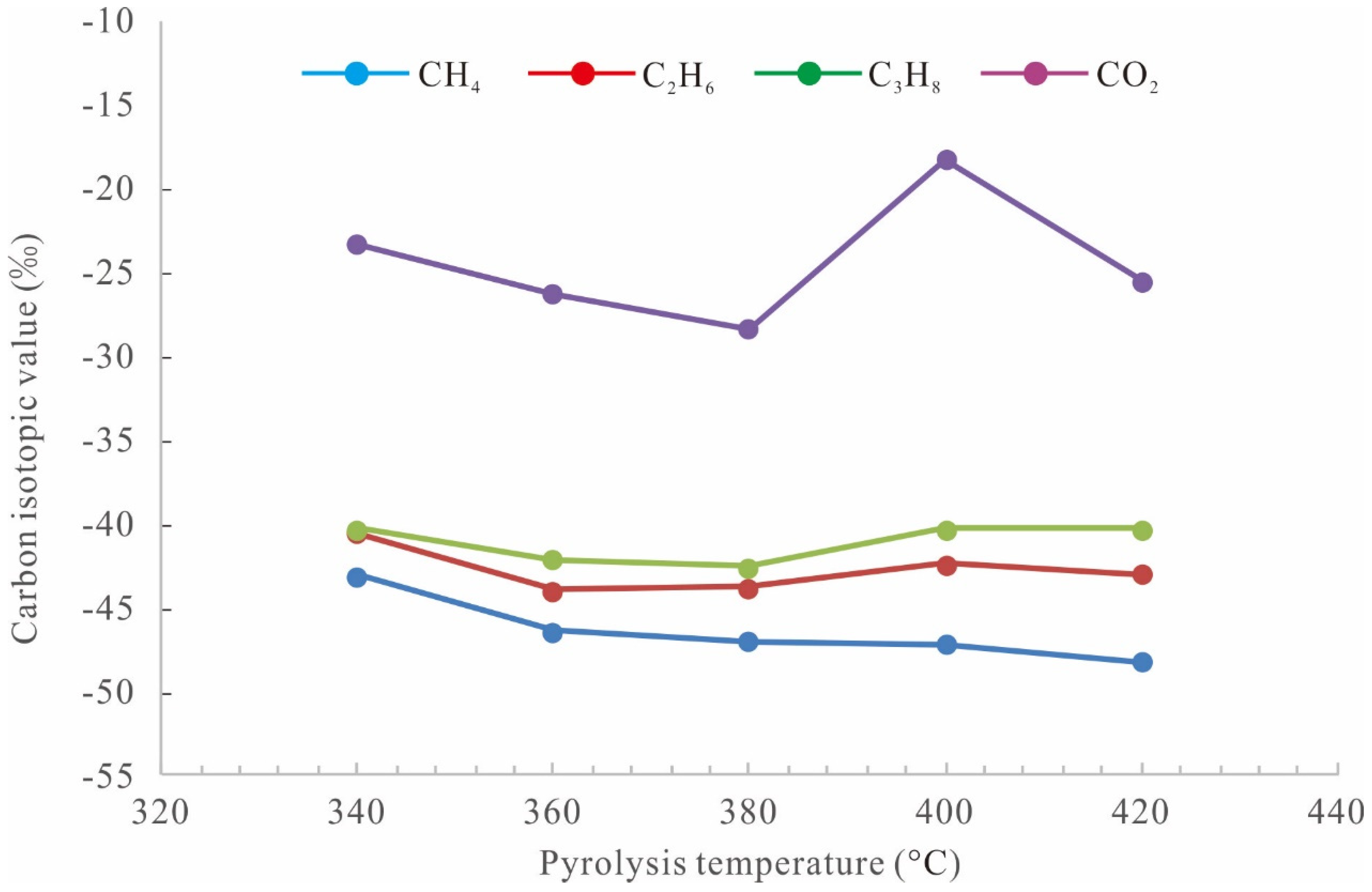
3.1. Quantification of Pyrolysis Products
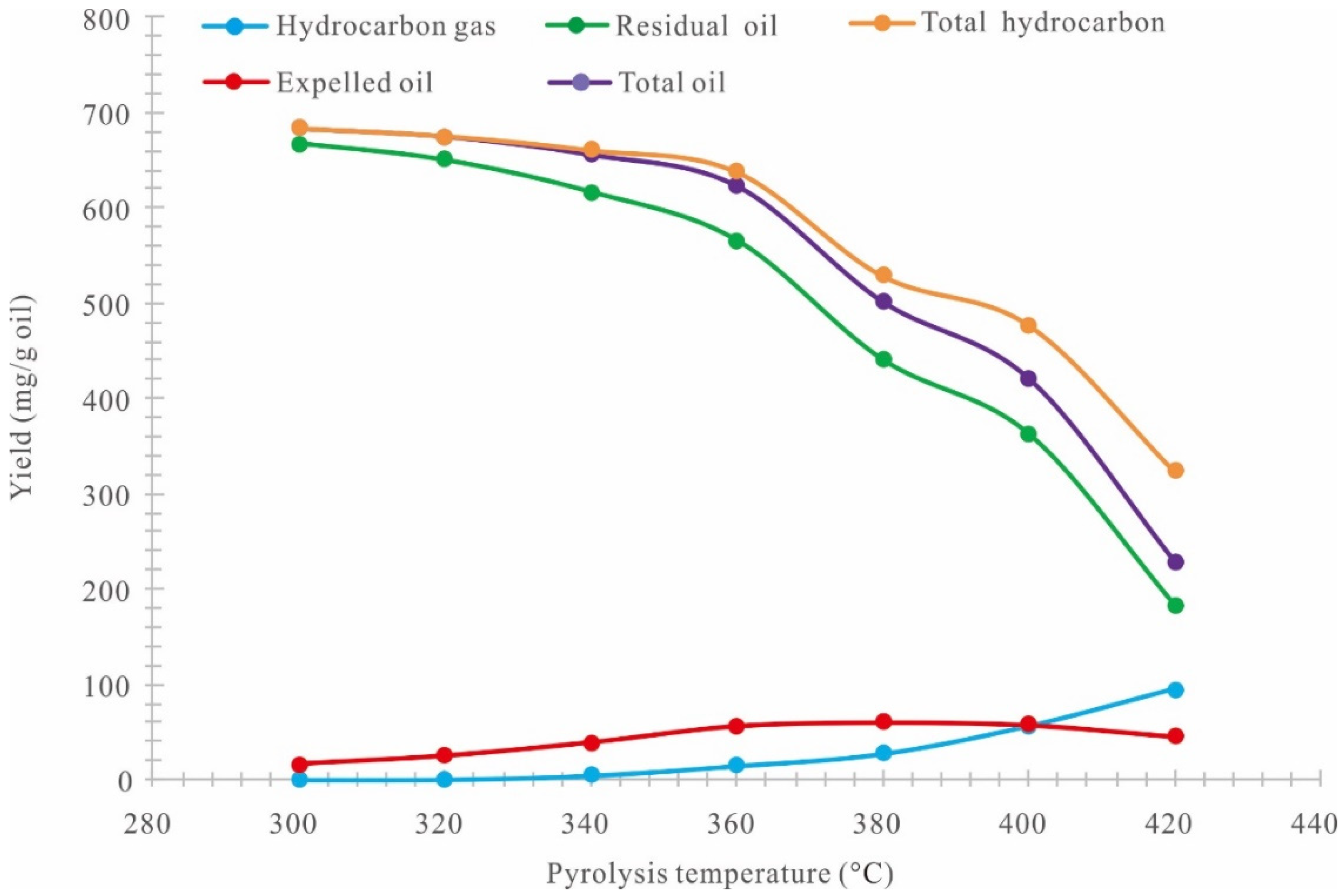
3.2. Accumulative Oil and Gas Yield
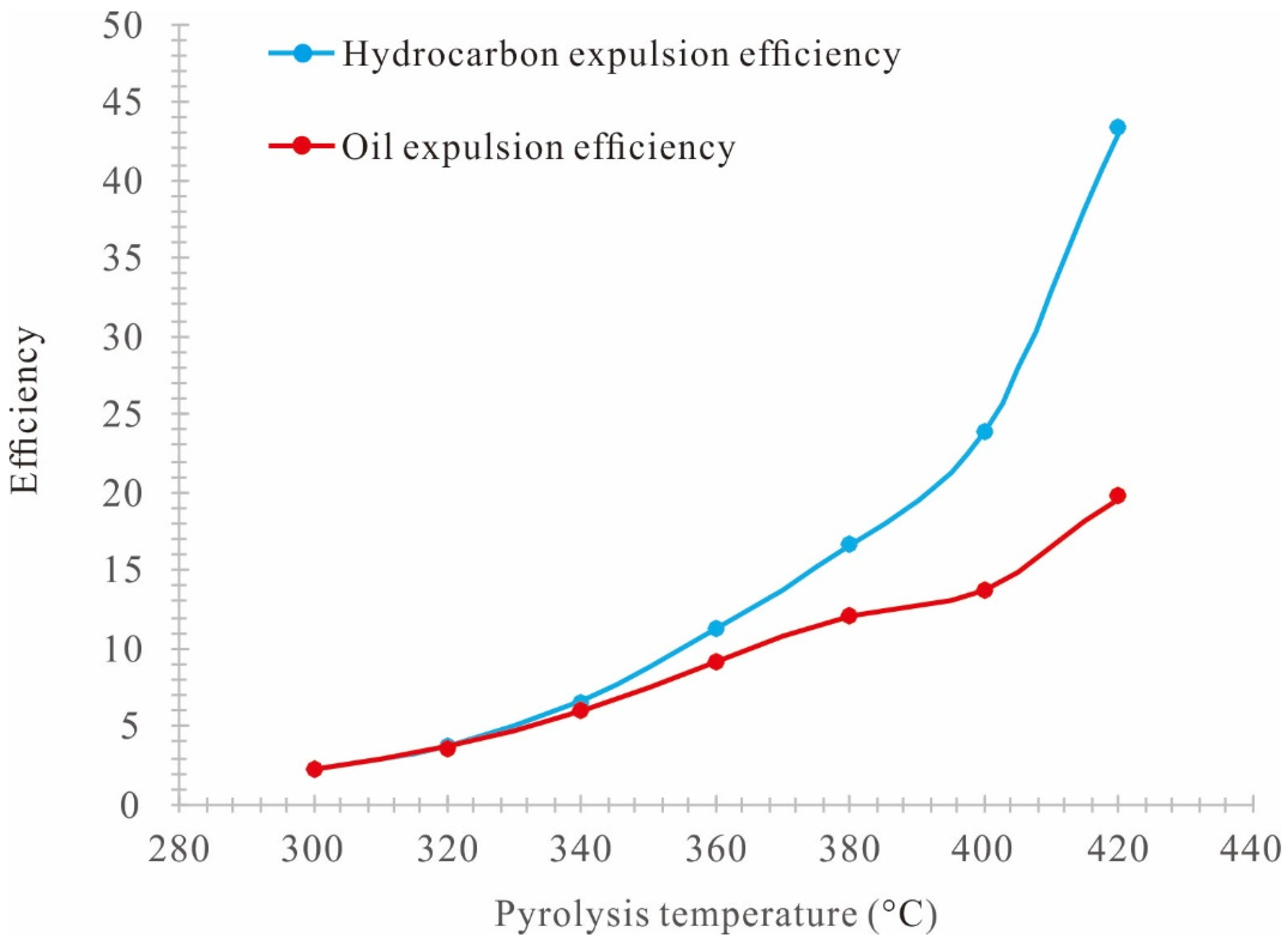
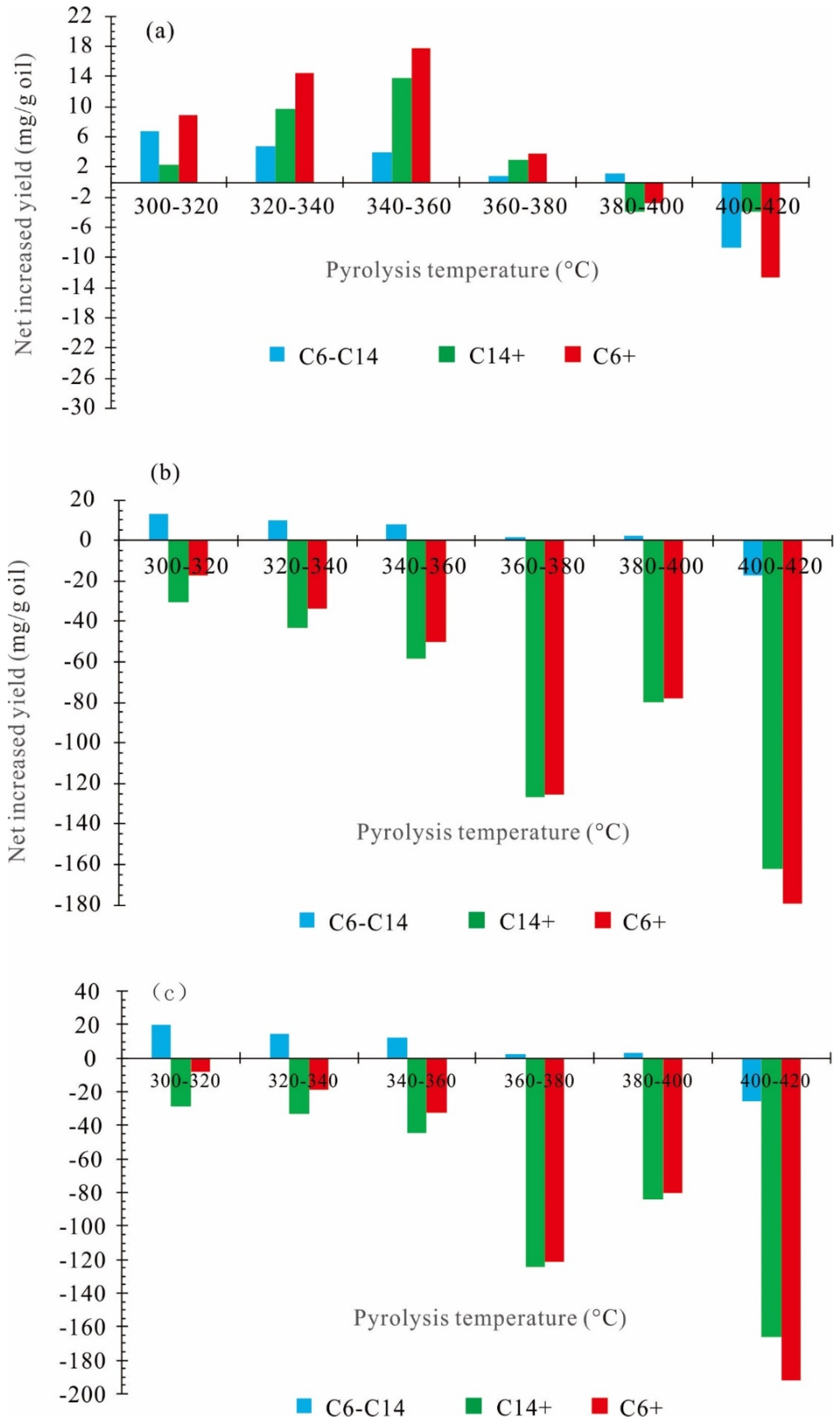
3.3. Net Increased Oil Yield
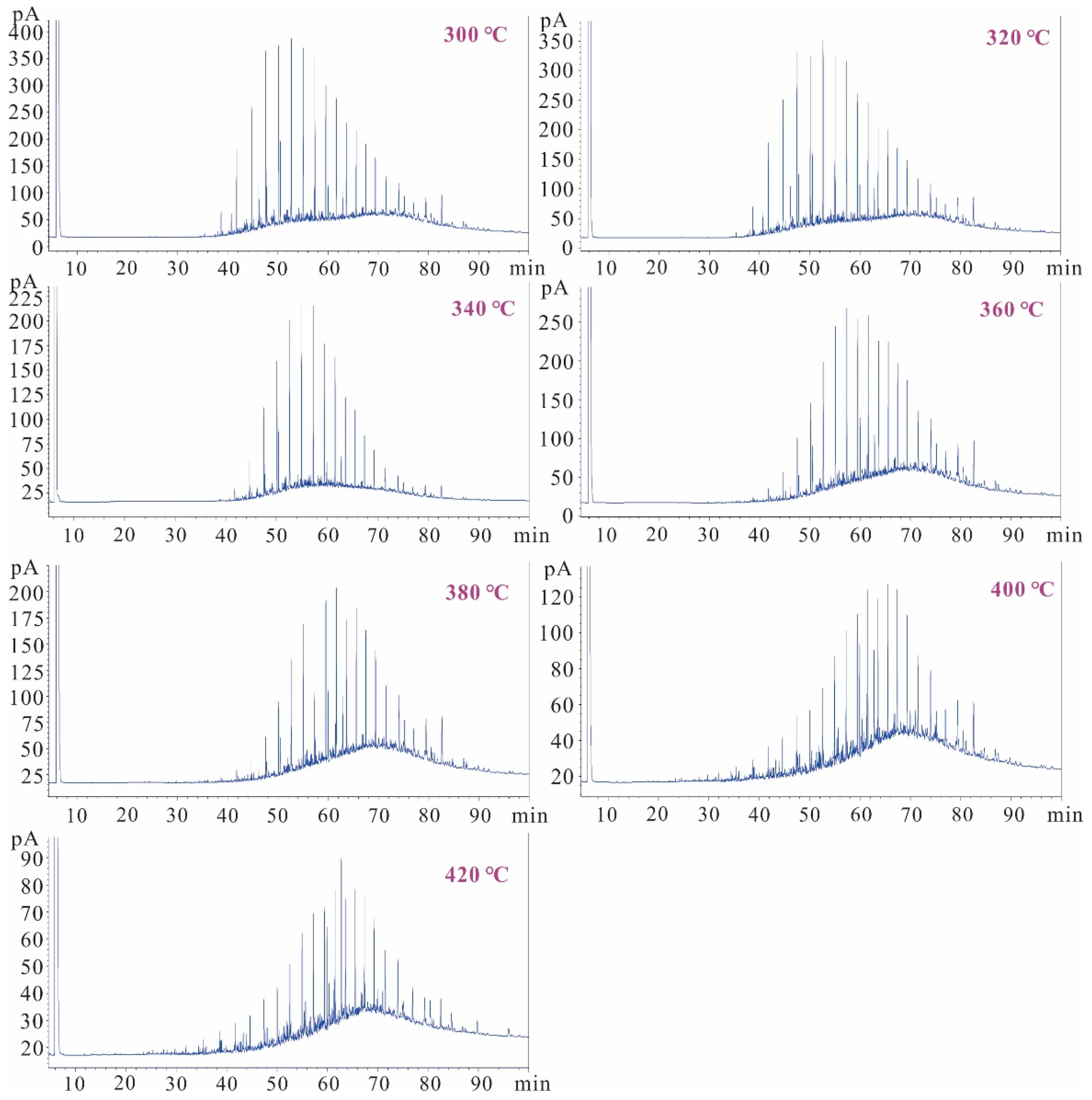
3.4. Chromatographic Characteristics of Generated Oil
3.5. Stages of Hydrocarbon Generation of Retained Oil
- (1)
- 300–320 °C (0.48%Ro–0.57%Ro): Slow hydrocarbon generation stage. In this stage, the increasing amplitude of the expelled oil rate, the decreasing amplitude of the residual oil rate, and the generation rate of gas hydrocarbons (C1–5) are smaller, and most retained oil remains in the alumina particles in the high-pressure reactor and can hardly be expelled.
- (2)
- 320–360 °C (0.57%Ro–0.79%Ro): Rapid hydrocarbon generation stage. In this stage, the expelled oil rate increases rapidly, the residual oil rate decreases rapidly, and the generation rate of gas hydrocarbons (C1–5) increases gradually.
- (3)
- 360–420 °C (0.79%Ro–1.47%Ro): Residual oil pyrolysis stage. The continuous increase of the temperature leads to the pyrolysis of the residual oil retained in the alumina particles in the reactor. As a result, the total residual oil decreases rapidly, the expelled oil continues to increases rapidly, and the generation rate of gas hydrocarbons (C1–5) increases rapidly.
4. Conclusions
- (1)
- 300–320 °C (0.48%Ro–0.57%Ro) stage is the slow hydrocarbon generation stage, when the increasing amplitude of the expelled oil rate and the decreasing amplitude of residual oil rate are smaller, and most of the retained oil remains in the alumina particles in the reactor and can hardly be expelled.
- (2)
- 320–360 °C (0.57%Ro–0.79%Ro) stage is the rapid hydrocarbon generation stage, when the expelled oil rate increases rapidly, the residual oil rate decreases rapidly, and the generation rate of gaseous hydrocarbons (C1–5) increases rapidly as well.
- (3)
- 360–420 °C (0.79%Ro–1.47%Ro) stage is the pyrolysis stage, when the continuous increase of the temperature leads to the pyrolysis of the residual oil retained in the alumina particles in the reactor.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Mao, Z.G.; Zhang, Z.Y.; Yuan, Y.L.; Chen, X.L.; Shi, Y.X.; Liu, G.L.; Shao, X.Z. Black shale formation environment and its control on shale oil enrichment in Triassic Chang 7 Member, Ordos Basin, NW China. Pet. Explor. Dev. 2021, 48, 1304–1314. [Google Scholar] [CrossRef]
- Cao, H.R.; Lei, Y.; Wang, X.Y.; Zou, Y.R.; Peng, P.A. Molecular structure evolution of Type I kerogen during pyrolysis: Case study from the Songliao Basin, NE China. Mar. Pet. Geol. 2021, 134, 105338. [Google Scholar] [CrossRef]
- Dieckmann, V.; Schenk, H.J.; Horsfield, B. Kinetics of petroleum generation and cracking by programmed-temperature closed-system pyrolysis of Toarcian Shales. Fuel 1998, 77, 23–31. [Google Scholar] [CrossRef]
- Gai, H.F.; Tian, H.; Cheng, P.; Zhou, Q.; Li, T.F.; Wang, X.; Xiao, X.M. Influence of retained bitumen in oil-prone shales on the chemical and carbon isotopic compositions of natural gases: Implications from pyrolysis experiments. Mar. Pet. Geol. 2019, 101, 148–161. [Google Scholar] [CrossRef]
- Hill, R.J.; Tang, Y.; Kaplan, I.R. Insights into oil cracking based on laboratory experiments. Org. Geochem. 2003, 34, 1651–1672. [Google Scholar] [CrossRef]
- Li, E.T.; Pan, C.C.; Yu, S.; Jin, X.D.; Liu, J.Z. Interaction of coal and oil in confined pyrolysis experiments: Insight from the yields and carbon isotopes of gas and liquid hydrocarbons. Mar. Pet. Geol. 2016, 69, 13–37. [Google Scholar] [CrossRef]
- Huang, W.K.; Zeng, L.F.; Pan, C.C.; Xiao, Z.Y.; Zhang, H.Z.; Huang, Z.B.; Zhao, Q.; Yu, S.; Xu, H.; Chen, C.S.; et al. Petroleum generation potentials and kinetics of coaly source rocks in the Kuqa Depression of Tarim Basin, northwest China. Org. Geochem. 2019, 133, 32–52. [Google Scholar] [CrossRef]
- Zeng, L.F.; Huang, W.K.; Pan, C.C.; Jin, J.; Ma, W.Y.; Yu, S.; Xu, H.; Liu, D.Y.; Liao, Y.H.; Liu, J.Z. Assessment of oil potentials for humic coals on the basis of flash Py-GC, Rock-Eval and confined pyrolysis experiments. Org. Geochem. 2020, 148, 104097. [Google Scholar] [CrossRef]
- Wang, Q.T.; Liu, W.H.; Pei, L.X.; Cai, Z.H.; Luo, H.Y.; Wang, X.F.; Zhang, D.D.; Liu, J.Z. Hydrocarbon generation from calcium stearate: Insights from closed-system pyrolysis. Mar. Pet. Geol. 2021, 126, 104923. [Google Scholar] [CrossRef]
- Jin, X.D.; Li, E.T.; Pan, C.C.; Yu, S.; Liu, J.Z. Interaction of coal and oil in confined pyrolysis experiments: Insight from the yield and composition of gas hydrocarbons. Mar. Pet. Geol. 2013, 48, 379–391. [Google Scholar] [CrossRef]
- Wang, Q.T.; Lu, H.; Greenwood, P.; Shen, C.C.; Liu, J.Z.; Peng, P.A. Gas evolution during kerogen pyrolysis of Estonian Kukersite shale in confined gold tube system. Org. Geochem. 2013, 65, 74–82. [Google Scholar] [CrossRef]
- Pan, C.C.; Jiang, L.L.; Liu, J.Z.; Zhang, S.C.; Zhu, G.Y. The effects of pyrobitumen on oil cracking in confined pyrolysis experiments. Org. Geochem. 2012, 45, 29–47. [Google Scholar] [CrossRef]
- Jia, W.; Wang, Q.; Liu, J.; Peng, P.; Li, B.; Lu, J. The effect of oil expulsion or retention on further thermal degradation of kerogen at the high maturity stage: A pyrolysis study of type II kerogen from Pingliang shale, China. Org. Geochem. 2014, 71, 17–29. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, X.M.; Cheng, P.; Tian, H. Formation and evolution of nanopores in shales and its impact on retained oil during oil generation and expulsion based on pyrolysis experiments. J. Pet. Sci. Eng. 2019, 176, 509–520. [Google Scholar] [CrossRef]
- Liu, C.; Lei, M.Y.; Sun, B.L.; Wang, X. Origin and process of carbon isotopic reversal of tight sandstone gas in the Shixi gas field, eastern margin of Ordos Basin. Int. J. Coal Geol. 2023, 276, 104327. [Google Scholar] [CrossRef]
- Su, K.M.; Chen, S.J.; Hou, Y.T.; Zhang, H.F.; Zhang, X.L.; Zhang, W.X.; Liu, G.L.; Hu, C.; Han, M.M. Geochemical characteristics, origin of the Chang 8 oil and natural gas in the southwestern Ordos Basin, China. J. Pet. Sci. Eng. 2021, 200, 108406. [Google Scholar] [CrossRef]
- Yang, S.R.; Gang, W.Z.; Cao, J.J.; Liu, J.P.; Gao, G.; Dang, W.L. Geochemical characteristics, origin and carbon isotope reversal of the presalt natural gas in the Lower Paleozoic Ordovician carbonates, Ordos Basin, China. Mar. Pet. Geol. 2022, 139, 105577. [Google Scholar] [CrossRef]
- Lotfy, N.M.; Qteishat, A.; Farouk, S.; Ahmad, F.; Al-Kahtany, K.; Hsu, C.S. Geochemical characteristics and genetic types of Ordovician tight gas in the Risha Gas Field, Eastern Jordan based on carbon and hydrogen isotope compositions. Mar. Pet. Geol. 2022, 143, 105810. [Google Scholar] [CrossRef]
- Hakimi, M.H.; Abass, A.N.; Lashin, A.; Gharib, A.F.; Radwan, A.E.; Rahim, A.; Ahmed, A.; Asiwaju, L.; Afify, W.E. Geochemical investigation and basin modelling of the Al renk shale formation in the Melut Basin, south Sudan: Implications for estimation of thermogenic gas generation potential. Mar. Pet. Geol. 2022, 146, 105926. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, X.M.; Cheng, P.; Wang, M.L.; Tian, H. The relationship between oil generation, expulsion and retention of lacustrine shales: Based on pyrolysis simulation experiments. J. Pet. Sci. Eng. 2021, 196, 107625. [Google Scholar] [CrossRef]
- Elzain, H.E.; Abdullatif, O.A.; Senapathi, V.; Chung, S.Y.; Sabarathinam, C.; Sekar, S. Lithofacies modeling of Late Jurassic in upper Ulayyah reservoir unit at central Saudi Arabia with inference of reservoir characterization. J. Pet. Sci. Eng. 2020, 185, 106664. [Google Scholar] [CrossRef]
- Jin, X.; Li, G.X.; Meng, S.W.; Wang, X.Q.; Liu, C.; Tao, J.P.; Liu, H. Microscale comprehensive evaluation of continental shale oil recoverability. Pet. Explor. Dev. 2021, 48, 256–268. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhang, G.S.; Tang, W.; Wang, D.H.; Wang, K.; Liu, J.Y.; Du, D. A review of commercial development of continental shale oil in China. Energy Geosci. 2022, 3, 282–289. [Google Scholar] [CrossRef]
- Guo, Q.H.; Li, S.X.; Jin, Z.K.; Zhou, X.P.; Liu, C.L. Characteristics and exploration targets of Chang 7 shale oil in Triassic Yanchang Formation, Ordos Basin, NW China. Pet. Explor. Dev. 2023, 50, 878–893. [Google Scholar] [CrossRef]
- Wang, G.P.; Zhang, Q.; Zhu, R.K.; Tang, X.; Liu, K.Q.; Jin, Z.J. Geological controls on the pore system of lacustrine unconventional shale reservoirs: The Triassic Chang 7 member in the Ordos Basin, China. Geoenergy Sci. Eng. 2023, 221, 111139. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.G.; Wu, Z.H. R Molecular and experimental study on hydrogen sulfide formation mechanism during Chang 7 type-II oil shale kerogen pyrolysis. Fuel 2023, 340, 127552. [Google Scholar] [CrossRef]
- Vandenbroucke, M.; Behar, F.; Espitalie, J. Characterization of sedimentary organic matter by preparative pyrolysis: Comparison with Rock Eval pyrolysis and pyrolysis-gas chromatography techniques. Energy Fuels 1988, 2, 252–258. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, J.C.; Jiang, Z.X.; Zhao, X.Z.; Liu, K.Y.; Zhang, R.F.; Xiong, J.Y.; Du, K.F.; Huang, Z.L.; Yu, J.D. Characteristics of solid residue, expelled and retained hydrocarbons of lacustrine marlstone based on semi-closed system hydrous pyrolysis: Implications for tight oil exploration. Fuel 2015, 162, 186–193. [Google Scholar] [CrossRef]
- Shao, D.Y.; Zhang, T.W.; Ko, L.T.; Li, Y.F.; Yan, J.P.; Zhang, L.L.; Luo, H.; Qiao, B. Experimental investigation of oil generation, retention, and expulsion within Type II kerogen-dominated marine shales: Insights from gold-tube nonhydrous pyrolysis of Barnett and Woodford Shales using miniature core plugs. Int. J. Coal Geol. 2020, 217, 103337. [Google Scholar] [CrossRef]
- Bowden, S.A.; Farrimond, P.; Snape, C.E.; Love, G.D. Compositional differences in biomarker constituents ofthe hydrocarbon, resin, asphaltene and kerogen fractions:An example from the Jet Rock (Yorkshire, UK). Org. Geochem. 2006, 37, 369–383. [Google Scholar] [CrossRef]
- Meredith, W.; Snape, C.E.; Carr, A.D.; Nytoft, H.P.; Love, G.D. The occurrence of unusual hopenes in hydropyrolysates generated from severely biodegraded oil seep asphaltenes. Org. Geochem. 2008, 39, 1243–1248. [Google Scholar] [CrossRef]
- Vuković, N.; Zivotic, D.; Filho, J.G.M.; Stevovic, T.K.; Vido, M.H.; Mendonca, J.D.O.; Stojanovic, K. The assessment of maturation changes of humic coal organic matter—Insights from closed-system pyrolysis experiments. Int. J. Coal Geol. 2016, 154, 213–239. [Google Scholar] [CrossRef]
- Chen, J.; Jia, W.L.; Yu, C.L.; Zhang, X.Y.; Peng, P.A. Bound hydrocarbons and structure of pyrobitumen rapidly formed byasphaltene cracking: Implications for oil–source correlation. Org. Geochem. 2020, 146, 104053. [Google Scholar] [CrossRef]

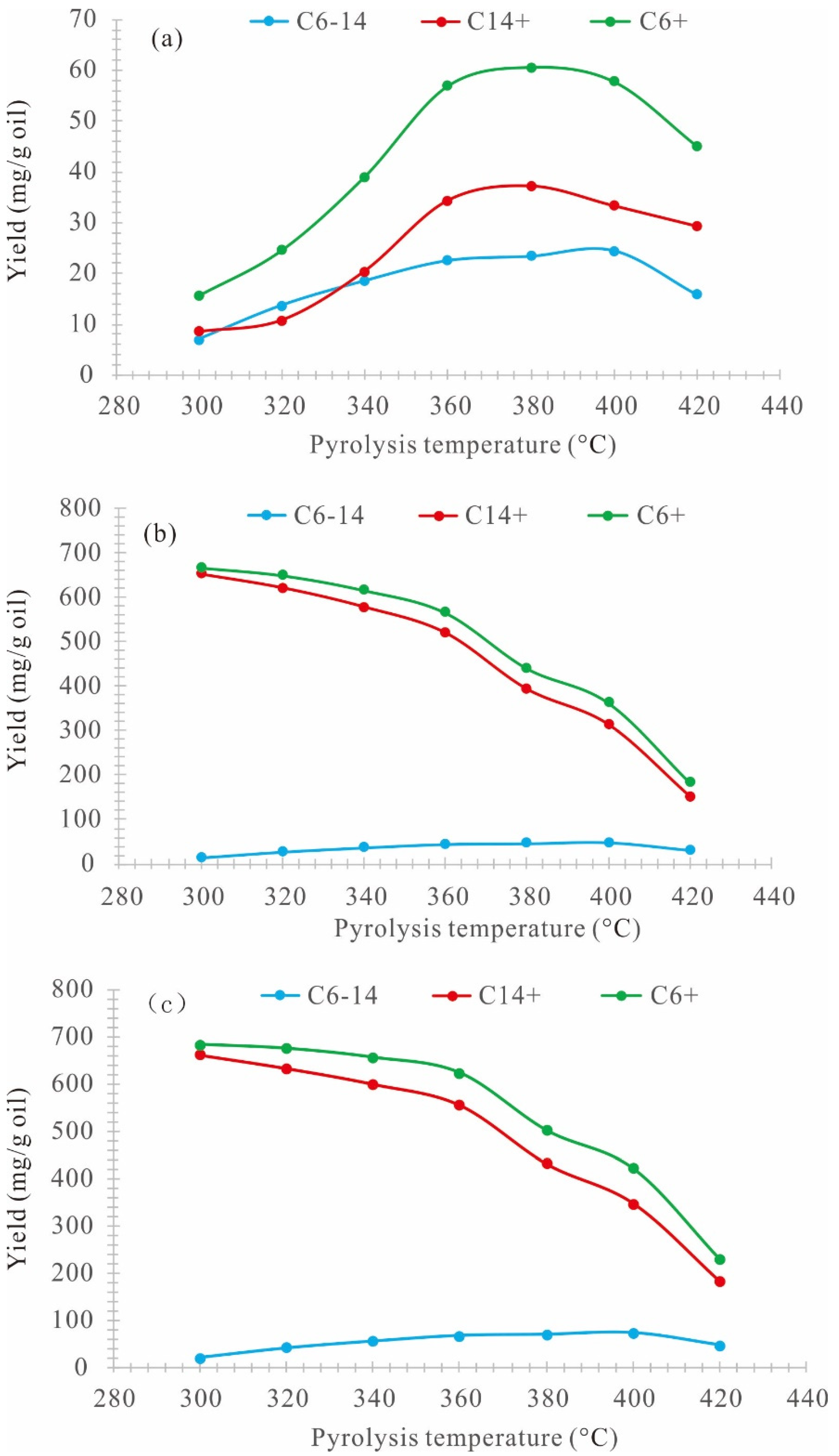

| Sample No. | Temperature | C6–14 | C14+ | C6+ |
|---|---|---|---|---|
| °C | mg/g Oil | mg/g Oil | mg/g Oil | |
| Expelled oil | ||||
| H-1E | 300 | 7.07 | 8.58 | 15.64 |
| H-2E | 320 | 13.76 | 10.84 | 24.60 |
| H-3E | 340 | 18.60 | 20.50 | 39.10 |
| H-4E | 360 | 22.59 | 34.37 | 56.96 |
| H-5E | 380 | 23.44 | 37.26 | 60.70 |
| H-6E | 400 | 24.55 | 33.37 | 57.92 |
| H-7E | 420 | 15.89 | 29.36 | 45.25 |
| Residual oil | ||||
| H-1R | 300 | 14.14 | 653.40 | 667.54 |
| H-2R | 320 | 27.53 | 622.68 | 650.20 |
| H-3R | 340 | 37.20 | 579.55 | 616.75 |
| H-4R | 360 | 45.18 | 521.13 | 566.31 |
| H-5R | 380 | 46.88 | 394.13 | 441.01 |
| H-6R | 400 | 49.11 | 314.11 | 363.21 |
| H-7R | 420 | 31.78 | 151.89 | 183.67 |
| Generated oil | ||||
| H-1T | 300 | 21.20 | 661.98 | 683.18 |
| H-2T | 320 | 41.29 | 633.52 | 674.81 |
| H-3T | 340 | 55.80 | 600.04 | 655.85 |
| H-4T | 360 | 67.77 | 555.50 | 623.27 |
| H-5T | 380 | 70.32 | 431.39 | 501.71 |
| H-6T | 400 | 73.66 | 347.47 | 421.14 |
| H-7T | 420 | 47.67 | 181.25 | 228.92 |
| Expelled Oil | |||
|---|---|---|---|
| Temperature Interval | C6–14 | C14+ | C6+ |
| °C | mg/g oil | mg/g oil | mg/g oil |
| 300–320 | 6.69 | 2.26 | 8.96 |
| 320–340 | 4.84 | 9.66 | 14.50 |
| 340–360 | 3.99 | 13.87 | 17.86 |
| 360–380 | 0.85 | 2.89 | 3.74 |
| 380–400 | 1.12 | −3.89 | −2.78 |
| 400–420 | −8.67 | −4.01 | −12.67 |
| Residual Oil | |||
| Temperature Interval | C6–14 | C14+ | C6+ |
| °C | mg/g oil | mg/g oil | mg/g oil |
| 300–320 | 13.39 | −30.73 | −17.34 |
| 320–340 | 9.68 | −43.13 | −33.46 |
| 340–360 | 7.98 | −58.42 | −50.44 |
| 360–380 | 1.70 | −126.99 | −125.30 |
| 380–400 | 2.23 | −80.03 | −77.80 |
| 400–420 | −17.33 | −162.21 | −179.54 |
| Generated Oil | |||
| Temperature Interval | C6–14 | C14+ | C6+ |
| °C | mg/g oil | mg/g oil | mg/g oil |
| 300–320 | 20.08 | −28.46 | −8.38 |
| 320–340 | 14.52 | −33.47 | −18.96 |
| 340–360 | 11.97 | −44.55 | −32.58 |
| 360–380 | 2.55 | −124.11 | −121.56 |
| 380–400 | 3.35 | −83.92 | −80.58 |
| 400–420 | −26.00 | −166.22 | −192.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Hou, L.; Zhao, Z.; Lin, S.; Pang, Z.; Zhang, L.; Han, W.; Li, Y. Exploitation and Utilization of Generated Oil and Gas by Pyrolysis Simulation Modeling of Shale Source Rocks under the Condition of In Situ Conversion. Processes 2024, 12, 121. https://doi.org/10.3390/pr12010121
Luo X, Hou L, Zhao Z, Lin S, Pang Z, Zhang L, Han W, Li Y. Exploitation and Utilization of Generated Oil and Gas by Pyrolysis Simulation Modeling of Shale Source Rocks under the Condition of In Situ Conversion. Processes. 2024; 12(1):121. https://doi.org/10.3390/pr12010121
Chicago/Turabian StyleLuo, Xia, Lianhua Hou, Zhongying Zhao, Senhu Lin, Zhenglian Pang, Lijun Zhang, Wenxue Han, and Yong Li. 2024. "Exploitation and Utilization of Generated Oil and Gas by Pyrolysis Simulation Modeling of Shale Source Rocks under the Condition of In Situ Conversion" Processes 12, no. 1: 121. https://doi.org/10.3390/pr12010121
APA StyleLuo, X., Hou, L., Zhao, Z., Lin, S., Pang, Z., Zhang, L., Han, W., & Li, Y. (2024). Exploitation and Utilization of Generated Oil and Gas by Pyrolysis Simulation Modeling of Shale Source Rocks under the Condition of In Situ Conversion. Processes, 12(1), 121. https://doi.org/10.3390/pr12010121