Rumex crispus Leaf Extract Inhibits Lipopolysaccharide-Induced Inflammatory Response in BV-2 Microglia Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. RLE Preparation
2.2. Cell Culture
2.3. Cytotoxicity
2.4. NO Production Assays
2.5. Quantitative PCR (qPCR) Analysis
2.6. Western Blotting
2.7. ELISA
2.8. Statistical Analysis
3. Results
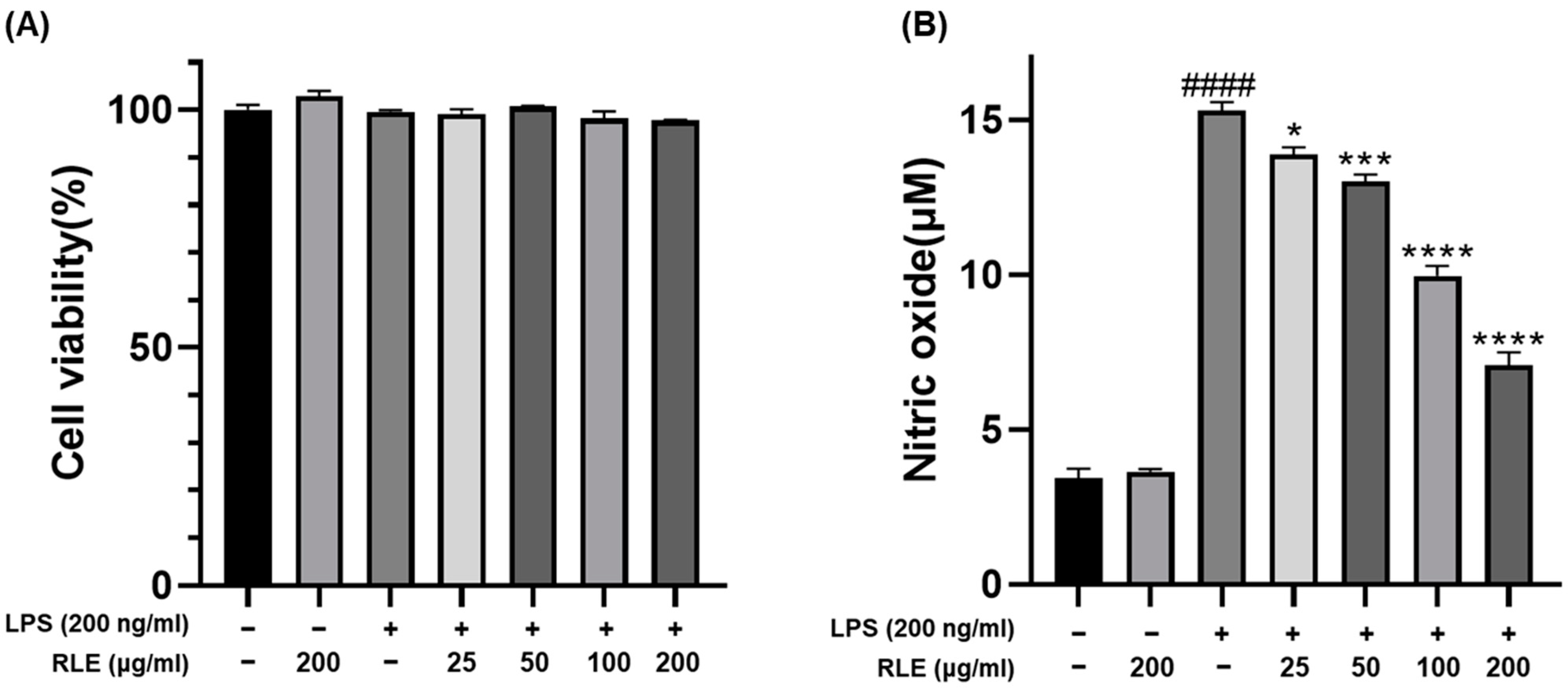
3.1. Effect of RLE on Cell Viability and NO Production in BV-2 Cells
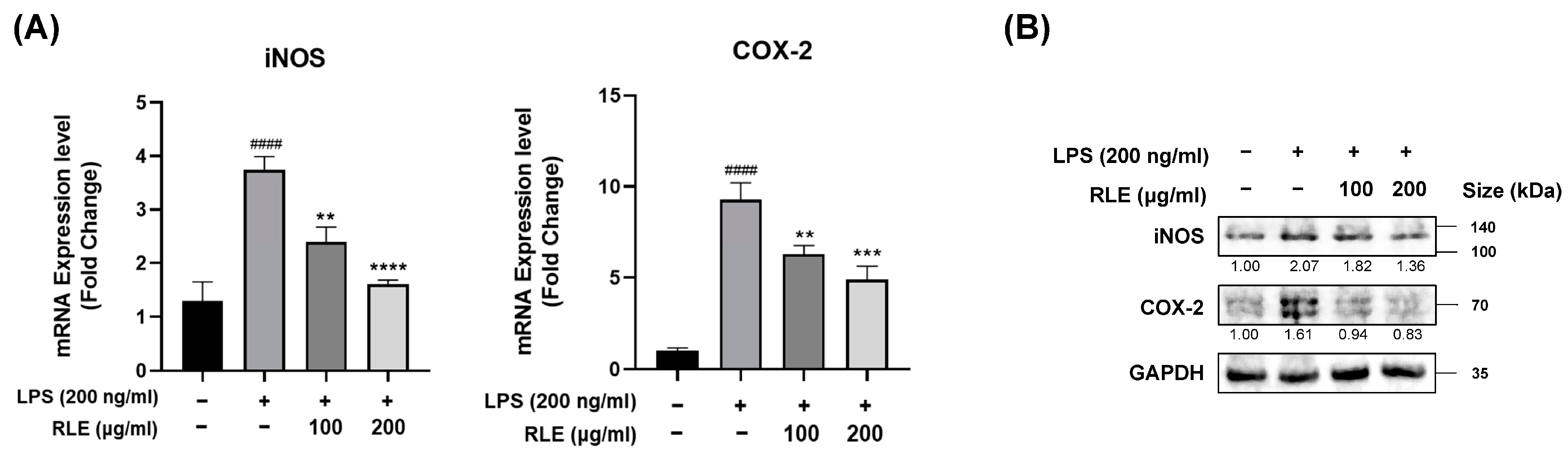
3.2. Effects of RLE on the Expression Levels of COX-2 and iNOS in Response to LPS Stimulation in BV-2 Cells
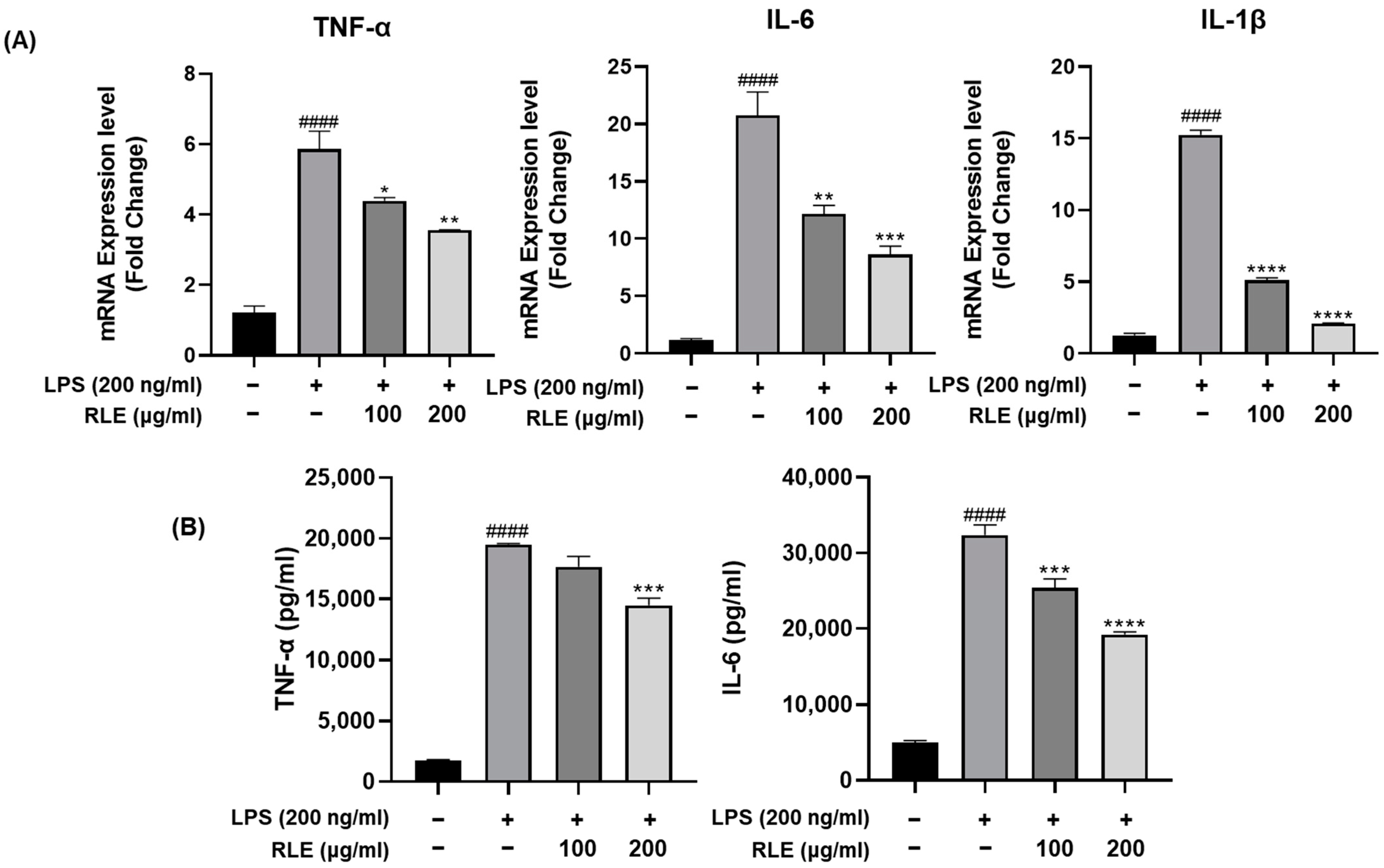
3.3. Effects of RLE on the Expression and Release of Pro-Inflammatory Cytokines in BV-2 Cells Stimulated by LPS
3.4. RLE Regulates NF-κB and MAPK Signaling Pathways Activated by LPS Stimulation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| MS | multiple sclerosis |
| CNS | central nervous system |
| NO | nitric oxide |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| RC | Rumex crispus |
| RLE | Rumex crispus leaf extract |
| LPS | lipopolysaccharide |
| NF-κB | nuclear factor kappa B subunit 1 |
| MAPK | mitogen-activated protein kinase |
| IκBα | NF-κB inhibitor alpha |
| iNOS | inducible nitric oxide synthase |
| COX-2 | cyclooxygenase-2 |
| PGE2 | prostaglandin E2 |
| ERK | extracellular signal-regulated kinases |
| JNK | c-Jun NH-2terminal kinases |
References
- Tang, Y.; Le, W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Min, J.-S.; Kim, B.; Chae, U.-B.; Yun, J.W.; Choi, M.-S.; Kong, I.-K.; Chang, K.-T.; Lee, D.-S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef]
- Shazhni, J.A.; Renu, A.; Vijayaraghavan, P. Insights of antidiabetic, anti-inflammatory and hepatoprotective properties of antimicrobial secondary metabolites of corm extract from Caladium × hortulanum. Saudi J. Biol. Sci. 2018, 25, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Olajide, O.A.; Aderogba, M.A.; Fiebich, B.L. Mechanisms of anti-inflammatory property of Anacardium occidentale stem bark: Inhibition of NF-κB and MAPK signalling in the microglia. J. Ethnopharmacol. 2013, 145, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yang, Y.; Xu, J.; Pan, Y.; Zhang, W.; Xing, Y.; Ni, H.; Sun, Y.; Hou, Y.; Li, N. Tamarix hohenackeri Bunge exerts anti-inflammatory effects on lipopolysaccharide-activated microglia in vitro. Phytomedicine 2018, 40, 10–19. [Google Scholar] [CrossRef]
- Eom, T.; Kim, E.; Kim, J.-S. In vitro antioxidant, antiinflammation, and anticancer activities and anthraquinone content from Rumex crispus root extract and fractions. Antioxidants 2020, 9, 726. [Google Scholar] [CrossRef]
- Maksimović, Z.; Kovačević, N.; Lakušić, B.; Ćebović, T. Antioxidant activity of yellow dock (Rumex crispus L., Polygonaceae) fruit extract. Phytother. Res. 2011, 25, 101–105. [Google Scholar] [CrossRef]
- Yıldırım, A.; Mavi, A.; Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001, 49, 4083–4089. [Google Scholar] [CrossRef]
- Choi, G.J.; Lee, S.-W.; Jang, K.S.; Kim, J.-S.; Cho, K.Y.; Kim, J.-C. Effects of chrysophanol, parietin, and nepodin of Rumex crispus on barley and cucumber powdery mildews. Crop Prot. 2004, 23, 1215–1221. [Google Scholar] [CrossRef]
- Shim, K.-S.; Lee, B.; Ma, J.Y. Water extract of Rumex crispus prevents bone loss by inhibiting osteoclastogenesis and inducing osteoblast mineralization. BMC Complement. Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Jeong, J.H.; Hyun, H.B.; Kim, J.H.; Kim, H.S.; Oh, H.I.; Wang, H.S.; Kim, H.H. Comparative analysis of the constituents of the leaves and roots of Rumex Crispus and their effects on the differentiation of human osteoblast-like MG-63 cells. Yakhak Hoeji 2014, 58, 307–313. [Google Scholar]
- Minh, T.N.; Van, T.M.; Andriana, Y.; Vinh, L.T.; Hau, D.V.; Duyen, D.H.; Guzman-Gelani, C.d. Antioxidant, xanthine oxidase, α-amylase and α-glucosidase inhibitory activities of bioactive compounds from Rumex crispus L. root. Molecules 2019, 24, 3899. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Wang, X.; Hu, D.; Zhang, L.; Lian, G.; Zhao, S.; Wang, C.; Yin, J.; Wu, C.; Yang, J. Gomisin A inhibits lipopolysaccharide-induced inflammatory responses in N9 microglia via blocking the NF-κB/MAPKs pathway. Food Chem. Toxicol. 2014, 63, 119–127. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, S.-C.; Chen, I.-C.; Chen, Y.-T.; Liu, P.-L.; Fang, S.-H.; Huang, S.-P.; Yeh, H.-C.; Liu, C.-C.; Lee, P.-Y. Protective effect of piplartine against LPS-induced sepsis through attenuating the MAPKs/NF-κB signaling pathway and NLRP3 inflammasome activation. Pharmaceuticals 2021, 14, 588. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 414–421. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 2012, 87, 10–20. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Smith, C.J.; Van Eldik, L.J. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1β production. Neurobiol. Aging 2004, 25, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019, 49, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Frank-Cannon, T.C.; Alto, L.T.; McAlpine, F.E.; Tansey, M.G. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol. Neurodegener. 2009, 4, 1–13. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Lee, S.Y.; Moon, E.; Kim, S.Y.; Lee, K.R. Quinic acid derivatives from Pimpinella brachycarpa exert anti-neuroinflammatory activity in lipopolysaccharide-induced microglia. Bioorg. Med. Chem. Lett. 2013, 23, 2140–2144. [Google Scholar] [CrossRef]
- Xu, F.; Wang, C.; Yang, L.; Luo, H.; Fan, W.; Zi, C.; Dong, F.; Hu, J.; Zhou, J. C-dideoxyhexosyl flavones from the stems and leaves of Passiflora edulis Sims. Food Chem. 2013, 136, 94–99. [Google Scholar] [CrossRef]
- Song, J.; Cheon, S.Y.; Jung, W.; Lee, W.T.; Lee, J.E. Resveratrol induces the expression of interleukin-10 and brain-derived neurotrophic factor in BV2 microglia under hypoxia. Int. J. Mol. Sci. 2014, 15, 15512–15529. [Google Scholar] [CrossRef]
- Wang, C.P.; Zhang, L.Z.; Li, G.C.; Shi, Y.W.; Li, J.L.; Zhang, X.C.; Wang, Z.W.; Ding, F.; Liang, X.M. Mulberroside a protects against ischemic impairment in primary culture of rat cortical neurons after oxygen–glucose deprivation followed by reperfusion. J. Neurosci. Res. 2014, 92, 944–954. [Google Scholar] [CrossRef]
- Bahar, E.; Kim, J.-Y.; Yoon, H. Quercetin attenuates manganese-induced neuroinflammation by alleviating oxidative stress through regulation of apoptosis, iNOS/NF-κB and HO-1/Nrf2 pathways. Int. J. Mol. Sci. 2017, 18, 1989. [Google Scholar] [CrossRef]
- Ruan, Q.; Hu, X.; Ao, H.; Ma, H.; Gao, Z.; Liu, F.; Kong, D.; Bao, Z.; Yu, Z. The neurovascular protective effects of huperzine A on D-galactose-induced inflammatory damage in the rat hippocampus. Gerontology 2014, 60, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Yang, L.; Sameshima, H. Galantamine, an acetylcholinesterase inhibitor, reduces brain damage induced by hypoxia-ischemia in newborn rats. Int. J. Dev. Neurosci. 2014, 37, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, J.; Song, Z.; Pan, X.; Chen, L.; Cui, X.; Wang, M. Berberine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signalling pathways. J. Pharm. Pharmacol. 2012, 64, 1510–1521. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef]
- Kim, H.; Mani, I.; Iversen, L.; Ziboh, V. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot. Essent. Fat. Acids 1998, 58, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bureau, G.; Longpré, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef]
- Geraets, L.; Moonen, H.J.; Brauers, K.; Wouters, E.F.; Bast, A.; Hageman, G.J. Dietary flavones and flavonoles are inhibitors of poly (ADP-ribose) polymerase-1 in pulmonary epithelial cells. J. Nutr. 2007, 137, 2190–2195. [Google Scholar] [CrossRef]
- Ghosh, B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-α production in murine macrophages. Int. J. Immunopharmacol. 1999, 21, 435–443. [Google Scholar]
- Lüderitz, O.; Freudenberg, M.A.; Galanos, C.; Lehmann, V.; Rietschel, E.T.; Shaw, D.H. Lipopolysaccharides of gram-negative bacteria. In Current Topics in Membranes and Transport; Elsevier: Amsterdam, The Netherlands, 1982; Volume 17, pp. 79–151. [Google Scholar]
- Panthi, S.; Manandhar, S.; Gautam, K. Hydrogen sulfide, nitric oxide, and neurodegenerative disorders. Transl. Neurodegener. 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From clinical significance to quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Baltimore, D. NF-κB: Ten years after. Cell 1996, 87, 13–20. [Google Scholar] [CrossRef]
- Yoshimura, A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006, 97, 439–447. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-W.; Kim, W.; Choi, C.Y.; Kim, S.-J. Rumex crispus Leaf Extract Inhibits Lipopolysaccharide-Induced Inflammatory Response in BV-2 Microglia Cells. Processes 2023, 11, 2756. https://doi.org/10.3390/pr11092756
Park J-W, Kim W, Choi CY, Kim S-J. Rumex crispus Leaf Extract Inhibits Lipopolysaccharide-Induced Inflammatory Response in BV-2 Microglia Cells. Processes. 2023; 11(9):2756. https://doi.org/10.3390/pr11092756
Chicago/Turabian StylePark, Ji-Woong, Woong Kim, Chul Yung Choi, and Seok-Jun Kim. 2023. "Rumex crispus Leaf Extract Inhibits Lipopolysaccharide-Induced Inflammatory Response in BV-2 Microglia Cells" Processes 11, no. 9: 2756. https://doi.org/10.3390/pr11092756
APA StylePark, J.-W., Kim, W., Choi, C. Y., & Kim, S.-J. (2023). Rumex crispus Leaf Extract Inhibits Lipopolysaccharide-Induced Inflammatory Response in BV-2 Microglia Cells. Processes, 11(9), 2756. https://doi.org/10.3390/pr11092756






