Abstract
The global trend towards net-zero carbon emissions from burning fuels in combustion engines alerts us to the alternative role of biodiesel. The manufacturing cost of biodiesel hinders the fast development of various types of biofuels. Feedstock cost is one of the major determining factors of biodiesel cost and thus the extent of its competitiveness in the fuel market with other available alternative fuels or fossil fuels. Some low-cost feedstocks such as high-acid oil, which is produced from the acidifying processes of soybean soapstock, frequently contain high contents of free fatty acids (FFAs) and water. Hence, those feedstocks cannot be used to produce biodiesel through strong alkaline catalyst transesterification on an industrial scale. In contrast, the water can be converted to hydroxyl radicals to enhance the formation of esters from the dissociation of the FFA in a supercritical reacting tank. Hence, cheap high-acid oils containing high amounts of water and FFAs were used to produce biodiesel through a supercritical transesterification reaction system. The engine emission characteristics of using the biodiesel produced in this study were analyzed and compared with those of commercial biodiesel and super-low sulfur diesel (SLSD). A naturally aspirated, direct-injection, four-stroke, four-cylinder marine diesel engine associated with an eddy-current dynamometer was used to carry out the engine emission measurement. In comparison with super-low sulfur diesel (SLSD), the biodiesel had lower CO2 and CO emissions and black smoke opacity but higher emissions of O2 and NOx. The higher engine speed caused lower emissions of O2 and NOx but higher CO2 emissions. The supercritical-transesterification biodiesel appeared to be a competitive alternative fuel to fossil diesel.
1. Introduction
Biodiesel is composed of short chain monoalkyl esters of fatty acids and is frequently termed fatty acid methyl esters (FAME). Biodiesel is regarded as an environmentally friendly alternative to traditional fossil diesel because of its rather low amount of toxic compounds, biodegradability, lack of sulfur content, low pollutant emissions, superior burning efficiency [1], etc. Nautiyal et al. [2] found that Spirulina platensis biodiesel reduced CO and HC engine emissions from 3.1 g/kWh and 0.06 g/kWh with diesel to 1.2 g/kWh and 0.04 g/kWh, respectively. Rajan et al. [3] applied microwave heating to assist the production of corn oil biodiesel (briefly termed CBDMW). They observed that in comparison with traditional corn oil biodiesel (CBD), the NO emissions decreased from 1.5 g/kWh to 0.8 g/kWh and smoke emissions from 0.6 BSU to 0.4 BSU, respectively. The existence of free fatty acids (FFAs) and monoglycerides in biodiesel enhances its lubrication characteristics [4]. However, biodiesel raises the controversial issue of the compatibility of vehicle fuel with human food since vegetable oils or animal fats are generally used as feedstocks [5]. Although used cooking oil is considered a low-cost and inedible feedstock [6], its high contents of water, impurities, and free fatty acids complicate the production procedures and result in intense saponification reactions, inferior fuel characteristics, high consumption of the alkaline catalyst, and low biodiesel yield. A series of pretreatment processes such as acid-esterification were used prior to subsequent strong alkaline catalyst transesterification to produce the biodiesel. The application of adequate production methods using low-cost feedstocks is considered one of the deterministic factors for the extent of the competitiveness of biodiesel in the fuel market [7]. About 70–90% of biodiesel production costs depend on the feedstock materials, and over 90% of raw materials come from refined plant oils or animal lipids [8]. Hence, applying cheap inedible feedstocks such as byproducts of refining processes for vegetable oil for second-generation biodiesel production can greatly reduce the production cost [9] and enhance the competitiveness of the biodiesel product with petro-diesel. In addition, more varieties of available feedstocks require further systematic exploration to improve their fuel properties and develop their specific economic manufacturing methods and processes.
Soybean soapstock is a purer lipid-rich compound in which neutral oil and free fatty acids are used to manufacture biodiesel. A strong mineral acid such as hydrochloric or sulphuric acid is generally used to acidulate the soybean soapstock to produce high-acid oil, which is composed of FFAs, sterols, pigments, tocopherols, triacylglycerol (TAG), diacylglycerol (DAG), and monoacylglycerol (MAG) [10]. The high-acid oil’s TAG, DAG, and MAG amounts might reach 28.0 wt. %, 4.4 wt. %, and less than 1.0 wt. %, respectively [11]. High-acid oil made through acidifying processes from discarded soybean soapstock is regarded as a potential cheap feedstock source for biodiesel production [12]. The manufacturing methods for those cheap, inferior, inedible vegetable oils containing high amounts of water and FFAs have not been investigated in order to improve their fuel characteristics [13]. When the moisture content in raw vegetable oil amounted to 2.5 wt. %, the conversion rate of biodiesel from triglyceride was reduced to only 80% through a strong-alkaline catalyst transesterification reaction [14]. Moreover, only a 35% conversion rate can be achieved from vegetable oil with 20 wt. % free fatty acids through the same reaction [15]. Typical production approaches might include ultrasound-irradiated, supercritical fluid, super-gravity centrifugal reactions and microwave-assisted transesterification, which would enhance the reaction rate to various extents.
The temperature and pressure of a fluid exceed its corresponding critical temperature, and pressure is termed a supercritical fluid (SCF). The relevant properties of an SCF, such as kinematic viscosity, thermal conductivity, density, and heat capacity, are highly sensitive to variations in pressure and temperature [16]. For example, a supercritical fluid has an excellent diffusion coefficient, superior permeating capability, and relatively lower viscosity [17]. This results in more contact surface area with other reacting compounds and a higher mass transfer rate. Supercritical fluid technology has been widely applied as green solvents [18] to remove toxic materials and enhancers to facilitate chemical reactions [19]. Supercritical methanol technology is well developed to produce biodiesel, which carries major advantages, including that no catalyst is required, the susceptible separation between crude biodiesel and glycerol, that no wastewater is produced after the process, an accelerated reaction rate, and the much shorter reaction time to complete the biodiesel production [20].
The supercritical methanol biodiesel production technology has not yet been successfully commercialized, primarily due to its low economic competitiveness, particularly for large-scale biodiesel production compared to conventional fossil fuels [21]. More systematic evaluation, particularly with regard to the environmental benefits of the biodiesel produced through the supercritical methanol biodiesel production technology, is required to promote its achievable advantages and facilitate future commercial development [22]. However, the engine emission characteristics of burning the high-acid oil biodiesel produced from soybean soapstock have not been investigated as of yet [23,24]. The environmental benefit of the high-acid oil biodiesel instead of biodiesels from other feedstocks has not been analyzed either. Hence, the engine emission characteristics of the biodiesel through the supercritical methanol biodiesel technology were analyzed and compared with commercial FAME from waste cooking oil through traditional alkaline-catalyst transesterification and super-low sulfur diesel (SLSD) in order to systematically evaluate the adequacy and competency of biodiesel.
2. Experimental Details
2.1. Biodiesel Production Processes
High-acid oil was made from the acidulation reaction of the soybean soapstock left after the oil extraction processes of soybean oil. The methanol and high-acid oil at a molar ratio of 42 were poured into a supercritical methanol reactor at a pressure and temperature of 20 MPa and 623 K, which significantly exceeded the critical pressure and temperature, i.e., 7.95 MPa and 513.2 K, respectively, to react for 30 min. The volumetric capacity of the reacting vessel was 5000 cm3. The specifications of the supercritical methanol reacting tank are shown in Table 1. The maximum working pressure and temperature of the reactor are 275.7 bar and 450 °C, respectively. The crude biodiesel product mixture was divided into upper crude biodiesel and lower glycerol due to the great difference between the densities of these two fluids. The crude biodiesel was washed with fresh water and evaporated by a rotary evaporator to remove unseparated glycerol, residual methanol, unevaporated water, volatile organic compounds, and other impurities.

Table 1.
Specifications of the supercritical methanol reactor.
The engine emission characteristics of the biodiesel from supercritical transesterification were analyzed and compared with a biodiesel product from waste cooking oil through conventional alkaline-catalyst transesterification and conventional fossil super-low sulfur diesel fuel (SLSD).
2.2. Appearance and Image of Carbon Residue from Burning Biodiesel
The appearance of the carbon residue left in the crucible within the oxygen bomb was observed by an SEM (scanning electron microscope, S-4100 model, Hitachi Ltd., Chiyoda-ku, Tokyo, Japan) at a magnification of 20 thousand. An electron beam of 5~30 kV was scanned onto the surface of the specimen to produce signals. After being enlarged and managed, the collected signals were inputted into the cathode ray tube to show the image of the specimens.
2.3. Comparison of Diesel Engine Emissions
A compression-ignition, four-stroke, four-cylinder, and naturally aspirated diesel engine was used to analyze and compare the diesel emission characteristics of the biodiesel through a supercritical methanol reaction, a commercial biodiesel made from used cooking oil, and fossil super-low sulfur diesel (SLSD). In this experimental study, an eddy-current engine dynamometer was aligned with the transmission shaft to control the engine torque. An engine dynamometer control system was used to regulate the engine torque, engine power, and engine operation modes. A data acquisition system for diesel engines attained the engine performance and emission data.
A portable gas analyzer (CA-NSX model, Bacharach Ltd., Pittsburgh city, PA, USA) was used to analyze the emissions of the compression-ignition engine powered with various sample fuels. The emissions, including NOx, O2, CO, CO2, etc., were measured in this study. The emission units were expressed in % or ppm, which can also be converted to g/kWh based on the conversion formulae [25]. A K-type thermocouple was connected to the gas analyzer probe to measure the engine’s exhaust gas temperature. A black smoke detector (DSM-20A model, Zexel Manufacture Ltd., Ibaraki, Japan) was applied to analyze the opacity (%) of black smoke in the exhaust gas of the diesel engine. The particles of black smoke were first collected on the quartz filter paper TissuquartzTM (2500QAT-UP model) with a diameter of 47 mm inhaled by an oil-sealed vacuum pump installed in the exhaust gas manifold. The photoelectric detector then irradiated the filter paper, which accumulated the particulate matter to measure the black smoke opacity in a range between 0 and 100%. Higher black smoke opacity indicated more accumulated amounts of black smoke particulates to hinder the transmission of irradiated light from the photoelectric detector. A greater extent of black smoke formation from the burning process during the operating processes of the compression-ignition engine implies inferior combustion efficiency due to the incomplete oxidation of hydrocarbon fuel. Each experiment was repeated 3 to 5 times to calculate their mean values. The experimental uncertainties of the NOx, CO, CO2, O2, black smoke opacity, and carbon residue size were 3.25%, 1.83%, 1.69%, 2.37%, 0.65%, and 4.94%, respectively.
3. Results and Discussion
3.1. Emission of Nitrogen Oxides
The production of nitrogen oxides (NOx) is primarily determined by the in-cylinder high gas temperature, residence time of the high-temperature flame, and availability of oxygen and nitrogen in the combustion chamber [26]. Gao et al. [27] observed that the biodiesel increases in the blend proportion of natural gas caused an obvious increase in NOx emissions. Varghese et al. [28] proposed that the degree of unsaturation, chain length, and number of biodiesel double bonds greatly influenced the NOx emissions. The higher NOx emissions from biodiesel combustion presents a noticeable hindrance preventing its wide use as an alternative fuel for internal combustion engines [29]. The drop in the degree of unsaturation of the feedstock compounds resulted in an increase in NOx emissions. The NOx emissions were observed to decrease with the rise of engine speeds, as shown in Figure 1. This is due to the increased engine volumetric efficiency and peak flame pressure at high engine speeds. NO emissions from fossil fuel combustion include thermal NO, fuel NO, and prompt NO. The formation rate of thermal NO is considerably lower than that of prompt NO [30]. The speed of gas flow was also facilitated by intensely stirring the reactant mixture in the combustion cylinder. The ignition delay and elapse of burning time were thus significantly reduced at higher engine speeds. The NOx formation was enhanced with the increase of burning gas temperature, residence time, and availability of oxygen compounds. The residence time of high-temperature burning gas was accordingly shortened with the rise of the engine speed. In consequence, decreased NOx formation with the increase of engine speed was observed, as can be seen in Figure 1. The experimental result agreed well with that of Dhande et al. [31]. Moreover, the two biodiesels appeared to have higher NOx emissions than the SLSD because the biodiesels contain about 10 wt. % more oxygen than the SLSD [32].
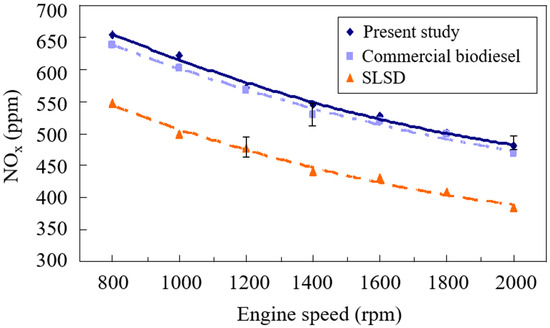
Figure 1.
Comparison of the NOx emissions of the biodiesels from the present study and waste cooking oil and super-low sulfur diesel.
Lin and Wu [33] considered that biodiesel with a lower cetane number tends to increase its ignition delay, accumulating an unburned reactant mixture of liquid fuel and inlet air in the combustion chamber. Higher-temperature burning gas was thus produced to release higher NOx emissions. Hence, the biodiesel sample made from this study, which bears a lower cetane index, was shown to have the highest NOx emission, as displayed in Figure 1. A lower cetane index implies a longer ignition delay and thus a greater accumulated amount of injected liquid fuel, resulting in a higher temperature and NOx emissions. The result conformed to that of Simsek [34]. The slightly higher NOx emissions of the present biodiesel made through supercritical methanol transesterification was also due to its lower weight percentage of unsaturated fatty acids, which amounted to 67.12 wt. %, compared to the commercial biodiesel from waste cooking oil, which was 71.29 wt. %.
3.2. Emission of Carbon Monoxide (CO)
The carbon monoxide (CO) emissions increased with the increase of engine speeds from 800 rpm to 1400 rpm, as shown in Figure 2. This is ascribed to the rise of the engine speed, which reduced the oxidation time, resulting in insufficient time for CO to convert to CO2 and leading to increased CO emissions. The burning gas temperature is also one of the key parameters for determining CO concentration. A low burning gas temperature will elongate the required oxidation time of CO with oxygen to produce CO2 [35], and thus higher CO emissions appear. Abed et al. [36] found that the increase in the blending proportion of Jatropha oil biodiesel in diesel fuel resulted in the reduction of the concentration of CO emissions, ascribed to its decreased elemental carbon content in the fuel mixture. In addition, they also observed that an increase in engine power decreased CO emissions.
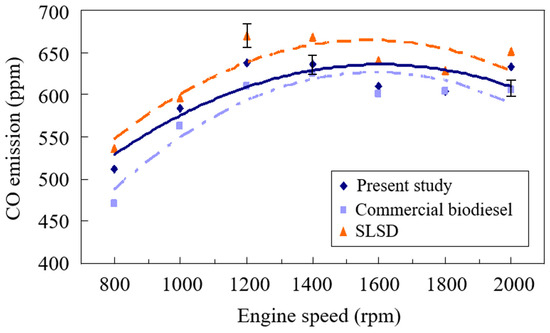
Figure 2.
Comparison of the CO emissions of the biodiesels from the present study and waste cooking oil and super-low sulfur diesel.
A lower conversion time from CO to CO2 at higher engine speeds resulted in higher CO emissions, as shown in Figure 2, when the engine speed was increased from 800 rpm to 1400 rpm. The flame gas temperature significantly increased to accelerate the formation rate of CO2 from the conversion of CO when the engine speed was raised from 1400 rpm to 2000 rpm. Decreased CO concentration with the engine speed was thus observed. The CO emission from burning the biodiesel was found to be significantly lower than that from the SLSD in Figure 2. This is primarily due to the higher elemental oxygen of the biodiesel uniting with CO to form CO2. Chuah et al. [37] also observed that the CO emissions from burning waste frying oil biodiesel were reduced by 17.15% compared to diesel fuel. The lower elemental carbon of the biodiesel was also responsible for the lower CO of the biodiesels. The biodiesel sample from this study was found to have higher CO emissions than the biodiesel made from used cooking oil primarily because of the former biodiesel’s lower elemental oxygen and higher elemental carbon. The weight percentage of the fatty acid compositions of carbon number 18, which included C18:0, C18:1, C18:2, and C18:3 of the present study made from high-acid oil, amounted to 77.02 wt. %, which was higher than the 74.31 wt. % of those of the commercial biodiesel from waste cooking oil, which was also responsible for the higher CO emissions from burning the former biodiesel.
3.3. Emission of Carbon Dioxide
Carbon dioxide (CO2) is a primary detrimental greenhouse gas. According to the Paris Agreement’s global target of 1.5 °C, the global mission of net-zero CO2 emissions is anticipated to be achieved by 2050. The carbon dioxide (CO2) emissions from the compression-ignition engine powered with different test samples at various engine speeds are shown in Figure 3. The CO2 emissions were decreased due to dilution with increased excess air at engine speeds from 800 rpm to 1000 rpm. The CO2 concentration was then raised with the increase in engine speeds. This is ascribed to more liquid fuel and air entering the combustion chamber to incur higher burning gas temperatures and release a larger amount of the complete reaction product—CO2. It followed that the increased CO2 concentration occurred at higher engine speeds for those three test fuels. CO2 emission is also governed by the equivalence ratio and elemental carbon [38]. Compared with SLSD, biodiesel has a lower equivalence ratio and elemental carbon by around 10 wt. % [39]. Hence, biodiesel was shown to have lower CO2 emissions in Figure 3. The experimental results of Gad et al. [40] showed that the CO2 emissions increased with the engine power increase because of the higher fuel consumption rate accompanied by the increase in engine load. Moreover, lower CO2 emissions were associated with a higher percentage of biodiesel blended in a diesel fuel mixture [41] due to its higher oxygen content.
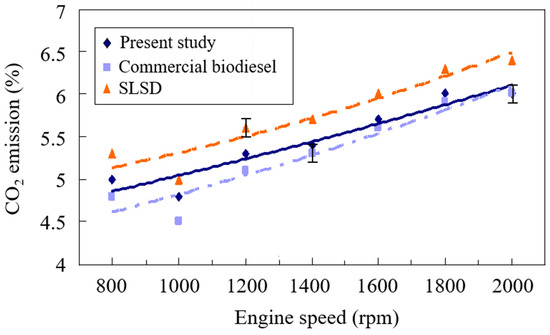
Figure 3.
Comparison of the CO2 emissions of the biodiesels from the present study and waste cooking oil and super-low sulfur diesel.
The CO2 emissions were expected to reach their highest value when the equivalence ratio approached one. The diesel engine is frequently operated at fuel-lean combustion conditions, which implies an equivalence ratio of less than one. Hence, the CO2 emissions were raised with the increase of the equivalence ratio in the range between zero and one, as shown in Figure 3. The equivalence ratio of commercial biodiesel is lower than that of the biodiesel made in this study, resulting in the lower CO2 emissions of the former biodiesel. The present study had a higher weight percentage of saturated fatty acids, which amounted to 32.88 wt. % compared to commercial biodiesel and is also the cause of the higher CO2 emissions from the former biodiesel. The CO2 emissions from burning the biodiesel would return to the ecological system. Vegetables or plants might repeatedly absorb the CO2 to grow vegetable or plant oils for manufacturing biodiesel. In consequence, no increase in net carbon dioxide value arises. The seventh Sustainable Development Goal (SDG) [42] moves towards both the development of renewable energy and the reduction of the emission of greenhouse gases so that the economic growth and environmental protection of the current generation can be assured together with the development of resources for future generations. Biodiesel produced through transesterification is one of the green energies of the SDGs which might reduce 10–75 g CO2/MJ [43]. This implies that the use of a mixture of biodiesel with diesel fuel can mitigate the extent of the environmental impact of the excessive consumption of fossil fuels.
3.4. Emission of Oxygen
The oxygen (O2) emissions of the exhaust gas from the compression-ignition engine powered by different fuels at varied engine speeds and constant engine torque are observed in Figure 4. The oxygen emissions appeared to increase slightly at engine speeds between 800 rpm and 1000 rpm. This is ascribed to the low extent of a complete reaction at reduced engine speeds [44], which results in the greater release of excess air and oxygen concentrations. The oxygen concentration thereafter increased with the increase of the engine speed from 1000 rpm to 2000 rpm. This is due to the increase in the amount of injected liquid fuel at the raised engine speed, causing the rise of the equivalence ratio. Hence, more oxygen was consumed, resulting in decreased oxygen content with the increase in engine speeds. A larger extent of complete burning was also achieved at higher engine speeds, which consumed more oxygen, leading to decreased O2 emissions. The experimental study of Emaish et al. [45] showed that the rise in the biodiesel blending ratio in the diesel fuel mixture resulted in higher O2 emissions, and the rise of engine load resulted in the reduction of O2 emissions. The maximum O2 emissions can reach 15.3% at the minimum engine load, while the minimum O2 emissions were 4.3%, which occurred at the maximum load. Odibi et al. [46] found that the increase in engine speeds caused the increase in the equivalence ratio, leading to the decrease of oxygen concentrations in the exhaust gas. Their results agreed well with the current findings.
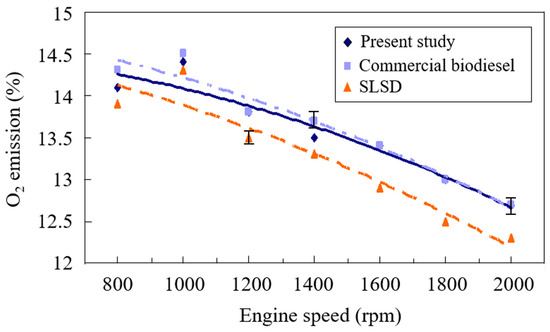
Figure 4.
Comparison of the O2 emissions of the biodiesels from the present study and waste cooking oil and super-low sulfur diesel.
A diesel engine is mostly run under inlet air-rich combustion states, leading to an adverse trend of oxygen concentrations to equivalence ratio. SLSD, which has the highest heating value, had a higher equivalence ratio and thus the lowest O2 emissions among those three test fuels. A lower equivalence ratio of the commercial biodiesel through conventional alkaline-catalyst transesterification from used cooking oil compared to that of the present biodiesel was obtained, leading to the highest oxygen emissions from burning the commercial biodiesel, as shown in Figure 4. In comparison with those of the commercial biodiesel, the compositions of fatty acids of higher carbon numbers, particularly the composition of carbon number 18, which reached 77.02 wt. %, consumed more available oxygen in the surrounding air, leading to fewer O2 emissions from burning the present biodiesel.
3.5. Black Smoke Opacity
Black smoke is produced due to the inferior burning efficiency of liquid fuel. The combustion of heavier liquid fuel, such as residual heavy fuel oil, which is composed of chemical compounds of longer carbon chains, might release high black smoke due to its low burning efficiency. A cyclone particle collector was installed at the end of the exhaust gas pipe to collect small particle matter on the quartz filter paper TissuquartzTM (Zefon International Ltd., Ocala city, FL, USA) with a diameter of 47 mm for 1 min during the engine operation under unvaried engine torque and different engine speeds. A black smoke detector was applied to analyze the black smoke opacity (%) of the emission gas released from the compression-ignition engine. The photoelectric detector irradiated the filter paper covered with black particulate matter to determine the black smoke opacity in a range between 0 and 100% [47]. The 0% black smoke opacity indicates a lack of shielding of the small, collected particles from the irradiating light emitted from the detector, while 100% black smoke opacity represents the collected particles totally blocking the irradiated light.
At high exhaust gas temperatures from the diesel engine, condensed water was found to agglomerate with particulate matter, which might influence the result of black smoke opacity to some extent. The black smoke opacity was observed to decrease with the rise of the engine speed, as shown in Figure 5. This is owing to the higher burning efficiency at increased engine speeds [48], leading to a decrease in black smoke opacity and an increase in CO2 emissions, as shown in Figure 3. The decrease of the equivalence ratio to less than one caused the inhalation of more air, which reacted with atomized fuel droplets so that the formation of black smoke was reduced [49] as well. In addition, the about 10 wt. % oxygen compounds of the biodiesels promoted the degree of complete burning. Hence, the biodiesels appeared to have lower black smoke opacity than SLSD. The biodiesel produced from used cooking oil under atmospheric pressure was shown to have slightly less black smoke opacity than the biodiesel made from supercritical methanol transesterification due to the higher heating value accompanied by the smaller equivalence ratio of the used cooking oil biodiesel. The results of the experimental study of Sutheerasak et al. [50] agree well with the present findings. They found that the increase of biofuel percentages such as ethanol and butanol in the diesel fuel mixture decreases the black smoke opacity due to the enhancement of burning efficiency, leaving less carbon residue after the fuel burning. They also observed that the increase in engine power in terms of mean effective pressure resulted in an increase in black smoke opacity.
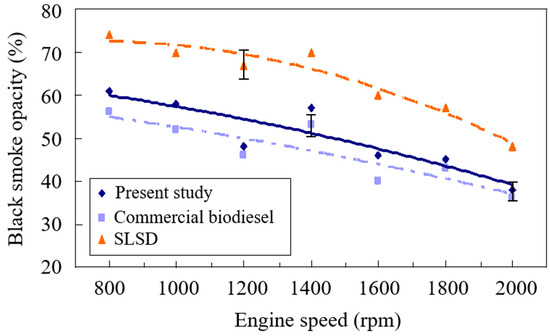
Figure 5.
Comparison of the black smoke opacity of the biodiesels from the present study and waste cooking oil and super-low sulfur diesel.
3.6. Comparison of Carbon Residue Size
The appearance of the image of the carbon residue left after the combustion process of the fuel samples in the crucible within the oxygen bomb observed by an SEM (scanning electron microscope) at a magnification of twenty thousand is observed in Figure 6. The diameters of carbon residue after burning the SLSD were obviously smaller than those of the biodiesels because biodiesel contains more gums, fatty acids, unseparated glycerol, impurities, absorbed water, and foreign residue, as observed by Dong et al. [51]. Carmona-Cabello et al. [52] considered that the existence of other residues, such as carotenoids in biodiesel, might result in more carbon residue after burning the biodiesel. In addition, biodiesel is a hydrophilic substance that is prone to absorbing water from the environment. The burning of biodiesel with higher water content tends to produce carbon residue of a larger diameter.
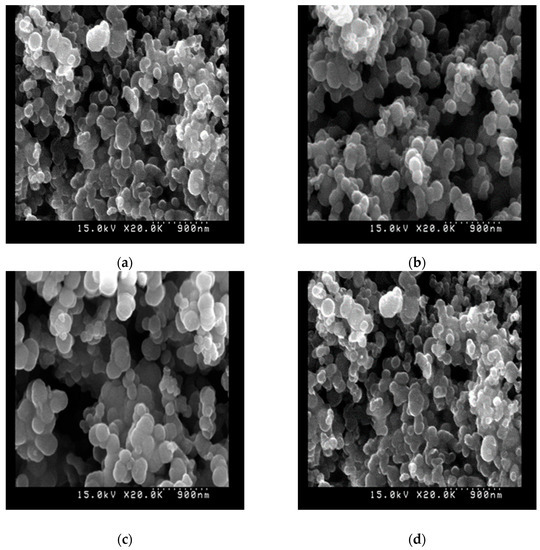
Figure 6.
The photographs from the SEM at a magnification of × 20.0 K of the carbon residue after burning the fuel samples of (a) SLSD, (b) biodiesel from waste cooking oil, (c) present biodiesel through supercritical methanol transesterification, and (d) high-acid oil.
The carbon residue that remained at the end of burning the present biodiesel through supercritical methanol transesterification appeared to have a larger diameter in comparison with the biodiesel made from used cooking oil under atmospheric pressure. This was ascribed to the higher impurity, heavier fatty acids, and water contents of the high-acid oil feedstock compared to those of the used cooking oil. Moreover, the distribution of the carbon residue after burning high-acid oil was shown to be much more scattered and connected together, primarily owing to its higher contents of gum and mineral impurities.
The photographs from the SEM of the high-acid oil appear to be composed of many smaller carbon particles and cluttered more densely among each other. This is ascribed to heavier compounds and more impurities in the high-acid oil, leading to a larger amount of carbon residue after its burning.
4. Conclusions
The biodiesel from high-acid oil, a biodiesel product made from used cooking oil, and super-low sulfur diesel (SLSD) were used to analyze and compare their engine emission characteristics. The major results obtained from this study are summed up below.
- An increase in engine speed raised CO2 emissions from the diesel engine while decreasing NOx emissions, O2 emissions, and black smoke opacity primarily because of the accompanying increase of injected liquid fuel and burning efficiency, leading to higher CO2 emissions and lower NOx, O2, and black smoke emissions. In addition, the CO emissions increased with engine speeds from 800 rpm to 1400 rpm and then decreased with the engine speeds afterward.
- The biodiesel made from the high-acid oil through supercritical methanol transesterification was observed to have higher NOx emissions and O2 emissions and a lower equivalence ratio, CO emissions, CO2 emissions, and black smoke opacity than SLSD. This is due to the higher elemental carbon and lower elemental oxygen in the biodiesel, which enhanced its combustion efficiency. It was also determined that the biodiesel’s lower heating value requires more injected liquid fuel to attain the same engine power output as SLSD.
- The size of carbon residue left after the burning of the biodiesel made from the high-acid oil of soybean soapstock through supercritical methanol transesterification was observed to be significantly larger than that of SLSD and the commercial biodiesel produced from used cooking oil due to its larger content of gums, heavier compounds of impurities, and absorbed water, according to the photographs from the scanning electron microscope (SEM). The biodiesel composed of hydrophilic substances also caused larger sizes of carbon residue than SLSD.
Author Contributions
Conceptualization, C.-Y.L.; methodology, C.-Y.L.; formal analysis, Y.-W.L. and H.Y.; investigation, C.-Y.L. and Y.-W.L.; data curation, Y.-W.L. and H.Y.; writing—original draft preparation, C.-Y.L. and Y.-W.L.; writing—review and editing, C.-Y.L.; supervision, C.-Y.L.; project administration, C.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council of Science and Technology, Taiwan, under the contract number NCST 109-2221-E-019-024.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within this article.
Acknowledgments
The authors would like to gratefully acknowledge the financial support from the National Council of Science and Technology, Taiwan, under the contract number NCST 109-2221-E-019-024.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ong, H.C.; Tiong, Y.W.; Goh, B.H.H.; Gan, Y.Y.; Mofijur, M.; Fattah, I.R.; Mahlia, T.M.I. Recent advances in biodiesel production from agricultural products and microalgae using ionic liquids: Opportunities and challenges. Energy Convers. Manag. 2021, 228, 113647. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G.; Kumar, A. Experimental assessment of performance, combustion and emissions of a compression ignition engine fuelled with Spirulina platensis biodiesel. Energy 2020, 193, 116861. [Google Scholar] [CrossRef]
- Rajan, K.; Rajaram Narayanan, M.; Ashraff Ali, K.S.; Prasanna, B.; Vinayagam, M. Analysis on the properties and emission characteristics of corn biodiesel subjected to improved transesterification. Int. J. Ambient Energy 2022, 43, 1695–1700. [Google Scholar] [CrossRef]
- De Araujo-Silva, R.; Vieira, A.C.; de Campos Giordano, R.; Fernandez-Lafuente, R.; Tardioli, P.W. Enzymatic synthesis of fatty acid isoamyl monoesters from soybean oil deodorizer distillate: A renewable and ecofriendly base stock for lubricant industries. Molecules 2022, 27, 2692. [Google Scholar] [CrossRef]
- Lima, M.G.B.; Gupta, J. The policy context of biofuels: A case of non-governance at the global level? Glob. Environ. Politics 2013, 13, 46–64. [Google Scholar] [CrossRef]
- Roick, C.; Otun, K.O.; Diankanua, N.; Joshua, G. Non-edible feedstock for biodiesel production. In Biodiesel Technology and Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 285–309. [Google Scholar]
- Sen, K.Y.; Baidurah, S. Renewable biomass feedstocks for production of sustainable biodegradable polymer. Curr. Opin. Green Sustain. Chem. 2021, 27, 100412. [Google Scholar] [CrossRef]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Basheer, S.; Watanabe, Y. Enzymatic conversion of acid oils to biodiesel. Lipid Technol. 2016, 28, 16–18. [Google Scholar] [CrossRef]
- Chew, S.C.; Nyam, K.L. Kenaf (Hibiscus cannabinus L.) seed oil. In Fruit Oils: Chemistry and Functionality; Springer: Berlin/Heidelberg, Germany, 2019; pp. 451–494. [Google Scholar]
- Ferrero, G.O.; Faba, E.M.S.; Eimer, G.A. Biodiesel production from alternative raw materials using a heterogeneous low ordered biosilicified enzyme as biocatalyst. Biotechnol. Biofuels 2021, 14, 67. [Google Scholar] [CrossRef]
- Veljković, V.B.; Banković-Ilić, I.B.; Stamenković, O.S.; Hung, Y.T. Waste Vegetable oils, fats, and cooking oils in biodiesel production. In Integrated Natural Resources Research; Springer: Cham, Switzerland, 2021; pp. 147–263. [Google Scholar]
- Hsiao, M.C.; Lin, W.T.; Chiu, W.C.; Hou, S.S. Two-Stage Biodiesel synthesis from used cooking oil with a high acid value via an ultrasound-assisted method. Energies 2021, 14, 3703. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Kadry, G.A.; Abdel-Samad, H.A.; Awad, M.E. High operative heterogeneous catalyst in biodiesel production from waste cooking oil. Egypt. J. Pet. 2020, 29, 59–65. [Google Scholar] [CrossRef]
- Li, K.; Xu, Z. A review of current progress of supercritical fluid technologies for e-waste treatment. J. Clean. Prod. 2019, 227, 794–809. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Lu, X.; Zhang, X.; He, H.; Pan, F.; Zhang, S. Cascade utilization of lignocellulosic biomass to high-value products. Green Chem. 2019, 21, 3499–3535. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- Matos, R.L.; Lu, T.; Leeke, G.; Prosapio, V.; McConville, C.; Ingram, A. Single-step coprecipitation and coating to prepare curcumin formulations by supercritical fluid technology. J. Supercrit. Fluids 2020, 159, 104758. [Google Scholar] [CrossRef]
- Qadeer, M.U.; Ayoub, M.; Komiyama, M.; Daulatzai, M.U.K.; Mukhtar, A.; Saqib, S.; Bokhari, A. Review of biodiesel synthesis technologies, current trends, yield influencing factors and economical analysis of supercritical process. J. Clean. Prod. 2021, 309, 127388. [Google Scholar] [CrossRef]
- Ortiz, F.J.G. Techno-economic assessment of supercritical processes for biofuel production. J. Supercrit. Fluids 2020, 160, 104788. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; Ortiz-Martínez, V.M.; García-Martínez, N.; de los Ríos, A.P.; Hernández-Fernández, F.J.; Quesada-Medina, J. Production of biodiesel under supercritical conditions: State of the art and bibliometric analysis. Appl. Energy 2020, 264, 114753. [Google Scholar] [CrossRef]
- Niyas, M.M.; Shaija, A. Effect of repeated heating of coconut, sunflower, and palm oils on their fatty acid profiles, biodiesel properties and performance, combustion, and emission, characteristics of a diesel engine fueled with their biodiesel blends. Fuel 2022, 328, 125242. [Google Scholar] [CrossRef]
- Palani, Y.; Devarajan, C.; Manickam, D.; Thanikodi, S. Performance and emission characteristics of biodiesel-blend in diesel engine: A review. Environ. Eng. Res. 2022, 27, 200338. [Google Scholar] [CrossRef]
- Ağbulut, Ü.; Sarıdemir, S.; Albayrak, S. Experimental investigation of combustion, performance and emission characteristics of a diesel engine fuelled with diesel–biodiesel–alcohol blends. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 1–12. [Google Scholar] [CrossRef]
- Cai, T.; Becker, S.M.; Cao, F.; Wang, B.; Tang, A.; Fu, J.; Zhao, D. NOx emission performance assessment on a perforated plate-implemented premixed ammonia-oxygen micro-combustion system. Chem. Eng. J. 2021, 417, 128033. [Google Scholar] [CrossRef]
- Gao, J.; Tian, G.; Ma, C.; Balasubramanian, D.; Xing, S.; Jenner, P. Numerical investigations of combustion and emissions characteristics of a novel small scale opposed rotary piston engine 512 fuelled with hydrogen at wide open throttle and stoichiometric conditions. Energy Convers. Manag. 2020, 221, 113178. [Google Scholar] [CrossRef]
- Varghese, G.; Saeed, K.; Lu, X.; Rutt, K.J. Effects of biodiesel degree of unsaturation, chain length and physical properties on tailpipe oxides of nitrogen (NOx). J. Energy Inst. 2022, 105, 355–366. [Google Scholar] [CrossRef]
- Singh, M.; Sandhu, S.S. Performance, emission and combustion characteristics of multi-cylinder CRDI engine fueled with argemone biodiesel/diesel blends. Fuel 2020, 265, 117024. [Google Scholar] [CrossRef]
- Ilbas, M.; Yılmaz, İ.; Kaplan, Y. Investigations of hydrogen and hydrogen–hydrocarbon composite fuel combustion and NOx emission characteristics in a model combustor. Int. J. Hydrogen Energy 2005, 30, 1139–1147. [Google Scholar] [CrossRef]
- Dhande, D.Y.; Nighot, D.V.; Sinaga, N.; Dahe, K.B. Extraction of bioethanol from waste pomegranate fruits as a potential feedstock and its blending effects on a performance of a single cylinder SI engine. Renew. Sustain. Energy Rev. 2021, 149, 111349. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, C.T. Emulsification characteristics of three-phase emulsion of biodiesel-in nitromethane-in-diesel prepared by microwave irradiation. Fuel 2015, 158, 50–56. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wu, X.E. Determination of cetane number from fatty acid compositions and structures of Biodiesel. Processes 2022, 10, 1502. [Google Scholar] [CrossRef]
- Şimşek, S. Increasing cetane number of the diesel fuel by fuel additives. Int. J. Automot. Sci. Technol. 2020, 4, 300–306. [Google Scholar] [CrossRef]
- Choudhary, R.; Clees, S.; Boddapati, V.; Shao, J.; Davidson, D.F.; Hanson, R.K. Low-temperature oxidation of n-octane and n-decane in shock tubes: Differences in time histories of key intermediates. Combust. Flame 2023, 251, 112670. [Google Scholar] [CrossRef]
- Abed, K.A.; Gad, M.S.; El Morsi, A.K.; Sayed, M.M.; Elyazeed, S.A. Effect of biodiesel fuels on diesel engine emissions. Egypt. J. Pet. 2019, 28, 183–188. [Google Scholar] [CrossRef]
- Chuah, L.F.; Bokhari, A.; Asif, S.; Klemeš, J.J.; Dailin, D.J.; El Enshasy, H.; Yusof, A.H.M. A review of performance and emission characteristic of engine diesel fuelled by biodiesel. Chem. Eng. Trans. 2022, 94, 1099–1104. [Google Scholar]
- Gong, C.; Li, Z.; Yi, L.; Liu, F. Experimental investigation of equivalence ratio effects on combustion and emissions characteristics of an H2/methanol dual-injection engine under different spark timings. Fuel 2020, 262, 116463. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wu, Q.; Duan, D.; Liu, Y.; Ruan, R.; Yang, X. Microwave-assisted pyrolysis of vegetable oil soapstock: Comparative study of rapeseed, sunflower, corn, soybean, rice, and peanut oil soapstock. Int. J. Agric. Biol. Eng. 2019, 12, 202–208. [Google Scholar] [CrossRef]
- Gad, M.S.; El-Araby, R.; Abed, K.A.; El-Ibiari, N.N.; El Morsi, A.K.; El-Diwani, G.I. Performance and emissions characteristics of CI engine fueled with palm oil/palm oil methyl ester blended with diesel fuel. Egypt. J. Pet. 2018, 27, 215–219. [Google Scholar] [CrossRef]
- Fu, W.; Li, F.; Meng, K.; Liu, Y.; Shi, W.; Lin, Q. Experiment and analysis of spray characteristics of biodiesel blending with di-n-butyl ether in a direct injection combustion chamber. Energy 2019, 185, 77–89. [Google Scholar] [CrossRef]
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.H.; Nguyen, T.H.P. Sustainability of the four generations of biofuels–a review. Int. J. Energy Res. 2020, 44, 9266–9282. [Google Scholar] [CrossRef]
- Sun, C.H.; Fu, Q.; Liao, Q. Life-cycle assessment of biofuel production from microalgae via various bioenergy conversion systems. Energy 2019, 171, 1033–1045. [Google Scholar] [CrossRef]
- Han, D.; Jiaqiang, E.; Deng, Y.; Chen, J.; Leng, E.; Liao, G.; Zhang, F. A review of studies using hydrocarbon adsorption material for reducing hydrocarbon emissions from cold start of gasoline engine. Renew. Sustain. Energy Rev. 2021, 135, 110079. [Google Scholar] [CrossRef]
- Emaish, H.; Abualnaja, K.M.; Kandil, E.E.; Abdelsalam, N.R. Evaluation of the performance and gas emissions of a tractor diesel engine using blended fuel diesel and biodiesel to determine the best loading stages. Sci. Rep. 2021, 11, 9811. [Google Scholar] [CrossRef] [PubMed]
- Odibi, C.; Babaie, M.; Zare, A.; Nabi, M.N.; Bodisco, T.A.; Brown, R.J. Exergy analysis of a diesel engine with waste cooking biodiesel and triacetin. Energy Convers. Manag. 2019, 198, 111912. [Google Scholar] [CrossRef]
- Chen, R.H.; Ong, H.C.; Wang, W.C. The optimal blendings of diesel, biodiesel and gasoline with various exhaust gas recirculations for reducing NO x and smoke emissions from a diesel engine. Int. J. Environ. Sci. Technol. 2020, 17, 4623–4654. [Google Scholar] [CrossRef]
- Sahoo, S.; Srivastava, D.K. Effect of compression ratio on engine knock, performance, combustion and emission characteristics of a bi-fuel CNG engine. Energy 2021, 233, 121144. [Google Scholar] [CrossRef]
- Bayat, Y.; Ghazikhani, M. Experimental investigation of compressed natural gas using in an indirect injection diesel engine at different conditions. J. Clean. Prod. 2020, 271, 122450. [Google Scholar] [CrossRef]
- Sutheerasak, E.; Pirompugd, W.; Ruengphrathuengsuka, W.; Sanitjai, S. Comparative investigation of using DEB oil and supercharging syngas and DEB oil as a dual fuel in a DI diesel engine. Eng. Appl. Sci. Res. 2019, 46, 26–36. [Google Scholar]
- Dong, Z.; Li, F.; Zhang, H.; Duan, Y.; Zhao, Z.; Zheng, Y.; Wang, W. Effects of oxidation degree and antioxidant addition on the carbon deposition tendency of jatropha biodiesel. Energy Sources, Part A 2023, 45, 4917–4930. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; García, I.L.; Papadaki, A.; Tsouko, E.; Koutinas, A.; Dorado, M.P. Biodiesel production using microbial lipids derived from food waste discarded by catering services. Bioresour. Technol. 2021, 323, 124597. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).