Effect of the Encapsulation Process on the Viability of Probiotics in a Simulated Gastrointestinal Tract Model Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacteria Cultivation
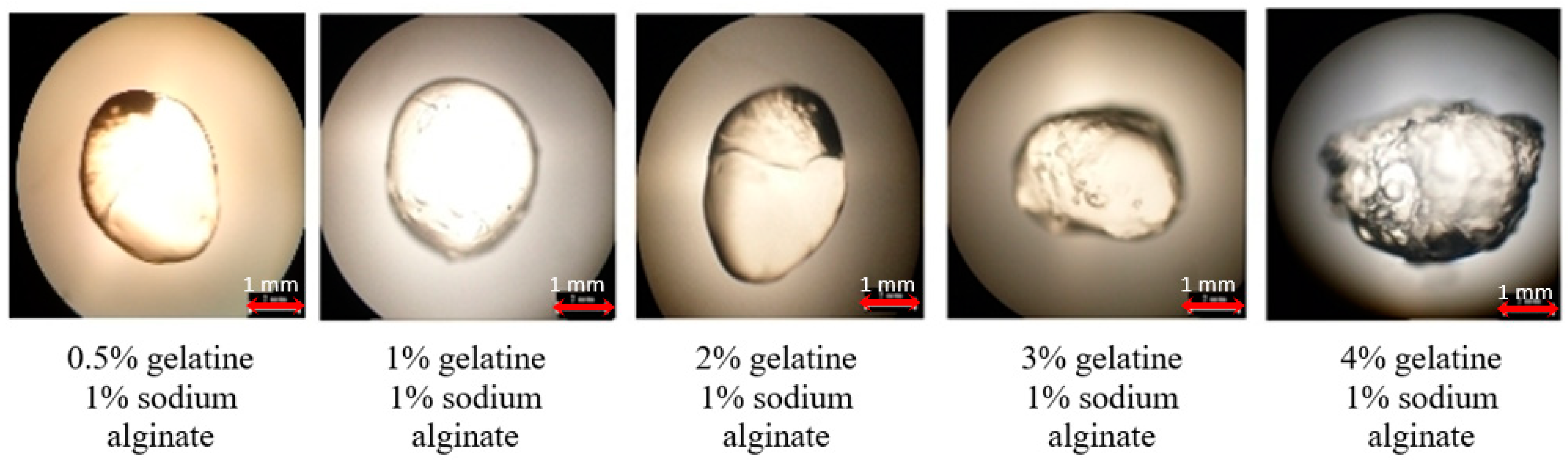
2.3. Preparation of Encapsulated Probiotics
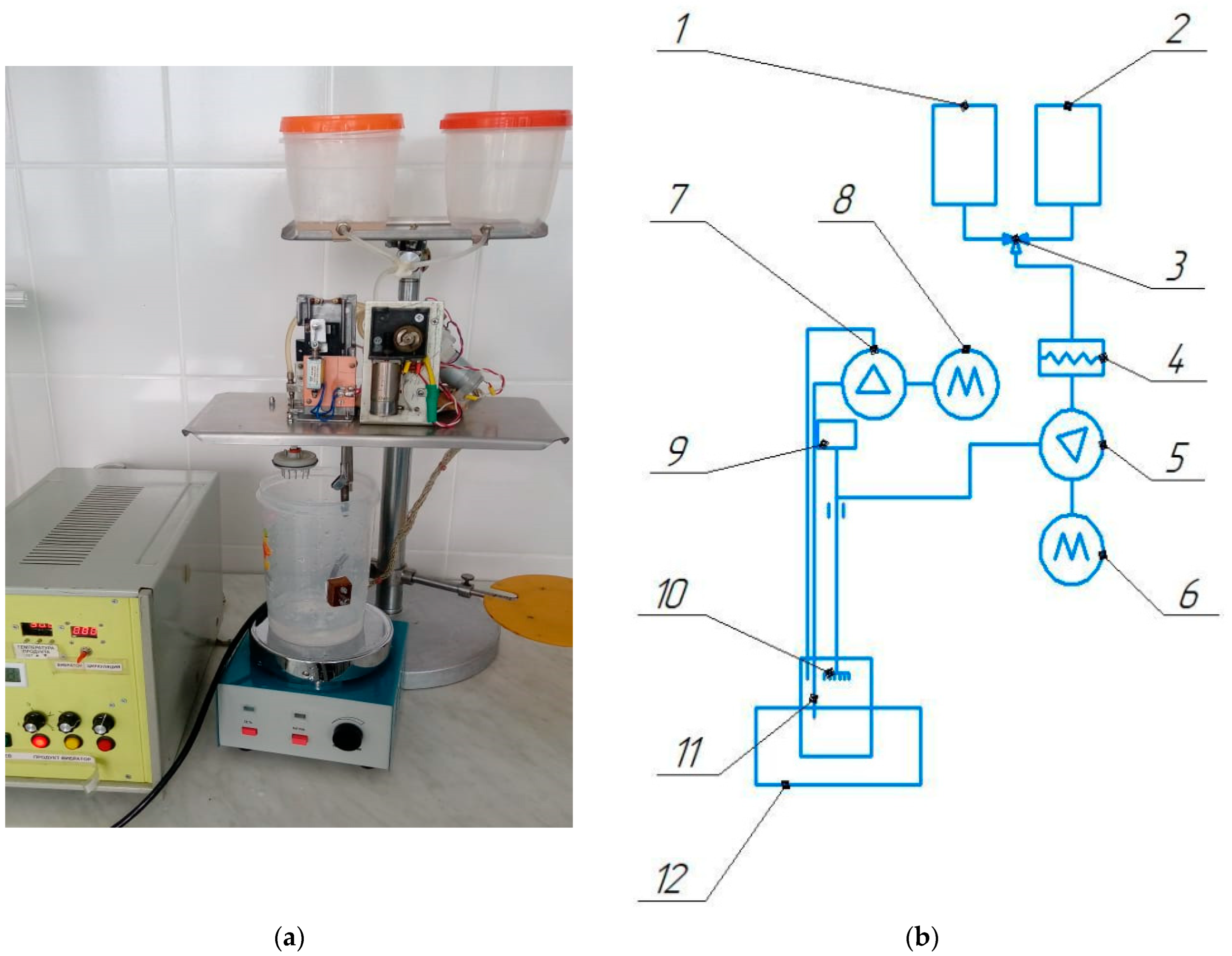
2.4. Development of Technology for the Production of Encapsulated Probiotics
2.5. Determination of Probiotic Cell Resistance to the Conditions of a Model Environment Simulating the Human Gastrointestinal Tract
2.6. Determination of Probiotic Cell Release from Capsules
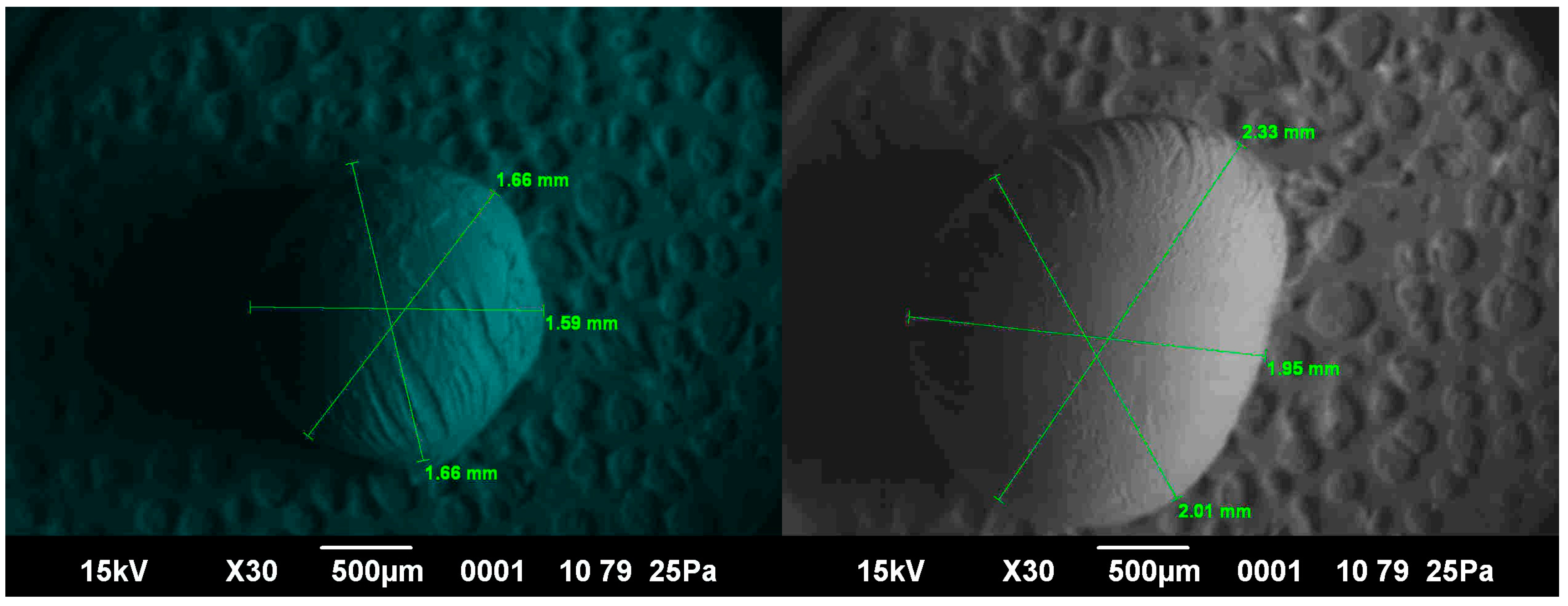
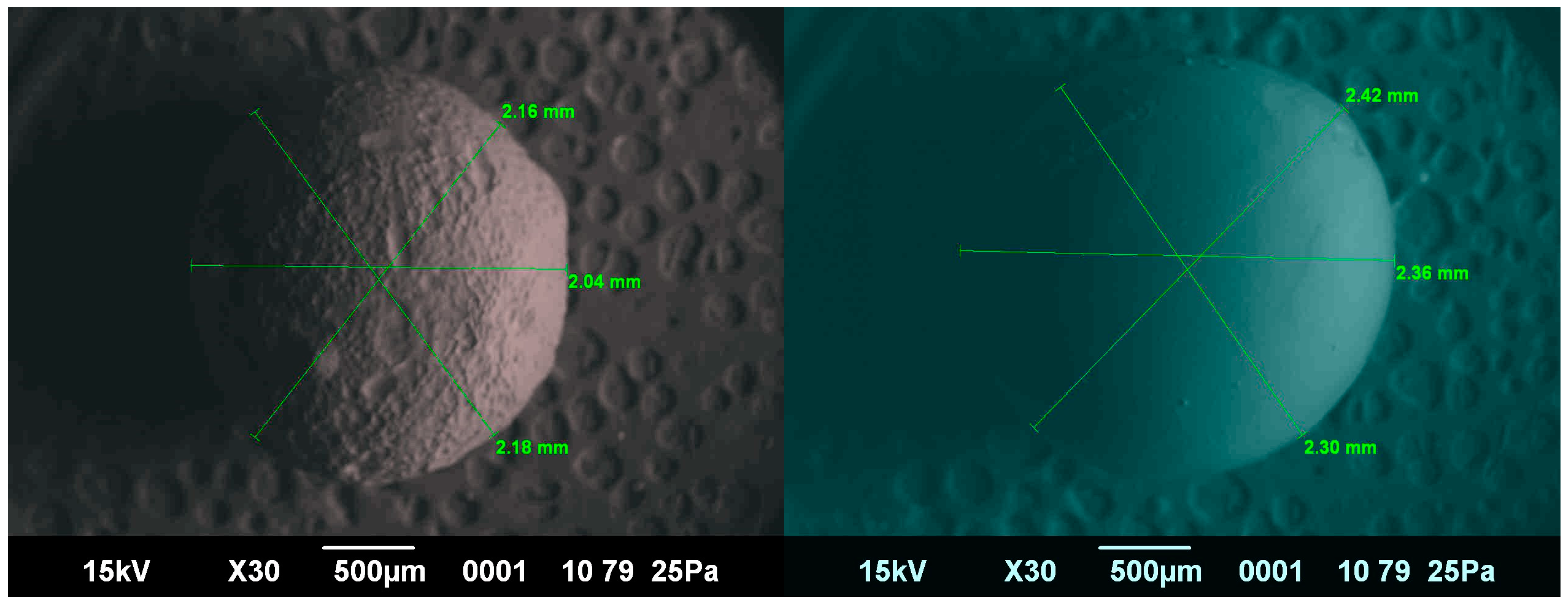
2.7. Microscopic Examination
- The capsules are placed sequentially in solutions of 30, 50, and 70% isopropyl alcohol. The capsules are soaked for 3 h in each solution and then placed in a vessel with 100% isopropyl alcohol where they are kept for at least 6 h to dehydrate the capsules.
- The capsules are then placed in solutions of tert-butyl alcohol at 30, 50, and 70% concentration, in the sequence indicated. Then they are placed into 100% tert-butyl alcohol to replace the isopropyl alcohol. Taking into account that 100% tert-butyl alcohol is able to freeze at a temperature of +25 °C, the capsule structure is fixed in a “frozen” form.
- The “frozen” capsule fixed in tert-butyl alcohol is placed on the freezing table of the MS-2 luge microtome, where the layers of the capsule are cut for access to the internal structure of the capsule.
- Next, lyophilization is performed. In the lyophilic dryer JFD-320 of “JEOL” company (Japan), under vacuum, there is a transition of tert-butyl alcohol from the frozen state immediately to the vapor state. This makes it possible to remove tert-butyl alcohol from the capsule and keep the capsule structure unchanged.
- Then, in the vacuum coating equipment JEE-420 of “JEOL” (Japan), the carbon layer is sprayed on the capsule slice’s surface, making it possible to obtain more contrasting images.
- The prepared capsules are fixed on the slide using double-sided carbon tape. Then, the slide is placed in the microscope chamber and the surface of the samples is scanned.
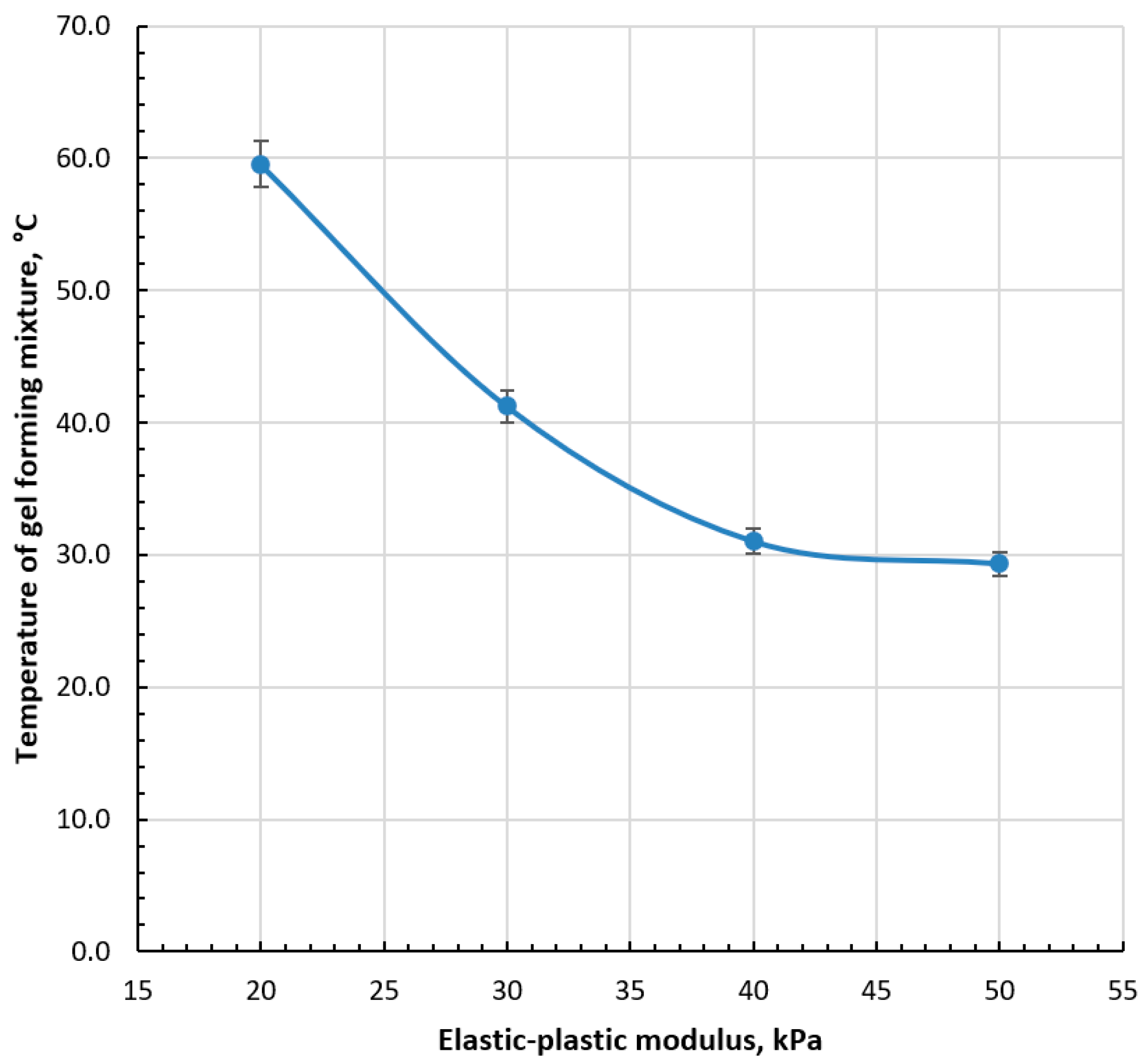
2.8. Determination of Elastic–Plastic Deformation of the Capsule Shells
2.9. Statistics
3. Results
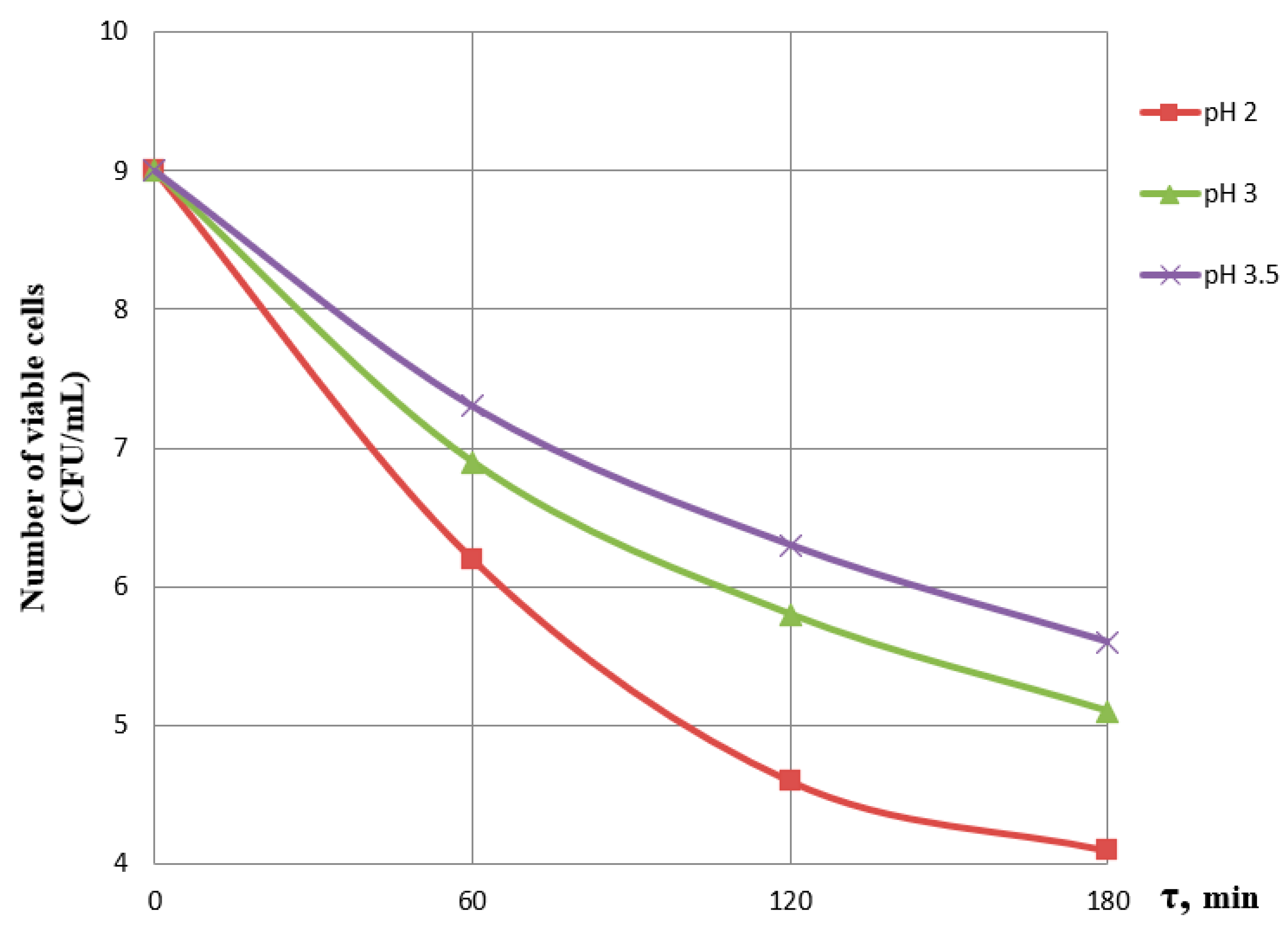
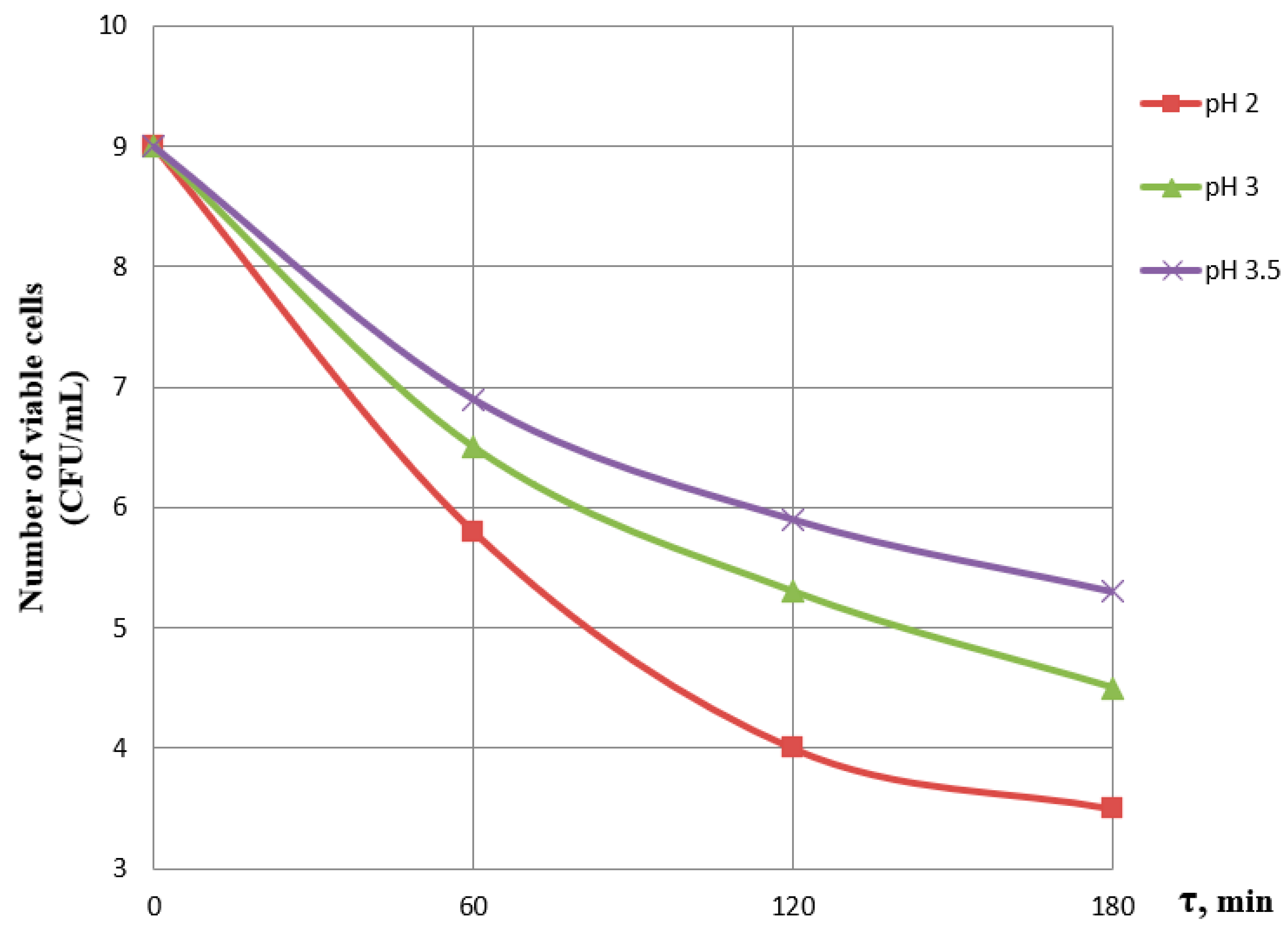
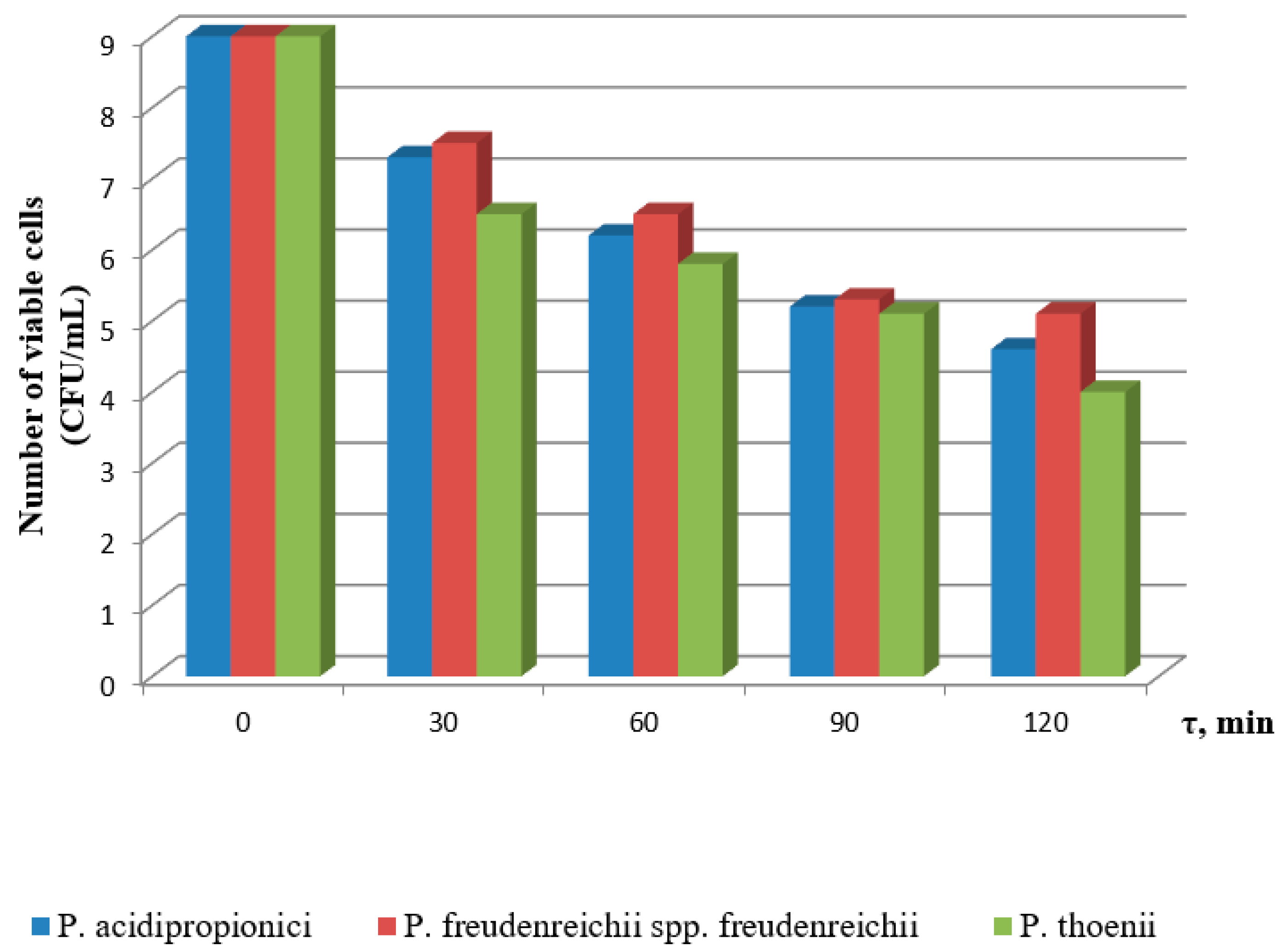
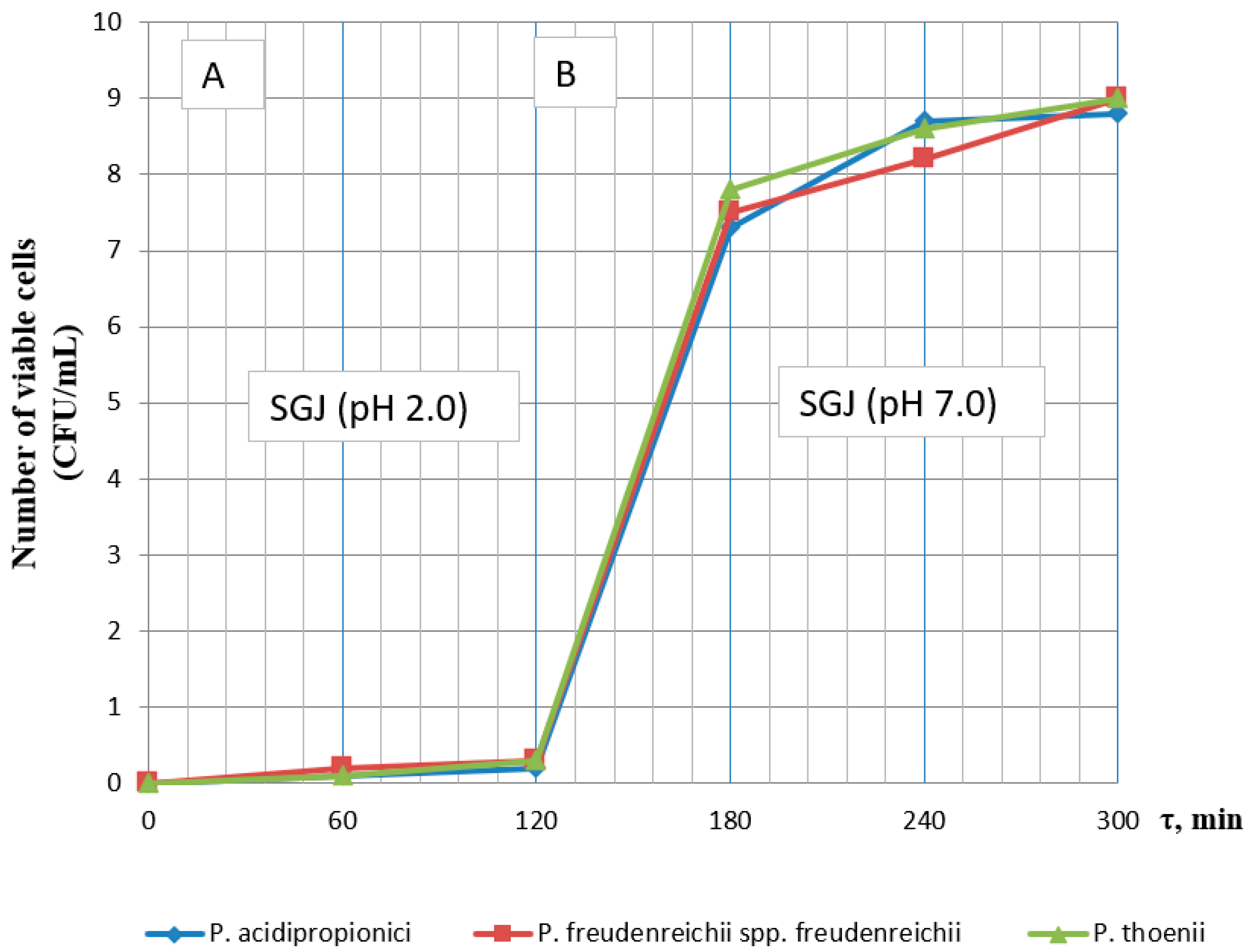
3.1. Effect of Incubation Time and pH on the Viability of Probiotics
3.2. Selection of Gel-Forming Mixture for Capsule Production
3.3. Investigation of the Viability of Encapsulated Probiotics in a Model Medium Simulating the Gastrointestinal Tract
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Terpou, A.; Nigam, P.; Bosnea, L.; Kanellaki, M. Evaluation of Chios mastic gum as antimicrobial agent and matrix-forming material targeting probiotic cell encapsulation for functional fermented milk production. LWT 2018, 97, 109–116. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, Z.; Pan, C.; Goulette, T.R.; Zhang, R.; Hendricks, G.; McClements, D.J.; Xiao, H. Encapsulation of Bifidobacterium pseudocatenulatum G7 in gastroprotective microgels: Improvement of the bacterial viability under simulated gastrointestinal conditions. Food Hydrocoll. 2019, 91, 283–289. [Google Scholar] [CrossRef]
- Yuan, Y.; Yin, M.; Zhai, Q.; Chen, M. The encapsulation strategy to improve the survival of probiotics for food application: From rough multicellular to single-cell surface engineering and microbial mediation. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Adeel, M.; Afzaal, M.; Saeed, F.; Ahmed, A.; Mahmood, K.; Abbas Shah, Y.; Ateeq, H.; Sibat, A.; Khan, M.R.; Busquets, R. Encapsulation of probiotic bacteria using polyelectrolytes stabilized nanoliposomes for improved viability under hostile conditions. J. Food Sci. 2023, 88, 3839–3848. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P.S. The Gut Microbiota Influenced by the Intake of Probiotics and Functional Foods with Prebiotics Can Sustain Wellness and Alleviate Certain Ailments like Gut-Inflammation and Colon-Cancer. Microorganisms 2022, 10, 665. [Google Scholar] [CrossRef]
- Wilkinson, M.G. Flow cytometry as a potential method of measuring bacterial viability in probiotic products: A review. Trends Food Sci. Technol. 2018, 78, 1–10. [Google Scholar] [CrossRef]
- Samedi, L.; Charles, A.L. Viability of 4 Probiotic Bacteria Microencapsulated with Arrowroot Starch in the Simulated Gastrointestinal Tract (GIT) and Yoghurt. Foods 2019, 8, 175. [Google Scholar] [CrossRef]
- Harel, M.; Tang, Q. Protection and delivery of probiotics for use in foods. In Microencapsulation in the Food Industry; Academic Press: Cambridge, MA, USA, 2023; pp. 463–480. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Bai, L.; Deng, J.; Zhou, Q. Biomaterial-based encapsulated probiotics for biomedical applications: Current status and future perspectives. Mater. Des. 2021, 210, 110018. [Google Scholar] [CrossRef]
- Bepeyeva, A.; de Barros, J.M.; Albadran, H.; Kakimov, A.K.; Kakimova, Z.K.; Charalampopoulos, D.; Khutoryanskiy, V.V. Encapsulation of Lactobacillus casei into calcium pectinate-chitosan beads for enteric delivery. J. Food Sci. 2017, 82, 954–2959. [Google Scholar] [CrossRef]
- Koh, W.Y.; Lim, X.X.; Tan, T.-C.; Kobun, R.; Rasti, B. Encapsulated probiotics: Potential techniques and coating materials for non-dairy food applications. Appl. Sci. 2022, 12, 10005. [Google Scholar] [CrossRef]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-based hydrogels: Potential biomaterials for remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef]
- Sun, Q.; Yin, S.; He, Y.; Cao, Y.; Jiang, C. Biomaterials and Encapsulation Techniques for Probiotics: Current Status and Future Prospects in Biomedical Applications. Nanomaterials 2023, 13, 2185. [Google Scholar] [CrossRef]
- Jankowski, T.; Zielinska, M.; Wysakowska, A. Encapsulation of lactic acid bacteria with alginate/starch capsules. Biotechnol. Tech. 1997, 11, 31–34. [Google Scholar] [CrossRef]
- Chandramouli, V.; Kailasapathy, K.; Peiris, P.; Jones, M. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J. Microbiol. Methods 2004, 56, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, P.S.; Blunt, K.W.; Bocke, C. Physical studies on cell immobilization using calcium alginate gels. Biotechnol. Bioeng. 1979, 21, 2155–2168. [Google Scholar] [CrossRef]
- Zeashan, M.; Afzaal, M.; Saeed, F.; Ahmed, A.; Tufail, T.; Ahmed, A.; Anjum, F.M. Survival and behavior of free and encapsulated probiotic bacteria under simulated human gastrointestinal and technological conditions. Food Sci. Nutr. 2020, 8, 2419–2426. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Gómez-Guillén, M.C. A state-of-the-art review on the elaboration of fish gelatin as bioactive packaging: Special emphasis on nanotechnology-based approaches. Trends Food Sci. Technol. 2018, 79, 125–135. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, X.; Zhang, H.; Zhang, Y.; Gänzle, M.; Yang, N.; Nishinari, K.; Fang, Y. Probiotic encapsulation in water-in-water emulsion via heteroprotein complex coacervation of type-A gelatin/sodium caseinate. Food Hydrocoll. 2020, 105, 105790. [Google Scholar] [CrossRef]
- King, A. Encapsulation of Food Ingredients: A review of available technology, focusing on hydrocolloids. In Encapsulation and Controlled Release of Food Ingredients, ACS Symposium Series 590; American Chemical Society: Washington, DC, USA, 1995; pp. 26–39. [Google Scholar] [CrossRef]
- Antone, U.; Ciprovica, I.; Zolovs, M.; Scerbaka, R.; Liepins, J. Propionic Acid Fermentation—Study of Substrates, Strains, and Antimicrobial Properties. Fermentation 2023, 9, 26. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Rutten, G.; Bruinenberg, P.; Sesma, F.; de Giori, G.S.; Smid, E.J. A novel dairy product fermented with Propionibacterium freudenreichii improves the riboflavin status of deficient rats. Nutrition 2006, 22, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Teneva, D.; Denev, P. Biologically Active Compounds from probiotic microorganisms and plant extracts used as biopreservatives. Microorganisms 2023, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Yerlikaya, O.; Akpinar, A.; Saygili, D.; Karagozlu, N. Incorporation of Propionibacterium shermanii subsp. freudenreichii in probiotic dairy drink production: Physicochemical, rheological, microbiological and sensorial properties. Int. J. Dairy Technol. 2019, 73, 392–402. [Google Scholar] [CrossRef]
- Khamagaeva, I.S.; Kachanina, L.M.; Tumurova, S.M. Biotechnology of Propionic Acid Bacteria Starter; VSGTU Publishing House: Ulan-Ude, Russia, 2006; 172p. [Google Scholar]
- Kakimov, A.; Ibragimov, N.; Zhumadilova, G.; Muratbayev, A.; Jumazhanova, M.; Soltanbekov, Z.; Yessimbekov, Z.; Mayorov, A. Design of equipment for probiotics encapsulation. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 468–471. [Google Scholar]
- GOST 34372-2017; Bacterial Starters for the Production of Dairy Products. General Technical Conditions. Standardinform: Moscow, Russia, 2018.
- Chae, J.K.; Han, S.; Kim, D.H.; Park, S.H.; Ha, S.D. Growth characterization of Propionibacterium and propionic acid production capabilities at different temperatures and pH levels. Food Sci. Biotechnol. 2022, 31, 357–364. [Google Scholar] [CrossRef] [PubMed]
- de Assis, D.A.; Machado, C.; Matte, C.; Ayub, M.A.Z. High cell density culture of dairy propionibacterium sp. and acidipropionibacterium sp.: A review for food industry applications. Food Bioprocess Technol. 2022, 15, 734–749. [Google Scholar] [CrossRef]
- Koga, Y. Microbiota in the stomach and application of probiotics to gastroduodenal diseases. World J. Gastroenterol. 2022, 28, 6702–6715. [Google Scholar] [CrossRef]
- Vecchione, A.; Celandroni, F.; Mazzantini, D.; Senesi, S.; Lupetti, A.; Ghelardi, E. Compositional quality and potential gastrointestinal behavior of probiotic products commercialized in Italy. Front. Med. 2018, 5, 59. [Google Scholar] [CrossRef]
- Sahadeva, R.P.K.; Leong, S.F.; Chua, K.H.; Tan, C.H.; Chan, H.Y.; Tong, E.V.; Wong, S.Y.W.; Chan, H.K. Survival of commercial probiotic strains to pH and bile. Int. Food Res. J. 2011, 18, 1515–1522. [Google Scholar]
- Suomalainen, T.; Sigvart-Mattila, P.; Mättö, J.; Tynkkynen, S. In vitro and in vivo gastrointestinal survival, antibiotic susceptibility and genetic identification of Propionibacterium freudenreichii ssp. shermanii JS. Int. Dairy J. 2008, 18, 271–278. [Google Scholar] [CrossRef]
- Darilmaz, D.O.; Beyatli, Y. Acid-bile, antibiotic resistance and inhibitory properties of propionibacteria isolated from Turkish traditional home-made cheeses. Anaerobe 2012, 18, 122–127. [Google Scholar] [CrossRef]
- Zárate, G.; Chaia, A.P.; González, S.; Oliver, G. Viability and β-galactosidase activity of dairy propionibacteria subjected to digestion by artificial gastric and intestinal fluids. J. Food Prot. 2000, 63, 1214–1221. [Google Scholar] [CrossRef]
- Jumazhanova, M.M.; Kakimov, A.K.; Muratbaev, A.M. Research and justification of the choice of probiotics for encapsulation. In Proceedings of the XV International Scientific and Practical Conference “Scientific Industry of the European Continent—2019”, Prague, Czech Republic, 7–9 December 2019; pp. 3–7. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Santamaría, E.; Maestro, A.; González, C. Encapsulation of Carvacrol-Loaded Nanoemulsion Obtained Using Phase Inversion Composition Method in Alginate Beads and Polysaccharide-Coated Alginate Beads. Foods 2023, 12, 1874. [Google Scholar] [CrossRef]
- Kakimov, A.; Kakimova, Z.; Jumazhanova, M.; Muratbayev, A.; Zhumadilova, G.; Mirasheva, G.; Soltanbekov, Z.; Mayorov, A. Experimental study of capsules formation using different types of polymers. ARPN J. Eng. Appl. Sci. 2019, 14, 1855–1862. [Google Scholar]
- Vemuri, S. Measurement of soft elastic gelatin capsule firmness with a universal testing machine. Drug Dev. Ind. Pharm. 1984, 10, 409–423. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; García-Carvajal, Z.Y.; Jiménez-Palomar, I.; Jiménez-Avalos, J.A.; Espinosa-Andrews, H. Development of gelatin/chitosan/PVA hydrogels: Thermal stability, water state, viscoelasticity, and cytotoxicity assays. J. Appl. Polym. Sci. 2019, 136, 47149. [Google Scholar] [CrossRef]
- Karimi, M.; Sekhavatizadeh, S.S.; Hosseinzadeh, S. Milk dessert containing Lactobacillus reuteri (ATCC 23272) encapsulated with sodium alginate, Ferula assa-foetida and Zedo (Amygdalus scoparia) gum as three layers of wall materials. Food Bioprod. Process. 2021, 127, 244–254. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Rønhave Laursen, R.; Ouwehand, A.C. The production and delivery of probiotics: A review of a practical approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef]
- Felton, L.A.; Haase, M.M.; Shah, N.H.; Zhang, G.; Infeld, M.H.; Malick, A.W.; McGinity, J.W. Physical and enteric properties of soft gelatin capsules coated with eudragit® L 30 D-55. Int. J. Pharm. 1995, 113, 17–24. [Google Scholar] [CrossRef]
- Pinto, J.T.; Wutscher, T.; Stankovic-Brandl, M.; Zellnitz, S.; Biserni, S.; Mercandelli, A.; Kobler, M.; Buttini, F.; Andrade, L.; Daza, V.; et al. Evaluation of the physico-mechanical properties and electrostatic charging behavior of different capsule types for inhalation under distinct environmental conditions. AAPS PharmSciTech 2020, 21, 128. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jumazhanova, M.; Kakimova, Z.; Zharykbasov, Y.; Kassymov, S.; Zhumadilova, G.; Muratbayev, A.; Tashybayeva, M.; Suychinov, A. Effect of the Encapsulation Process on the Viability of Probiotics in a Simulated Gastrointestinal Tract Model Medium. Processes 2023, 11, 2757. https://doi.org/10.3390/pr11092757
Jumazhanova M, Kakimova Z, Zharykbasov Y, Kassymov S, Zhumadilova G, Muratbayev A, Tashybayeva M, Suychinov A. Effect of the Encapsulation Process on the Viability of Probiotics in a Simulated Gastrointestinal Tract Model Medium. Processes. 2023; 11(9):2757. https://doi.org/10.3390/pr11092757
Chicago/Turabian StyleJumazhanova, Madina, Zhaynagul Kakimova, Yerlan Zharykbasov, Samat Kassymov, Gulmira Zhumadilova, Alibek Muratbayev, Marzhan Tashybayeva, and Anuarbek Suychinov. 2023. "Effect of the Encapsulation Process on the Viability of Probiotics in a Simulated Gastrointestinal Tract Model Medium" Processes 11, no. 9: 2757. https://doi.org/10.3390/pr11092757
APA StyleJumazhanova, M., Kakimova, Z., Zharykbasov, Y., Kassymov, S., Zhumadilova, G., Muratbayev, A., Tashybayeva, M., & Suychinov, A. (2023). Effect of the Encapsulation Process on the Viability of Probiotics in a Simulated Gastrointestinal Tract Model Medium. Processes, 11(9), 2757. https://doi.org/10.3390/pr11092757






