Abstract
Betulin is a lupane-type pentacyclic triterpene. It is characterized by a range of biological properties, including anti-cancer and anti-inflammatory activities. It is also an origin compound for obtaining derivatives with higher biological activity and better bioavailability. Chronic inflammation stimulates the formation of a pro-cancer microenvironment, promoting tumor growth, cell migration, and neoangiogenesis. Many factors, immune system cells, and cytokines and chemokines released by them are involved in this process. Therefore, it has been suggested that the optimal target for anti-cancer drugs in this disease could be substances showing anti-inflammatory activity. The aim of the study was to indicate the direction of changes in the expression of genes related to the inflammatory state in colorectal cancer cells promoted by betulin and its selected alkynyl derivatives. Cytotoxicity assessment was carried out using a sulforhodamine B (SRB) test, whereas lipophilicity was determined by reversed-phase thin-layer chromatography (RP-TLC). The analysis of the gene expression profile in colon adenocarcinoma cells treated with betulin and its derivatives was performed using oligonucleotide microarrays HG-U133A. Based on the conducted analysis, it can be stated that betulin and its derivatives 1–3 influence the change in the expression profile of genes related to inflammatory processes in the HT-29 colon adenocarcinoma cell lines. The highest expression changes (FC > 2) were observed for HMOX1 (compound 1 vs. control) and TMED7 (compound 3 vs. control) mRNAs. An important observation is the comparison of the profile of changes in the expression of the studied genes in the compared compounds. Derivative 1 showed the greatest similarity to control cells, whereas betulin showed similarity to cisplatin. These observations indicate the necessity further research on the impact of betulin and its derivatives on inflammatory processes and the possible direction of chemical modification of compounds.
1. Introduction
In recent years, one of the most rapidly developing research directions is the search for substances with anti-cancer activity. Researchers mainly focus on the new application possibilities of well-known drugs, but they are also constantly looking for new, more effective compounds with anti-cancer activity. Attention is also given to the need to individualize treatment according to the type of cancer and its etiopathogenesis. Currently used chemotherapy is not a selective treatment and is associated with a high risk of systemic undesired effects. The rapid development of molecular biology has led to the discovery of proto-oncogenes and transcription factors conditioning tumor transformation. The action mechanism of targeted drugs involves inhibiting specific signaling pathways for processes related to tumor development: infiltration, proliferation, angiogenesis, and metastasis formation [1].
1.1. Betulin
Betulin (lup-20(29)-en-3β,28-diol) is a pentacyclic triterpene of the lupane type. It consists of four six-membered rings arranged in a trans configuration and one five-membered ring. It has two hydroxyl groups in its structure: a primary one at the C-28 atom and a secondary one at the C-3 atom, as well as an isopropenyl group at the C-19 carbon (Figure 1). Betulin alcohol can undergo a variety of chemical reactions such as: oxidation, isomerization, esterification, dehydrogenation, dehydration, and hydrogenation. Many biological activities of betulin derivatives have been demonstrated, it has also been noticed how modifications of the structure of this compound can affect the decrease or increase of desired biological properties [2,3,4,5].
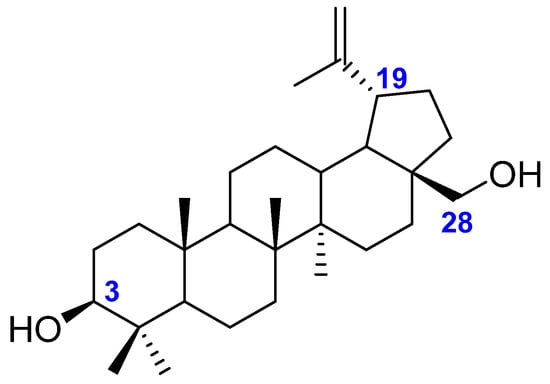
Figure 1.
Chemical structure of betulin.
1.2. Antitumor Activity
The anticancer properties of betulin have been confirmed based on many cancer cell lines, including primary ones and in vivo models. However, the mechanism of action of betulin has remained unclear for many years. Only in recent years has the ability to induce apoptosis in cancer cells been recognized as one of the mechanisms of the antiproliferative and cytotoxic action of betulin. Morphological changes occurring under the influence of betulin indicate the transition of cells to apoptosis. These observations were confirmed on melanoma cells (B164A5), lung cancer (A549), T lymphoblastic leukemia (Jurkat), and cervical cancer (HeLa) [2]. In one study on a cell line derived from cervical cancer HeLa, after incubation with betulin at a concentration of 10 μg/mL, single cells showed morphological features characteristic of cells undergoing apoptosis: cell rounding and cell membrane blebbing. In addition, cell staining using DAPI (4′,6-diamidino-2-phenylindole) showed that chromatin condensation and DNA fragmentation (deoxyribonucleic acid) occurred in the cells [6]. A clear increase in the activity of caspase 9 and caspase 3/7 was also observed. The activity of caspase 8 remained unchanged, suggesting a lack of extrinsic apoptosis activation. This confirms that betulin activates the process of a programmed cell death through the intrinsic pathway. After the initial increase in caspase 9 activity, the release of cytochrome c/Smac protein from the perimitochondrial space, mitochondrial membrane depolarization, and translocation of the Bax and Bak proteins occur. However, the involvement of Bcl-2 family proteins in betulin-stimulated apoptosis is not unequivocal [2,6]. Studies on the anticancer mechanism of betulin were also conducted on the kidney cancer cells RCC. They showed that the mechanism of action of betulin is related to the mTOR pathway [7]. Yim, Jung et al. in their study on the same type of RCC4 cancer line confirmed the antiproliferative activity of betulin was dependent on the dose and time of action. In the cells exposed to the action of the compound, an increase in the level of caspases 3, 7, 8, and 9 was observed, as well as increased expression of proteins associated with apoptosis processes such as PARP and the Bcl-2 family. Moreover, betulin has been shown to be able to activate death receptors (TRAIL, TNFR1) and regulate the level of anti- and pro-apoptotic proteins. These observations suggest that the studied compound can induce the apoptotic process in RCC4 kidney cancer cells both through the intracellular pathway and the extracellular pathway [8]. The mechanism of action of betulin is also related to its effect on cell cycle regulating factors. In the lung cancer cells of the A549 and NCI-292 cell lines, an increase in the expression of the factors p21 and p27 was confirmed, with a simultaneous decrease in the concentration of the cyclins B, D1, and E. However, it should be noted that the impact of betulin on genes regulating the cell cycle is not always identical and to a large extent depends on the type of cells [2,9].
Gastrointestinal cancers are among the most common types of cancers in humans. The increase in incidences of stomach, liver, pancreas, and colon cancers, as well as their relatively late detection, present a significant clinical problem. Betulin also demonstrates anti-cancer activity against cell lines of gastrointestinal system cancers. This compound retains high cytotoxic activity (IC50 = 18.7 μM) against stomach cancer EPG85-257P, as well as towards the atypical line resistant to mitoxantrone treatment (EPG85-257RNOV; IC50 = 12.3 μM) and resistant to daunorubicin treatment (EPG85-257RDB; IC50 = 11 μM). Similar activity has been demonstrated against pancreatic cancer lines (EPP85-181P; IC50 = 21.1 μM) and variants resistant to mitoxantrone (EPP85-181RNOV; IC50 = 20.6 μM) and daunorubicin treatments (EPP85-181RDB; IC50 = 26.5 μM). In the case of colon cancers, betulin’s activity varies depending on the type of cell line. The highest activity was observed for the DLD-1 colon adenocarcinoma cell line (IC50 = 6.6 μM) and the HT-29 cell line (IC50 = 4.3 μM) [2]. In the Hep2G liver cancer cell line, it was shown that betulin induces apoptotic death through an intrinsic pathway involving the activation of caspases 3 and 9 [10]. Against SGC7901 stomach cancer cells, the antiproliferative action is based on the translocation of the Bak and Bax proteins, the release of cytochrome c, and the activation of caspase 3 and caspase 9. The observed changes and lack of influence on the level of caspase 8 again suggest the activation of the mitochondrial apoptosis pathway. Additionally, betulin reduces the expression of the gene encoding the anti-apoptotic protein Bcl-2 as well as XIAP, which blocks the activation of procaspase 9 and caspase 3/9. An important observation was also the increase in ROS levels in the cells treated with betulin in a dose- and time-dependent manner [11]. In colon cancer cells, the induction of an intrinsic apoptosis mechanism by betulin has also been confirmed. The study involved HT-29 and HCT116 colon adenocarcinoma cells and a line of normal intestinal epithelial cells. Betulin showed no toxicity towards normal cells, whereas it significantly reduced the proliferation of cancer cells depending on the applied dose [12].
1.3. Anti-Inflammatory Action
One of the presumed mechanisms of betulin’s anti-inflammatory action is the inhibition of phospholipase A2 (PLA2) activity, one of the key enzymes involved in the inflammatory process [13,14]. The conducted studies also showed that the mechanism of betulin’s anti- inflammatory action may also be associated with the transcriptional nuclear factor κB (NF-κB) [13,15]. In a study using AC16 heart muscle cells, it was proven that the presence of betulin leads to the blockade of the transcriptional activity of the RelA protein. Using AC16 cells, it was confirmed that betulin stimulates the phosphorylation of residue 705 tyrosine in the STAT3 factor. It also caused an increase in mRNA levels for the cytokine signaling suppressor 3 (SOCS3) and stimulated the expression of the anti-apoptotic gene Bcl-xL. In AC16 heart muscle cells subjected to the action of betulin, a decrease in the levels of IL-6, MCP-1, and IL-1 cytokines, considered to be pro-inflammatory, was also observed. Wan et al., in their studies using liver stellate cells (Ito), also proved that the compound in question increases STAT3 phosphorylation [16,17,18].
In an in vivo experiment with induced acute liver inflammation using LPS, the application of betulin significantly caused a decrease in the level of liver enzymes (ALT and AST) in the plasma. Furthermore, it demonstrated an inhibitory effect on the secretion of MPO, IL-1β, and TNF-α in liver tissue [19].
The theory regarding the anti-inflammatory action of betulin has been confirmed in many studies on various models with induced inflammation both in vitro and in vivo. The immunomodulatory action shown by betulin seems particularly interesting in combination with its antitumor activity and indicates its potential in regulating the tumorigenesis processes that are related to inflammatory processes [20].
1.4. Lipophilicity
Lipophilicity is one of the most important physicochemical properties of biologically active compounds, affecting their biological activity, bioavailability, degree of degradation, and toxicity. Its determination enables the prediction of absorption, distribution, metabolism, and excretion profiles, i.e., the behavior of a substance in the human body after administration. Therefore, determining the lipophilicity value has become crucial in predicting the biological activity and behavior of drugs in the human body. The optimal lipophilicity of a compound aims to ensure its affinity for biological membranes and the ability to reach specific target structures. Excessive lipophilicity tends to reduce the biological activity of the substance. A lipophilicity above 5 (log P) often contributes to high metabolism, low solubility, and poor oral absorption. The general condition for the optimal absorption of substances from the gastrointestinal tract by passive diffusion after oral administration is a moderate log P value (range 0–3). Moreover, highly lipophilic compounds tend to bind to other hydrophobic targets than the desired one. Low lipophilicity can negatively affect permeability and potency, resulting in low bioavailability and, consequently, effectiveness [21,22,23].
1.5. Colorectal Cancer and Inflammation
Pro-inflammatory cytokines (TGFβ, TNFα, INF-γ, IL1, IL6) secreted as a result of disturbances in the interactions between tumor cells and the immune system cells (lymphocytes and monocytes or host macrophages) change the transcriptional activity of many genes involved in apoptosis, proliferation, and angiogenesis [24]. An inflammatory background, not only in the case of coexisting obesity, has its significant share in the initiation and progression of colorectal cancer. More than 20% of people with ulcerative colitis develop bowel cancer as a result of the inflammation. Similarly, patients with Crohn’s disease are twice as likely to develop bowel cancer as the general population. Long-term processes related to the chronic inflammation caused by bacterial infections, chemical, and mechanical damage can lead to disturbances in control over regeneration processes and lead to increased cell proliferation and tumor transformation [25,26]. The mechanisms related to the overlap between inflammation and carcinogenesis are currently an interesting area of research for the development of new therapies for cancer patients. The inflammatory state, created and maintained by genetic dysregulation and stimulating stimuli, both exogenous and endogenous, influences the transformation of immune cells from anti-tumor to pro-tumor. Certain types of inflammatory cells and cytokines are responsible for establishing and maintaining the tumor microenvironment. These include M2 phenotype macrophages, DCs, regulatory T (Treg) and Th17 lymphocytes, and the lymphokines they secrete IL-6, IL-13, IL-17, IL-22, IL-10, and TGF-β factor [27].
It turns out that autoimmune diseases associated with Th1 lymphocytes (IL-12, IFN-γ, anti-angiogenic CXCR3 ligands) and/or type 1-IFN immunization protect against the activation of the inflammatory tumor pathway in tissues. However, inflammatory diseases associated with Th2 and the interleukins IL-6, IL-23, and IL-22 and progressing with the activation of NFκB, STAT3, and Akt pathways, significantly more often lead to the malignant transformation of cells within the tissues affected by inflammatory processes [28,29].
The aim of this study is to evaluate the cytotoxicity of betulin and its derivatives 1–3 against colorectal adenocarcinoma cells (HT-29) and normal cells (NHDF), as well as to assess changes in gene expression related to the regulation of inflammation in colorectal cancer cells exposed to the selected compounds. An additional aim was to determine the bioavailability descriptor of selected compounds (lipophilicity) and correlate it with their biological activity, which is an important parameter in the selection of potential drugs.
2. Materials and Methods
The transcriptional activity of genes associated with inflammatory processes in colorectal cancer cells was determined in the HT-29 cell line. The methodological part consisted of several stages. In the first stage, a culture of colorectal cancer cells (HT-29) and normal fibroblasts (NHDF) was maintained and the cytotoxicity of betulin and its derivatives (1–3) or cisplatin as a reference compound was evaluated. The lipophilicity of the compounds was also determined to assess their physicochemical parameters. The next stage of molecular analysis included the isolation of total RNA and the determination of the transcriptome in cells treated with betulin and its derivatives using oligonucleotide microarray technology.
2.1. Compound Synthesis
Betulin (purity 98%) purchased from Sigma Aldrich (Poznań, Poland) was used as a starting compound in the synthesis of derivatives 1, 2, and betulonic acid. 28-O-Propynoylbetulin 1 was obtained by introducing a solution of DCC (N,N’-dicyclohexylcarbodiimide, 0.23 g, 1.12 mmol) and DMAP (4-dimethylaminopyridine, 0.01 g, 0.08 mmol) in methylene chloride (1 mL) to a mixture of betulin (0.44 g, 1 mmol) and propynoic acid (0.08 g, 1.10 mmol) in a methylene chloride (CH2Cl2, 5 mL) (Figure 2). The course of the reaction was monitored using thin-layer chromatography (TLC). The reaction was conducted at −10 °C for 5 h, then at room temperature for 24 h until the disappearance of betulin.
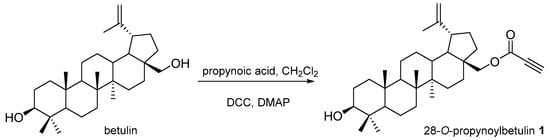
Figure 2.
Synthesis scheme of 28-O-propynoilobetulin 1.
28-O-Propargyloxycarbonylobetulin 2 was obtained by adding a solution of propargyl chloroformate (0.29 mL, 3 mmol) in chloroform (5 mL) to a mixture of betulin (0.44 g, 1 mmol) in benzene (6 mL) and pyridine (2.5 mL) (Figure 3). The course of the reaction was monitored using thin-layer chromatography (TLC). The reaction was conducted for 4 h so that the temperature of the mixture did not rise above 5 °C. After this time, the reaction mixture was stirred at room temperature for another 24 h until the disappearance of betulin.
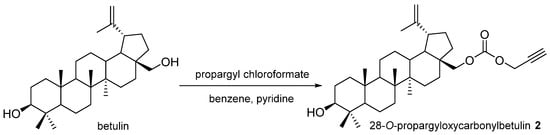
Figure 3.
Synthesis scheme of 28-O-propargyloxycarbonylobetulin 2.
Crude reaction products were purified by gel column chromatography (SiO2, chloroform/ethanol, 20:1, v/v, and chloroform/ethanol, 40:1, v/v for compounds 1 and 2, respectively). The spectroscopic data (1H NMR and 13C NMR) (Bruker AVANCE III HD 600, Billerica, MA, USA, deuterated chloroform) and melting points (Electrothermal IA 9300, Bibby Scientific Limited, Stone, Southampton, UK) for alkynyl derivatives 1 and 2 were consistent with the previously described literature values [4].
In the reaction of betulonic acid (0.15 g, 0.33 mmol) with 4-bromobut-1-yne (0.065 mL, 0.70 mmol) in the presence of potassium carbonate (K2CO3, 0.14 g, 1 mmol) in a dimethylformamide (DMF, 2.3 mL), an ester derivative of betulonic acid was obtained that was then subjected to a reduction reaction using sodium borohydride (NaBH4, 0.40 g, 1,10 mmol) in tetrahydrofuran (THF, 10 mL) to a derivative of betulinic acid 3 (Figure 4).
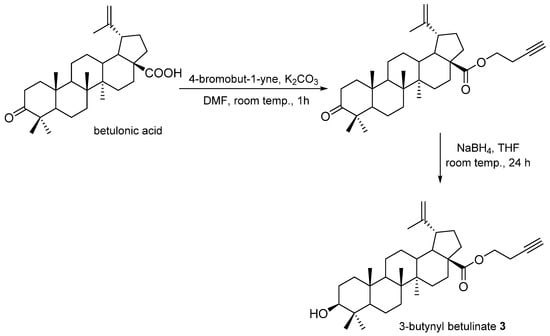
Figure 4.
Synthesis scheme of 3-butynyl betulinate 3.
The crude reaction product was purified by gel column chromatography (SiO2, chloroform/ethanol, 40:1, v/v). The spectroscopic data (1H NMR and 13C NMR) of compound 3 were consistent with the literature information [30]. The spectroscopic data for tested compounds 1, 2, and 3 are shown in the Supplementary Materials (Table S1).
2.2. Cell Lines
HT-29 is a human colon adenocarcinoma cell line. They represent a valuable model due to their similarities with enterocytes of the small intestine. They have also been frequently used to study the intestinal immune response to various factors. Comparative studies on other colorectal cancer cell lines are also planned.
The culture of HT-29 ((85061109) human Caucasian colon carcinoma, Homo sapiens, human, Sigma-Aldrich, St. Louis, MO, USA) and NHDF ((CC-2511), normal human dermal fibroblasts, Homo sapiens, human, Lonza Group Ltd., Basel, Switzerland]) cells was maintained in 25 mL culture vessels with a Nunclon surface and equipped with bacterial filters (Nunc, Wiesbaden, Germany). The HT-29 cell line was cultured in McCoy’s 5A medium (Sigma Aldrich) enriched with L-glutamine and gentamicin. Additionally, the medium was enriched with 10% FBS for 24 h after cell passage. Cells from the 4th to the 6th passage were used for the experiments.
NHDF cells were cultured in FBM (Fibroblast Basal Medium; Lonza, Basel, Switzerland), enriched with FGF-B (fibroblast growth factor, Fibroblast Growth Factor-basic), L-glutamine, and gentamicin (FGM-2 TM Single Quots TM; Lonza, Basel, Switzerland). Additionally, the medium was enriched with 2% FBS for 24 h after cell passage. Cells from the 4th to the 6th passage were used for the experiments. Experiments were conducted during the logarithmic phase of cell growth and at a viability of ≥98%.
2.3. Cytotoxicity
The cytotoxicity of betulin and its alkynyl derivatives, as well as cisplatin, was measured using the In Vitro Toxicology Assay Kit, Sulforhodamine B based (TOX6, Sigma- Aldrich, St. Louis, MO, USA) according to the manufacturer’s recommendations. This test measures cell growth inhibition using the dye sulforhodamine B. Sulforhodamine B is an anionic dye that binds to the essential amino acids of cell proteins. The determination of cytotoxic activity is carried out by measuring the amount of cellular protein.
The two cell lines, HT-29 and NHDF, were passaged into 96-well plates (Nunc, Wiesbaden, Germany) at a density of approximately 6 × 103/well and incubated 24 h before the addition of the test factors. The cells were then incubated in a medium containing test compounds at a concentration of 0.1, 1, 10, or 100 μg/mL for 72 h. After 72 h, an assay was performed using the sulforhodamine B dye. Absorbance was measured at 565 nm using an MRX Revelation microplate reader (Dynex Technologies, Chantilly, VA, USA).
The derivatives of betulin and betulin were dissolved in 10% DMSO to a concentration of 1 mg/mL and next diluted in culture medium to reach the required concentrations. DMSO as a solvent did not exert any inhibitory effect on cell proliferation. The compounds in given concentrations were examined in triplicate for each experiment, which was repeated 3–5 times. The results of in vitro cytotoxic activity are expressed as an IC50 value in µg/mL. In the test, controls were untreated cells that were cultured under the same conditions. Cisplatin was used as the reference compound.
The results of the in vitro cytotoxic activity were expressed as an IC50 value (μg/mL), i.e., the concentration of a compound that inhibits the proliferation of 50% of tumor cells as compared with the control untreated cells.
2.4. Lipophilicity
RP-18F254S plates (10 × 10 cm) for lipophilicity determination were prepared by applying a starting line 1 cm from the bottom edge of the plate with a 1 cm gap from the right and left edges. The same amount of chloroform solutions of the tested substances was applied at equal distances along the starting line. The developing system used for the determinations was acetone: Tris buffer at pH 7.4. The determinations were performed in the following concentration ranges: 90%, 85%, 80%, 75%, 70%, 65%, and 60% acetone. The plates were dried under a fume hood. Then, chromatograms were visualized by spraying the plates with a solution of 10% concentrated sulfuric acid in ethanol and then heated up to 100 °C. Based on the available literature formulas, the partition coefficient (Rf), retention (RM), and normalized retention coefficient (RM0) values were calculated for the tested alkynyl derivatives of betulin. The experimental lipophilicity was also determined for the reference substances acetanilide, 4-bromoacetophenone, benzophenone, anthracene, and DDT. Then, based on the obtained values and the literature log P values a standard curve was made. The equations for calculating Rf, RM, and RM0 and received values are shown in the Supplementary Materials (Ad.2; Table S2).
2.5. Transcriptome Determination Using Oligonucleotide Microarray Technology
Total RNA isolation from the HT-29 and NHDF cell lines was performed using TRIzol® reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. To eliminate any potential genomic DNA contamination, total RNA extracts were treated with DNase I (MBI Fermentas, Vilnius, Lithuania) on RNeasy Mini Kit columns (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The quality of the extracts was evaluated by agarose gel electrophoresis using a 0.8% agarose gel stained with ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA). The concentration and purity of nucleic acid were determined using the GeneQuant II RNA/DNA spectrophotometer (Pharmacia Biotech, Cambridge, UK). The analysis of gene expression profiles in colorectal cancer cells treated with betulin and its derivatives was performed using HG-U133A oligonucleotide microarrays (Affymetrix, Santa Clara, CA) according to the manufacturer’s instructions. Based on literature data and the Affymetrix NetAffx TM Analysis Center database (http://www.affymetrix.com/analysis/index.affx; accessed on 31 August 2019), 973 mRNA gene IDs related to inflammatory processes that can be analyzed using HG-U133A microarray plates were selected. The criterion for considering a gene as differentiating required the absolute fold change (FC) value, which is the multiple of the fluorescence signal difference between the compared samples, to be greater than 1.1 (minimum 1.1-fold increase or decrease in signal intensity) and the significance level to be p < 0.05.
2.6. Statistical analysis
The analysis of the cytotoxicity results used Statistica 13.1 (StatSoft).
The analysis of the results obtained using the oligonucleotide microarray technology was carried out using the MicroArray Suite 5.0 and Data Mining Tool (Affymetrix, Santa Clara, CA) programs. The normalization of the results using the robust multi-array average (RMA) method, which involves logarithmic transformation of fluorescence signal values for each transcript (log2), was performed using the RMA Express program. Statistical analysis was performed using the PL-Grid Infrastructure (http://www.plgrid.pl/; accessed on 31 August 2019) with GeneSpring 12.0 software (Agilent Technologies UK Limited, South Queensferry, UK) and the PANTHER program (Protein ANalysis THrough Evolutionary Relationships). The samples were grouped using the hierarchical clustering method, using the measure of urban distance.
Based on the literature data and the Affymetrix NetAffx TM Analysis Center database (http://www.affymetrix.com/analysis/index.affx; accessed on 31 August 2019), 973 mRNA IDs of genes related to inflammatory processes that can be analyzed using HG-U133A microarray plates were selected. The criterion for considering a gene as differentiating required the absolute fold change (FC) value, which is the multiple of the fluorescence signal difference between the compared samples, to be greater than 1.1 (minimum 1.1-fold increase or decrease in signal intensity) and the significance level to be p < 0.05.
3. Results
3.1. Cytotoxicity Evaluation
The obtained cytotoxicity values are presented in Table 1. The viability analysis results for HT-29 and NHDF cells treated with the analyzed compounds at concentrations of 0.1 μg/mL, 1 μg/mL, 10 μg/mL, and 100 μg/mL, relative to control cells (100%), are shown in Figures S1–S5 in the Supplementary Materials.

Table 1.
Comparison of IC50 values for HT-29 and NHDF cell lines treated with betulin, its alkynyl derivatives, and cisplatin; mean value (µg/mL) ± standard deviation.
Betulin exhibited significant cytotoxic activity at low concentrations in the colorectal cancer cell line (HT-29). In comparison with normal fibroblast cells (NHDF), even at a concentration of 100 μg/mL betulin did not cause a 50% decrease in cell viability.
28-O-Propynoylbetulin 1 showed significant cytotoxicity against the cancer cell line HT-29 only at a concentration of 10 μg/mL, lower concentrations did not reduce cell proliferation. However, for the NHDF cell line, a concentration of 1 μg/mL of 28-O-propynoylbetulin 1 exhibited significant cytotoxicity. 28-O-Propargyloxycarbonylbetulin 2 showed the lowest cytotoxicity against NHDF cells, significantly reducing the number of cancer cells in the tested samples at concentrations of 10 μg/mL and 100 μg/mL. 3-Butynyl betulinate 3 showed noticeable cytotoxic activity only when cells were exposed to a concentration of 10 μg/mL.
3.2. Lipophilicity
Lipophilicity parameters were determined using reversed-phase thin-layer chromatography (RP-TLC). The stationary phase consisted of a nonpolar silicone oil deposited on a silica-gel-coated plate (RP-18F254S plates from Merck). The mobile phase was a mixture of acetone and an aqueous Tris buffer solution (0.2 M) at pH 7.4 in the following acetone concentration ratios: 90:10, 85:15, 80:20, 75:25, 70:30, 65:35, and 60:40. The first step was to calculate the retardation factor (Rf). Based on the obtained Rf values, the RM parameter values were calculated, which is a chromatographic parameter indicating lipophilicity. The relationship between RM values and acetone concentration in the mobile phase showed a linear character with a high correlation coefficient, allowing for the determination of the RM0 parameter. To determine the lipophilicity parameter log PTLC in the studied system, a calibration curve was necessary. For this purpose, reference standards such as acetanilide, 4-bromoacetophenone, benzophenone, anthracene, and DDT were selected, whose log P values in the literature ranged from 1.21 to 6.38. The obtained calibration curve (Figure 5) exhibited a high correlation coefficient, which enabled the calculation of the log PTLC values for the tested alkynyl derivatives of betulin based on its equation.
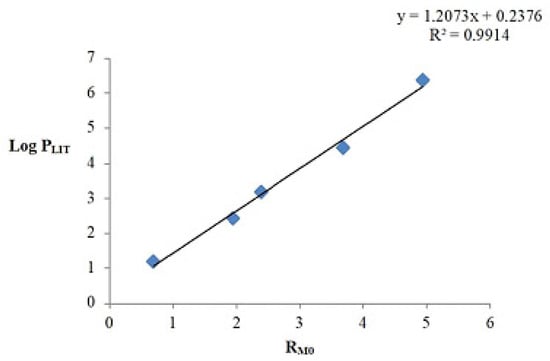
Figure 5.
Standard curve for the acetone system: Tris buffer.
The values of the obtained RM0 and log PTLC parameters for the tested compounds are summarized in Table 2.

Table 2.
Values of RM0 and log PTLC parameters for compounds 1–3 in the acetonitrile: Tris buffer system.
Figure 6 shows the correlation between the experimentally determined lipophilicity parameter log PTLC and the biological activity of the tested compounds.
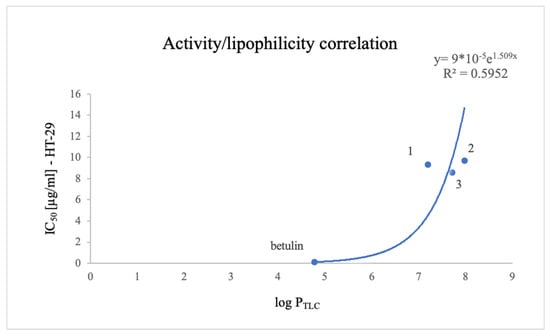
Figure 6.
Correlation between the cytotoxic activity of the tested compounds on HT-29 cells and the experimentally determined lipophilicity parameter log PTLC.
Despite the low correlation coefficient, it was observed that a lower lipophilicity may be associated with a higher biological activity for the compounds.
3.3. Evaluation of Changes in the Gene Expression Profile of Proteins Involved in Inflammatory Processes in Colorectal Cancer Cells
For the subsequent stage of the study, HT-29 cells were seeded in 6-well plates and incubated for 72 h in the presence of betulin, its derivatives, and cisplatin at a concentration corresponding to the experimentally determined IC25 value. The total RNA necessary for the microarray experiment was then extracted.
To determine the expression profile, among the 22,283 probes present on the HG- U133A microarray, those that determine the expression of genes involved in inflammatory processes (973 mRNA IDs) were selected for transcriptome analysis. The names of the corresponding probes and the information on the biological function of the genes were obtained from the Affymetrix NetAffxTM Analysis Center (http://www.affymetrix.com/analysis/index.affx; accessed on 31 August 2019). A sample quality control was performed using a 3D PCA (3D principal component analysis) test, analysis of normalized fluorescence signal values for hybridization control probes, and 3′/5′ ratios for internal control probes. The aim of the analysis was to identify differences and similarities in the expression levels of the selected genes. The set of transcripts of genes involved in inflammatory processes, which included numerical fluorescence signal values for the modified gene expression due to exposure to the tested compounds, was analyzed to find genes with the highest FC values compared with control samples and a significance level of p < 0.05. Comparisons were made between the following groups:
- HT-29 cells treated with betulin vs. control NHDF cells;
- HT-29 cells treated with derivative 1 vs. control NHDF cells;
- HT-29 cells treated with derivative 2 vs. control NHDF cells;
- HT-29 cells treated with derivative 3 vs. control NHDF cells;
- HT-29 cells treated with cisplatin vs. control NHDF cells.
In the first stage, a cluster analysis was applied. The analyzed transcriptomes were clustered using hierarchical clustering with the Euclidean distance measure.
Clustering (Figure 7) allowed for the comparison of changes in the expression of genes associated with inflammation in the individual analyzed groups. The group subjected to betulin treatment showed the highest similarity to the reference compound cisplatin in terms of changes in gene expression. A clear similarity in the level of gene expression was observed between the group treated with derivative 1 and the control group. The level of expression of the analyzed set of genes associated with inflammatory processes in cells exposed to derivatives 2 and 3 was different but most similar to the betulin and cisplatin groups.
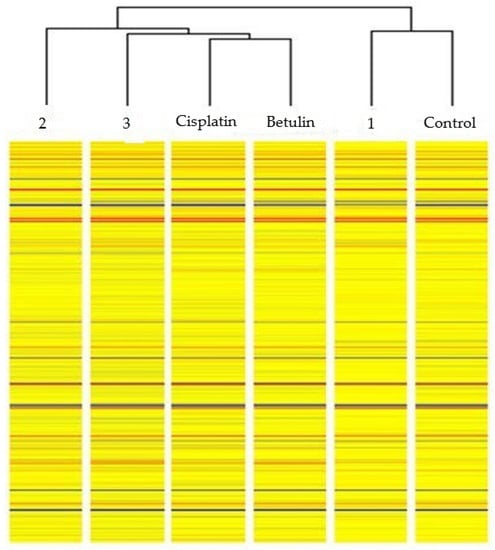
Figure 7.
Hierarchical clustering of the analyzed transcriptomes based on the expression profile of genes involved in inflammatory processes in HT-29 cell line.
The next step was to perform a sample correlation analysis (Figure 8). It revealed the strongest correlation between changes in the gene expression profile in samples exposed to betulin and cisplatin. The largest differences were observed between the control group and the changes induced by derivative 2.
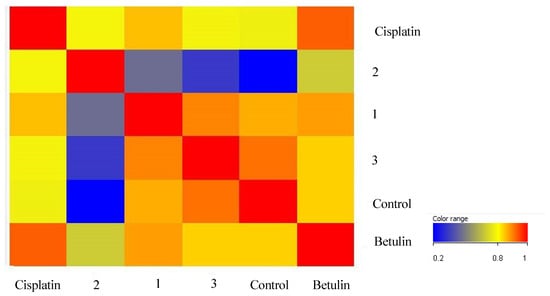
Figure 8.
Sample correlation analysis based on the expression profile of genes involved in inflammatory processes in the HT-29 cell line.
The next step in the comparative analysis of the transcriptome groups was to assess the statistical significance of the observed differences in the mRNA concentration profiles between the control groups and those exposed to betulin derivatives. The one-way analysis of variance (ANOVA) test was used to determine the set of genes that differentiated the compound-treated cells from the control cells. One-way ANOVA, with a significance threshold of p < 0.05, identified 68 mRNA probes associated with inflammatory processes that showed significantly altered transcriptional activity compared with the control group (Table 3).

Table 3.
One-way analysis of variance (ANOVA) for the 973 selected mRNA probes associated with inflammatory processes in the HT-29 cell line, number of differentiating mRNA probes in the transcriptome groups treated with betulin, compounds 1, 2, or 3, and the control group, depending on the chosen criteria of differentiation strength.
In the subsequent statistical analysis, a post-hoc test—Tukey’s test—was performed to indicate the number of representative mRNA probes differentiating between the transcriptome groups (Table 4).

Table 4.
Results of the post-hoc multiple comparisons test—Tukey’s test—indicating the number of representative differentiating mRNA probes between the transcriptome groups for the HT-29 cell line.
The results obtained indicate that the largest number of differentiating mRNA probes was found in the group treated with compound 2 (18 mRNA probes). Slightly fewer probes, specifically 14 and 10 mRNA probes, were identified for the groups treated with compound 3 and cisplatin, respectively. The lowest number of differentiating mRNA probes from the control group was found for betulin. The mRNA probes identified for each group are listed in Table 5.

Table 5.
List of representative mRNA probes differentiating between the transcriptome groups for the HT-29 cell line that were identified using the post-hoc multiple comparisons test—Tukey’s test.
The greatest expression changes (FC > 2) were observed for the HMOX1 and TMED7 mRNA IDs. In the case of cells treated with betulin, using the criteria FC > 1.1 and p < 0.05, the number of differentiating mRNA IDs was five. Among these genes, three showed increased expression and two showed reduced expression. For cells exposed to derivative 1, the number of differentiating genes was six, with four IDs showing reduced expression and two showing increased expression. Among the 18 mRNA IDs identified as differentiating the group treated with derivative 2 from the control group, 8 IDs showed increased expression, whereas the remaining 10 IDs showed decreased expression. In cells treated with derivative 3, 14 differentiating mRNA IDs were identified. Among these, seven IDs showed downregulation and seven IDs showed upregulation. In the cisplatin-treated group, out of 10 mRNA IDs, the majority (7 IDs) showed decreased expression.
The next step of the statistical analysis was to perform an overrepresentation test to determine the biological processes associated with inflammatory processes, assuming that the calculated binomial test p-value was less than 0.05.
Table 6 summarizes several biological processes involving the differentiating genes in the analyzed groups compared with the control. The most prominent differences are observed in the group of genes involved in immune cell chemotaxis, followed by genes responsible for the defense response of the immune system. The other processes show similar levels of involvement.

Table 6.
Biological processes involving the genes associated with inflammatory processes differentiating HT-29 colorectal adenocarcinoma cells depending on the used chemical compound, determined using the PANTHER program based on the binomial test analysis with p < 0.05.
4. Discussion
Carcinogenesis in non-specific inflammatory bowel diseases is associated with multiple processes contributing to DNA damage, apoptosis, and the increased proliferation of cancer cells. Chronic inflammatory conditions promote the formation of a pro-tumor microenvironment that supports tumor growth, cell migration, and neoangiogenesis. Many factors, immune cells, and the cytokines and chemokines released by them are involved in this process. Inflammatory cytokines play a key role in tumor progression through multiple signaling pathways and direct effects on tumor cells, interactions with the chemokine system, promotion of epithelial–mesenchymal transition, and enhancement of metastasis. Cytokines and pro-inflammatory mediators secreted into the tumor microenvironment contribute to sustained inflammation and tumor progression by regulating transcription factors, which in turn influence the synthesis of their protein targets, thereby affecting the functions of both colorectal epithelial cells and the immune system [31].
One of the major challenges in the successful targeted treatment of colorectal cancer is avoiding drug absorption and degradation in the upper gastrointestinal tract. Additionally, it is crucial to develop drugs that exhibit high bioavailability and low toxicity towards normal cells. In the case of colorectal cancer, a dual action of antitumor and immunomodulatory effects would be ideal, inhibiting the proliferation of cancer cells by inducing apoptosis and modulating the immune system and tumor microenvironment that promote tumor progression [32].
Betulin is a compound that demonstrates multifunctionality. It possesses a range of biological properties, including anti-inflammatory and anticancer, as well as antioxidant, hepatoprotective, antiviral, and antiparasitic activities. New derivatives with different chemical modifications are synthesized to achieve compounds with improved biological activity. These derivatives were synthesized at the Department of Organic Chemistry of the Medical University of Silesia in Katowice. The derivatives were: 28-O-propynoyloxybetulin 1, 28-O-propargyloxycarbonyloxybetulin 2, and 3-butynyl betulinate. Compounds 1 and 3 were tested by Boryczka et al. for cytotoxic activity against five cell lines. The monoester derivative of betulin 2 was found to be 3–4 times more active than betulin itself on all tested cell lines (T47D—breast cancer, CCRF/CEM—lymphocytic leukemia, SW707—colorectal cancer, P388—mouse leukemia, and Balb3T3—mouse fibroblasts). Compound 1 exhibited very high cytotoxic activity against the lymphocytic leukemia cell line (IC50 = 0.02 μg/mL) and slightly lower activity against the breast cancer cell line T47D [4]. Compounds 1 and 2 were also tested for antitumor activity on the G-361 melanoma line. Compound 1 showed the highest activity, inducing apoptosis more than twice that of the reference betulin by activating caspase 3 [30,33]. An experiment evaluating the activity of compound 1 was conducted on the C32 melanoma cell line and amelanotic melanoma cell line A2058. A higher activity was observed on the A2058 line [33]. The same derivative 1, with a propynoyl group at position C28, exhibited strong activity against human promyelocytic leukemia cell line HL-60 that was over 24 times higher than betulin [34]. Similar results were obtained in a study involving the endometrial cancer cell line. 28-O-Propynoylbetulin 1 showed activity similar to cisplatin. It increased NF-κB p65 expression, increased caspase 3 concentration, and inhibited collagen synthesis in endometrial cancer cells [35].
In the first stage of the conducted research, the cytotoxicity of alkynyl derivatives of betulin was tested using sulforhodamine B dye. Among the tested compounds, betulin (IC50 = 0.1 µg/mL) and compound 3 (IC50 = 8.57 µg/mL) showed the most antiproliferative activity against the HT-29 cell line. The lipophilicity of the compounds was also considered, which is important for bioavailability [36]. All compounds had a logarithm of the partition coefficient (log P) higher than five, except for betulin, which had an experimentally determined log P value below five. Therefore, the tested compounds are more suitable for local application.
The main stage of the experiment was the evaluation of changes in the expression of genes associated with inflammatory processes in HT-29 colorectal adenocarcinoma cells treated with betulin and its alkynyl derivatives 1–3.
We selected representative genes for which the significance parameter was p < 0.05 and the fold-change parameter was FC > 1.1, as a threshold to differentiate genes with altered and unaltered expression. In the HT-29 cell line, we observed an increase in fluorescence signal for three mRNA IDs and a decrease in the fluorescence signal for two mRNA IDs concerning betulin. We identified differential expression of six mRNA IDs compared with control cells when HT-29 cells were treated with compound 1. We observed a significant decrease in the fluorescence signal for the ICAM1 mRNA ID, signifying a reduction in the expression of this gene. In the group of cells treated with compound 2, the expression of 18 mRNA IDs altered significantly in the studied cell line. Compound 3 caused alterations in the expression of 13 mRNA IDs. The final group comprised cells that were exposed to cisplatin, which was used as a reference compound. Its activity caused changes in the expression of 10 mRNA IDs.
In the groups treated with derivatives 2 and 3, betulin, and cisplatin, a single mutual CHUK mRNA ID was selected, demonstrating increased expression in all groups in comparison with the control. For the group treated with substance 1, two mRNA IDs were exclusive to this group: HMOX1 and CXCR4. The expression of HMOX1 mRNA is stimulated by a variety of molecular and physical signals. The significance of HMOX1 in the pathogenesis of colorectal cancer remains unclear. These pathways are activated by the biologically active catabolites of heme degradation and promote disease progression through anti-apoptotic, antioxidant, and anti-inflammatory effects in addition to proangiogenic and prometastatic effects. There are instances in the literature where the protein encoded by HMOX plays an opposing anti-tumoral role. However, it is assumed that HMOX1 acts as a conditional tumor promoter in the pathogenesis of colorectal cancer [37,38].
The C-X-C chemokine receptor 4 (CXCR4) has been suggested to play an important role in several types of cancer and has been implicated in biological behaviors associated with tumor progression. In human pancreatic ductal adenocarcinoma and colorectal cancer, CXCR4 signaling can suppress the immune system and impair the function of the chemokine receptors that mediate the intratumoral accumulation of immune cells [39,40].
Three ID mRNAs were selected as specific for cells incubated with betulin (CD59, DARC, and CEBPB) and one ID mRNA (IL25) was downregulated by both betulin and compound 2. CD59 encodes a glycoprotein on the cell surface that regulates cell lysis mediated via the complement system and is involved in lymphocyte signal transduction. CD59 additionally regulates the function, infiltration, and phenotypes of various immune cells in the tumor microenvironment. Increased CD59 expression on malignant colonic cells may protect against complement-mediated damage, thereby enabling higher local invasiveness and metastasizing potential. An increased level is a disadvantageous treatment factor [41,42].
The role of DARC in accelerated inflammation and malignant transformation remains understudied. Multiple studies show that the expression of DARC is downregulated in colorectal cancer and is associated with the clinical and pathological characteristics of this disease [43,44].
IL-25 might play a multifarious role in cancer progression or regression. Although IL-25 inhibits tumor growth in some cases, it has been shown in several studies that IL-25 also plays a crucial role in cancer progression. The tumor-suppressive role of this interleukin is mainly correlated with the infiltration of eosinophils and B cells into the tumor microenvironment and induction of apoptosis. In contrast, its tumor-supportive roles rely on the deviation of immune responses and cell growth [45].
Ten ID mRNAs are specific only for a group of genes associated with inflammatory processes after the action of compound 2, which differentiates them from the others. Reduced expression of the ICAM1 gene was observed for three of the study groups: 3 vs. control, 2 vs. control, and 1 vs. control. Intracellular adhesion molecule-1 (ICAM-1) is a potential therapeutic target to enhance therapeutic effectiveness for colorectal cancer. Recent studies have shown that it promotes malignancy in several carcinomas. ICAM-1 may further accelerate SRC signaling, promoting the malignant potential of cancer, so it can be considered as a novel therapeutic anticancer target [46].
In the group with the third derivative vs. control, seven mRNA IDs were isolated specific to this group.
In the overrepresentation analysis, nine mRNA IDs related to oxidative stress response, antimicrobial response, immune defense, chemotaxis, and cytokine signaling pathway were selected. Among them was the CHUK gene encoding the NF-κB kinase inhibitor. The role of the NF-κB pathway has been confirmed in the development of many cancers. It is activated by various stimuli, including pro-inflammatory cytokines, bacterial and viral products, DNA damage, and oxidative stress. It activates the transcription of hundreds of genes involved in immune response, growth control, and protection against apoptosis. It is also responsible for chemotherapy and radiotherapy resistance. The increased expression of the NF-κB inhibitor observed under the action of betulin and compounds 2 and 3 will prevent the activation of NF-κB molecules and their translocation into the cell nucleus. This is a confirmed direction of the actions in colorectal cancer therapy [47]. Another gene highlighted in the overrepresentation analysis was MAP2K3. It encodes the protein kinase mitogen-activated protein kinase 3, belonging to the MAP kinase family. Activation of MAP kinases leads to cell proliferation and survival. Many studies emphasize the role of MAP2K3 in tumor progression and invasiveness, indicating it as a promising target for anticancer therapy. Silencing this gene reduces the viability of cancer cells without affecting normal cells. Moreover, it reduces tumor growth and improves the biological response to chemotherapy agents [48]. In the presented study, in the microarray experiment, a decrease in mRNA expression of MAP2K3 was observed in HT-29 cells subjected to the action of compound 2 and cisplatin.
The analysis of individual changes does not allow us to clearly determine the effect of individual derivatives on colorectal adenocarcinoma cells of the HT-29 cell line. However, it shows the differences in the changes that occur under the influence of individual alterations in the chemical structure.
5. Conclusions
The analysis of all the changes in the expression of genes associated with inflammatory processes that are induced by betulin and its derivatives 1–3 in a colorectal adenocarcinoma cell line leads to the conclusion that these compounds significantly affect the examined gene profile. The number of observed changes depends on the used compound. Each compound exerts its specific influence on different groups of genes. However, the direction of the changes seems to be consistent in most analyzed alterations. The identified changes in gene expression confirm the prospective application of betulin and its derivatives 1–3 in the therapy of colorectal adenocarcinoma due to their potential anticancer effects and influence on inflammatory processes. Undoubtedly, an appropriate step in selecting anticancer therapy should be to supplement surgical–morphological assessment with an evaluation of the molecular phenotype of the tumor undergoing treatment. This would allow for the selection of appropriate procedures and cytostatics for the disease, increasing treatment effectiveness and reducing the side effects of therapy. The physicochemical parameters of the tested compounds also make them suitable for use as drugs; however, due to their high lipophilicity, they are recommended for local treatment. Moreover, further research and analysis are needed to understand the mechanism of action of betulin and its derivatives 1–3 in relation to inflammation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr11092676/s1, Table S1. The spectroscopic data for tested compounds 1, 2 and 3; Table S2. RM0, b, R parameters for betulin and compounds 1–3 in acetone: Tris buffer aqueous solution; Figure S1. Viability of HT-29, NHDF after exposure to betulin at concentrations of 0.1 μg/mL; 1 μg/mL; 10 μg/mL; 100 μg/mL compared to control cells (0 μM). The absorbance value obtained in control cell cultures was taken as 100%. Cell survival is shown as a percentage of control values ± standard deviation of % cell growth. ANOVA, post hoc Tukey; Figure S2. Viability of HT-29, NHDF after exposure to 28-O-propynoylbetulin 1 at concentrations of 0.1 μg/mL; 1 μg/mL; 10 μg/mL; 100 μg/mL compared to control cells (0 μM). The absorbance value obtained in control cell cultures was taken as 100%. Cell survival is shown as a percentage of control values ± standard deviation of % cell growth. ANOVA, post hoc Tukey; Figure S3. Viability of HT-29, NHDF after exposure to 28-O-propargyloxycarbonylbetulin 2 at concentrations of 0.1 μg/mL; 1 μg/mL; 10 μg/mL; 100 μg/mL compared to control cells (0 μM). The absorbance value obtained in control cell cultures was taken as 100%. Cell survival is shown as a percentage of control values ± standard deviation of % cell growth. ANOVA, post hoc Tukey; Figure S4. Viability of HT-29, NHDF after exposure to betulinic acid 3-butynyl ester 3 at concentrations of 0.1 μg/mL; 1 μg/mL; 10 μg/mL; 100 μg/mL compared to control cells (0 μM). The absorbance value obtained in control cell cultures was taken as 100%. Cell survival is shown as a percentage of control values ± standard deviation of % cell growth. ANOVA, post hoc Tukey; Figure S5. Viability of HT-29, NHDF after exposure to cisplatin at concentrations of 0.1 μg/mL; 1 μg/mL; 10 μg/mL; 100 μg/mL compared to control cells (0 μM). The absorbance value obtained in control cell cultures was taken as 100%. Cell survival is shown as a percentage of control values ± standard deviation of % cell growth. ANOVA, post hoc Tukey;
Author Contributions
All authors were responsible for the concept and design of the study. A.L. conceived the study, carried out the experiments, contributed to the interpretation of the results, analyzed the data, wrote the manuscript, and designed the figures; E.B. synthesized betulin derivatives, contributed to the interpretation of the results, and designed the figures; D.W.-D. and A.G. conceived and planned the experiments, verified the analytical methods, and worked on the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Medical University of Silesia.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors are grateful to Urszula Mazurek for her valuable contribution to the creation of the presented study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef]
- Król, S.K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive review on betulin as a potent anticancer agent. Biomed. Res. Int. 2015, 2015, 584189. [Google Scholar] [CrossRef]
- Alakurtti, S.; Mäkelä, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Boryczka, S.; Bębenek, E.; Wietrzyk, J.; Kempińska, K.; Jastrzębska, M.; Kusz, J.; Nowak, M. Synthesis, structure and cytotoxic activity of new acetylenic derivatives of betulin. Molecules 2013, 18, 4526–4543. [Google Scholar] [CrossRef]
- Tolstikov, G.A.; Flekhter, O.B.; Shultz, E.; Baltina, L.A.; Tolstikov, A.G. Betulin and Its Derivatives. Chemistry and Biological Activity. Chem. Sustain. Dev. 2005, 13, 1–29. [Google Scholar]
- Li, Y.; He, K.; Huang, Y.; Zheng, D.; Gao, C.; Cui, L.; Jin, Y.H. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010, 49, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Soica, C.; Ledeţi, I.; Aluaş, M.; Zupko, I.G.; Luşcan, A.; Munteanu, M. Study of the betulin enriched birch bark extracts effects on human carcinoma cells and ear inflammation. Chem. Cent. J. 2012, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Yim, N.H.; Jung, Y.P.; Kim, A.; Kim, T.; Ma, J.Y. Induction of apoptotic cell death by betulin in multidrug-resistant human renal carcinoma cells. Oncol. Rep. 2015, 34, 1058–1064. [Google Scholar] [CrossRef]
- Oh, S.H.; Choi, J.E.; Lim, S.C. Protection of betulin against cadmium-induced apoptosis in hepatoma cells. Toxicology 2006, 220, 1–12. [Google Scholar] [CrossRef]
- Yang, L.; Taiyi, S.J.; Chang, G.; Qing, L.; Ying-hua, J. Caspase-9 Activation—Critical for Betulin-induced Apoptosis of Human Hepatoma Cells. Chem. Res. Chin. Univ. 2010, 26, 792–797. [Google Scholar]
- Li, Y.; Liu, X.; Jiang, D.; Lin, Y.; Wang, Y.; Li, Q.; Jin, Y.H. Betulin induces reactive oxygen species-dependent apoptosis in human gastric cancer SGC7901 cells. Arch. Pharm. Res. 2016, 39, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, C.; Cai, Z.; Zhao, F.; He, L.; Lou, X.; Qi, X. Betulin induces cytochrome c release and apoptosis in colon cancer cells via NOXA. Oncol. Lett. 2018, 15, 7319–7327. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [CrossRef]
- Bernard, P.; Scior, T.; Didier, B.; Hiber, M.; Berthon, J.Y. Ethnopharmacology and bioinformatic combination for leads discovery: Application to phospholipase A(2) inhibitors. Phytochemistry 2001, 58, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Nennig, S.E.; Schank, J.R. The Role of NFkB in Drug Addiction: Beyond Inflammation. Alcohol Alcohol. 2017, 52, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Zhao, Q.F.; Fang, N.N.; Yu, J.G. Betulin inhibits pro-inflammatory cytokines expression through activation STAT3 signaling pathway in human cardiac cells. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 455–460. [Google Scholar]
- Wan, Y.; Jiang, S.; Lian, L.H.; Bai, T.; Cui, P.H.; Sun, X.T.; Nan, J.X. Betulinic acid and betulin ameliorate acute ethanol-induced fatty liver via TLR4 and STAT3 in vivo and in vitro. Int. Immunopharmacol. 2013, 17, 184–190. [Google Scholar] [CrossRef]
- Zatorski, H.; Sałaga, M.; Zielińska, M. Czynniki genetyczne w patogenezie, przebiegu i leczeniu nieswoistych chorób zapalnych jelit. Postepy Hig. Med. Dosw. 2015, 69, 335–344. [Google Scholar] [CrossRef]
- Laavola, M.; Haavikko, R.; Hämäläinen, M.; Leppänen, T.; Nieminen, R.; Alakurtti, S.; Moilanen, E. Betulin Derivatives Effectively Suppress Inflammation in Vitro and in Vivo. J. Nat. Prod. 2016, 79, 274–280. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Jóźwiak, K.; Szumiło, H.; Soczewiński, E. Lipofilowość, metody wyznaczania i rola w działaniu biologicznym substancji chemicznych. Wiadomości Chem. 2001, 55, 1048–1074. [Google Scholar]
- Alqahtani, S. In silico ADME-Tox modeling: Progress and prospects. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Tsopelas, F.; Giaginis, C.; Tsantili-Kakoulidou, A. Lipophilicity and biomimetic properties to support drug discovery. Expert. Opin. Drug Discov. 2017, 12, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Płużański, A. Kryteria oceny odpowiedzi na leczenie RECIST 1.1. J. Oncol. 2014, 64, 331–335. [Google Scholar] [CrossRef]
- Platts, J. Insulin therapy and cancer risk in diabetes mellitus. Clin. Med. 2010, 10, 509–512. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Protti, M.P.; De Monte, L. Cross-talk within the tumor microenvironment mediates Th2-type inflammation in pancreatic cancer. Oncoimmunology 2012, 1, 89–91. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Bębenek, E.; Chrobak, E.; Wietrzyk, J.; Kadela, M.; Chrobak, A.; Kusz, J.; Książek, M.; Jastrzębska, M.; Boryczka, S. Synthesis, structure and cytotoxic activity of acetylenic derivatives of betulonic and betulinic acids. J. Mol. Struct. 2016, 1106, 210–219. [Google Scholar] [CrossRef]
- Kaps, A.; Chodurek, E.; Orchel, A.; Jaworska-Kik, M.; Bębenek, E.; Boryczka, S.; Kasperczyk, J. Influence of 28-O-propynoylbetulin on proliferation and apoptosis of melanotic and amelanotic human melanoma cells. Postepy. Hig. Med. Dosw. 2016, 70, 1404–1408. [Google Scholar]
- Banerjee, A.; Pathak, S.; Subramanium, V.D.; Murugesan, R.; Verma, R.S. Strategies for targeted drug delivery in treatment of colon cancer: Current trends and future perspectives. Drug Discov. Today 2017, 22, 1224–1232. [Google Scholar] [CrossRef]
- Drąg-Zalesińska, M.; Wysocka, T.; Borska, S.; Drąg, M.; Poręba, M.; Choromańska, A.; Saczko, J. The new esters derivatives of betulin and betulinic acid in epidermoid squamous carcinoma treatment—In vitro studies. Biomed. Pharmacother. 2015, 72, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Bębenek, E.; Chodurek, E.; Orchel, A.; Dzierżewicz, Z.; Boryczka, S. Antiproliferative Activity of Novel Acetylenic Derivatives of Betulin against G-361 Human Melanoma Cells. Acta Pol. Pharm. 2015, 72, 699–703. [Google Scholar]
- Bębenek, E.; Kadela-Tomanek, M.; Chrobak, E.; Wietrzyk, J.; Sadowska, J.; Boryczka, S. New acetylenic derivatives of betulin and betulone, synthesis and cytotoxic activity. Med. Chem. Res. 2017, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Szoka, L.; Karna, E.; Hlebowicz-Sarat, K.; Karaszewski, J.; Boryczka, S.; Palka, J.A. Acetylenic derivative of betulin induces apoptosis in endometrial adenocarcinoma cell line. Biomed. Pharmacother. 2017, 95, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Lalani, R.; Bardoliwala, D.; Ghosh, S.; Misra, A. Lipid-Based Oral Formulation Strategies for Lipophilic Drugs. AAPS PharmSciTech 2018, 19, 3609–3630. [Google Scholar] [CrossRef] [PubMed]
- Luu Hoang, K.N.; Anstee, J.E.; Arnold, J.N. The Diverse Roles of Heme Oxygenase-1 in Tumor Progression. Front. Immunol. 2021, 12, 658315. [Google Scholar] [CrossRef]
- Gamage, S.M.K.; Nanayakkara, S.; Macfarlane, L.; Hewage, D.; Cheng, T.; Aktar, S.; Lu, C.T.; Dissabandara, L.; Islam, F.; Lam, A.K.; et al. Heme oxygenase-1 & 2 and their potential contribution in heme induced colorectal carcinogenesis. Pathol. Res. Pract. 2022, 233, 153885. [Google Scholar] [CrossRef]
- Biasci, D.; Smoragiewicz, M.; Connell, C.M.; Wang, Z.; Gao, Y.; Thaventhiran, J.E.D.; Basu, B.; Magiera, L.; Johnson, T.I.; Bax, L.; et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc. Natl. Acad. Sci. USA 2020, 117, 28960–28970. [Google Scholar] [CrossRef]
- Qiu, L.; Xu, Y.; Xu, H.; Yu, B. The clinicopathological and prognostic value of CXCR4 expression in patients with lung cancer: A meta-analysis. BMC Cancer 2022, 22, 681. [Google Scholar] [CrossRef]
- Bjørge, L.; Vedeler, C.A.; Ulvestad, E.; Matre, R. Expression and function of CD59 on colonic adenocarcinoma cells. Eur. J. Immunol. 1994, 24, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Q.; Liao, Q.; Zhao, Y. CD59: A promising target for tumor immunotherapy. Future Oncol. 2018, 14, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Jinna, N.; Rida, P.; Su, T.; Gong, Z.; Yao, S.; LaBarge, M.; Natarajan, R.; Jovanovic-Talisman, T.; Ambrosone, C.; Seewaldt, V. The DARC Side of Inflamm-Aging: Duffy Antigen Receptor for Chemokines (DARC/ACKR1) as a Potential Biomarker of Aging, Immunosenescence, and Breast Oncogenesis among High-Risk Subpopulations. Cells 2022, 11, 3818. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, M.; Hu, Y.; An, W.; Liang, X.; Yu, W.; Piao, F. Expression of Duffy antigen receptor for chemokines (DARC) is down-regulated in colorectal cancer. J. Recept. Signal Transduct. Res. 2015, 35, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Gowhari Shabgah, A.; Amir, A.; Gardanova, Z.R.; Olegovna Zekiy, A.; Thangavelu, L.; Ebrahimi Nik, M.; Ahmadi, M.; Gholizadeh Navashenaq, J. Interleukin-25: New perspective and state-of-the-art in cancer prognosis and treatment approaches. Cancer Med. 2021, 10, 5191–5202. [Google Scholar] [CrossRef]
- Lim, E.J.; Kang, J.H.; Kim, Y.J.; Kim, S.; Lee, S.J. ICAM-1 promotes cancer progression by regulating SRC activity as an adapter protein in colorectal cancer. Cell Death Dis. 2022, 13, 417. [Google Scholar] [CrossRef]
- Hassanzadeh, P. Colorectal cancer and NF-κB signaling pathway. Gastroenterol. Hepatol. Bed Bench 2011, 4, 127–132. [Google Scholar]
- Warr, N.; Siggers, P.; Carré, G.A.; Wells, S.; Greenfield, A. Genetic Analyses Reveal Functions for MAP2K3 and MAP2K6 in Mouse Testis Determination. Biol. Reprod. 2016, 94, 103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).