High Dimensional Model Representation Approach for Prediction and Optimization of the Supercritical Water Gasification System Coupled with Photothermal Energy Storage
Abstract
1. Introduction
- (1)
- The SCWG system driven using solar energy coupled with molten salt energy storage is established to solve the intermittent problem of solar energy and provide a new method for biomass hydrogen production.
- (2)
- In this paper, the HDMR model is used to predict and analyze the supercritical water gasification characteristics of five different types of biomass, and the multi-objective optimization analysis of the HDMR model is carried out.
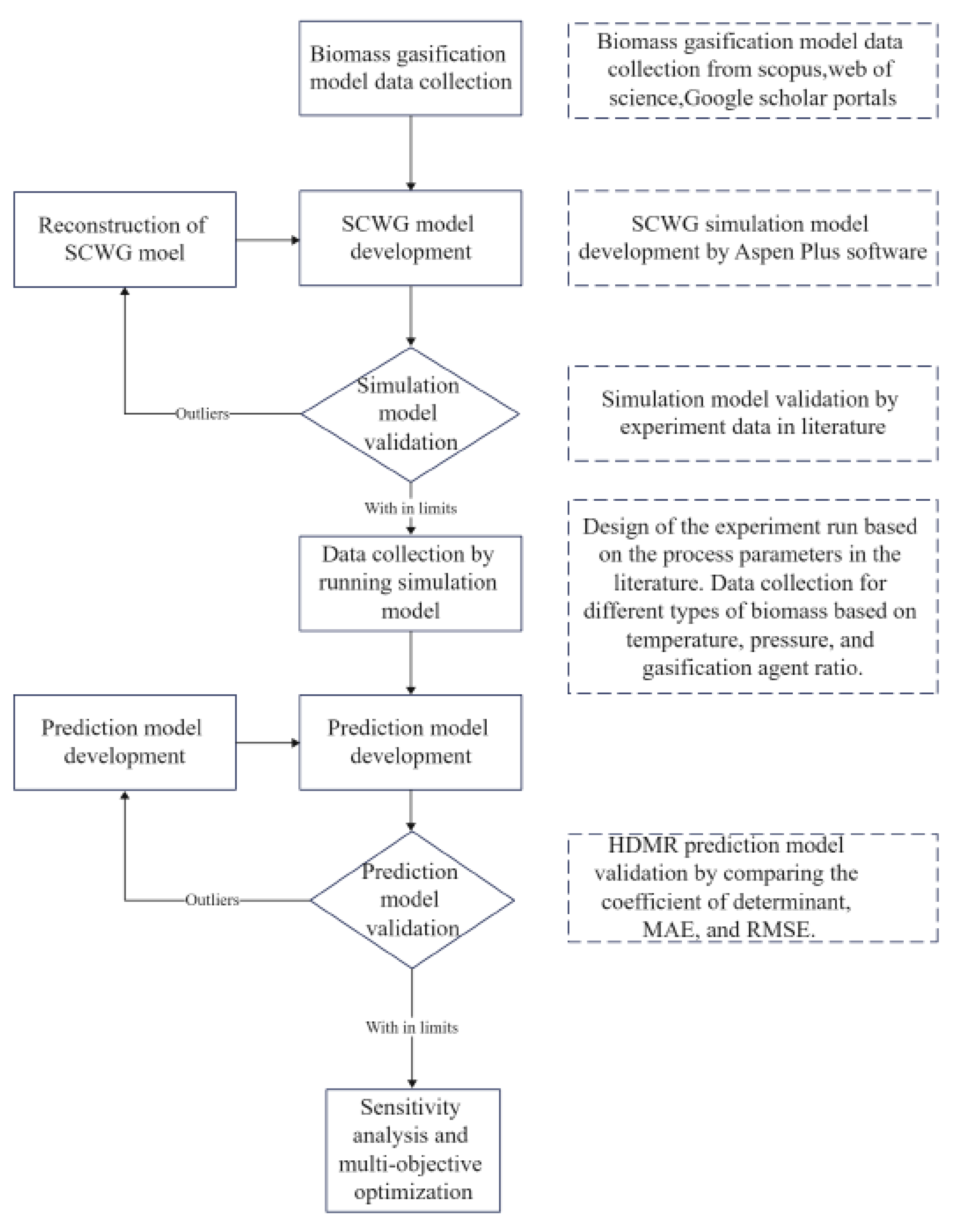
2. Methodology
2.1. Process Flow
2.2. Process Simulation Model
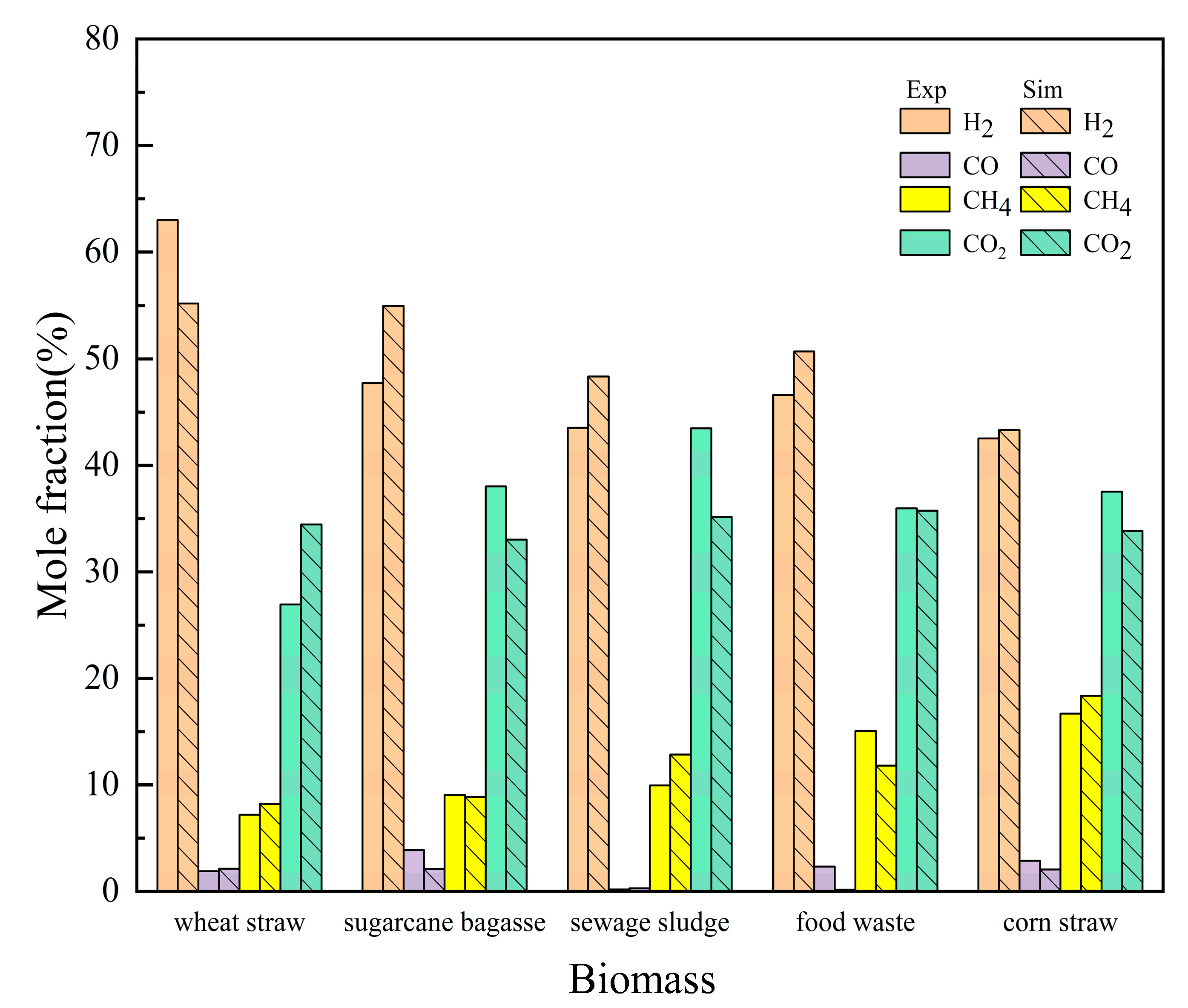
2.2.1. Validation of the Simulation Model
2.2.2. Data Collection from the Validated Simulation Models
2.3. High-Dimensional Model Representation (HDMR)
2.4. Multi-Objective Optimization
s.t. g(x) ≤ 0
h(x) = 0
3. Results and Discussions
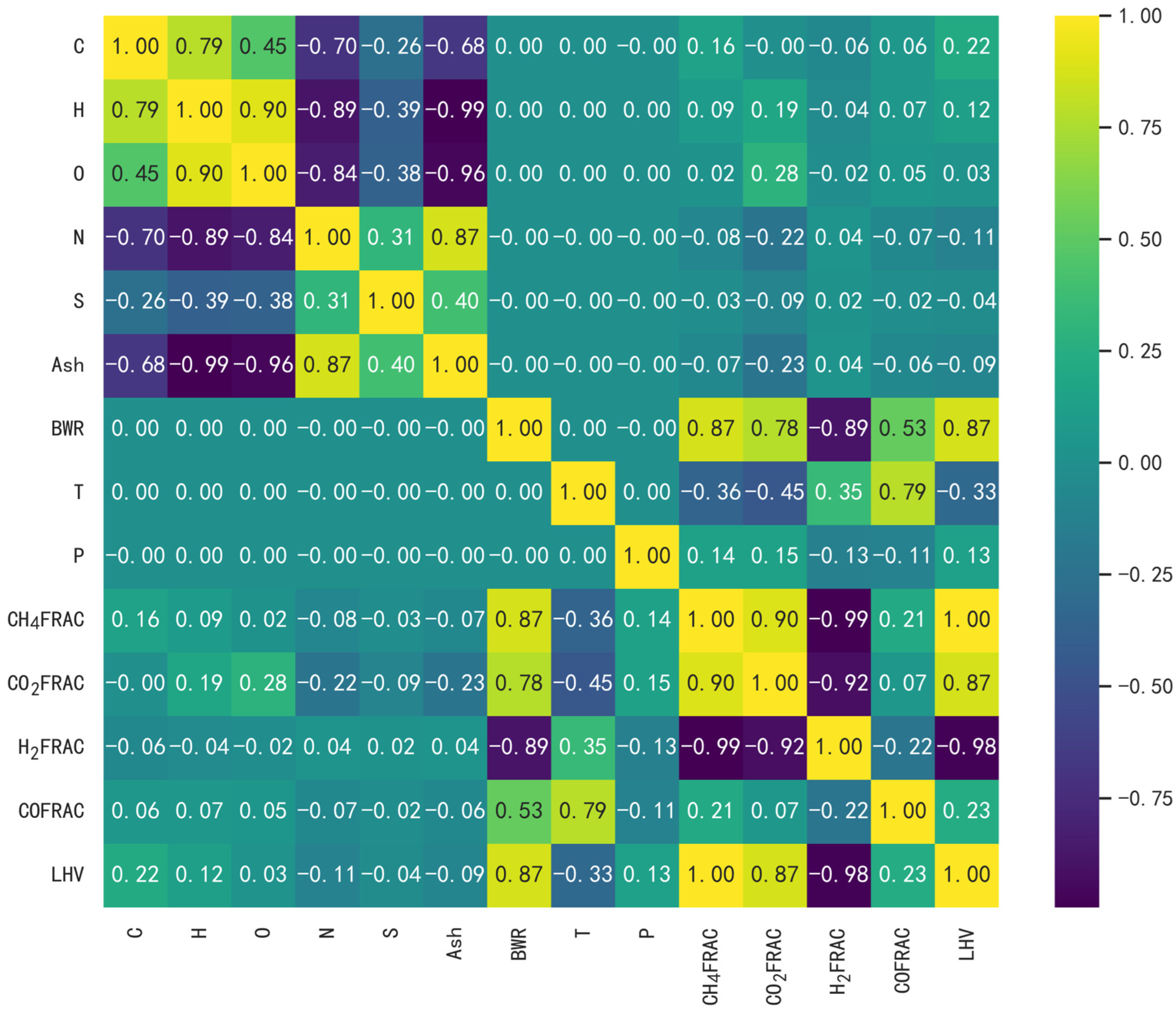
3.1. Statistical Results and Analysis of a Linear Relationship between Any Two Variables
3.2. The Performance of the Prediction Models
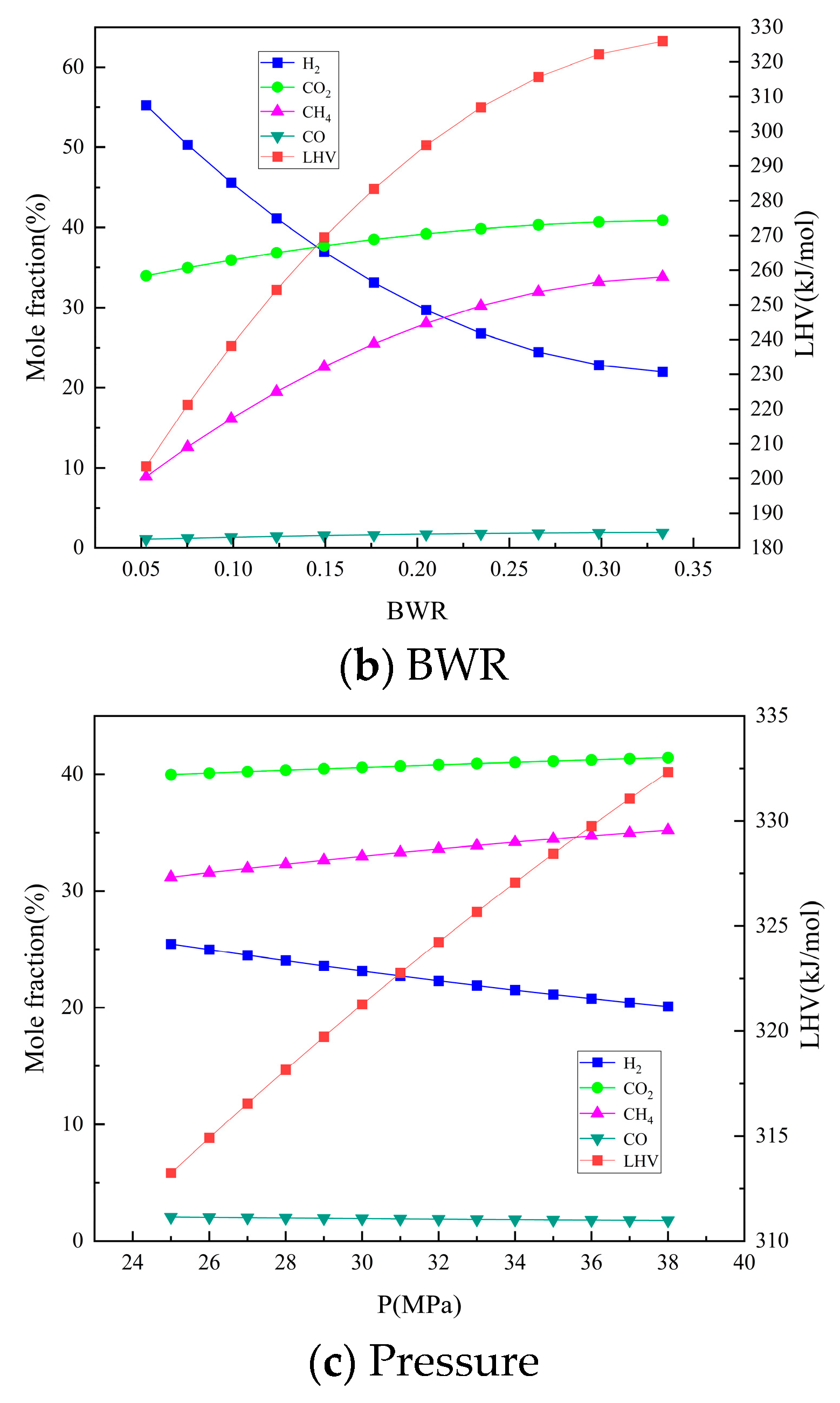
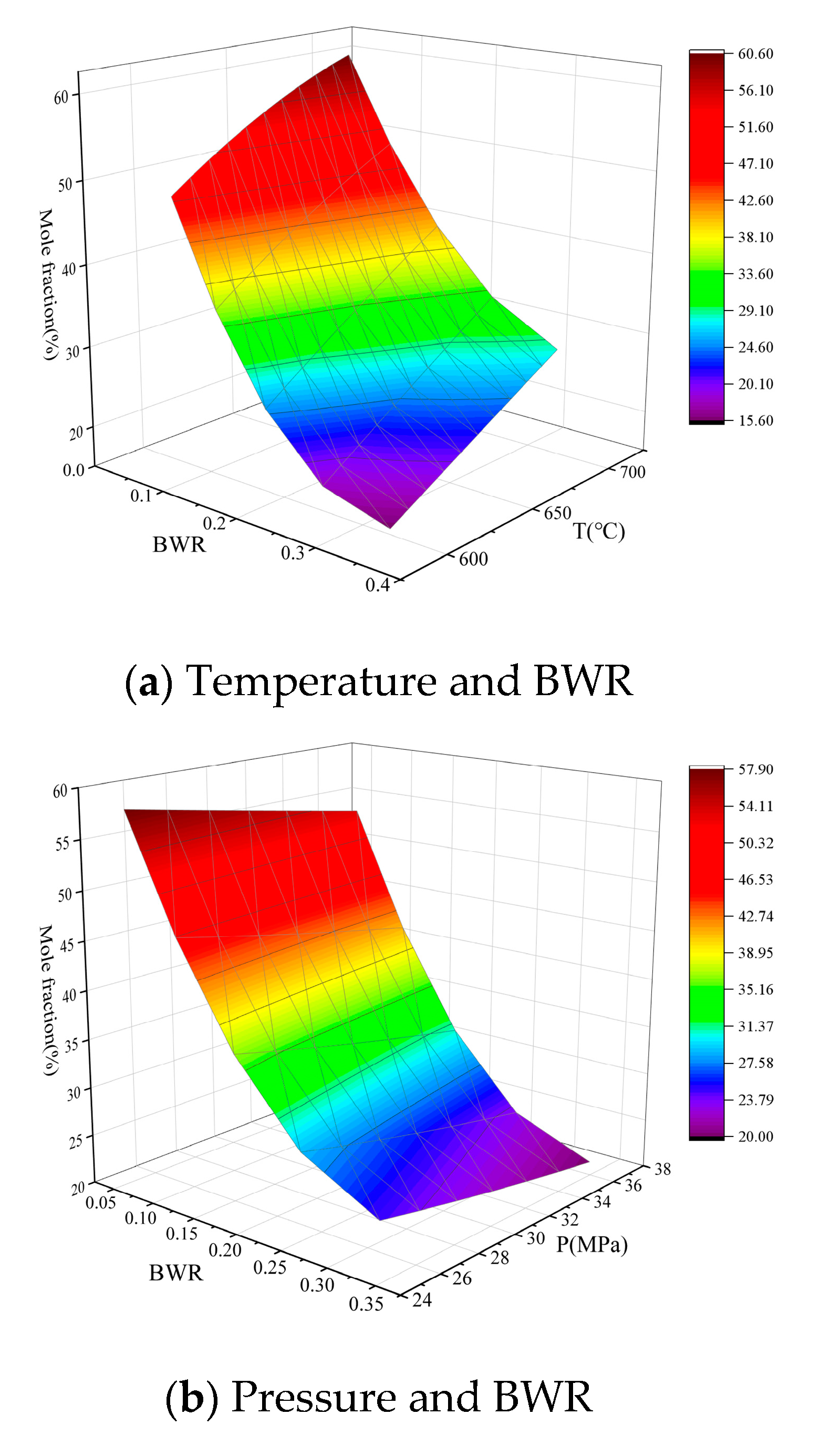
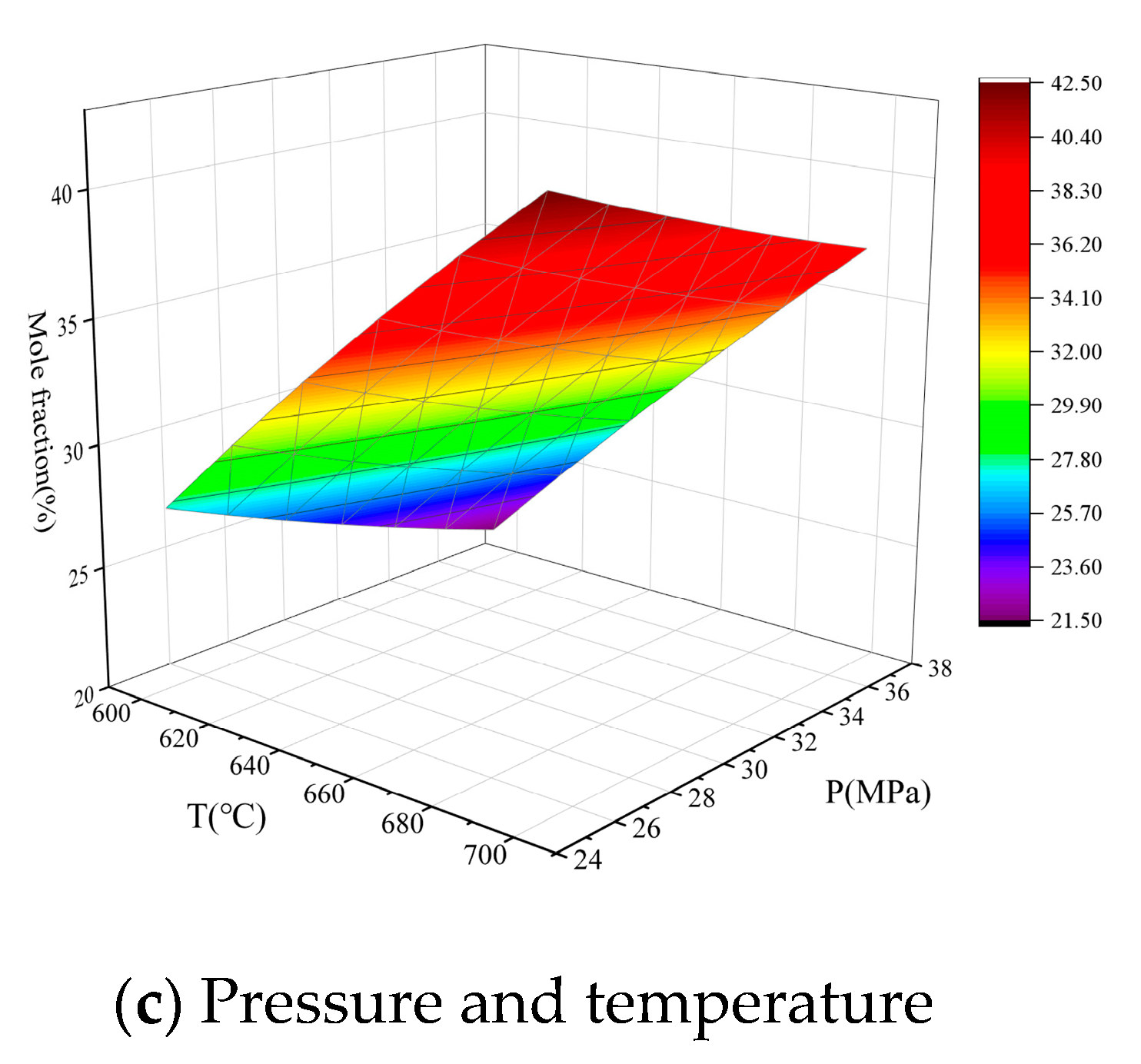
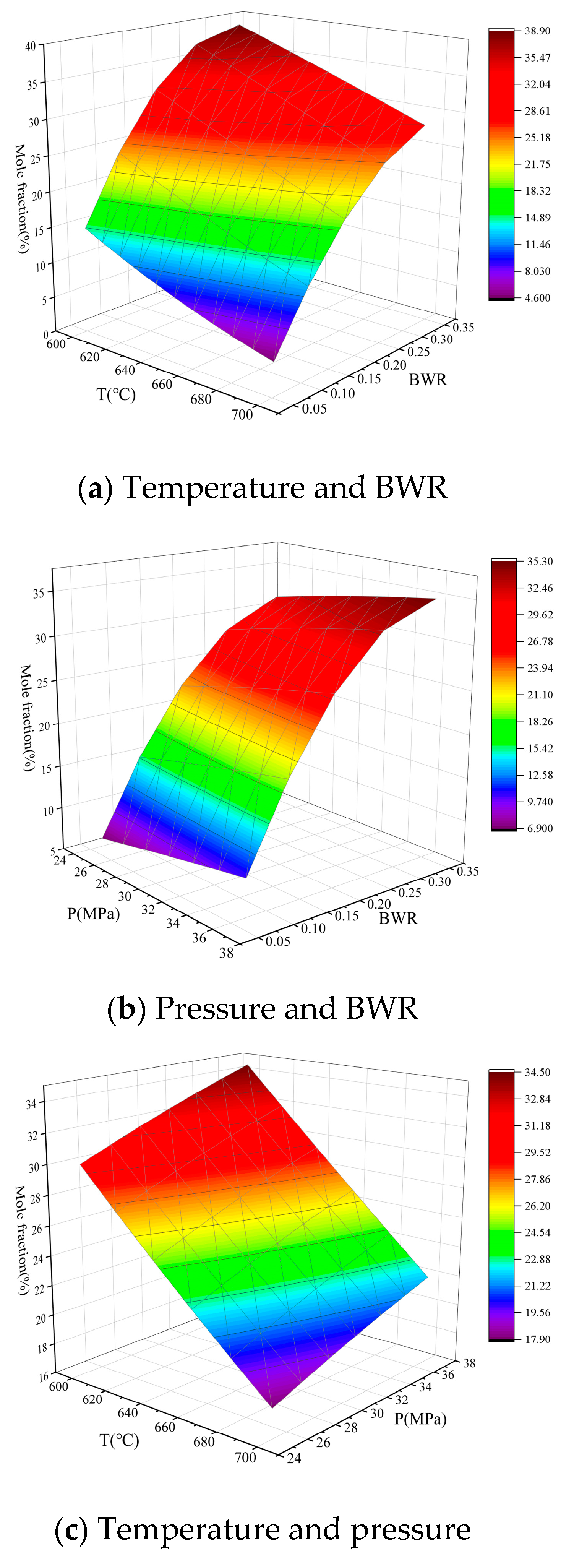
3.3. Analysis of the effects of Single Operation Parameters on Syngas Properties
3.4. Analysis of the effects of Operation Parameter Interactions on Syngas Properties
3.5. Multi-Objective Optimization
Max LHV of syngas = fHDMR (T, BWR, P)
0.052632 ≤ BWR ≤ 0.33
25 MPa ≤ P ≤ 38 MPa
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramalingam, S.; Ezhumalai, M.; Govindasamy, M. Syngas: Derived from biodiesel and its influence on CI engine. Energy 2019, 189, 116–189. [Google Scholar] [CrossRef]
- Hanif, I.; Raza, S.M.F.; Gago-de-Santos, P.; Abbas, Q. Fossil fuels, foreign direct investment, and economic growth has triggered CO2 emissions in emerging Asian economies: Some empirical evidence. Energy 2019, 171, 493–501. [Google Scholar] [CrossRef]
- Darmawan, A.; Ajiwibowo, M.W.; Yoshikawa, K.; Aziz, M.; Tokimatsu, K. Energy-efficient recovery of black liquor through gasification and syngas chemical looping. Appl. Energy 2018, 219, 290–298. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Zhu, M.-Q.; Wen, J.-L.; Sun, R.-C. Gasification of bio-oil: Effects of equivalence ratio and gasifying agents on product distribution and gasification efficiency. Bioresour. Technol. 2016, 211, 164–172. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, H.; Liu, Y.; Dang, H.; Ye, L.; Conejio, A.N.; Xu, R. Review on biomass metallurgy: Pretreatment technology, metallurgical mechanism and process design. Int. J. Miner. Metall. Mater. 2022, 29, 1133–1149. [Google Scholar] [CrossRef]
- Taghizadeh-Alisaraei, A.; Tatari, A.; Khanali, M.; Keshavarzi, M. Potential of biofuels production from wheat straw biomass, current achievements and perspectives: A review. Biofuels 2022, 14, 79–92. [Google Scholar] [CrossRef]
- Shah, S.; Venkatramanan, V. Advances in microbial technology for upscaling sustainable biofuel production. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–76. [Google Scholar]
- Mazaheri, N.; Akbarzadeh, A.; Madadian, E.; Lefsrud, M. Systematic review of research guidelines for numerical simulation of biomass gasification for bioenergy production. Energy Convers. Manag. 2019, 183, 671–688. [Google Scholar] [CrossRef]
- Safarian, S.; Unnthorsson, R.; Richter, C. Performance investigation of biomass gasification for syngas and hydrogen production using Aspen Plus. Open J. Model. Simul. 2022, 10, 71–87. [Google Scholar] [CrossRef]
- Moretti, L.; Arpino, F.; Cortellessa, G.; Dell’Isola, M.; Ficco, G.; Grossi, G.; Zuena, F.; Di Palma, M.; Vanoli, L. Analytical and numerical modelling of biomass gasification in downdraft gasifiers. J. Phys. Conf. Ser. 2022, 2177, 012028. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2022, 387, 224–236. [Google Scholar] [CrossRef]
- Kanthasamy, R.; Ali, I.; Ayodele, B.V.; Maddah, H.A. Bio-hydrogen production from the photocatalytic conversion of wastewater: Parametric analysis and data-driven modelling using nonlinear autoregressive with exogeneous input and back-propagated multilayer perceptron neural networks. Fuel 2023, 344, 128026. [Google Scholar] [CrossRef]
- Meier, T.R.W.; Cremonez, P.A.; Maniglia, T.C.; Maddah, H.A. Production of biohydrogen by an anaerobic digestion process using the residual glycerol from biodiesel production as additive to cassava wastewater. J. Clean. Prod. 2020, 258, 120833. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, M.; Ding, L.; Su, Y. Energy recovery and phosphorus phase behaviour from catalytic gasification of cyanobacterial biomass in supercritical water. Biomass Bioenergy 2020, 134, 105477. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chen, W.-H.; Lee, D.-J.; Chang, J.-S. Supercritical water gasification (SCWG) as a potential tool for the valorization of phycoremediation-derived waste algal biomass for biofuel generation. J. Hazard. Mater. 2021, 418, 126278. [Google Scholar] [CrossRef]
- Wei, N.; Xu, D.; Hao, B.; Guo, S.; Guo, Y.; Wang, S. Chemical reactions of organic compounds in supercritical water gasification and oxidation. Water Res. 2021, 190, 116634. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Wu, X.; Jiaqiang, E.; Leng, E.; Zhang, F.; Liao, G. Thermodynamic, environmental analysis and comprehensive evaluation of supercritical water gasification of biomass fermentation residue. J. Clean. Prod. 2022, 361, 132126. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Cheng, Z.; Lu, L.; Guo, L.; Jin, H.; Zhang, D.; Wang, R.; Liu, S. Performance simulation and thermodynamics analysis of hydrogen production based on supercritical water gasification of coal. Int. J. Hydrogen Energy 2021, 46, 28474–28485. [Google Scholar] [CrossRef]
- Rahbari, A.; Venkataraman, M.B.; Pye, J. Energy and exergy analysis of concentrated solar supercritical water gasification of algal biomass. Appl. Energy 2018, 228, 1669–1682. [Google Scholar] [CrossRef]
- Jiang, P.; Dong, J.; Huang, H. Optimal integrated demand response scheduling in regional integrated energy system with concentrating solar power. Appl. Therm. Eng. 2020, 166, 114754. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, Q.; Lei, J.; Hong, H.; Jin, H. New solar-biomass power generation system integrated a two-stage gasifier. Appl. Energy 2017, 194, 310–319. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.-P.; Zhao, X.-Y.; Yang, F.-L.; Wei, X.-Y. Recent advances in syngas production from biomass catalytic gasification: A critical review on reactors, catalysts, catalytic mechanisms and mathematical models. Renew. Sustain. Energy Rev. 2019, 116, 109426. [Google Scholar] [CrossRef]
- Srinivas, T.; Gupta, A.; Reddy, B.V. Thermodynamic equilibrium model and exergy analysis of a biomass gasifier. J. Energy Resour. Technol. 2009, 131, 031801. [Google Scholar] [CrossRef]
- Formica, M.; Frigo, S.; Gabbrielli, R. Development of a new steady state zero-dimensional simulation model for woody biomass gasification in a full scale plant. Energy Convers. Manag. 2016, 120, 358–369. [Google Scholar] [CrossRef]
- Mendiburu, A.Z.; Carvalho, J.A., Jr.; Zanzi, R.; Coronado, C.R.; Silveira, J.L. Thermochemical equilibrium modeling of a biomass downdraft gasifier: Constrained and unconstrained non-stoichiometric models. Energy 2014, 71, 624–637. [Google Scholar] [CrossRef]
- Ding, G.; He, B.; Yao, H.; Kuang, Y.; Song, J.; Su, L. Synergistic effect, kinetic and thermodynamics parameters analyses of co-gasification of municipal solid waste and bituminous coal with CO2. Waste Manag. 2021, 119, 342–355. [Google Scholar] [CrossRef]
- Rabitz, H.; Aliş, Ö.F. General foundations of high-dimensional model representations. J. Math. Chem. 1999, 25, 197–233. [Google Scholar] [CrossRef]
- Li, G.Y.; Artamonov, M.; Rabitz, H.; Wang, S.W.; Georgopoulos, P.G.; Demiralp, M. High-dimensional model representations generated from low order Terms—1p-RS-HDMR. J. Comput. Chem. 2003, 24, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Kargbo, H.O.; Zhang, J.; Phan, A.N. Optimisation of two-stage biomass gasification for hydrogen production via artificial neural network. Appl. Energy 2021, 302, 117567. [Google Scholar] [CrossRef]
- Sezer, S.; Özveren, U. Investigation of syngas exergy value and hydrogen concentration in syngas from biomass gasification in a bubbling fluidized bed gasifier by using machine learning. Int. J. Hydrogen Energy 2021, 46, 20377–20396. [Google Scholar] [CrossRef]
- Chinas-Palacios, C.; Vargas-Salgado, C.; Aguila-Leon, J.; Hurtado-Pérez, E. A cascade hybrid PSO feed-forward neural network model of a biomass gasification plant for covering the energy demand in an AC microgrid. Energy Convers. Manag. 2021, 232, 113896. [Google Scholar] [CrossRef]
- Musharavati, F.; Khoshnevisan, A.; Alirahmi, S.M.; Ahmadi, P.; Khanmohammadi, S. Multi-objective optimization of a biomass gasification to generate electricity and desalinated water using Grey Wolf Optimizer and artificial neural network. Chemosphere 2022, 287, 131980. [Google Scholar] [CrossRef] [PubMed]
- Sobol’, I.M.; Safety, S. Theorems and examples on high dimensional model representation. Reliab. Eng. Syst. Saf. 2003, 79, 187–193. [Google Scholar] [CrossRef]
- Alış, Ö.F.; Rabitz, H.J. Efficient implementation of high dimensional model representations. J. Math. Chem. 2001, 29, 127–142. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Lu, L.; Qiao, P. An Adaptive Dendrite-HDMR Metamodeling Technique for High-Dimensional Problems. J. Mech. Des. 2022, 144, 081701. [Google Scholar] [CrossRef]
- Huang, Z.; Qiu, H.; Zhao, M.; Cai, X.; Gao, L. An adaptive SVR-HDMR model for approximating high dimensional problems. Eng. Comput. 2015, 32, 643–667. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, H.; Bo, D.; He, C.; Pan, M. Data-driven Modeling and Optimization of Complex Chemical Processes Using a Novel HDMR Methodology. Comput. Aided Chem. Eng. 2018, 44, 835–840. [Google Scholar]
- Liu, S.; Yang, Y.; Yu, L.; Li, X. Thermodynamic and environmental analysis of solar-driven supercritical water gasification of algae for ammonia synthesis and power production. Energy Convers. Manag. 2021, 243, 114409. [Google Scholar]
- Striūgas, N.; Zakarauskas, K.; Džiugys, A.; Navakas, R.; Paulauskas, R. An evaluation of performance of automatically operated multi-fuel downdraft gasifier for energy production. Appl. Therm. Eng. 2014, 73, 1151–1159. [Google Scholar] [CrossRef]
- Chen, J.; Fan, Y.; Zhao, X.; Jiaqiang, E.; Xu, W.; Zhang, F.; Liao, G.; Leng, E.; Liu, S. Experimental investigation on gasification characteristic of food waste using supercritical water for combustible gas production: Exploring the way to complete gasification. Fuel 2020, 263, 116735. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, L.; Cao, W.; Jin, H.; Guo, S.; Zhang, X. Hydrogen production by sewage sludge gasification in supercritical water with a fluidized bed reactor. Energy 2013, 38, 12991–12999. [Google Scholar]
- Cao, W.; Guo, L.; Yan, X.; Zhang, D.; Yao, X. Assessment of sugarcane bagasse gasification in supercritical water for hydrogen production. Int. J. Hydrogen Energy 2018, 43, 13711–13719. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Liu, X.; Huang, G.; Xiao, W.; Han, L.J. The composition characteristics of different crop straw types and their multivariate analysis and comparison. Waste Manag. 2020, 110, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Louw, J.; Schwarz, C.E.; Knoetze, J.H.; Burger, A. Thermodynamic modelling of supercritical water gasification: Investigating the effect of biomass composition to aid in the selection of appropriate feedstock material. Bioresour. Technol. 2014, 174, 11–23. [Google Scholar] [CrossRef]
- Mutlu, Ö.Ç.; Zeng, T. Challenges and opportunities of modeling biomass gasification in Aspen Plus: A review. Chem. Eng. Technol. 2020, 43, 1674–1689. [Google Scholar] [CrossRef]
- Ruya, P.M.; Purwadi, R.; Lim, S.S. Supercritical water gasification of sewage sludge for power generation–thermodynamic study on auto-thermal operation using Aspen Plus. Energy Convers. Manag. 2020, 206, 112458. [Google Scholar] [CrossRef]
- Gimenez, P.; Fereres, S. Effect of heating rates and composition on the thermal decomposition of nitrate based molten salts. Energy Procedia 2015, 69, 654–662. [Google Scholar] [CrossRef]
- Bernagozzi, M.; Panesar, A.S.; Morgan, R. Molten salt selection methodology for medium temperature liquid air energy storage application. Appl. Energy 2019, 248, 500–511. [Google Scholar] [CrossRef]
- Kenisarin, M.M. High-temperature phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2010, 14, 955–970. [Google Scholar] [CrossRef]
- Zhao, Q.G.; Hu, C.X.; Liu, S.J.; Guo, H.; Wu, Y.T. The thermal conductivity of molten NaNO3, KNO3, and their mixtures. Energy Procedia 2017, 143, 774–779. [Google Scholar] [CrossRef]
- Gong, Q.; Shi, H.; Chai, Y.; Yu, R.; Weisenburger, A.; Wang, D.; Bonk, A.; Bauer, T.; Ding, W.J. Molten chloride salt technology for next-generation CSP plants: Compatibility of Fe-based alloys with purified molten MgCl2-KCl-NaCl salt at 700 °C. Appl. Energy 2022, 324, 119708. [Google Scholar] [CrossRef]
- Villada, C.; Ding, W.; Bonk, A.; Bauer, T. Engineering molten MgCl2–KCl–NaCl salt for high-temperature thermal energy storage: Review on salt properties and corrosion control strategies. Sol. Energy Mater. Sol. Cells 2021, 232, 111344. [Google Scholar] [CrossRef]
- Villada, C.; Ding, W.; Bonk, A.; Bauer, T. Simulation-assisted determination of the minimum melting temperature composition of MgCl2–KCl–NaCl salt mixture for next-generation molten salt thermal energy storage. Front. Energy Res. 2022, 10, 181. [Google Scholar] [CrossRef]
- Cao, C.; Xu, L.; He, Y.; Guo, L.; Jin, H.; Huo, Z.J. High-efficiency gasification of wheat straw black liquor in supercritical water at high temperatures for hydrogen production. Energy Fuels 2017, 31, 3970–3978. [Google Scholar] [CrossRef]
- Yan, M.; Su, H.; Hantoko, D.; Kanchanatip, E.; Hamid, F.B.S.; Zhang, S.; Wang, G.; Xu, Z. Experimental study on the energy conversion of food waste via supercritical water gasification: Improvement of hydrogen production. Int. J. Hydrogen Energy 2019, 44, 4664–4673. [Google Scholar] [CrossRef]
- Freitas, A.C.; Guirardello, R. Use of CO2 as a co-reactant to promote syngas production in supercritical water gasification of sugarcane bagasse. J. CO2 Util. 2015, 9, 66–73. [Google Scholar] [CrossRef]
- Moghaddam, E.M.; Goel, A.; Siedlecki, M.; Michalska, K.; Yakaboylu, O. Supercritical water gasification of wet biomass residues from farming and food production practices: Lab-scale experiments and comparison of different modelling approaches. Sustain. Energy Fuels 2021, 5, 1521–1537. [Google Scholar] [CrossRef]
- Macrì, D.; Catizzone, E.; Molino, A.; Migliori, M. Supercritical water gasification of biomass and agro-food residues: Energy assessment from modelling approach. Renew. Energy 2020, 150, 624–636. [Google Scholar] [CrossRef]
- Yakaboylu, O.; Harinck, J.; Smit, K.G. Supercritical water gasification of manure: A thermodynamic equilibrium modeling approach. Biomass Bioenergy 2013, 59, 253–263. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.-W.; Rabitz, H. Practical approaches to construct RS-HDMR component functions. J. Phys. Chem. A 2002, 106, 8721–8733. [Google Scholar] [CrossRef]
- Horvat, A.; Pandey, D.S.; Kwapinska, M.; Mello, B.B.; Gómez-Barea, A.; Fryda, L.E.; Rabou, L.P. Tar yield and composition from poultry litter gasification in a fluidised bed reactor: Effects of equivalence ratio, temperature and limestone addition. RSC Adv. 2019, 9, 13283–13296. [Google Scholar] [CrossRef]
- Ziegel, E.R. The elements of statistical learning. Technometrics 2003, 45, 267–268. [Google Scholar] [CrossRef]
- Lu, Y.; Jin, H.; Guo, L.; Zhang, X.; Cao, C.; Guo, X. Hydrogen production by biomass gasification in supercritical water with a fluidized bed reactor. J. Supercrit. Fluids 2008, 33, 6066–6075. [Google Scholar] [CrossRef]




| Temperature °C (T) | Pressure MPa (P) | Biomass-to-Water Ratio (BWR) | Model Data |
|---|---|---|---|
| 600 | 25 | 0.0523 | Tij × Pij × BWRi = 70 |
| 610 | 26 | 0.111 | Tij × Pij × BWRi = 70 |
| 620 | 27 | 0.176 | Tij × Pij × BWRi = 70 |
| 630 | 28 | 0.250 | Tij × Pij × BWRi = 70 |
| 640 | 29 | 0.333 | Tij × Pij × BWRi = 70 |
| 650 | 30 | — | Tij × Pij × BWRi = 70 |
| 660 | 31 | — | Tij × Pij × BWRi = 70 |
| 670 | 32 | — | Tij × Pij × BWRi = 70 |
| 680 | 33 | — | Tij × Pij × BWRi = 70 |
| 690 | 34 | — | Tij × Pij × BWRi = 70 |
| 700 | 35 | — | Tij × Pij × BWRi = 70 |
| — | 36 | — | Tij × Pij × BWRi = 70 |
| — | 37 | — | Tij × Pij × BWRi = 70 |
| — | 38 | — | Tij × Pij × BWRi = 70 |
| Ultimate Analysis (%) | Wheat Straw | Sewage Sludge | Corn Waste | Sugarcane Bagasse | Food Waste |
|---|---|---|---|---|---|
| C | 42.270 | 38.180 | 47.000 | 47.090 | 44.223 |
| H | 5.330 | 3.400 | 5.100 | 6.160 | 6.134 |
| O | 41.696 | 23.740 | 29.400 | 42.500 | 46.15 |
| N | 0.596 | 4.670 | 2.100 | 0.520 | 1.651 |
| S | 0.368 | 1.050 | 0.300 | 1.080 | 0.128 |
| Ash | 9.740 | 28.960 | 16.100 | 3.650 | 1.714 |
| Molten Salt | Melting Temperature (°C) | Degradation Temperature (°C) | Heat Capacity (kJ/kg·K) | Density (kg/m3) |
|---|---|---|---|---|
| MgCl2-NaCl-KCl | 385 | 800+ | 0.8246 | 1680 |
| Model | MAE | RMSE |
|---|---|---|
| H2 (fraction) | 0.00624 | 0.00272 |
| CH4 (fraction) | 0.00469 | 0.00577 |
| CO (fraction) | 0.00235 | 0.00306 |
| CO2 (fraction) | 0.00191 | 0.00987 |
| H2 (mol/kg) | 0.00128 | 0.00161 |
| LHV (kJ/mol) | 0.00241 | 0.00294 |
| Biomass | T (°C) | BWR | P (MPa) | H2 (mol/kg) | LHV (kJ/mol) |
|---|---|---|---|---|---|
| Wheat straw | 613 | 0.05304 | 37.6 | 9.78 | 225 |
| Sugarcane bagasse | 620 | 0.05263 | 37.7 | 9.96 | 231 |
| Sewage sludge | 609 | 0.05263 | 36.4 | 10.05 | 217 |
| Food waste | 600 | 0.0528 | 28.1 | 10.12 | 220 |
| Corn straw | 624 | 0.05268 | 37.8 | 10.07 | 235 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Lei, J.; Jia, M.; Xu, H.; Wu, S. High Dimensional Model Representation Approach for Prediction and Optimization of the Supercritical Water Gasification System Coupled with Photothermal Energy Storage. Processes 2023, 11, 2313. https://doi.org/10.3390/pr11082313
Li H, Lei J, Jia M, Xu H, Wu S. High Dimensional Model Representation Approach for Prediction and Optimization of the Supercritical Water Gasification System Coupled with Photothermal Energy Storage. Processes. 2023; 11(8):2313. https://doi.org/10.3390/pr11082313
Chicago/Turabian StyleLi, Haoxing, Jianhong Lei, Ming Jia, Hongpeng Xu, and Shaohua Wu. 2023. "High Dimensional Model Representation Approach for Prediction and Optimization of the Supercritical Water Gasification System Coupled with Photothermal Energy Storage" Processes 11, no. 8: 2313. https://doi.org/10.3390/pr11082313
APA StyleLi, H., Lei, J., Jia, M., Xu, H., & Wu, S. (2023). High Dimensional Model Representation Approach for Prediction and Optimization of the Supercritical Water Gasification System Coupled with Photothermal Energy Storage. Processes, 11(8), 2313. https://doi.org/10.3390/pr11082313









