Peptide Diversification through Addition Reaction of Free Carboxylic Acids to Ynamides
Abstract
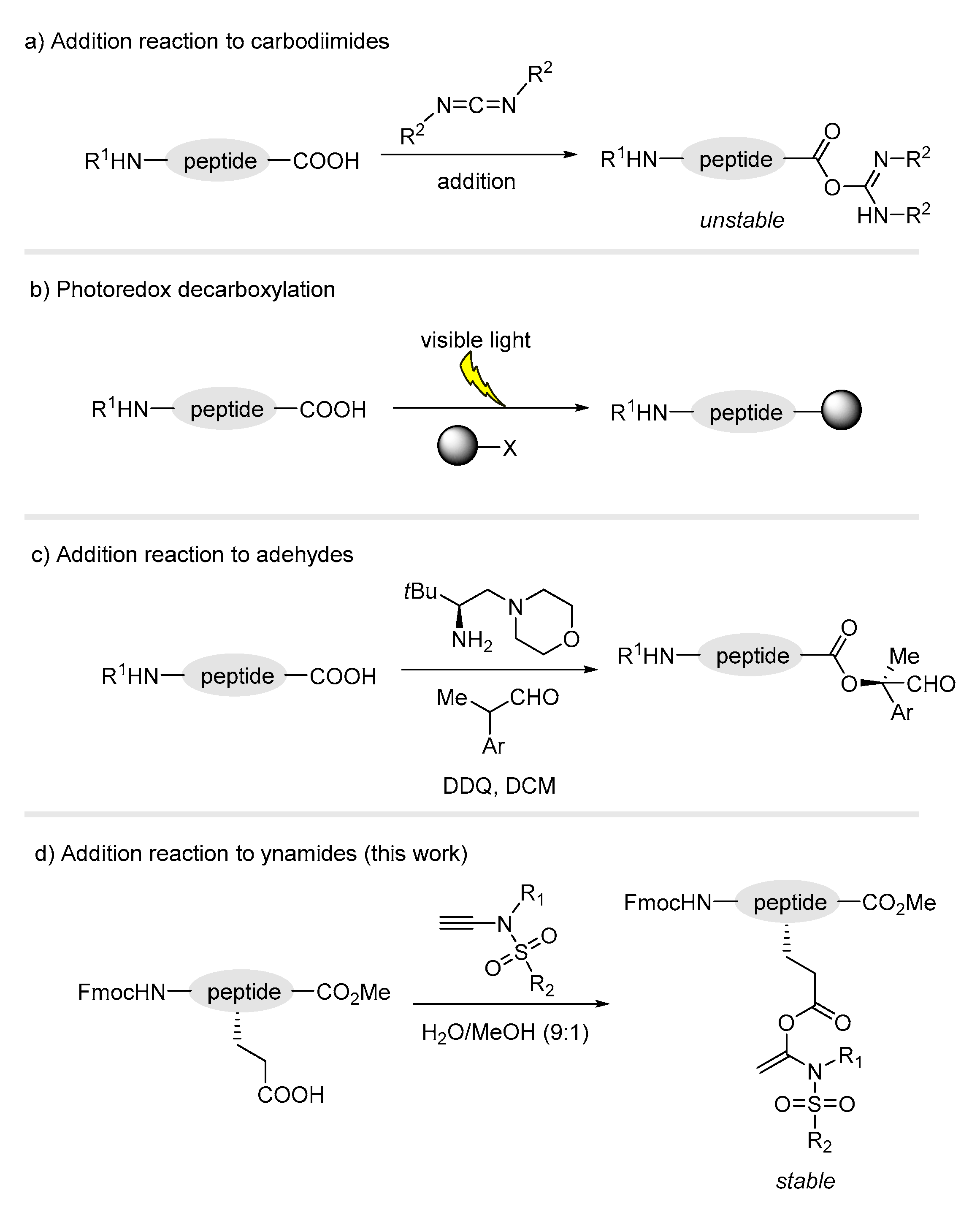
1. Introduction
2. Materials and Methods
2.1. General Information
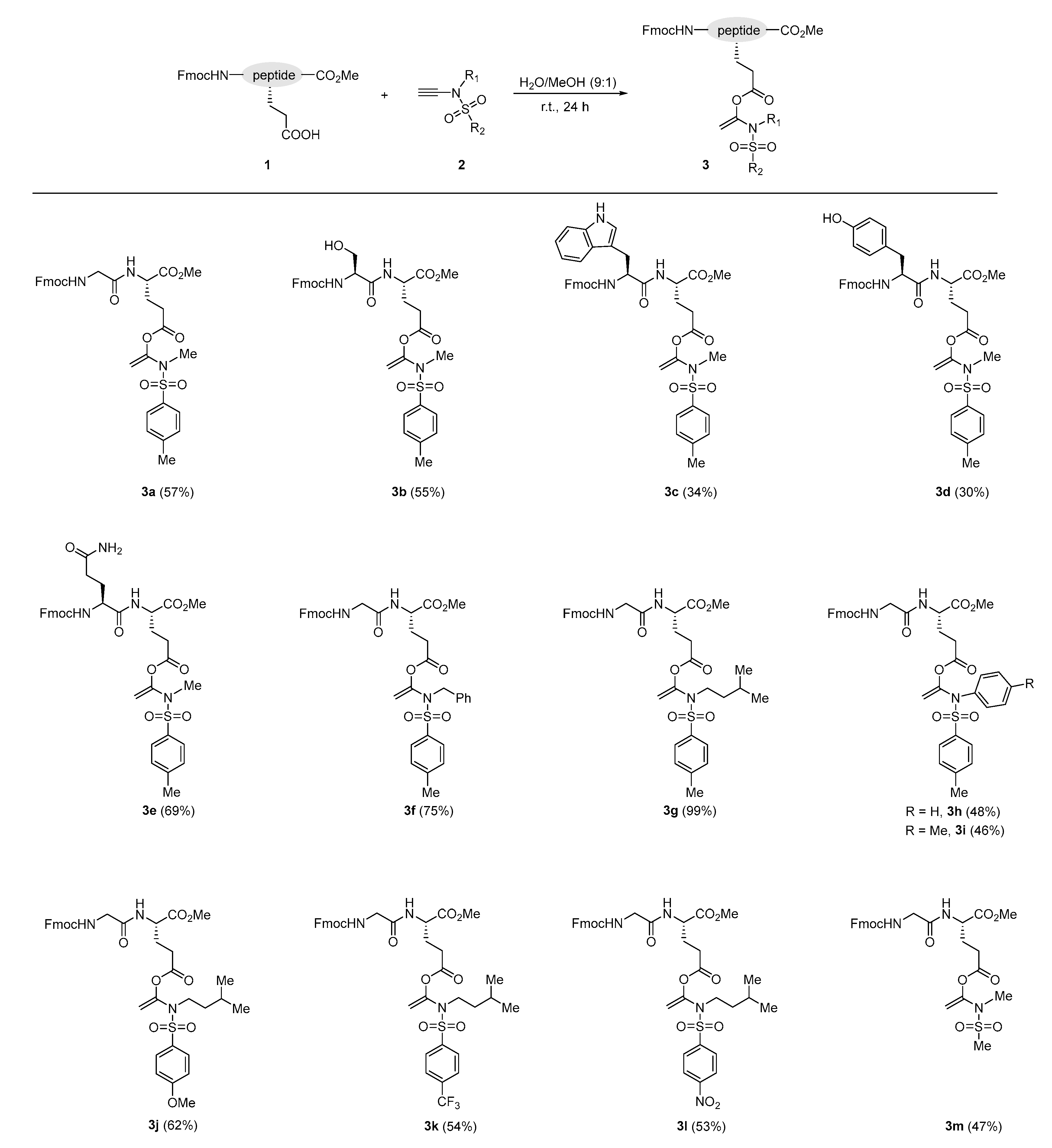
2.2. Synthesis of Product 3
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Albericio, F.; Kruger, H.G. Therapeutic peptides. Future Med. Chem. 2012, 4, 1527–1531. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, A.; Dawson, P.E. Expanding the scope of chemoselective peptide ligations in chemical biology. Curr. Opin. Chem. Biol. 2008, 12, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Xing, B.; Loh, T.-P. Allenamides as Orthogonal Handles for Selective Modification of Cysteine in Peptides and Proteins. Angew. Chem. Int. Ed. 2014, 53, 7491–7494. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Ojeda-Carralero, G.M.; Parmar, D.; González-Martínez, D.A.; Van Meervelt, L.; Van der Eycken, J.; Goeman, J.; Rivera, D.G.; Van der Eycken, E.V. Chemoselective Peptide Backbone Diversification and Bioorthogonal Ligation by Ruthenium-Catalyzed C−H Activation/Annulation. Adv. Synth. Catal. 2021, 363, 3297–3304. [Google Scholar] [CrossRef]
- deGruyter, J.N.; Malins, L.R.; Baran, P.S. Residue-Specific Peptide Modification: A Chemist’s Guide. Biochemistry 2017, 56, 3863–3873. [Google Scholar] [CrossRef]
- Song, L.; Lv, Z.; Li, Y.; Zhang, K.; Van der Eycken, E.V.; Cai, L. Construction of Peptide–Isoquinolone Conjugates via Rh(III)-Catalyzed C–H Activation/Annulation. Org. Lett. 2023, 25, 2996–3000. [Google Scholar] [CrossRef]
- Chatterjee, J.; Rechenmacher, F.; Kessler, H. N-Methylation of Peptides and Proteins: An Important Element for Modulating Biological Functions. Angew. Chem. Int. Ed. 2013, 52, 254–269. [Google Scholar] [CrossRef]
- Gracia, S.R.; Gaus, K.; Sewald, N. Synthesis of chemically modified bioactive peptides: Recent advances, challenges and developments for medicinal chemistry. Future Med. Chem. 2009, 1, 1289–1310. [Google Scholar] [CrossRef]
- Rosen, C.B.; Francis, M.B. Targeting the N terminus for site-selective protein modification. Nat. Chem. Biol. 2017, 13, 697–705. [Google Scholar] [CrossRef]
- Rivera, D.G.; Ojeda-Carralero, G.M.; Reguera, L.; Van der Eycken, E.V. Peptide macrocyclization by transition metal catalysis. Chem. Soc. Rev. 2020, 49, 2039–2059. [Google Scholar] [CrossRef]
- Hu, L.; Xu, S.; Zhao, Z.; Yang, Y.; Peng, Z.; Yang, M.; Wang, C.; Zhao, J. Ynamides as Racemization-Free Coupling Reagents for Amide and Peptide Synthesis. J. Am. Chem. Soc. 2016, 138, 13135–13138. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Wang, P.; Zhao, J. Allenone-Mediated Racemization/Epimerization-Free Peptide Bond Formation and Its Application in Peptide Synthesis. J. Am. Chem. Soc. 2021, 143, 10374–10381. [Google Scholar] [CrossRef]
- El-Faham, A.; Albericio, F. Peptide Coupling Reagents, More than a Letter Soup. Chem. Rev. 2011, 111, 6557–6602. [Google Scholar] [CrossRef]
- Mix, K.A.; Raines, R.T. Optimized Diazo Scaffold for Protein Esterification. Org. Lett. 2015, 17, 2358–2361. [Google Scholar] [CrossRef] [PubMed]
- Mix, K.A.; Lomax, J.E.; Raines, R.T. Cytosolic Delivery of Proteins by Bioreversible Esterification. J. Am. Chem. Soc. 2017, 139, 14396–14398. [Google Scholar] [CrossRef] [PubMed]
- Tobiesen, H.N.; Leth, L.A.; Iversen, M.V.; Næsborg, L.; Bertelsen, S.; Jørgensen, K.A. Stereoselective Oxidative Bioconjugation of Amino Acids and Oligopeptides to Aldehydes. Angew. Chem. Int. Ed. 2020, 59, 18490–18494. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Cornella, J.; Li, C.; Malins, L.R.; Edwards, J.T.; Kawamura, S.; Maxwell, B.D.; Eastgate, M.D.; Baran, P.S. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 2016, 352, 801–805. [Google Scholar] [CrossRef]
- Malins, L.R. Decarboxylative couplings as versatile tools for late-stage peptide modifications. Pept. Sci. 2018, 110, e24049. [Google Scholar] [CrossRef]
- Mondal, S.; Chowdhury, S. Recent Advances on Amino Acid Modifications via C–H Functionalization and Decarboxylative Functionalization Strategies. Adv. Synth. Catal. 2018, 360, 1884–1912. [Google Scholar] [CrossRef]
- Bloom, S.; Liu, C.; Kölmel, D.K.; Qiao, J.X.; Zhang, Y.; Poss, M.A.; Ewing, W.R.; MacMillan, D.W.C. Decarboxylative alkylation for site-selective bioconjugation of native proteins via oxidation potentials. Nat. Chem. 2018, 10, 205–211. [Google Scholar] [CrossRef]
- McCarver, S.J.; Qiao, J.X.; Carpenter, J.; Borzilleri, R.M.; Poss, M.A.; Eastgate, M.D.; Miller, M.M.; MacMillan, D.W.C. Decarboxylative Peptide Macrocyclization through Photoredox Catalysis. Angew. Chem. Int. Ed. 2017, 56, 728–732. [Google Scholar] [CrossRef]
- Lang, S.B.; O’Nele, K.M.; Douglas, J.T.; Tunge, J.A. Dual Catalytic Decarboxylative Allylations of α-Amino Acids and Their Divergent Mechanisms. Chem. Eur. J. 2015, 21, 18589–18593. [Google Scholar] [CrossRef]
- Dodd, R.H.; Cariou, K. Ketenimines Generated from Ynamides: Versatile Building Blocks for Nitrogen-Containing Scaffolds. Chem. Eur. J. 2018, 24, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- DeKorver, K.A.; Li, H.; Lohse, A.G.; Hayashi, R.; Lu, Z.; Zhang, Y.; Hsung, R.P. Ynamides: A Modern Functional Group for the New Millennium. Chem. Rev. 2010, 110, 5064–5106. [Google Scholar] [CrossRef]
- Evano, G.; Coste, A.; Jouvin, K. Ynamides: Versatile Tools in Organic Synthesis. Angew. Chem. Int. Ed. 2010, 49, 2840–2859. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tan, T.-D.; Zhu, X.-Q.; Shang, M.; Ye, L.-W. Reversal of Regioselectivity in Ynamide Chemistry. ACS Catal. 2019, 9, 6393–6406. [Google Scholar] [CrossRef]
- Liu, T.; Xu, S.; Zhao, J. Recent Advances in Ynamide Coupling Reagent. Chin. J. Org. Chem. 2021, 41, 873–887. [Google Scholar] [CrossRef]
- Tian, X.; Song, L.; Rudolph, M.; Rominger, F.; Oeser, T.; Hashmi, A.S.K. Sulfilimines as Versatile Nitrene Transfer Reagents: Facile Access to Diverse Aza-Heterocycles. Angew. Chem. Int. Ed. 2019, 58, 3589–3593. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Mallick, R.K.; Prasad, R.; Gandon, V.; Sahoo, A.K. Alkyne Versus Ynamide Reactivity: Regioselective Radical Cyclization of Yne-Ynamides. Angew. Chem. Int. Ed. 2019, 58, 2289–2294. [Google Scholar] [CrossRef]
- Song, L.; Tian, G.; Blanpain, A.; VanMeervelt, L.; Van der Eycken, E.V. Diversification of peptidomimetics and oligopeptides through microwave-assisted rhodium (III)-catalyzed intramolecular annulation. Adv. Synth. Catal. 2019, 361, 4442–4447. [Google Scholar] [CrossRef]
- Song, L.; Liu, C.; Tian, G.; Van Meervelt, L.; Van der Eycken, J.; Van der Eycken, E.V. Late-stage diversification of peptidomimetics and oligopeptides via gold-catalyzed post-Ugi cyclization. Mol. Catal. 2022, 522, 112240. [Google Scholar] [CrossRef]
- Liu, C.; Bolognani, A.; Song, L.; Van Meervelt, L.; Peshkov, V.A.; Van der Eycken, E.V. Gold(I)-catalyzed intramolecular bicyclization: Divergent construction of quinazolinone and ampakine analogues. Org. Lett. 2022, 24, 8536–8541. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Moegle, B.; Ghosh, S.; Perfetto, A.; Luise, D.; Ciofini, I.; Miesch, L. Copper-catalyzed synthesis of terminal vs. fluorine-substituted N-allenamides via addition of diazo compounds to terminal ynamides. Chem. A Eur. J. 2022, 28, e202103598. [Google Scholar] [CrossRef] [PubMed]
- Schlimpen, F.; Ast, T.; Bénéteau, V.; Pale, P.; Chassaing, S. From A3/KA2 to AYA/KYA multicomponent coupling reactions with terminal ynamides as alkyne surrogates—A direct, green route to γ-amino-ynamides. Green Chem. 2022, 24, 6467–6475. [Google Scholar] [CrossRef]
- Yudasaka, M.; Shimbo, D.; Maruyama, T.; Tada, N.; Itoh, A. Synthesis, characterization, and reactivity of an ethynyl benziodoxolone (EBX)–acetonitrile complex. Org. Lett. 2019, 21, 1098–1102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Cai, L.; Song, L. Peptide Diversification through Addition Reaction of Free Carboxylic Acids to Ynamides. Processes 2023, 11, 2262. https://doi.org/10.3390/pr11082262
Zhang Z, Cai L, Song L. Peptide Diversification through Addition Reaction of Free Carboxylic Acids to Ynamides. Processes. 2023; 11(8):2262. https://doi.org/10.3390/pr11082262
Chicago/Turabian StyleZhang, Zhefan, Lingchao Cai, and Liangliang Song. 2023. "Peptide Diversification through Addition Reaction of Free Carboxylic Acids to Ynamides" Processes 11, no. 8: 2262. https://doi.org/10.3390/pr11082262
APA StyleZhang, Z., Cai, L., & Song, L. (2023). Peptide Diversification through Addition Reaction of Free Carboxylic Acids to Ynamides. Processes, 11(8), 2262. https://doi.org/10.3390/pr11082262







