Concurrent Biocatalytic Oxidation of 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic Acid by Merging Galactose Oxidase with Whole Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strain Cultivation
2.3. Preparation of GO M3–5 CFE
2.4. Enzyme Assay
2.5. Biocatalytic Oxidation of HMF into FDCA
2.6. Preparative Synthesis of FDCA
2.7. HPLC Analysis
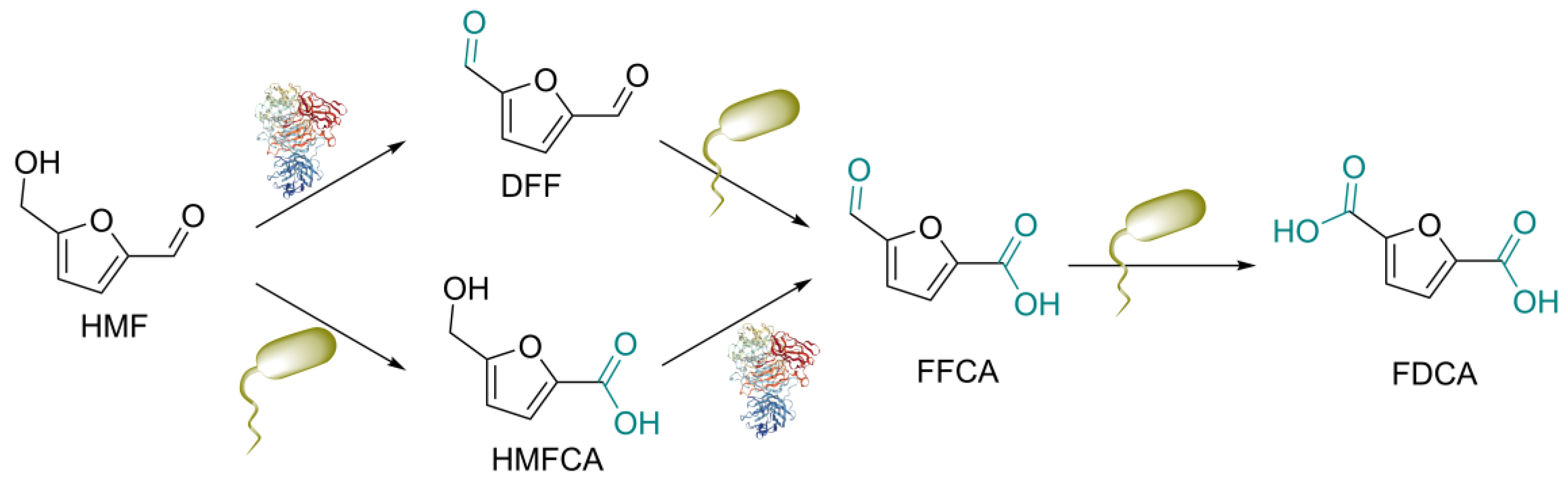
3. Results and Discussion
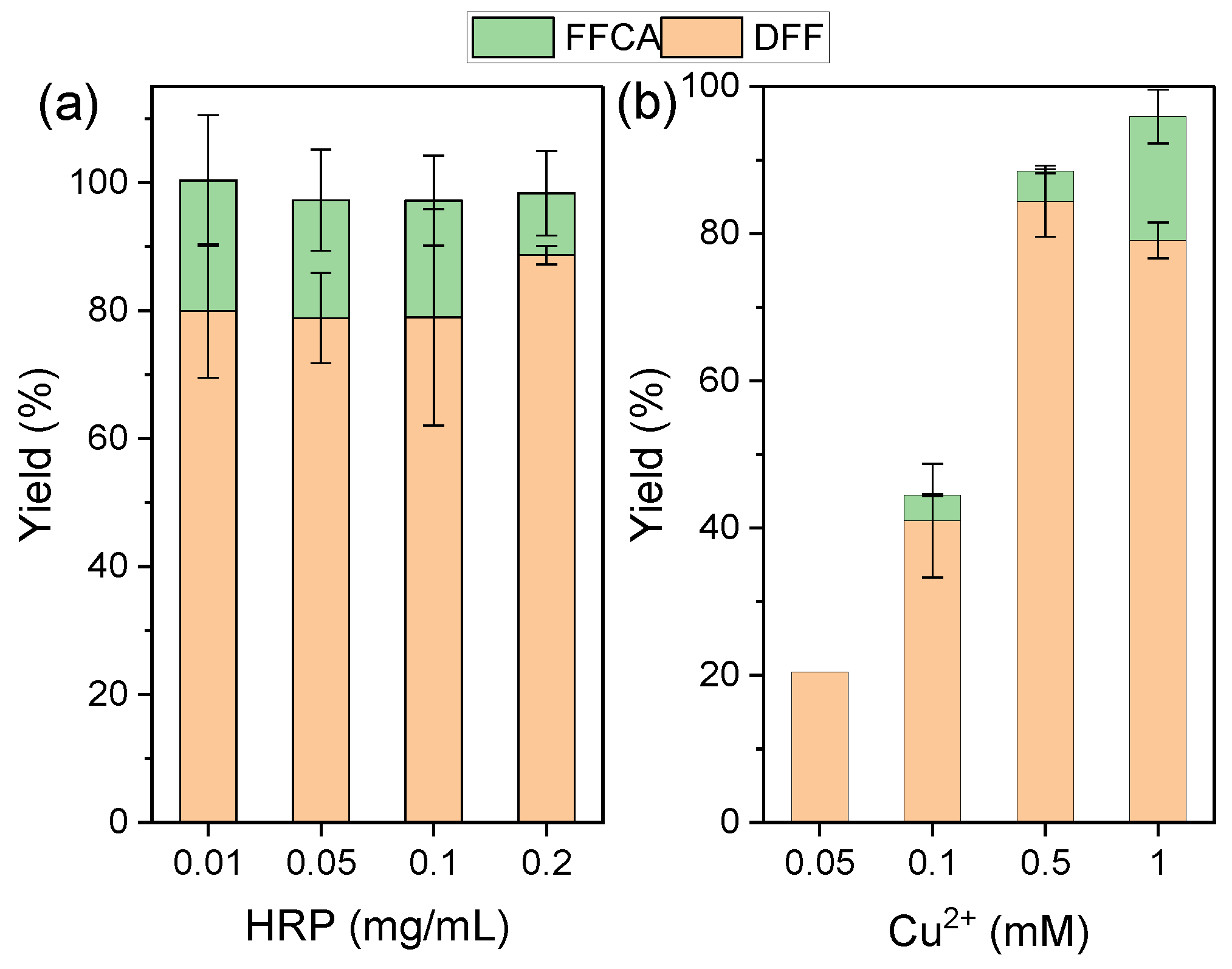
3.1. Optimization of GO-Catalyzed HMF Oxidation
3.2. Whole-Cell Catalytic Oxidation of DFF and FFCA
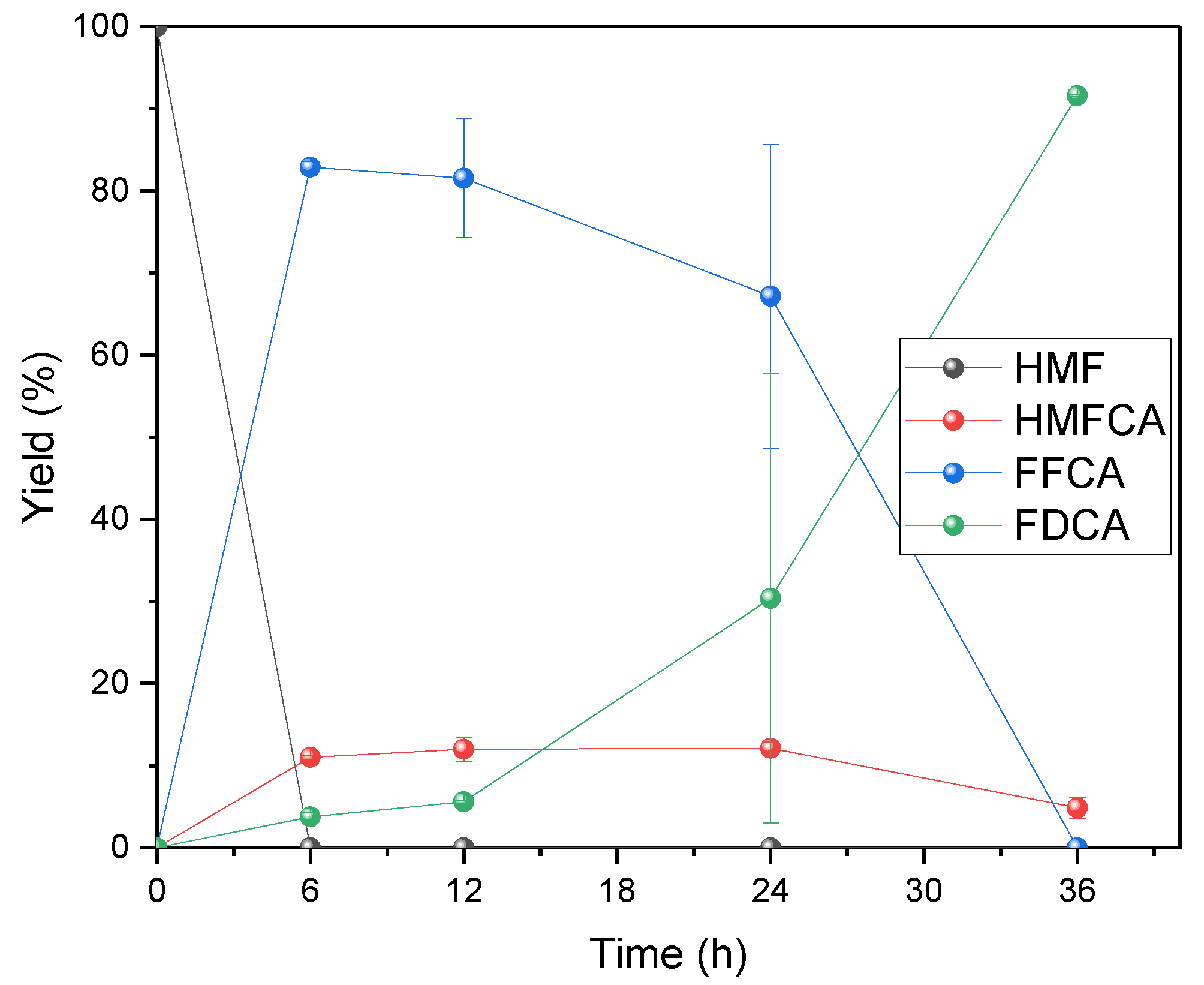
3.3. Biocatalytic HMF Oxidation into FDCA
3.4. Biocatalytic Preparative Synthesis of FDCA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Figueirêdo, M.B.; Hita, I.; Deuss, P.J.; Venderbosch, R.H.; Heeres, H.J. Pyrolytic lignin: A promising biorefinery feedstock for the production of fuels and valuable chemicals. Green Chem. 2022, 24, 4680–4702. [Google Scholar] [CrossRef]
- Bielski, R.; Grynkiewicz, G. Furan platform chemicals beyond fuels and plastics. Green Chem. 2021, 23, 7458–7487. [Google Scholar] [CrossRef]
- Sheldon, R.A. Chemicals from renewable biomass: A renaissance in carbohydrate chemistry. Curr. Opin. Green Sustain. Chem. 2018, 14, 89–95. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, R.; Itabaiana, I. Engineering the future: Perspectives in the 2,5-furandicarboxylic acid synthesis. Catal. Today 2020, 354, 211–217. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Winkler, C.K.; Schrittwieser, J.H.; Kroutil, W. Power of Biocatalysis for Organic Synthesis. ACS Cent. Sci. 2021, 7, 55–71. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Dominguez de Maria, P.; Guajardo, N. Biocatalytic valorization of furans: Opportunities for inherently unstable substrates. ChemSusChem 2017, 10, 4123–4134. [Google Scholar] [CrossRef]
- Li, N.; Zong, M.-H. (Chemo)biocatalytic upgrading of biobased furanic platforms to chemicals, fuels, and materials: A comprehensive review. ACS Catal. 2022, 12, 10080–10114. [Google Scholar] [CrossRef]
- Koopman, F.; Wierckx, N.; de Winde, J.H.; Ruijssenaars, H.J. Efficient whole-cell biotransformation of 5-(hydroxymethyl) furfural into FDCA, 2,5-furandicarboxylic acid. Bioresour. Technol. 2010, 101, 6291–6296. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, W.P.; Groothuis, D.E.; Fraaije, M.W. Enzyme-catalyzed oxidation of 5-hydroxymethylfurfural to furan-2,5-dicarboxylic acid. Angew. Chem. Int. Ed. 2014, 53, 6515–6518. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.-Z.; Li, Y.-M.; Zong, M.-H.; Wu, H.; Li, N. Enzyme-catalyzed selective oxidation of 5-hydroxymethylfurfural (HMF) and separation of HMF and 2,5-diformylfuran using deep eutectic solvents. Green Chem. 2015, 17, 3718–3722. [Google Scholar] [CrossRef]
- McKenna, S.M.; Leimkuhler, S.; Herter, S.; Turner, N.J.; Carnell, A.J. Enzyme cascade reactions: Synthesis of furandicarboxylic acid (FDCA) and carboxylic acids using oxidases in tandem. Green Chem. 2015, 17, 3271–3275. [Google Scholar] [CrossRef]
- Carro, J.; Ferreira, P.; Rodriguez, L.; Prieto, A.; Serrano, A.; Balcells, B.; Arda, A.; Jimenez-Barbero, J.; Gutierrez, A.; Ullrich, R. 5-Hydroxymethylfurfural conversion by fungal aryl-alcohol oxidase and unspecific peroxygenase. FEBS J. 2015, 282, 3218–3229. [Google Scholar] [CrossRef]
- Hsu, C.-T.; Kuo, Y.-C.; Liu, Y.-C.; Tsai, S.-L. Green conversion of 5-hydroxymethylfurfural to furan-2,5-dicarboxylic acid by heterogeneous expression of 5-hydroxymethylfurfural oxidase in Pseudomonas putida S12. Microb. Biotechnol. 2020, 13, 1094–1102. [Google Scholar] [CrossRef]
- Pham, N.N.; Chen, C.-Y.; Li, H.; Nguyen, M.T.T.; Nguyen, P.K.P.; Tsai, S.-L.; Chou, J.-Y.; Ramli, T.C.; Hu, Y.-C. Engineering stable Pseudomonas putida S12 by CRISPR for 2,5-furandicarboxylic acid (FDCA) production. ACS Synth. Biol. 2020, 9, 1138–1149. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.-Y.; Zong, M.-H.; Li, N. Sacrificial substrate-free whole-cell biocatalysis for the synthesis of 2,5-furandicarboxylic acid by engineered Escherichia coli. ACS Sustain. Chem. Eng. 2020, 8, 4341–4345. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, Y.; Li, J.; Shin, H.-d.; Du, G.; Shi, Z.; Chen, J.; Liu, L. Combinatorial synthetic pathway fine-tuning and comparative transcriptomics for metabolic engineering of Raoultella ornithinolytica BF60 to efficiently synthesize 2,5-furandicarboxylic acid. Biotechnol. Bioeng. 2018, 115, 2148–2155. [Google Scholar] [CrossRef]
- Hossain, G.S.; Yuan, H.; Li, J.; Shin, H.-d.; Wang, M.; Du, G.; Chen, J.; Liu, L. Metabolic engineering of Raoultella ornithinolytica BF60 for production of 2,5-furandicarboxylic acid from 5-hydroxymethylfurfural. Appl. Environ. Microbiol. 2017, 83, e02312-16. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Wen, M.; Zong, M.-H.; Li, N. Synergistic chemo/biocatalytic synthesis of 2,5-furandicarboxylic acid from 5-hydroxymethylfurfural. Catal. Commun. 2020, 139, 105979. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, Z.; Tan, H.; Xu, Q.; Ouyang, J. Synthesis of 2,5-furandicarboxylic acid by a TEMPO/laccase system coupled with Pseudomonas putida KT2440. RSC Adv. 2020, 10, 21781–21788. [Google Scholar] [CrossRef] [PubMed]
- Payne, K.A.P.; Marshall, S.A.; Fisher, K.; Cliff, M.J.; Cannas, D.M.; Yan, C.; Heyes, D.J.; Parker, D.A.; Larrosa, I.; Leys, D. Enzymatic carboxylation of 2-furoic acid yields 2,5-furandicarboxylic acid (FDCA). ACS Catal. 2019, 9, 2854–2865. [Google Scholar] [CrossRef]
- Kawanabe, K.; Aono, R.; Kino, K. 2,5-Furandicarboxylic acid production from furfural by sequential biocatalytic reactions. J. Biosci. Bioeng. 2021, 132, 18–24. [Google Scholar] [CrossRef]
- Lopez-Lorenzo, X.; Asem, H.; Stamm, A.; Subramaniyan, S.; Hakkarainen, M.; Syrén, P.-O. Whole-cell Mediated Carboxylation of 2-Furoic Acid Towards the Production of Renewable Platform Chemicals and Biomaterials. ChemCatChem 2023, 15, e202201483. [Google Scholar] [CrossRef]
- McKenna, S.M.; Mines, P.; Law, P.; Kovacs-Schreiner, K.; Birmingham, W.R.; Turner, N.J.; Leimkuhler, S.; Carnell, A.J. The continuous oxidation of HMF to FDCA and the immobilisation and stabilisation of periplasmic aldehyde oxidase (PaoABC). Green Chem. 2017, 19, 4660–4665. [Google Scholar] [CrossRef]
- Tan, H.; Zhou, F.; Liao, D.; Ouyang, J.; Zheng, Z. Improved biosynthesis of 2,5-furandicarboxylic acid through coupling of heterologous pathways in Escherichia coli and native pathways in Pseudomonas putida. Biochem. Eng. J. 2020, 161, 107657. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zong, M.-H.; Li, N. Whole-cell biocatalytic selective oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid. Green Chem. 2017, 19, 4544–4551. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Ou, X.-Y.; Fu, Y.-J.; Zong, M.-H.; Li, N. Efficient synthesis of 5-hydroxymethyl-2-furancarboxylic acid by Escherichia coli overexpressing aldehyde dehydrogenases. J. Biotechnol. 2020, 307, 125–130. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Wang, X.; Li, N.-W.; Guo, Z.-W.; Zong, M.-H.; Li, N. Furan carboxylic acids production with high productivity by cofactor-engineered whole-cell biocatalysts. ChemCatChem 2020, 12, 3257–3264. [Google Scholar] [CrossRef]
- Parikka, K.; Tenkanen, M. Oxidation of methyl-α-D-galactopyranoside by galactose oxidase: Products formed and optimization of reaction conditions for production of aldehyde. Carbohydr. Res. 2009, 344, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, W.R.; Toftgaard Pedersen, A.; Dias Gomes, M.; Bøje Madsen, M.; Breuer, M.; Woodley, J.M.; Turner, N.J. Toward scalable biocatalytic conversion of 5-hydroxymethylfurfural by galactose oxidase using coordinated reaction and enzyme engineering. Nat. Commun. 2021, 12, 4946. [Google Scholar] [CrossRef]
- Parikka, K.; Master, E.; Tenkanen, M. Oxidation with galactose oxidase: Multifunctional enzymatic catalysis. J. Mol. Catal. B Enzym. 2015, 120, 47–59. [Google Scholar] [CrossRef]
- Hoshino, T.; Yamabe, E.; Hawari, M.A.; Tamura, M.; Kanamaru, S.; Yoshida, K.; Koesoema, A.A.; Matsuda, T. Oxidation of aromatic and aliphatic aldehydes to carboxylic acids by Geotrichum candidum aldehyde dehydrogenase. Tetrahedron 2020, 76, 131387. [Google Scholar] [CrossRef]
- Serrano, A.; Calvino, E.; Carro, J.; Sanchez-Ruiz, M.I.; Canada, F.J.; Martinez, A.T. Complete oxidation of hydroxymethylfurfural to furandicarboxylic acid by aryl-alcohol oxidase. Biotechnol. Biofuels 2019, 12, 217. [Google Scholar] [CrossRef]
- He, A.; Dong, L.; Xu, N.; El-Hout, S.I.; Xia, J.; Qiu, Z.; He, J.; Deng, Y.; Liu, X.; Hu, L.; et al. Complete oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid by a novel enzyme–nanozyme hybrid catalyst. Chem. Eng. J. 2022, 449, 137797. [Google Scholar] [CrossRef]
- Dijkman, W.P.; Binda, C.; Fraaije, M.W.; Mattevi, A. Structure-based enzyme tailoring of 5-hydroxymethylfurfural oxidase. ACS Catal. 2015, 5, 1833–1839. [Google Scholar] [CrossRef]
- Cheng, A.-D.; Zong, M.-H.; Lu, G.-H.; Li, N. Solvent-promoted oxidation of aromatic alcohols/aldehydes to carboxylic acids by a laccase-TEMPO system: Efficient access to 2,5-furandicarboxylic acid and 5-methyl-2-pyrazinecarboxylic acid. Adv. Sustain. Syst. 2021, 5, 2000297. [Google Scholar] [CrossRef]
- Totaro, G.; Sisti, L.; Marchese, P.; Colonna, M.; Romano, A.; Gioia, C.; Vannini, M.; Celli, A. Current Advances in the Sustainable Conversion of 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic Acid. ChemSusChem 2022, 15, e202200501. [Google Scholar] [CrossRef]
- Liguori, F.; Barbaro, P.; Calisi, N. Continuous-Flow Oxidation of HMF to FDCA by Resin-Supported Platinum Catalysts in Neat Water. ChemSusChem 2019, 12, 2558–2563. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.R.; Cosgrove, S.C.; Turner, N.J.; Kapur, N.; Blacker, A.J. Highly productive oxidative biocatalysis in continuous flow by enhancing the aqueous equilibrium solubility of oxygen. Angew. Chem. Int. Ed. 2018, 57, 10535–10539. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Di, J.; He, Y. Efficient Synthesis of Furfuryl Alcohol from Corncob in a Deep Eutectic Solvent System. Processes 2022, 10, 1873. [Google Scholar] [CrossRef]
- He, W.; He, Y.; Ye, J. Efficient Synthesis of Biobased Furoic Acid from Corncob via Chemoenzymatic Approach. Processes 2022, 10, 677. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, F.-F.; Wang, J.-P.; Zong, M.-H.; Zheng, Z.-J.; Li, N. Concurrent Biocatalytic Oxidation of 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic Acid by Merging Galactose Oxidase with Whole Cells. Processes 2023, 11, 2261. https://doi.org/10.3390/pr11082261
Zhu F-F, Wang J-P, Zong M-H, Zheng Z-J, Li N. Concurrent Biocatalytic Oxidation of 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic Acid by Merging Galactose Oxidase with Whole Cells. Processes. 2023; 11(8):2261. https://doi.org/10.3390/pr11082261
Chicago/Turabian StyleZhu, Fan-Feng, Jian-Peng Wang, Min-Hua Zong, Zhao-Juan Zheng, and Ning Li. 2023. "Concurrent Biocatalytic Oxidation of 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic Acid by Merging Galactose Oxidase with Whole Cells" Processes 11, no. 8: 2261. https://doi.org/10.3390/pr11082261
APA StyleZhu, F.-F., Wang, J.-P., Zong, M.-H., Zheng, Z.-J., & Li, N. (2023). Concurrent Biocatalytic Oxidation of 5-Hydroxymethylfurfural into 2,5-Furandicarboxylic Acid by Merging Galactose Oxidase with Whole Cells. Processes, 11(8), 2261. https://doi.org/10.3390/pr11082261






