Effects of Centrifugation on the Oxidative Stability and Antioxidant Profile of Cold-Pressed Rapeseed Oil during Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
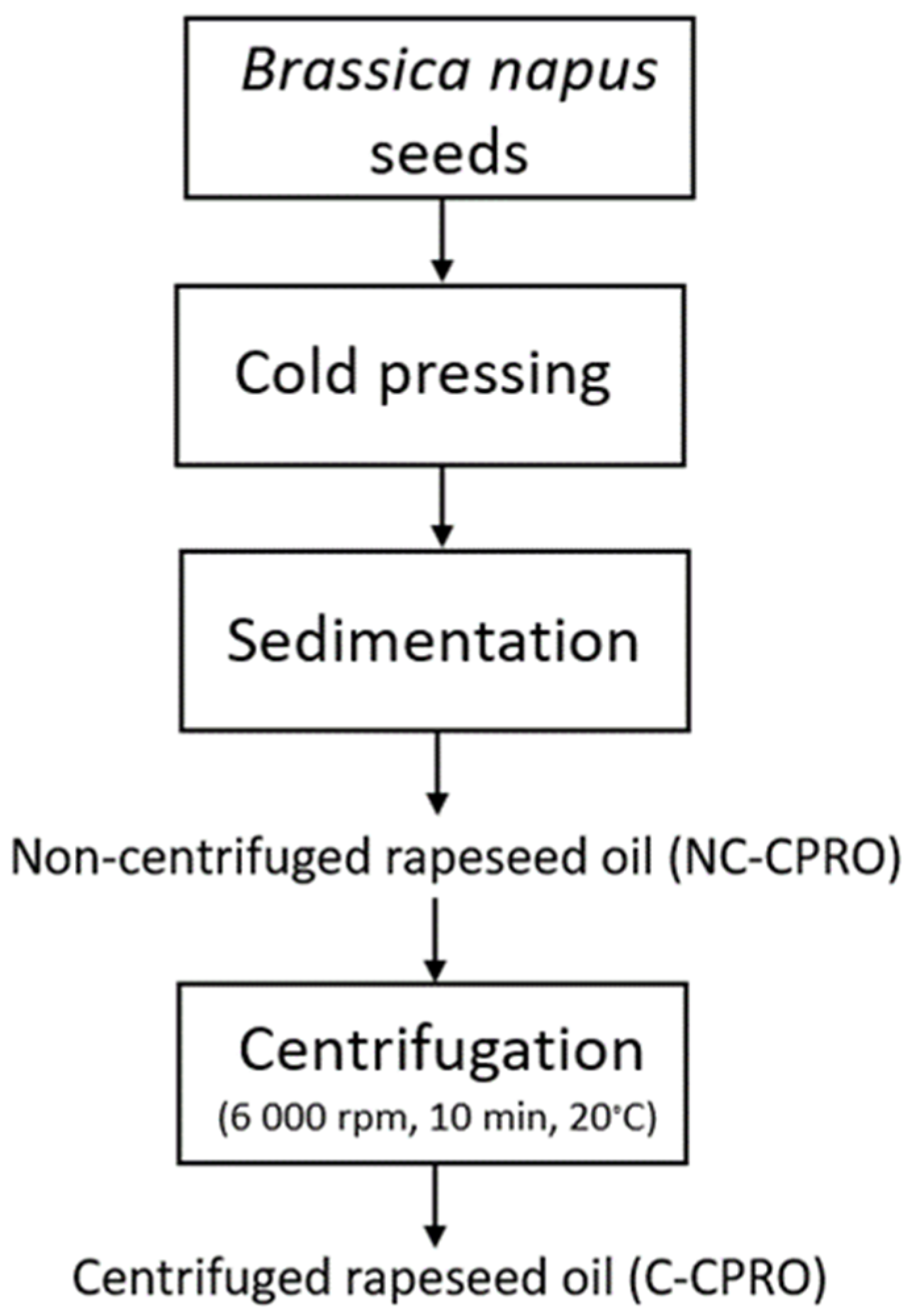
2.2. Methods
2.2.1. Preanalytical Oil Sample Preparation
2.2.2. Chemical Characteristic
Peroxide Value
Acid Value
2.2.3. Pigments and Total Polyphenols
Chlorophyll and Carotenoid Content
Total Polyphenols
2.2.4. Antioxidant Capability
FRAP (Ferric Reducing Antioxidant Power) Method
Determination of the Antioxidant Potential (DPPH)
ABTS Radical Scavenging Activity
3. Results and Discussion
3.1. Hydrolytic and Oxidative Profile of NC-CPRO and C-CPRO
3.2. Pigments and Total Phenols Content in NC-CPRO and C-CPRO
3.3. Antioxidant Properties of NC-CPRO and C-CPRO
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frieß, J.L.; Breckling, B.; Pascher, K.; Schröder, W. Case Study 2: Oilseed Rape (Brassica napus L.). In Gene Drives at Tipping Points; Von Gleich, A., Schröder, W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 103–145. ISBN 978-3-030-38933-8. [Google Scholar]
- Statista.Com. Available online: https://www.statista.com/statistics/263937/vegetable-oils-global-consumption/ (accessed on 20 June 2023).
- Symoniuk, E.; Ratusz, K.; Krygier, K. Evaluation of the Oxidative Stability of Cold-Pressed Rapeseed Oil by Rancimat and Pressure Differential Scanning Calorimetry Measurements. Eur. J. Lipid Sci. Technol. 2019, 121, 1800017. [Google Scholar] [CrossRef]
- Rabiej-Kozioł, D.; Tymczewska, A.; Szydłowska-Czerniak, A. Changes in Quality of Cold-Pressed Rapeseed Oil with Sinapic Acid Ester-Gelatin Films during Storage. Foods 2022, 11, 3341. [Google Scholar] [CrossRef]
- Chew, S.C. Cold-Pressed Rapeseed (Brassica napus) Oil: Chemistry and Functionality. Food Res. Int. 2020, 131, 108997. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F. Introduction to Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications. In Cold Pressed Oils; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–5. ISBN 978-0-12-818188-1. [Google Scholar]
- Shen, J.; Liu, Y.; Wang, X.; Bai, J.; Lin, L.; Luo, F.; Zhong, H. A Comprehensive Review of Health-Benefiting Components in Rapeseed Oil. Nutrients 2023, 15, 999. [Google Scholar] [CrossRef] [PubMed]
- Raboanatahiry, N.; Li, H.; Yu, L.; Li, M. Rapeseed (Brassica napus): Processing, Utilization, and Genetic Improvement. Agronomy 2021, 11, 1776. [Google Scholar] [CrossRef]
- Lin, L.; Allemekinders, H.; Dansby, A.; Campbell, L.; Durance-Tod, S.; Berger, A.; Jones, P.J. Evidence of Health Benefits of Canola Oil. Nutr. Rev. 2013, 71, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Frančáková, H.; Ivanišová, E.; Dráb, Š.; Krajčovič, T.; Tokár, M.; Mareček, J.; Musilová, J. Composition of Fatty Acids in Selected Vegetable Oils. Potravin. Slovak J. Food Sci. 2015, 9, 538–542. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Omega-6/Omega-3 Fatty Acid Ratio: Health Implications. Ol. Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Rokosik, E.; Dwiecki, K.; Siger, A. Nutritional Quality and Phytochemical Contents of Cold Pressed Oil Obtained from Chia, Milk Thistle, Nigella, and White and Black Poppy Seeds. Grasas Aceites 2020, 71, 368. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Konuskan, D.B.; Arslan, M.; Oksuz, A. Physicochemical Properties of Cold Pressed Sunflower, Peanut, Rapeseed, Mustard and Olive Oils Grown in the Eastern Mediterranean Region. Saudi J. Biol. Sci. 2019, 26, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Ghazani, S.M.; Marangoni, A.G. Minor Components in Canola Oil and Effects of Refining on These Constituents: A Review. J. Am. Oil Chem. Soc. 2013, 90, 923–932. [Google Scholar] [CrossRef]
- Grosshagauer, S.; Steinschaden, R.; Pignitter, M. Strategies to Increase the Oxidative Stability of Cold Pressed Oils. LWT 2019, 106, 72–77. [Google Scholar] [CrossRef]
- Kasprzak, M.; Rudzińska, M.; Przybylski, R.; Kmiecik, D.; Siger, A.; Olejnik, A. The Degradation of Bioactive Compounds and Formation of Their Oxidation Derivatives in Refined Rapeseed Oil during Heating in Model System. LWT 2020, 123, 109078. [Google Scholar] [CrossRef]
- Farhoosh, R.; Pazhouhanmehr, S. Relative Contribution of Compositional Parameters to the Primary and Secondary Oxidation of Canola Oil. Food Chem. 2009, 114, 1002–1006. [Google Scholar] [CrossRef]
- Jackson, V.; Penumetcha, M. Dietary Oxidised Lipids, Health Consequences and Novel Food Technologies That Thwart Food Lipid Oxidation: An Update. Int. J. Food Sci. Technol. 2019, 54, 1981–1988. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ratusz, K.; Ostrowska-Ligęza, E.; Krygier, K. Impact of Selected Chemical Characteristics of Cold-Pressed Oils on Their Oxidative Stability Determined Using the Rancimat and Pressure Differential Scanning Calorimetry Method. Food Anal. Methods 2018, 11, 1095–1104. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Ferreira, I.J.B.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M. Green Emerging Extraction Technologies to Obtain High-Quality Vegetable Oils from Nuts: A Review. Innov. Food Sci. Emerg. Technol. 2022, 76, 102931. [Google Scholar] [CrossRef]
- Symoniuk, E.; Wroniak, M.; Napiórkowska, K.; Brzezińska, R.; Ratusz, K. Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures. Foods 2022, 11, 1597. [Google Scholar] [CrossRef]
- Brkić Bubola, K.; Lukić, M.; Mofardin, I.; Butumović, A.; Koprivnjak, O. Filtered vs. Naturally Sedimented and Decanted Virgin Olive Oil during Storage: Effect on Quality and Composition. LWT 2017, 84, 370–377. [Google Scholar] [CrossRef]
- Guerrini, L.; Zanoni, B.; Breschi, C.; Angeloni, G.; Masella, P.; Calamai, L.; Parenti, A. Understanding Olive Oil Stability Using Filtration and High Hydrostatic Pressure. Molecules 2020, 25, 420. [Google Scholar] [CrossRef]
- Lozano-Sánchez, J.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Filtration Process of Extra Virgin Olive Oil: Effect on Minor Components, Oxidative Stability and Sensorial and Physicochemical Characteristics. Trends Food Sci. Technol. 2010, 21, 201–211. [Google Scholar] [CrossRef]
- Bubola, K.B.; Koprivnjak, O. Influence of Filtration on Composition of Olive Oils. In Processing and Impact on Active Components in Food; Elsevier: Amsterdam, The Netherlands, 2015; pp. 259–265. ISBN 978-0-12-404699-3. [Google Scholar]
- Kraljić, K.; Škevin, D.; Pospišil, M.; Obranović, M.; Neđeral, S.; Bosolt, T. Quality of Rapeseed Oil Produced by Conditioning Seeds at Modest Temperatures. J. Am. Oil Chem. Soc. 2013, 90, 589–599. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Loewen, S.; Tsao, R. Ultra-Performance Liquid Chromatographic Separation of Geometric Isomers of Carotenoids and Antioxidant Activities of 20 Tomato Cultivars and Breeding Lines. Food Chem. 2012, 132, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Use of Plantago Major Seed Mucilage as a Novel Edible Coating Incorporated with Anethum Graveolens Essential Oil on Shelf Life Extension of Beef in Refrigerated Storage. Int. J. Biol. Macromol. 2017, 94, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Sivarooban, T.; Hettiarachchy, N.S.; Johnson, M.G. Physical and Antimicrobial Properties of Grape Seed Extract, Nisin, and EDTA Incorporated Soy Protein Edible Films. Food Res. Int. 2008, 41, 781–785. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Di Pietro, M.E.; Mannu, A.; Mele, A. NMR Determination of Free Fatty Acids in Vegetable Oils. Processes 2020, 8, 410. [Google Scholar] [CrossRef]
- Wroniak, M.; Chlebowska-Śmigiel, A. Impact of Purity of Rapeseed and Oil Purification Method on Selected Properties of Cold-Pressed Oils. Zywnosc Nauka Technol. Jakosc Food Sci. Technol. Qual. 2013, 20, 133–149. [Google Scholar] [CrossRef]
- Poznań University of Life Sciences, Poland; Rokosik, E.; Dwiecki, K.; Siger, A. The Quality of Cold-Pressed Rapeseed Oil Obtained from Seeds of Brassica napus L. with Increased Moisture Content. Acta Sci. Pol. Technol. Aliment. 2019, 18, 205–218. [Google Scholar] [CrossRef]
- Madhujith, T.; Sivakanthan, S. Oxidative Stability of Edible Plant Oils. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 529–551. ISBN 978-3-319-78029-0. [Google Scholar]
- Issaoui, M.; Delgado, A.M. Grading, Labeling and Standardization of Edible Oils. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 9–52. ISBN 978-3-030-12472-4. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Lo Turco, V.; Litrenta, F.; Nava, V.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Potortì, A.G.; Di Bella, G. Effect of Filtration Process on Oxidative Stability and Minor Compounds of the Cold-Pressed Hempseed Oil during Storage; Chemistry and Materials Science. Antioxidants 2023, 12, 1231. [Google Scholar] [CrossRef] [PubMed]
- Mach, J. Chlorophyll Breakdown Branches Out: Identification of a Major Catabolic Route Involving Cytochrome P450 CYP89A9. Plant Cell 2013, 25, 1486. [Google Scholar] [CrossRef] [PubMed]
- Heaton, J.W.; Marangoni, A.G. Chlorophyll Degradation in Processed Foods and Senescent Plant Tissues. Trends Food Sci. Technol. 1996, 7, 8–15. [Google Scholar] [CrossRef]
- Eckardt, N.A. A New Chlorophyll Degradation Pathway. Plant Cell 2009, 21, 700. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.A.; nguez-Mosquera, M.I.M. Change in the Natural Ratio between Chlorophylls and Carotenoids in Olive Fruit during Processing for Virgin Olive Oil. J. Am. Oil Chem. Soc. 2001, 78, 133–138. [Google Scholar] [CrossRef]
- Wroniak, M.; Raczyk, M.; Kruszewski, B.; Symoniuk, E.; Dach, D. Effect of Deep Frying of Potatoes and Tofu on Thermo-Oxidative Changes of Cold Pressed Rapeseed Oil, Cold Pressed High Oleic Rapeseed Oil and Palm Olein. Antioxidants 2021, 10, 1637. [Google Scholar] [CrossRef] [PubMed]
- Daun, J.K. Spectrophotometric Analysis of Chlorophyll Pigments in Canola and Rapeseed Oils. Lipid Technol. 2012, 24, 134–136. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, C.; Zhou, Q.; Huang, F.; Liu, C.; Wang, H. Minor Components and Oxidative Stability of Cold-Pressed Oil from Rapeseed Cultivars in China. J. Food Compos. Anal. 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Fakourelis, N.; Lee, E.C.; Min, D.B. Effects of Chlorophyll and β-Carotene on the Oxidation Stability of Olive Oil. J. Food Sci. 1987, 52, 234–235. [Google Scholar] [CrossRef]
- Gandul-Rojas, B.; Roca, M.; Gallardo-Guerrero, L. Chlorophylls and Carotenoids in Food Products from Olive Tree. In Products from Olive Tree; Boskou, D., Clodoveo, M.L., Eds.; InTech: Garching, Germany, 2016; ISBN 978-953-51-2724-6. [Google Scholar]
- Vidal, A.M.; Alcalá, S.; De Torres, A.; Moya, M.; Espínola, F. Centrifugation, Storage, and Filtration of Olive Oil in an Oil Mill: Effect on the Quality and Content of Minority Compounds. J. Food Qual. 2019, 2019, 7381761. [Google Scholar] [CrossRef]
- Dordevic, D.; Dordevic, S.; Ćavar-Zeljković, S.; Kulawik, P.; Kushkevych, I.; Tremlová, B.; Kalová, V. Monitoring the Quality of Fortified Cold-Pressed Rapeseed Oil in Different Storage Conditions. Eur. Food Res. Technol. 2022, 248, 2695–2705. [Google Scholar] [CrossRef]
- Fadda, A.; Sanna, D.; Sakar, E.H.; Gharby, S.; Mulas, M.; Medda, S.; Yesilcubuk, N.S.; Karaca, A.C.; Gozukirmizi, C.K.; Lucarini, M.; et al. Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils. Sustainability 2022, 14, 849. [Google Scholar] [CrossRef]
- Ghanbari Shendi, E.; Sivri Ozay, D.; Ozkaya, M.T. Effects of Filtration Process on the Minor Constituents and Oxidative Stability of Virgin Olive Oil during 24 Months Storage Time. OCL 2020, 27, 37. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Georgiou, A.; Koidis, A.; Boskou, D. Loss of Stability of “Veiled” (Cloudy) Virgin Olive Oils in Storage. Food Chem. 2005, 93, 377–383. [Google Scholar] [CrossRef]
- Fortini, M.; Migliorini, M.; Cherubini, C.; Cecchi, L.; Guerrini, L.; Masella, P.; Parenti, A. Shelf Life and Quality of Olive Oil Filtered without Vertical Centrifugation. Eur. J. Lipid Sci. Technol. 2016, 118, 1213–1222. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A. Rapeseed and Its Products—Sources of Bioactive Compounds: A Review of Their Characteristics and Analysis. Crit. Rev. Food Sci. Nutr. 2013, 53, 307–330. [Google Scholar] [CrossRef]
- Hunyadi, A. The Mechanism(s) of Action of Antioxidants: From Scavenging Reactive Oxygen/Nitrogen Species to Redox Signaling and the Generation of Bioactive Secondary Metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef]
- Thanan, R.; Oikawa, S.; Hiraku, Y.; Ohnishi, S.; Ma, N.; Pinlaor, S.; Yongvanit, P.; Kawanishi, S.; Murata, M. Oxidative Stress and Its Significant Roles in Neurodegenerative Diseases and Cancer. Int. J. Mol. Sci. 2014, 16, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kathariya, R.; Bansal, S.; Singh, A.; Shahakar, D. Dietary Antioxidants and Their Indispensable Role in Periodontal Health. J. Food Drug Anal. 2016, 24, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Yoshida, M.; Arita, M. Omega-3 Fatty Acid-Derived Mediators That Control Inflammation and Tissue Homeostasis. Int. Immunol. 2019, 31, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Siger, A.; Kaczmarek, A.; Rudzińska, M. Antioxidant Activity and Phytochemical Content of Cold-pressed Rapeseed Oil Obtained from Roasted Seeds. Eur. J. Lipid Sci. Technol. 2015, 117, 1225–1237. [Google Scholar] [CrossRef]
- Othón-Díaz, E.D.; Fimbres-García, J.O.; Flores-Sauceda, M.; Silva-Espinoza, B.A.; López-Martínez, L.X.; Bernal-Mercado, A.T.; Ayala-Zavala, J.F. Antioxidants in Oak (Quercus sp.): Potential Application to Reduce Oxidative Rancidity in Foods. Antioxidants 2023, 12, 861. [Google Scholar] [CrossRef]
- Wang, S.; Yang, R.; Li, H.; Jiang, J.; Zhang, L.; Zhang, Q.; Li, P. Evaluation and Comparison of in Vitro Antioxidant Activities of Unsaponifiable Fraction of 11 Kinds of Edible Vegetable Oils. Food Sci. Nutr. 2018, 6, 2355–2362. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Shahinuzzaman, M.; Yaakob, Z.; Anuar, F.H.; Akhtar, P.; Kadir, N.H.A.; Hasan, A.K.M.; Sobayel, K.; Nour, M.; Sindi, H.; Amin, N.; et al. In Vitro Antioxidant Activity of Ficus carica L. Latex from 18 Different Cultivars. Sci. Rep. 2020, 10, 10852. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and Development of DPPH Method of Antioxidant Assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological Aspects about in Vitro Evaluation of Antioxidant Properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
| Months of Storage | NC-CPRO | C-CPRO | % of Mean Change after 3 Months of Storage * (NC-CPRO; C-CPRO) | % of Mean Change after 6 Months of Storage * (NC-CPRO; C-CPRO) |
|---|---|---|---|---|
| Acid Value [mg KOH/g of the oil] | ||||
| T0 (0 months) | 12.44 ± 9.53 a | 12.58 ± 7.42 a | -;- | -;- |
| T1 (3 months) | 49.69 ± 0.82 b | 20.63 ± 0.73 a | 299.44;63.99 | -;- |
| T2 (6 months) | 83.59 ± 2.38 c | 28.03 ± 1.57 a | -;- | 571.95;122.81 |
| Peroxide Value [meqO2/kg of the oil] | ||||
| T0 (0 months) | 1.77 ± 0.27 a | 1.72 ± 0.33 a | -;- | -;- |
| T1 (3 months) | 8.49 ± 0.13 c | 10.08 ± 0.15 d | 379.66;486.05 | -;- |
| T2 (6 months) | 29.89 ± 0.14 b | 29.20 ± 0.29 b | -;- | 1588.70;1597.67 |
| Months of Storage | NC-CPRO | C-CPRO | % of Mean Change after 3 Months of Storage * (NC-CPRO; C-CPRO) | % of Mean Change after 6 Months of Storage * (NC-CPRO; C-CPRO) |
|---|---|---|---|---|
| Chlorophyll [mg/kg of the oil] | ||||
| T0 (0 months) | 18.79 ± 0.20 a,c | 18.41 ± 0.32 a | -;- | -;- |
| T1 (3 months) | 18.90 ± 0.27 c | 17.11 ± 0.34 b | 0.59; −7.06 | -;- |
| T2 (6 months) | 18.90 ± 0.27 a,c | 16.61 ± 0.53 b | -;- | 0.59; −9.78 |
| Carotenes [mg/kg] | ||||
| T0 (0 months) | 180.75 ± 0.96 a | 178.10 ± 3.67 a | -;- | -;- |
| T1 (3 months) | 157.77 ± 1.83 b | 159.10 ± 0.30 b | −12.71; −10.67 | -;- |
| T2 (6 months) | 144.39 ± 4.37 c | 146.53 ± 4.54 c | -;- | −20.12; −17.73 |
| Total polyphenols [mg GAE/g of the oil] | ||||
| T0 (0 months) | 0.169 ± 0.088 a,b | 0.147 ± 0.012 a | -;- | -;- |
| T1 (3 months) | 0.167 ± 0.002 a | 0.425 ± 0.008 c | −1.18; 189.12 | -;- |
| T2 (6 months) | 0.024 ± 0.001 b | 0.011 ± 0.001 d | -;- | −85.80; −92.52 |
| Months of Storage | NC-CPRO | C-CPRO | % of Mean Change after 3 Months of Storage * (NC-CPRO; C-CPRO) | % of Mean Change after 6 Months of Storage * (NC-CPRO; C-CPRO) |
|---|---|---|---|---|
| DPPH inhibition [%] | ||||
| T0 (0 months) | 53.04 ± 1.90 a | 53.48 ± 1.80 a | -;- | -;- |
| T1 (3 months) | 46.34 ± 4.85 a,b | 39.99 ± 6.38 b | −12.63; −25.22 | -;- |
| T2 (6 months) | 17.57 ± 2.63 c | 15.73 ± 2.63 c | -;- | −66.87; −70.59 |
| FRAP [µg/mL] | ||||
| T0 (0 months) | 156.91 ± 10.92 a | 139.34 ± 9.18 a | -;- | -;- |
| T1 (3 months) | 194.33 ± 52.64 a | 141.43 ± 15.74 a | 23.85; 1.50 | -;- |
| T2 (6 months) | 46.21 ± 2.95 b | 18.33 ± 1.07 c | -;- | −70.55; −86.85 |
| ABTS [%] | ||||
| T0 (0 months) | 2.40 ±0.11 a | 2.50 ± 0.07 a,b | -;- | -;- |
| T1 (3 months) | 3.23 ± 0.47 b,c | 3.36 ± 0.22 c | 34.58;34.4 | -;- |
| T2 (6 months) | 1.10 ± 0.37 d | 1.94 ± 0.75 a,b,d | -;- | −54.17; −22.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dordevic, D.; Gablo, N.; Dordevic Janickova, S.; Tremlova, B. Effects of Centrifugation on the Oxidative Stability and Antioxidant Profile of Cold-Pressed Rapeseed Oil during Storage. Processes 2023, 11, 2224. https://doi.org/10.3390/pr11072224
Dordevic D, Gablo N, Dordevic Janickova S, Tremlova B. Effects of Centrifugation on the Oxidative Stability and Antioxidant Profile of Cold-Pressed Rapeseed Oil during Storage. Processes. 2023; 11(7):2224. https://doi.org/10.3390/pr11072224
Chicago/Turabian StyleDordevic, Dani, Natalia Gablo, Simona Dordevic Janickova, and Bohuslava Tremlova. 2023. "Effects of Centrifugation on the Oxidative Stability and Antioxidant Profile of Cold-Pressed Rapeseed Oil during Storage" Processes 11, no. 7: 2224. https://doi.org/10.3390/pr11072224
APA StyleDordevic, D., Gablo, N., Dordevic Janickova, S., & Tremlova, B. (2023). Effects of Centrifugation on the Oxidative Stability and Antioxidant Profile of Cold-Pressed Rapeseed Oil during Storage. Processes, 11(7), 2224. https://doi.org/10.3390/pr11072224








