Thermochemical Upgrading of Heavy Crude Oil in Reservoir Conditions
Abstract
1. Introduction
- The selection of catalysts and their optimal concentrations.
- The choice of surfactants with suitable physicochemical characteristics, such as thermal stability, salt resistance, etc.
- Investigation of the catalyst and surfactant’s potential for reservoir plugging and consideration of their environmental impact.
- Exploration and testing of more cost-effective methods for reservoir heating.
2. Materials and Methods
2.1. Surfactant Synthesis
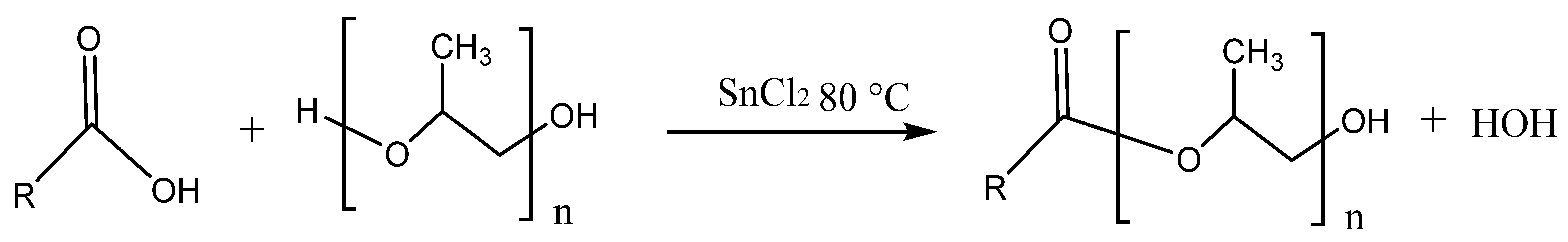
2.2. Thermal Analysis of Surfactants
2.3. Fourier-Transform Infrared (FT-IR) Spectral Analysis
2.4. Activity of the Synthesized Surfactant
2.4.1. Upgrading Experiments of Heavy Oil with Surfactant
2.4.2. SARA Analysis
2.4.3. Elemental Analysis
2.4.4. Viscosity Measurements
2.4.5. Analysis of Evolved Gases by Gas Chromatography (GC)
3. Results and Discussion
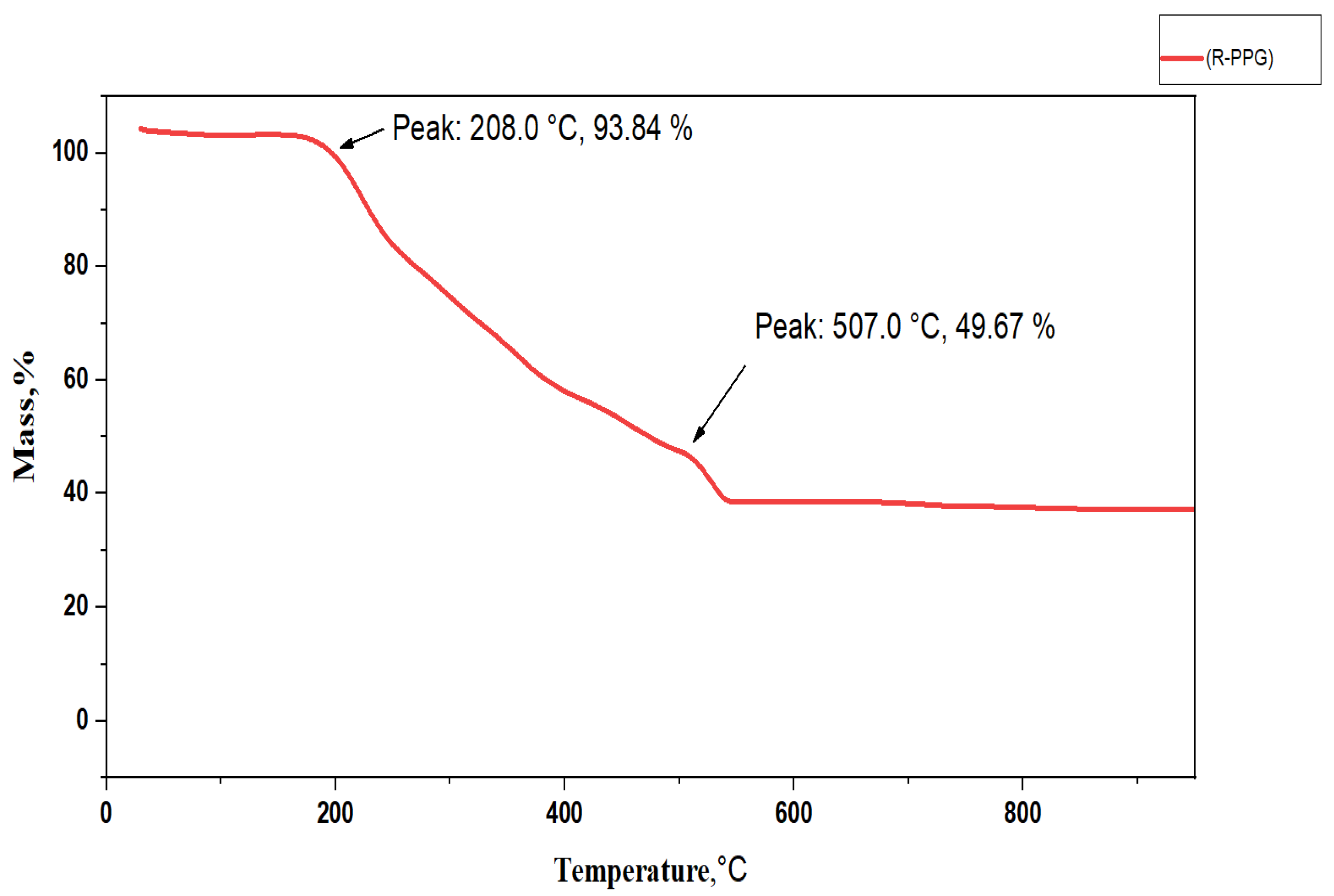
3.1. Thermal Stability of Surfactants (R-PEG)
3.2. FT-IR Analysis of Surfactants (R-PPG)
3.3. Analysis of Chemical Composition and Elemental Analysis
Gas Composition of the Products
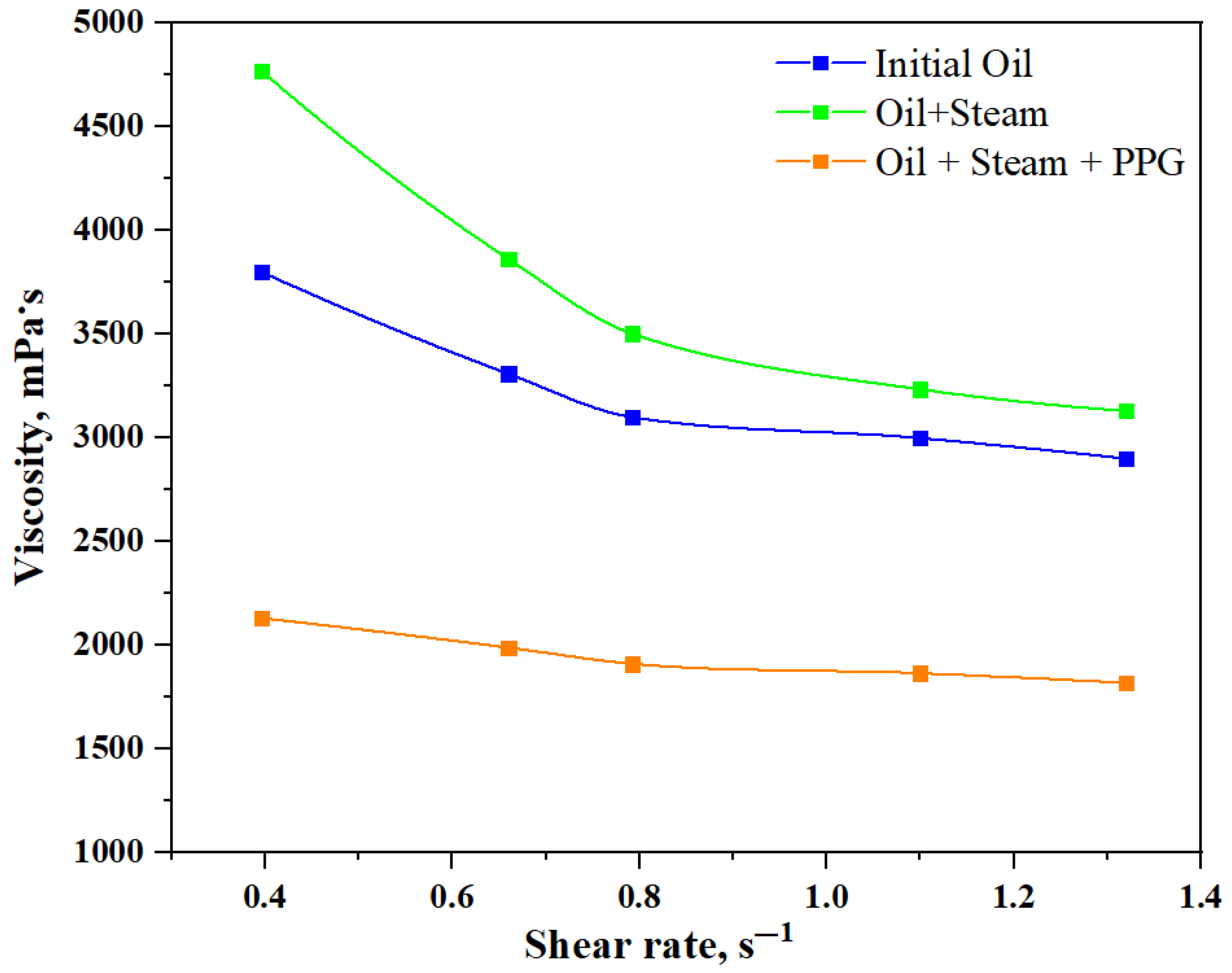
3.4. Dynamic Viscosity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chilingarian, G.V. Bitumens, Asphalts, and Tar Sands; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 0080868614. [Google Scholar]
- Wiehe, I.A.; Liang, K.S. Asphaltenes, resins, and other petroleum macromolecules. Fluid Phase Equilibria 1996, 117, 201–210. [Google Scholar] [CrossRef]
- Hughey, C.A.; Rodgers, R.P.; Marshall, A.G. Resolution of 11,000 Compositionally Distinct Components in a Single Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrum of Crude Oil. Anal. Chem. 2002, 74, 4145–4149. [Google Scholar] [CrossRef] [PubMed]
- Mullins, O.C.; Sheu, E.Y.; Hammami, A.; Marshall, A.G. Asphaltenes, Heavy Oils, and Petroleomics; Springer Science & Business Media: Berlin, Germany, 2007; ISBN 0387689036. [Google Scholar]
- Mullins, O.C.; Sheu, E.Y. Structures and Dynamics of Asphaltenes; Springer Science & Business Media: Berlin, Germany, 1999; ISBN 0306459302. [Google Scholar]
- Wang, Y.; Wang, Q.; Yang, D.; Hu, T.; Zhang, L.; Jiang, C. Synthesis and properties evaluation of novel Gemini surfactant with temperature tolerance and salt resistance for heavy oil. J. Mol. Liq. 2023, 382, 121851. [Google Scholar] [CrossRef]
- Kholmurodov, T.; Aliev, F.; Mirzaev, O.; Dengaev, A.; Tajik, A.; Vakhin, A. Hydrothermal In-Reservoir Upgrading of Heavy Oil in the Presence of Non-Ionic Surfactants. Processes 2022, 10, 2176. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. Aquathermolysis of heavy oil: A review and perspective on catalyst development. Fuel 2015, 157, 219–231. [Google Scholar] [CrossRef]
- Hyne, J.B. Aquathermolysis: A Synopsis of Work on the Chemical Reaction between Water (Steam) and Heavy Oil Sands during Simulated Steam Stimulation; AOSTRA Library and Information Service: Edmonton, Canada, 1986. [Google Scholar]
- Hyne, J.B.; Clark, P.D.; Clarke, R.A.; Koo, J.; Greidanus, J.W. Aquathermolysis of Heavy Oils Review Technology; INTEVEP: Caracas, Venezuela, 1982; p. 2. [Google Scholar]
- Qu, X.; Li, Y.; Li, S.; Wang, J.; Xu, H.; Li, Z. Thermal cracking, aquathermolysis, and their upgrading effects of Mackay River oil sand. J. Pet. Sci. Eng. 2021, 201, 108473. [Google Scholar] [CrossRef]
- Usman, M.; Galadima, A.; Faruq, U.Z.; Jibril, B.Y. Catalytic Influences of Dispersed Copper Complex Ion on Aquathermolysis of Heavy Crude Oil. Int. J. Sci. Glob. Sustain. 2016, 2, 10. [Google Scholar]
- Maity, S.K.; Ancheyta, J.; Marroquín, G. Catalytic Aquathermolysis Used for Viscosity Reduction of Heavy Crude Oils: A Review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Mukhamatdinov, I.I.; Vakhin, A.V.; Ivanova, A.G.; Voronina, E.V. Composition of Aquathermolysis Catalysts Forming in Situ from Oil-Soluble Catalyst Precursor Mixtures. J. Pet. Sci. Eng. 2018, 169, 44–50. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Sharifullin, A.V.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Catalytic Aquathermolysis of Boca de Jaruco Heavy Oil with Nickel-Based Oil-Soluble Catalyst. Processes 2020, 8, 532. [Google Scholar] [CrossRef]
- Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-Situ Heavy Oil Aquathermolysis in the Presence of Nanodispersed Catalysts Based on Transition Metals. Processes 2021, 9, 127. [Google Scholar] [CrossRef]
- Fan, H.; Liu, Y. Downhole Catalyst Upgrades Heavy Oil. Oil Gas J. 2002, 100, 60–62. [Google Scholar]
- Li, W.; Zhu, J.; Qi, J. Application of nano-nickel catalyst in the viscosity reduction of Liaohe extra-heavy oil by aqua-thermolysis. J. Fuel Chem. Technol. 2007, 35, 176–180. [Google Scholar] [CrossRef]
- Lin, D.; Zhu, H.; Wu, Y.; Lu, T.; Liu, Y.; Chen, X.; Peng, C.; Yang, C.; Feng, X. Morphological insights into the catalytic aquathermolysis of crude oil with an easily prepared high-efficiency Fe3O4-containing catalyst. Fuel 2019, 245, 420–428. [Google Scholar] [CrossRef]
- Aliev, F.; Mirzaev, O.; Kholmurodov, T.; Slavkina, O.; Vakhin, A. Utilization of Carbon Dioxide via Catalytic Hydrogenation Processes during Steam-Based Enhanced Oil Recovery. Processes 2022, 10, 2306. [Google Scholar] [CrossRef]
- Aliev, F.; Kholmurodov, T.; Mirzayev, O.; Tajik, A.; Mukhamadiev, N.; Slavkina, O.; Nourgalieva, N.; Vakhin, A. Enhanced Oil Recovery by In-Reservoir Hydrogenation of Carbon Dioxide Using Na-Fe3O4. Catalysts 2023, 13, 153. [Google Scholar] [CrossRef]
- Maini, B.B.; Ma, V. Thermal Stability of Surfactants for Steamflood Applications. In Proceedings of the SPE Oilfield and Geothermal Chemistry Symposium, Phoenix, Arizona, 9–11 April 1985; OnePetro: Richardson, TX, USA, 1985. [Google Scholar]
- Zeidani, K.; Gupta, S. Hydrocarbon Recovery from Bituminous Sands with Injection of Surfactant Vapour. U.S. Patent 20130081808A1, 4 April 2013. [Google Scholar]
- Szabo, G.H.; Peats, A.; Felix, J.; Samuel, M.; Abdallah, W. Chemically Enhanced Thermal Recovery of Heavy Oil. U.S. Patent 20090159288A1, 25 June 2009. [Google Scholar]
- Aoudia, M.; Al-Shibli, M.N.; Al-Kasimi, L.H.; Al-Maamari, R.; Al-Bemani, A. Novel surfactants for ultralow interfacial tension in a wide range of surfactant concentration and temperature. J. Surfactants Deterg. 2006, 9, 287–293. [Google Scholar] [CrossRef]
- Jarrahian, K.; Seiedi, O.; Sheykhan, M.; Sefti, M.V.; Ayatollahi, S. Wettability alteration of carbonate rocks by surfactants: A mechanistic study. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 1–10. [Google Scholar] [CrossRef]
- Mousavi, S.-P.; Hemmati-Sarapardeh, A.; Norouzi-Apourvari, S.; Jalalvand, M.; Schaffie, M.; Ranjbar, M. Toward mechanistic understanding of wettability alteration in calcite and dolomite rocks: The effects of resin, asphaltene, anionic surfactant, and hydrophilic nano particles. J. Mol. Liq. 2021, 321, 114672. [Google Scholar] [CrossRef]
- Mpelwa, M.; Tang, S.; Jin, L.; Hu, R. New sulfonate Gemini surfactants: Synthesis and evaluation for enhanced oil recovery applications. J. Dispers. Sci. Technol. 2020, 41, 2091–2099. [Google Scholar] [CrossRef]
- Sheng, J.J. Status of surfactant EOR technology. Petroleum 2015, 1, 97–105. [Google Scholar] [CrossRef]
- Ahmadi, M.; Chen, Z. Insight into the Interfacial Behavior of Surfactants and Asphaltenes: Molecular Dynamics Simulation Study. Energy Fuels 2020, 34, 13536–13551. [Google Scholar] [CrossRef]
- Ahmadi, M.; Chen, Z. Challenges and future of chemical assisted heavy oil recovery processes. Adv. Colloid Interface Sci. 2020, 275, 102081. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.A.; Sheng, J. Performance improvement of ionic surfactant flooding in carbonate rock samples by use of nanoparticles. Pet. Sci. 2016, 13, 725–736. [Google Scholar] [CrossRef]
- ASTM D5134-98; Standard Test Method for Detailed Analysis of Petroleum Naphthas through N-nonane by Capillary Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2008.
- Tirado, A.; Félix, G.; Al-Muntaser, A.A.; Chemam, M.S.; Yuan, C.; Varfolomeev, M.A.; Ancheyta, J. Molecular Asphaltene Transformations during Aquathermolysis of Heavy Crude Oil: Analysis of the Literature Data. Energy Fuels 2023, 37, 7927–7944. [Google Scholar] [CrossRef]
- Tirado, A.; Yuan, C.; Varfolomeev, M.A.; Ancheyta, J. Kinetic modeling of aquathermolysis for upgrading of heavy oils. Fuel 2022, 310, 122286. [Google Scholar] [CrossRef]
- Kholmurodov, T.; Vakhin, A.; Aliev, F.; Galyametdinov, Y.; Mirzayev, O.; Tajik, A.; Gafurov, M. Influence of Anionic and Amphoteric Surfactants on Heavy Oil Upgrading Performance with Nickel Tallate under Steam Injection Processes. Ind. Eng. Chem. Res. 2023, 62, 10277–10289. [Google Scholar] [CrossRef]



| Elemental Analysis, wt.% | ||||||
|---|---|---|---|---|---|---|
| Ashalcha Oil | C | H | N | S | O | H/C |
| 79.01 | 8.74 | 0.45 | 4.85 | 5.85 | 1.32 | |
| Oil + Steam | 81.69 | 11.96 | 0.00 | 4.76 | 1.59 | 1.74 |
| Oil + Steam + R-PPG | 80.80 | 12.15 | 0.00 | 4.57 | 2.48 | 1.79 |
| Gas Composition, wt.% | Samples | |
|---|---|---|
| Oil + Steam | Oil + Steam + R-PEG | |
| CH4 | 1.68 | 1.64 |
| C2–C4 | 5.06 | 9.75 |
| H2 | 1.72 | 0.19 |
| O2 | 2.43 | 6.5 |
| CO2 | 11.56 | 17.09 |
| H2S | 31.04 | 6.71 |
| Other gases | 46.50 | 58.07 |
| Total | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kholmurodov, T.; Mirzaev, O.; Affane, B.; Tajik, A.; Romanova, K.; Galyametdinov, Y.; Dengaev, A.; Vakhin, A. Thermochemical Upgrading of Heavy Crude Oil in Reservoir Conditions. Processes 2023, 11, 2156. https://doi.org/10.3390/pr11072156
Kholmurodov T, Mirzaev O, Affane B, Tajik A, Romanova K, Galyametdinov Y, Dengaev A, Vakhin A. Thermochemical Upgrading of Heavy Crude Oil in Reservoir Conditions. Processes. 2023; 11(7):2156. https://doi.org/10.3390/pr11072156
Chicago/Turabian StyleKholmurodov, Temurali, Oybek Mirzaev, Boudkhil Affane, Arash Tajik, Ksenia Romanova, Yuriy Galyametdinov, Aleksey Dengaev, and Alexey Vakhin. 2023. "Thermochemical Upgrading of Heavy Crude Oil in Reservoir Conditions" Processes 11, no. 7: 2156. https://doi.org/10.3390/pr11072156
APA StyleKholmurodov, T., Mirzaev, O., Affane, B., Tajik, A., Romanova, K., Galyametdinov, Y., Dengaev, A., & Vakhin, A. (2023). Thermochemical Upgrading of Heavy Crude Oil in Reservoir Conditions. Processes, 11(7), 2156. https://doi.org/10.3390/pr11072156









