Integrated a Fused Silica Capillary Cell and In Situ Raman Spectroscopy for Determining the Solubility of CO2 in n-Decane and n-Decane + n-Hexane System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
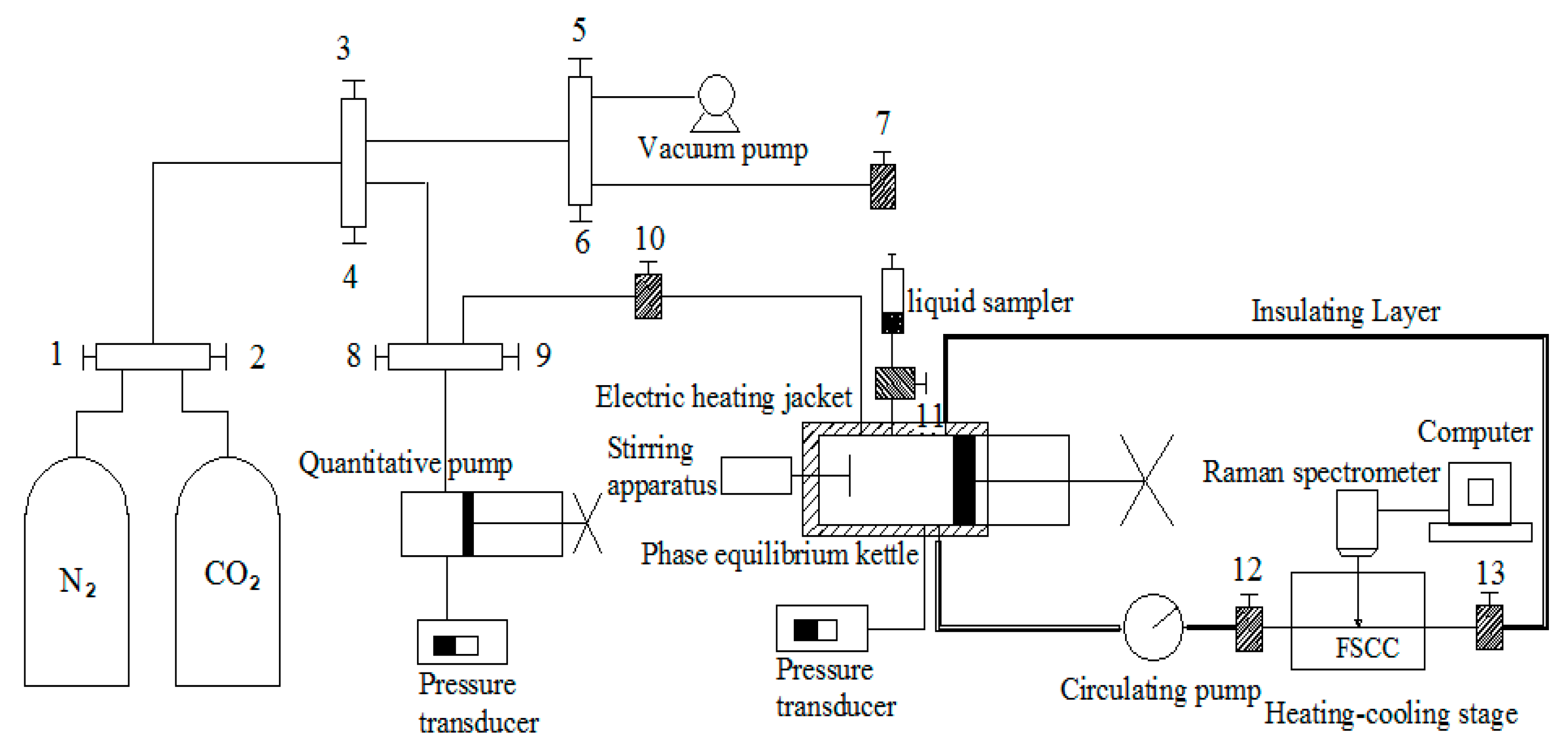
2.2. Experimental Apparatus
2.3. Experimental Procedure
2.4. Method of Analysis
3. Results and Discussion
3.1. Phase Equilibrium of CO2 + n-Decane or CO2 + n-Decane + n-Hexane
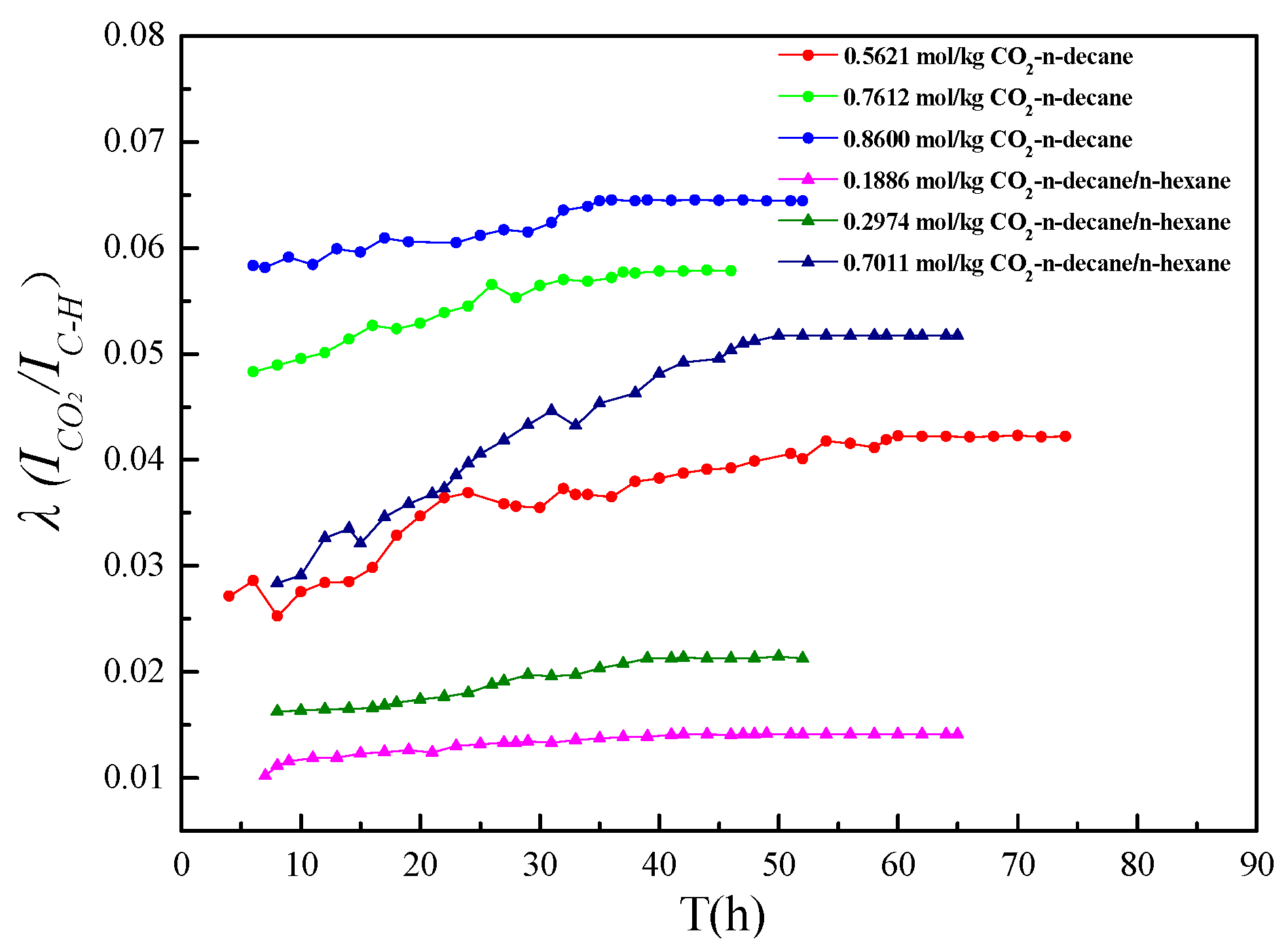
3.2. Relationship between CO2 Concentration and the Raman Peak Intensity Ratio
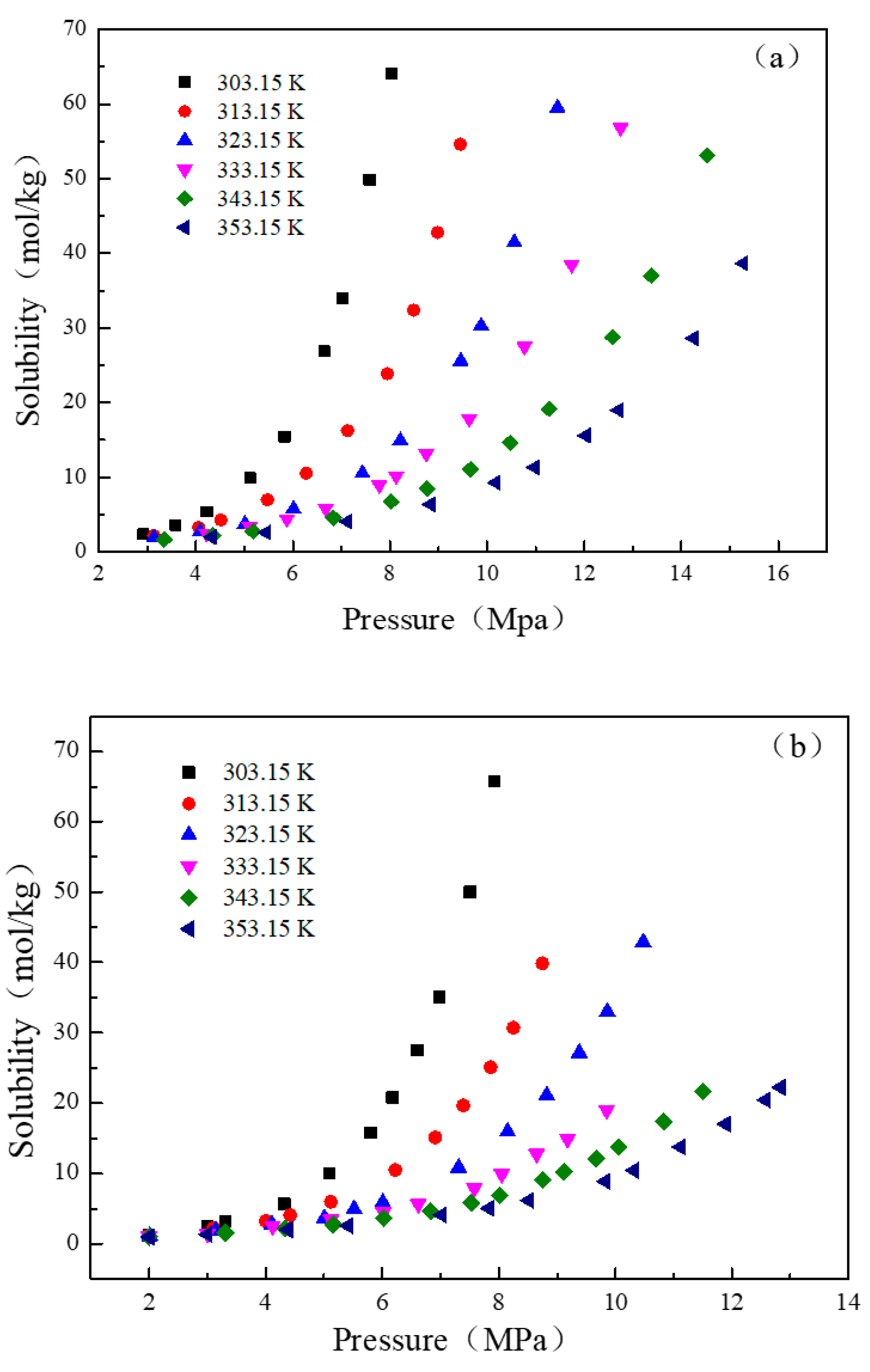
3.3. The Solubility of CO2 in n-Decane and n-Decane + n-Hexane Systems
3.4. The Solubility Prediction Model of CO2 + n-Decane System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Obama, B. The irreversible momentum of clean energy. Science 2017, 355, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Haszeldine, R.S.; Flude, S.; Johnson, G.; Scott, V. Negative emissions technologies and carbon capture and storage to achieve the Paris Agreement commitments. Phil. Trans. R. Soc. A 2018, 376, 20160447. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Henni, A. Solubility of carbon dioxide (CO2) in four bis (Trifluoromethyl-Sulfonyl) imide based ionic liquids. Fluid Phase Equilib. 2020, 524, 112757. [Google Scholar] [CrossRef]
- Boot-Handford, M.; Abanades, J.; Anthony, E.; Blunt, M. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Mosavat, N.; Abedini, A.; Torabi, F. Phase Behaviour of CO2-Brine and CO2-Oil Systems for CO2 Storage and Enhanced Oil Recovery: Experimental Studies. Energy Procedia 2014, 63, 5631–5645. [Google Scholar] [CrossRef]
- Vikara, D.; Shih, C.Y.; Lin, S.; Guinan, A.; Grant, T.; Morgan, D.; Remson, D. U.S. DOE’s Economic Approaches and Resources for Evaluating the Cost of Implementing Carbon Capture, Utilization, and Storage (CCUS). J. Sustain. Energy Eng. 2017, 5, 307–340. [Google Scholar] [CrossRef]
- Moody Mark, A.; Fukai Isis, M. Economic analysis of CO2-enhanced oil recovery in Ohio: Implications for carbon capture, utilization, and storage in the Appalachian Basin region. Int. J. Greenh. Gas Control 2016, 52, 357–377. [Google Scholar]
- Stuardi, F.M.; MacPherson, F.; Leclaire, J. Integrated CO2 capture and utilization: A priority research direction. Green Sus. Chem. 2019, 16, 71–76. [Google Scholar]
- Dowell, N.M.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Jia, B.; Tsau, J.-S.; Barati, R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Gui, X.; Wang, W.; Gao, Q.; Yun, Z.; Fan, M.H.; Chen, Z.H. Measurement and correlation of high pressure phase equilibria for CO2 + alkanes and CO2 + crude oil systems. J. Chem. Eng. Data 2017, 62, 3807–3822. [Google Scholar] [CrossRef]
- Panayiotou, C. Interfacial tension and interfacial profiles: An equation-of-state approach. J. Colloid Interface Sci. 2003, 267, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Arabloo, M.; Kamari, A.; Mohammadi, A.H. Modeling of CO2 solubility in crude oil during carbon dioxide enhanced oil recovery using gene expression programming. Fuel 2017, 210, 768–782. [Google Scholar] [CrossRef]
- Wang, X.; Strycker, A. Evaluation of CO2 Injection with Three Hydrocarbon Phases. In Proceedings of the 2000 SPE International Oil and Gas Conference and Exhibition, Beijing, China, 7–10 November 2000; pp. 2–11. [Google Scholar]
- Kavousi, A.; Torabi, F.; Chan, C.W.; Shirif, E. Experimental measurement and parametric study of CO2 solubility and molecular diffusivity in heavy crude oil systems. Fluid Phase Equilib. 2014, 371, 57–66. [Google Scholar] [CrossRef]
- Varet, G.; Montel, F.; Nasri, D.; Daridon, J.-L. Gas solubility measurement in heavy oil and extra heavy oil at vapor extraction (VAPEX) conditions. Energy Fuels 2013, 27, 2528–2535. [Google Scholar] [CrossRef]
- Eustaquio-Rincón, R.; Trejo, A. Solubility of n-octadecane in supercritical carbon dioxide at 310, 313, 333, and 353 K, in the range 10–20 MPa. Fluid Phase Equilib. 2001, 185, 231–239. [Google Scholar] [CrossRef]
- Liu, J.; Qin, Z.; Wang, G.; Hou, X.; Wang, J. Critical Properties of Binary and Ternary Mixtures of Hexane + Methanol, Hexane + Carbon Dioxide, Methanol + Carbon Dioxide, and Hexane + Carbon Dioxide + Methanol. J. Chem. Eng. Data 2003, 48, 1610–1613. [Google Scholar] [CrossRef]
- Mutelet, F.; Vitu, S.; Privat, R.; Jaubert, J.-N. Solubility of CO2 in branched alkanes in order to extend the PPR78 model (predictive 1978, Peng-Robinson EOS with temperature-dependent kij calculated through a group contribution method) to such systems. Fluid Phase Equilib. 2005, 238, 157–168. [Google Scholar] [CrossRef]
- Camacho-Camacho, L.E.; Galicia-Luna, L.A.; Elizalde-Solis, O.; Martínez-Ramírez, Z. New isothermal vapor–liquid equilibria for the CO2 + n-nonane, and CO2 + n-undecane systems. Fluid Phase Equilib. 2007, 259, 45–50. [Google Scholar] [CrossRef]
- Luther, S.K.; Schuster, J.J.; Leipertz, A.; Braeuer, A. Microfluidic Investigation into Mass Transfer in Compressible Multi-Phase Systems Composed of Oil, Water and Carbon Dioxide at Elevated Pressure. J. Supercrit. Fluids 2013, 84, 121–131. [Google Scholar] [CrossRef]
- Lang, S.; Frerich, S.; Pollak, S. Solubility of Pressurised Carbon Dioxide in Three Different Polydimethylsiloxanes. Fluid Phase Equilib. 2019, 491, 12–22. [Google Scholar] [CrossRef]
- Yang, Z.; Li, M.; Peng, B.; Lin, M.; Dong, Z. Volume expansion of CO2 + oil at near critical and supercritical conditions of CO2. Fuel 2013, 112, 283–288. [Google Scholar] [CrossRef]
- Han, H.; Yuan, S.; Li, S.; Liu, X.; Chen, X. Dissolving capacity and volume expansion of carbon dioxide in chain n-alkanes. Adv. Pet. Explor. Dev. 2015, 42, 97–103. [Google Scholar] [CrossRef]
- Liu, N.; Aymonier, C.; Lecoutre, C.; Garrabos, Y.; Marre, S. Microfluidic approach for studying CO2 solubility in water and brine using in situ Raman spectroscopy. Chem. Phys. Lett. 2012, 551, 139–143. [Google Scholar] [CrossRef]
- Belgodere, C.; Dubessy, J.; Vautrin, D.; Caumon, M.-C.; Sterpenich, J.; Pironon, J.; Robert, P.; Randi, A.; Birat, J.-P. Experimental determination of CO2 diffusion coefficient in aqueous solutions under pressure at room temperature via Raman spectroscopy: Impact of salinity (NaCl). J. Raman Spectrosc. 2015, 46, 1025–1032. [Google Scholar] [CrossRef]
- Aarnoutse, P.J.; Westerhuis, J.A. Quantitative Raman Reaction Monitoring Using the Solvent as Internal Standard. Anal. Chem. 2005, 77, 1228–1236. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.; Hu, Q.; Lu, W.; Ou, W.; Geng, L. Quantitative Raman spectroscopic investigation of geo-fluids high-pressure phase equilibria: Part, I. Accurate calibration and determination of CO2 solubility in water from 273.15 to 573.15 K and from 10 to 120 MPa. Fluid Phase Equilib. 2014, 382, 70–79. [Google Scholar] [CrossRef]
- Bei, K.; Junliang, W.; Shuyan, Z.; Guangna, X.; Yanmei, X.; Liang, W.; Zhuoran, J.; I-Ming, C.; Zhiyan, P. Determining the volume expansion of the CO2 + octane mixture using a fused silica capillary cell with in-situ Raman spectroscopy. J. CO2 Util. 2018, 24, 149–156. [Google Scholar] [CrossRef]
- Wang, J.; He, B.; Xie, L.; Bei, K.; Li, G.; Chen, Z.; Chou, I.-M.; Lin, C.; Pan, Z. Determination of CO2 solubility in water and NaCl solutions under geological sequestration conditions using a fused silica capillary cell with in Situ Raman spectroscopy. J. Chem. Eng. Data 2019, 64, 2484–2496. [Google Scholar] [CrossRef]
- Campbell, I.T.; Brand, U. Henry’s law. Enc. Earth Sci. 1998, 26, 315. [Google Scholar]
- Liu, Y.; Jiang, L.; Song, Y.; Zhao, Y.; Zhang, Y.; Wang, D. Estimation of minimum miscibility pressure (MMP) of CO2 and liquid n-alkane systems using an improved MRI technique. Magn. Reson. Imaging 2016, 34, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, M.; Peng, B.; Lin, M.; Dong, Z. Dispersion property of CO2 in oil. 1. Volume expansion of CO2+alkane at near critical and supercritical condition of CO2. J. Chem. Eng. Data 2012, 57, 882–889. [Google Scholar] [CrossRef]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Jou, F.Y.; Mather, A.E. Solubility of carbon dioxide in an aqueous mixture of methyldiethanolamine and N-methylpyrrolidone at elevated pressures. Fluid Phase Equilib. 2005, 228, 465–469. [Google Scholar] [CrossRef]
- Paninho, A.B.; Nunes, A.V.M.; Paiva, A.; Najdanovic-Visak, V. High pressure phase behavior of the binary system (ethyl lactate+ carbon dioxide). Fluid Phase Equilib. 2013, 360, 129–133. [Google Scholar] [CrossRef]
- Fornari, T.; Hernández, E.J.; Reglero, G. Solubility of supercritical gases in organic liquids. J. Supercrit. Fluids 2009, 51, 115–122. [Google Scholar] [CrossRef]
- Lashkarbolooki, M.; Eftekhari, M.J.; Najimi, S.; Ayatollahi, S. Minimum miscibility pressure of CO2 and crude oil during CO2 injection in the reservoir. J. Supercrit. Fluids 2017, 127, 121–128. [Google Scholar] [CrossRef]
- Bon, J.; Sarma, H.K.; Theophilos, A.M. An Investigation of Minimum Miscibility Pressure for CO2—Rich Injection Gases with Pentanes-Plus Fraction. In Proceedings of the SPE International Improved Oil Recovery Conference, Kuala Lumpur, Malaysia, 5–6 December 2005. [Google Scholar]



| 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | 7 h | |
|---|---|---|---|---|---|---|---|
| CO2 + n-decane | |||||||
| 303.15 K 0.5621 mol/kg | 0.04378 | 0.04538 | 0.04039 | 0.04185 | 0.04128 | 0.04136 | 0.04146 |
| 313.15 K 0.6606 mol/kg | 0.05143 | 0.04796 | 0.04657 | 0.04852 | 0.04834 | 0.04878 | 0.04865 |
| CO2 + n-decane + n-hexane | |||||||
| 303.15 K 0.1886 mol/kg | 0.01421 | 0.01552 | 0.01432 | 0.01408 | 0.01407 | 0.01408 | 0.01409 |
| 313.15 K 0.2974 mol/kg | 0.02235 | 0.02201 | 0.02169 | 0.02146 | 0.02148 | 0.02151 | 0.02142 |
| Temperature, Pressure | 100 μm | 200 μm | 300 μm | Standard Deviation SD |
|---|---|---|---|---|
| CO2 + n-decane | ||||
| 303.15 K, 2.82 MPa | 0.1657 | 0.1664 | 0.1667 | 0.00051 |
| 313.15 K, 3.93 MPa | 0.2045 | 0.2053 | 0.2054 | 0.00049 |
| 323.15 K, 4.81 MPa | 0.2237 | 0.2231 | 0.2238 | 0.00041 |
| 333.15 K, 6.53 MPa | 0.3754 | 0.3752 | 0.3761 | 0.00049 |
| 343.15 K, 6.64 MPa | 0.3085 | 0.3091 | 0.3084 | 0.00034 |
| 353.15 K, 10.08 MPa | 0.6693 | 0.6690 | 0.6702 | 0.00062 |
| CO2 + n-decane + n-hexane | ||||
| 303.15 K, 3.00 MPa | 0.1694 | 0.1694 | 0.1701 | 0.0004 |
| 313.15 K, 2.02 MPa | 0.08748 | 0.08775 | 0.08744 | 0.0002 |
| 323.15 K, 3.10 MPa | 0.1315 | 0.1317 | 0.1318 | 0.0001 |
| 333.15 K, 6.53 MPa | 0.4146 | 0.4146 | 0.4147 | 0.0001 |
| 343.15 K, 8.32 MPa | 0.5902 | 0.5904 | 0.5913 | 0.0006 |
| 353.15 K, 10.45 MPa | 0.6732 | 0.6733 | 0.6737 | 0.0003 |
mol/kg | P MPa | Peak Intensity Ratio | SD | mol/kg | P MPa | Peak Intensity Ratio | SD |
|---|---|---|---|---|---|---|---|
| 303.15 K | 323.15 K | ||||||
| 0.5621 | 4.03 | 0.04120 | 0.00024 | 0.7612 | 6.15 | 0.05486 | 0.00022 |
| 5.79 | 0.04114 | 7.78 | 0.05430 | ||||
| 7.31 | 0.04130 | 9.07 | 0.05469 | ||||
| 8.65 | 0.04169 | 10.16 | 0.05476 | ||||
| 9.81 | 0.04104 | 11.81 | 0.05449 | ||||
| 11.21 | 0.04146 | 13.25 | 0.05441 | ||||
| 343.15 K | 333.15 K | ||||||
| 0.8600 | 7.35 | 0.05990 | 0.00026 | 0.9596 | 5.48 | 0.06731 | 0.00027 |
| 10.13 | 0.06014 | 6.65 | 0.06785 | ||||
| 11.85 | 0.06032 | 8.10 | 0.06757 | ||||
| 13.11 | 0.06027 | 9.31 | 0.06782 | ||||
| 14.42 | 0.06064 | 10.50 | 0.06799 | ||||
| 15.75 | 0.06052 | 11.43 | 0.06802 | ||||
| T (K) | 303.15 | 313.15 | 323.15 | 333.15 | 343.15 | 353.15 | SD | |
|---|---|---|---|---|---|---|---|---|
| (mol/kg) | ||||||||
| 0.5621 | 0.04169 | 0.04062 | 0.04029 | 0.03927 | 0.03874 | 0.03729 | 0.0015 | |
| 0.6606 | 0.04953 | 0.04878 | 0.04819 | 0.04769 | 0.04710 | 0.04638 | 0.0011 | |
| 0.7612 | 0.05563 | 0.05483 | 0.05429 | 0.05410 | 0.05353 | 0.05315 | 0.00089 | |
| 0.8600 | 0.06305 | 0.06240 | 0.06170 | 0.06125 | 0.06051 | 0.06004 | 0.0011 | |
| 0.9596 | 0.07023 | 0.06934 | 0.06853 | 0.06785 | 0.06706 | 0.06673 | 0.0013 | |
| T (K) | 303.15 | 313.15 | 323.15 | 333.15 | 343.15 | 353.15 | SD | |
|---|---|---|---|---|---|---|---|---|
| (mol/kg) | ||||||||
| 0.1886 | 0.01409 | 0.01370 | 0.01350 | 0.01304 | 0.01272 | 0.01251 | 0.0006 | |
| 0.2974 | 0.02260 | 0.02150 | 0.02118 | 0.02082 | 0.02041 | 0.02009 | 0.0009 | |
| 0.7011 | 0.05162 | 0.05114 | 0.05048 | 0.05005 | 0.04940 | 0.04911 | 0.0010 | |
| T (K) | Fitting Equation |
|---|---|
| 303.15 | λ = 0.0710 × CCO2 + 0.0020 (R2 = 0.9983) |
| 313.15 | λ = 0.0715 × CCO2 + 0.0008 (R2 = 0.9977) |
| 323.15 | λ = 0.0704 × CCO2 + 0.0011 (R2 = 0.9982) |
| 333.15 | λ = 0.0711 × CCO2 − 0.00006 (R2 = 0.9970) |
| 343.15 | λ = 0.0704 × CCO2 − 0.0002 (R2 = 0.9969) |
| 353.15 | λ = 0.0729 × CCO2 − 0.0028 (R2 = 0.9944) |
| Temperature (K) | Pressure (MPa) | Solubility (mol/kg) | Temperature (K) | Pressure (MPa) | Solubility (mol/kg) |
|---|---|---|---|---|---|
| 303.15 | 2.92 | 2.3741 | 313.15 | 3.14 | 2.1676 |
| 3.59 | 3.5654 | 4.06 | 3.2723 | ||
| 4.23 | 5.3615 | 4.52 | 4.2598 | ||
| 5.12 | 9.8819 | 5.48 | 6.9693 | ||
| 5.83 | 15.4430 | 6.28 | 10.5335 | ||
| 6.65 | 26.8551 | 7.13 | 16.2304 | ||
| 7.03 | 33.9367 | 7.95 | 23.8631 | ||
| 7.58 | 49.8425 | 8.49 | 32.3855 | ||
| 8.03 | 64.0084 | 8.98 | 42.8045 | ||
| - | - | 9.45 | 54.6061 | ||
| 323.15 | 3.14 | 1.9191 | 333.15 | 4.12 | 2.4537 |
| 4.09 | 2.6978 | 5.11 | 3.4143 | ||
| 5.01 | 3.7191 | 5.98 | 4.3792 | ||
| 6.01 | 5.7333 | 6.68 | 5.8202 | ||
| 7.43 | 10.5475 | 7.88 | 8.9228 | ||
| 8.21 | 14.9078 | 8.23 | 10.1629 | ||
| 9.46 | 25.5872 | 8.75 | 13.1587 | ||
| 9.88 | 30.2865 | 9.63 | 17.8441 | ||
| 10.56 | 41.4610 | 10.77 | 27.5098 | ||
| —— | —— | 11.74 | 38.4256 | ||
| —— | —— | 12.85 | 56.8202 | ||
| 343.15 | 3.35 | 1.6355 | 353.15 | 4.33 | 1.9795 |
| 4.35 | 2.2014 | 5.45 | 2.5699 | ||
| 5.18 | 2.7581 | 7.12 | 4.0630 | ||
| 6.83 | 4.5781 | 8.83 | 6.3712 | ||
| 8.02 | 6.7266 | 10.18 | 9.2589 | ||
| 8.76 | 8.5032 | 10.98 | 11.3027 | ||
| 9.66 | 11.0490 | 12.03 | 15.5671 | ||
| 10.48 | 14.6504 | 12.71 | 18.9835 | ||
| 11.28 | 19.1455 | 14.25 | 28.6054 | ||
| 12.58 | 28.7775 | 15.28 | 38.6424 | ||
| 13.38 | 37.0169 | —— | —— |
| Temperature (K) | Pressure (MPa) | Solubility (mol/kg) | Temperature (K) | Pressure (MPa) | Solubility (mol/kg) |
|---|---|---|---|---|---|
| 303.15 | 2.00 | 1.2874 | 313.15 | 2.00 | 1.1989 |
| 3.00 | 2.3823 | 3.10 | 2.2014 | ||
| 3.31 | 3.1934 | 4.00 | 3.2599 | ||
| 4.32 | 5.6807 | 4.42 | 4.0249 | ||
| 5.1 | 9.9944 | 5.12 | 5.9455 | ||
| 5.8 | 15.7693 | 6.23 | 10.4441 | ||
| 6.18 | 20.7623 | 6.91 | 15.1131 | ||
| 6.60 | 27.4936 | 7.39 | 19.6187 | ||
| 6.98 | 35.1055 | 7.86 | 25.0726 | ||
| 7.51 | 49.9972 | 8.25 | 30.7025 | ||
| 7.92 | 65.72856 | 8.75 | 39.8393 | ||
| 323.15 | 2.01 | 1.1464 | 333.15 | 2.00 | 1.0737 |
| 3.14 | 1.9025 | 3.00 | 1.4642 | ||
| 4.11 | 2.7663 | 4.12 | 2.5187 | ||
| 5.01 | 3.6824 | 5.12 | 3.4375 | ||
| 5.52 | 4.9262 | 6.02 | 4.4820 | ||
| 6.01 | 5.8865 | 6.62 | 5.6446 | ||
| 7.31 | 10.8312 | 7.58 | 7.8596 | ||
| 8.15 | 15.9135 | 8.05 | 9.8820 | ||
| 8.82 | 21.0780 | 8.65 | 12.7374 | ||
| 9.38 | 27.1475 | 9.18 | 14.8694 | ||
| 9.86 | 32.9816 | 9.85 | 18.9972 | ||
| 10.48 | 42.8794 | ||||
| 343.15 | 2.00 | 1.0414 | 353.15 | 2.00 | 1.0127 |
| 3.31 | 1.6115 | 3.00 | 1.3549 | ||
| 4.33 | 2.2012 | 4.38 | 2.0044 | ||
| 5.16 | 2.7349 | 5.42 | 2.5910 | ||
| 6.02 | 3.6346 | 7.02 | 4.0900 | ||
| 6.83 | 4.6598 | 7.83 | 5.0452 | ||
| 7.53 | 5.7991 | 8.52 | 6.1411 | ||
| 8.02 | 6.8687 | 9.83 | 8.8753 | ||
| 9.12 | 10.1991 | 10.32 | 10.4260 | ||
| 9.67 | 12.1169 | 11.12 | 13.7493 | ||
| 10.06 | 13.7765 | 11.89 | 17.0370 | ||
| 10.83 | 17.3594 | 12.58 | 20.4384 | ||
| 11.50 | 21.6204 | 12.83 | 22.2329 |
| T (K) | Fitting Equations | R2 |
|---|---|---|
| 303.15 | P = 1.5326lnS + 1.6242 | 0.9998 |
| 313.15 | P = 1.9485lnS + 1.6966 | 0.9996 |
| 323.15 | P = 2.3796lnS + 1.7591 | 0.9991 |
| 333.15 | P = 2.7238lnS + 1.8194 | 0.9989 |
| 343.15 | P = 3.1907lnS + 1.8954 | 0.9997 |
| 353.15 | P = 3.6666lnS + 1.9661 | 0.9996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, Z.; Wang, Q.; Lou, T.; Pan, Z.; Hu, M. Integrated a Fused Silica Capillary Cell and In Situ Raman Spectroscopy for Determining the Solubility of CO2 in n-Decane and n-Decane + n-Hexane System. Processes 2023, 11, 1137. https://doi.org/10.3390/pr11041137
Wang J, Zhang Z, Wang Q, Lou T, Pan Z, Hu M. Integrated a Fused Silica Capillary Cell and In Situ Raman Spectroscopy for Determining the Solubility of CO2 in n-Decane and n-Decane + n-Hexane System. Processes. 2023; 11(4):1137. https://doi.org/10.3390/pr11041137
Chicago/Turabian StyleWang, Junliang, Zhenzhen Zhang, Quanyuan Wang, Tianhong Lou, Zhiyan Pan, and Mian Hu. 2023. "Integrated a Fused Silica Capillary Cell and In Situ Raman Spectroscopy for Determining the Solubility of CO2 in n-Decane and n-Decane + n-Hexane System" Processes 11, no. 4: 1137. https://doi.org/10.3390/pr11041137
APA StyleWang, J., Zhang, Z., Wang, Q., Lou, T., Pan, Z., & Hu, M. (2023). Integrated a Fused Silica Capillary Cell and In Situ Raman Spectroscopy for Determining the Solubility of CO2 in n-Decane and n-Decane + n-Hexane System. Processes, 11(4), 1137. https://doi.org/10.3390/pr11041137








