Production and Characterization of Kombucha Tea from Different Sources of Tea and Its Kinetic Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Kombucha Culture and Media
2.2. Experimental Design
2.3. Preparation of Tea Extracts, Inoculation, and Fermentation
2.4. Analysis
2.5. Kinetic Parameters
2.6. Kinetic Modeling
2.7. Statistical Analysis
3. Results and Discussion
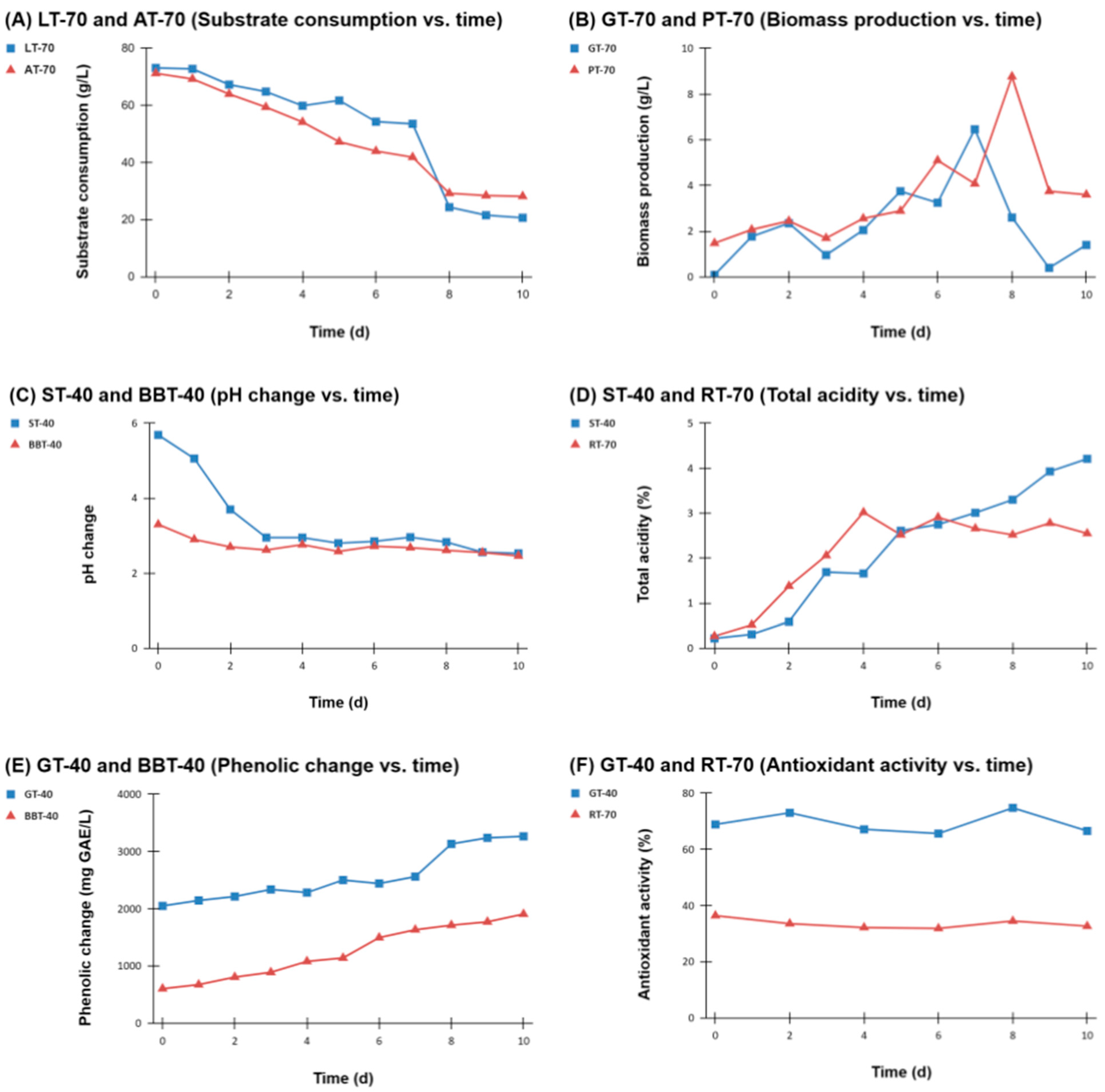
3.1. Sugar Consumption and Biomass Production
3.2. pH and Total Acidity
3.3. Total Phenolic Compounds and Antioxidant Activity
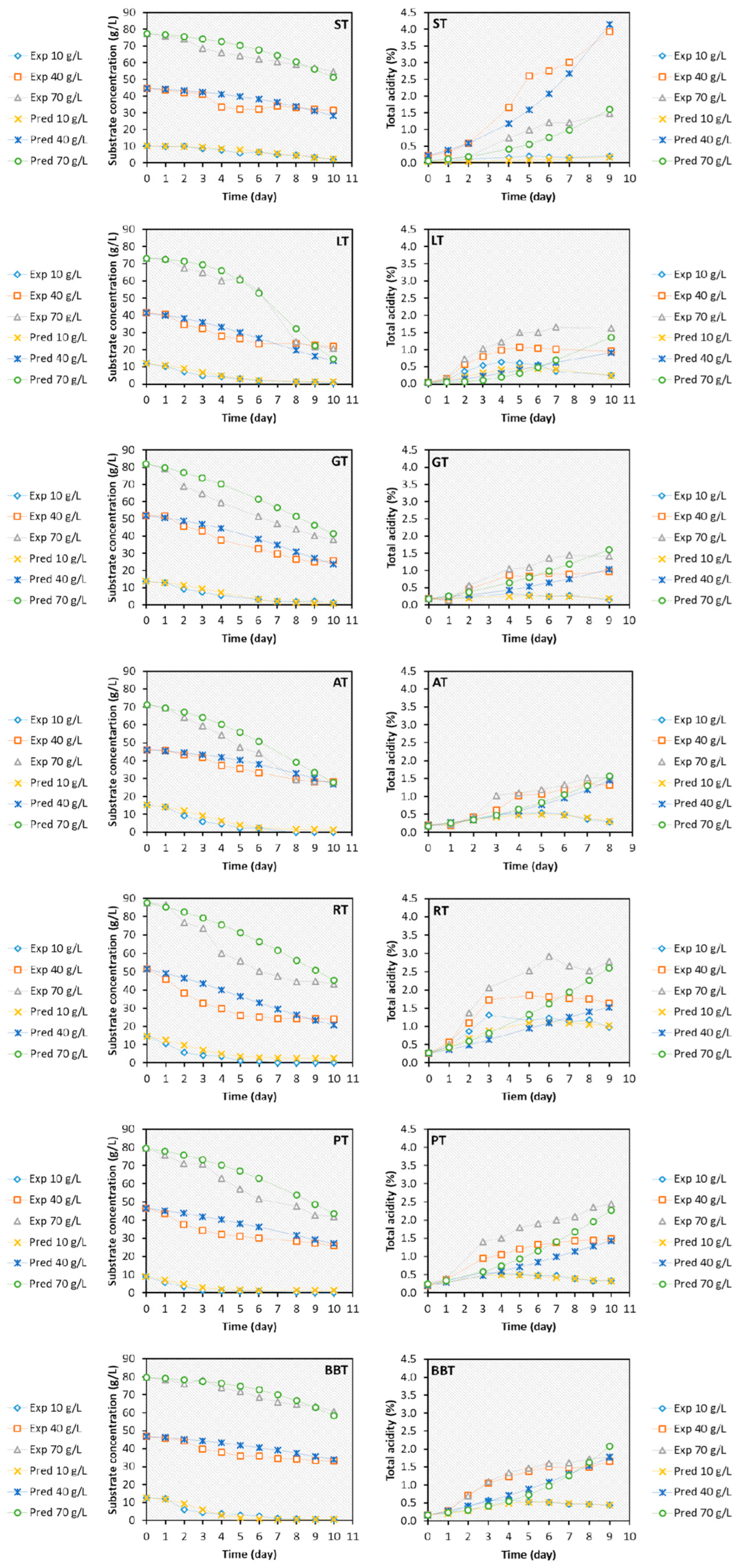
3.4. Kinetic Characterization
3.5. Kinetic Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Full Name |
| ISC | Initial sucrose concentration |
| AAB | Acetic acid bacteria |
| R2 | Determination of coefficient |
| α and β | Empirical constants |
| N | Normal |
| HCl | Hydrochloric acid |
| LPM | Luedeking–Piret model |
| LM | Logistic model |
| GT | Green tea |
| ST | Sage tea |
| LT | Linden tea |
| BT | Black tea |
| BBT | Bilberry tea |
| RT | Rosehip tea |
| AT | Apple tea |
| PT | Pomegranate tea |
| NaOH | Sodium hydroxide |
| GAE | Gallic acid equivalents |
| DPPH | α, α-diphenyl-β-picrylhydrazyl |
| ∆S | Substrate consumption, g/L |
| QS | Maximum substrate consumption rate, g/L/d |
| η | Substrate utilization yield, % |
| ∆X | Biomass production, g/L |
| QX | Maximum biomass production rate, g/L/d |
| YX/S | Biomass yield, g biomass/g substrate |
| TA | Total acidity, % |
| QTA | Maximum total acidity production rate, %/d |
| ∆PH | Phenolic production, mg/L |
| QPH | Maximum phenolic production rate, mg/L/d |
| YPH/S | Phenolic yield, mg phenolic/g substrate |
| −dS/dt | Substrate consumption rate, g/L/d |
| µm, S | Specific sugar consumption rate, 1/d |
| S | Residual substrate concentration at the time “t”, g/L |
| Sm | Maximum substrate concentration, g/L |
| Sf | Final substrate concentration, g/L |
| dP/dt | Total acidity rate, %/d |
| SAS | Statistical Analysis System |
References
- Dutta, H.; Paul, S.K. Kombucha drink: Production, quality, and safety aspects. In Production and Management of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–288. [Google Scholar]
- Jayabalan, R.; Malbasa, R.V.; Loncar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea: Microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.L.; Heard, G.; Cox, J. Yeast ecology of Kombucha fermentation. Int. J. Food Microbiol. 2004, 95, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Malbaša, R.; Lončar, E.; Djurić, M. Comparison of the products of Kombucha fermentation on sucrose and molasses. Food Chem. 2008, 106, 1039–1045. [Google Scholar] [CrossRef]
- Şafak, S.; Mercan, N.; Aslim, B.; Beyatli, Y. A study on the production of poly-β-hydroxybutyrate by some eukaryotic microorganisms. Turk. Electron. J. Biotechnol. 2002, 1, 11–17. [Google Scholar]
- Mayser, P.; Fromme, S.; Leitzmann, G.; Gründer, K. The yeast spectrum of the ‘tea fungus Kombucha’. Mycoses 1995, 38, 289–295. [Google Scholar] [CrossRef]
- Tsilo, P.H.; Basson, A.K.; Ntombela, Z.G.; Maliehe, T.S.; Pullabhotla, R.V. Isolation and optimization of culture conditions of a bioflocculant-producing fungi from Kombucha tea SCOBY. Microbiol. Res. 2021, 12, 950–966. [Google Scholar] [CrossRef]
- Greenwalt, C.; Steinkraus, K.; Ledford, R. Kombucha, the fermented tea: Microbiology, composition, and claimed health effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Robnett, C.J.; Basehoar-Powers, E. Zygosaccharomyces kombuchaensis, a new ascosporogenous yeast from ‘Kombucha tea’. FEMS Yeast Res. 2001, 1, 133–138. [Google Scholar] [CrossRef]
- Liu, C.-H.; Hsu, W.-H.; Lee, F.-L.; Liao, C.-C. The isolation and identification of microbes from a fermented tea beverage, Haipao, and their interactions during Haipao fermentation. Food Microbiol. 1996, 13, 407–415. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Morales-de la Peña, M.; Miranda-Mejía, G.A.; Martín-Belloso, O. Recent Trends in Fermented Beverages Processing: The Use of Emerging Technologies. Beverages 2023, 9, 51. [Google Scholar] [CrossRef]
- Nummer, B.A. Kombucha brewing under the Food and Drug Administration Model Food Code: Risk analysis and processing guidance. J. Environ. Health 2013, 76, 8–11. [Google Scholar] [PubMed]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Malbasa, R.V.; Loncar, E.S.; Vitas, J.S.; Canadanovic-Brunet, J.M. Influence of starter cultures on the antioxidant activity of kombucha beverage. Food Chem. 2011, 127, 1727–1731. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Ledford, R.A.; Steinkraus, K.H. Determination and characterization of the antimicrobial activity of the fermented tea kombucha. LWT-Food Sci. Technol. 1998, 31, 291–296. [Google Scholar] [CrossRef]
- Battikh, H.; Bakhrouf, A.; Ammar, E. Antimicrobial effect of kombucha analogues. LWT-Food Sci. Technol. 2012, 47, 71–77. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Bhattacharya, S.; Patra, M.M.; Chakravorty, S.; Sarkar, S.; Chakraborty, W.; Koley, H.; Gachhui, R. Antibacterial activity of polyphenolic fraction of kombucha against enteric bacterial pathogens. Curr. Microbiol. 2016, 73, 885–896. [Google Scholar] [CrossRef]
- Chu, S.C.; Chen, C.S. Effects of origins and fermentation time on the antioxidant activities of kombucha. Food Chem. 2006, 98, 502–507. [Google Scholar] [CrossRef]
- Malbasa, R.; Loncar, E.; Djuric, M.; Dosenovic, I. Effect of sucrose concentration on the products of kombucha fermentation on molasses. Food Chem. 2008, 108, 926–932. [Google Scholar] [CrossRef]
- Zubaidah, E.; Yurista, S.; Rahmadani, N. Characteristic of physical, chemical, and microbiological kombucha from various varieties of apples. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2018; Volume 1, p. 012040. [Google Scholar]
- Tarhan, K. Use of Different Substrate Resources in the Production of Kombucha Tea. 2017. Available online: https://agris.fao.org/agris-search/search.do?recordID=TR2019000120 (accessed on 18 February 2023).
- Tamer, C.E.; Temel, Ş.G.; Suna, S.; Karabacak, A.Ö.; Özcan, T.; Ersan, L.Y.; Kaya, B.T.; Çopur, Ö.U. Evaluation of bioaccessibility and functional properties of kombucha beverages fortified with different medicinal plant extracts. Turk. J. Agric. For. 2021, 45, 13–32. [Google Scholar]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Battikh, H.; Chaieb, K.; Bakhrouf, A.; Ammar, E. Antibacterial and antifungal activities of black and green kombucha teas. J. Food Biochem. 2013, 37, 231–236. [Google Scholar] [CrossRef]
- Lončar, E.S.; Kanurić, K.G.; Malbaša, R.V.; Đurić, M.S.; Milanović, S.D. Kinetics of saccharose fermentation by Kombucha. Chem. Ind. Chem. Eng. Q. 2014, 20, 345–352. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Bassyouni, R.H.; Kamel, Z.; Gabr, S.M. Detoxification of patulin by kombucha tea culture. CyTA-J. Food 2016, 14, 271–279. [Google Scholar] [CrossRef]
- Germec, M.; Karhan, M.; Demirci, A.; Turhan, I. Kinetic modeling, sensitivity analysis, and techno-economic feasibility of ethanol fermentation from non-sterile carob extract-based media in Saccharomyces cerevisiae biofilm reactor under a repeated-batch fermentation process. Fuel 2022, 324, 124729. [Google Scholar] [CrossRef]
- Alboreadi, M.A.; Al-Najdawi, M.M.; Jarrar, Q.B.; Moshawih, S. Evaluation of hair growth properties of topical kombucha tea extracts. Adv. Tradit. Med. 2021, 22, 155–161. [Google Scholar] [CrossRef]
- Kruk, M.; Trząskowska, M.; Ścibisz, I.; Pokorski, P. Application of the “SCOBY” and Kombucha tea for the production of fermented milk drinks. Microorganisms 2021, 9, 123. [Google Scholar] [CrossRef]
- Cemeroğlu, B. Gıda Analizleri, Gıda Teknolojisi Derneği Yayınları; Bizim Grup Basımevi: Ankara, Turkey, 2010. [Google Scholar]
- Germec, M.; Karahalil, E.; Yatmaz, E.; Tari, C.; Turhan, I. Effect of process parameters and microparticle addition on polygalacturonase activity and fungal morphology of Aspergillus sojae. Biomass Convers. Biorefin. 2021, 12, 5329–5344. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Mahdinia, E.; Mamouri, S.J.; Puri, V.M.; Demirci, A.; Berenjian, A. Modeling of vitamin K (Menaquinoe-7) fermentation by Bacillus subtilis natto in biofilm reactors. Biocatal. Agric. Biotechnol. 2019, 17, 196–202. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Health, wellness, and safety aspects of the consumption of kombucha. J. Chem. 2015, 2015, 591869. [Google Scholar] [CrossRef]
- Muhialdin, B.; Osman, F.; Muhamad, R.; Che Wan Sapawi, C.; Anzian, A.; Voon, W.; Hussin, A. Effects of sugar sources and fermentation time on the properties of tea fungus (kombucha) beverage. Int. Food Res. J. 2019, 26, 481–487. [Google Scholar]
- Kallel, L.; Desseaux, V.; Hamdi, M.; Stocker, P.; Ajandouz, E. Insights into the fermentation biochemistry of kombucha teas and potential impacts of kombucha drinking on starch digestion. Food Res. Int. 2012, 49, 226–232. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. Effect of kombucha, a fermented black tea in attenuating oxidative stress mediated tissue damage in alloxan induced diabetic rats. Food Chem. Toxicol. 2013, 60, 328–340. [Google Scholar] [CrossRef]
- Emiljanowicz, K.E.; Malinowska-Pańczyk, E. Kombucha from alternative raw materials—The review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3185–3194. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.L.; Yan, F.; Cao, Z.L.; Xie, F.Y.; Lin, J. Antioxidant activities of kombucha prepared from three different substrates and changes in content of probiotics during storage. Food Sci. Technol. 2014, 34, 123–126. [Google Scholar] [CrossRef]
- Carlson, H.A. Check your confidence: Size really does matter. J. Chem. Inf. Model. 2013, 53, 1837–1841. [Google Scholar] [CrossRef]
- Ilgın, M.; Germec, M.; Turhan, I. Statistical and kinetic modeling of Aspergillus niger inulinase fermentation from carob extract and its partial concentration. Ind. Crops Prod. 2020, 156, 112866. [Google Scholar] [CrossRef]
- Germec, M.; Turhan, I. Kinetic modeling and sensitivity analysis of inulinase production in large-scale stirred tank bioreactor with sugar beet molasses-based medium. Biochem. Eng. J. 2021, 176, 108201. [Google Scholar] [CrossRef]
| Tea Origin | Tea | Initial Sugar Concentration (g/L) | ||
|---|---|---|---|---|
| 10 | 40 | 70 | ||
| Herbal tea | Sage tea (ST) | ST-10 | ST-40 | ST-70 |
| Linden tea (LT) | LT-10 | LT-40 | LT-70 | |
| Green tea (GT) | GT-10 | GT-40 | GT-70 | |
| Black tea (BT) | BT-10 | BT-40 | BT-70 | |
| Fruit tea | Apple tea (AT) | AT-10 | AT-40 | AT-70 |
| Rosehip tea (RT) | RT-10 | RT-40 | RT-70 | |
| Pomegranate tea (PT) | PT-10 | PT-40 | PT-70 | |
| Bilberry tea (BBT) | BBT-10 | BBT-40 | BBT-70 | |
| Tea | [Substrate] | Kinetic Parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ∆S (g/L) | QS (g/L/d) | η (%) | ∆X (g/L) | QX (g/L/d) | YX/S (g/g) | ∆TA (%) | QTA (%/d) | ∆PH (mg/L) | QPH (mg/L/d) | YPH/S (mg/g) | ||
| ST | 10 g/L | 8.52 | 1.29 | 79.48 | 0.72 | 0.12 | 0.08 | 0.17 | 0.08 | 445.96 | 97.18 | 52.34 |
| 40 g/L | 13.10 | 3.71 | 29.39 | 1.74 | 0.51 | 0.13 | 4.10 | 0.60 | 883.17 | 169.75 | 67.42 | |
| 70 g/L | 26.62 | 4.05 | 32.71 | 3.96 | 0.94 | 0.15 | 1.51 | 0.28 | 900.31 | 189.07 | 33.82 | |
| LT | 10 g/L | 11.66 | 2.72 | 97.25 | 1.02 | 0.22 | 0.09 | 0.59 | 0.17 | 242.12 | 26.80 | 20.77 |
| 40 g/L | 19.85 | 4.00 | 47.40 | 2.72 | 1.20 | 0.14 | 1.04 | 0.27 | 399.65 | 49.54 | 20.13 | |
| 70 g/L | 55.40 | 11.28 | 72.78 | 3.80 | 0.14 | 0.07 | 1.63 | 0.31 | 227.06 | 48.14 | 4.10 | |
| GT | 10 g/L | 12.13 | 2.40 | 88.80 | 0.74 | 0.26 | 0.06 | 0.35 | 0.15 | 1217.60 | 410.68 | 100.38 |
| 40 g/L | 32.43 | 4.90 | 56.65 | 2.52 | 0.78 | 0.08 | 0.87 | 0.33 | 1215.90 | 345.69 | 37.49 | |
| 70 g/L | 44.07 | 6.34 | 53.72 | 6.46 | 1.22 | 0.15 | 1.42 | 0.37 | 1268.40 | 264.16 | 28.78 | |
| AT | 10 g/L | 15.75 | 2.82 | 100.00 | 1.09 | 0.34 | 0.07 | 0.34 | 0.15 | 321.98 | 64.14 | 20.44 |
| 40 g/L | 18.61 | 3.07 | 39.89 | 3.76 | 1.39 | 0.20 | 1.18 | 0.27 | 785.45 | 159.90 | 42.21 | |
| 70 g/L | 48.39 | 5.28 | 63.15 | 4.28 | 1.37 | 0.09 | 1.64 | 0.41 | 466.38 | 74.71 | 9.64 | |
| RT | 10 g/L | 15.43 | 3.65 | 100.00 | 2.01 | 0.23 | 0.13 | 1.07 | 0.36 | 130.66 | 21.39 | 8.47 |
| 40 g/L | 28.74 | 6.37 | 54.33 | 4.71 | 2.17 | 0.16 | 1.61 | 0.49 | 295.36 | 76.28 | 10.28 | |
| 70 g/L | 45.66 | 8.32 | 51.38 | 5.60 | 1.31 | 0.12 | 2.77 | 0.82 | 255.86 | 56.83 | 5.60 | |
| PT | 10 g/L | 10.33 | 2.47 | 100.00 | 2.20 | 0.91 | 0.21 | 0.39 | 0.20 | 549.89 | 95.36 | 53.23 |
| 40 g/L | 21.04 | 3.67 | 44.69 | 3.25 | 1.43 | 0.15 | 1.30 | 0.30 | 800.04 | 101.66 | 38.02 | |
| 70 g/L | 42.92 | 6.36 | 50.61 | 8.77 | 1.66 | 0.20 | 2.18 | 0.34 | 930.94 | 166.20 | 21.69 | |
| BBT | 10 g/L | 12.67 | 2.54 | 95.62 | 0.81 | 0.31 | 0.06 | 0.44 | 0.18 | 655.20 | 95.51 | 51.71 |
| 40 g/L | 16.05 | 3.07 | 32.56 | 2.07 | 0.70 | 0.13 | 1.52 | 0.39 | 1311.92 | 187.92 | 81.74 | |
| 70 g/L | 21.41 | 3.24 | 26.02 | 3.55 | 1.40 | 0.17 | 1.73 | 0.41 | 782.90 | 178.13 | 36.57 | |
| Tea | [Substrate] | Kinetics for Substrate Consumption | Kinetics for Acidity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| µm, S (1/d) | Sm (g/L) | Sf (g/L) | R2 | β (%/gS.d) | α (%/gS) | A > β Fold | R2 | ||
| ST | 10 (g/L) | 0.4738 | 10.24 | 0.34 | 0.9438 | 0.0001 | 0.0201 | 360.19 | 0.5675 |
| 40 (g/L) | 0.2752 | 44.58 | 1.87 | 0.6038 | 0.0076 | 0.2584 | 34.00 | 0.9064 | |
| 70 (g/L) | 0.2864 | 77.45 | 2.54 | 0.8572 | 0.0013 | 0.0662 | 49.85 | 0.8481 | |
| LT | 10 (g/L) | 0.7821 | 11.99 | 1.19 | 0.9672 | −0.0058 | 0.0631 | 10.91 | 0.8060 |
| 40 (g/L) | 0.3462 | 41.42 | 3.89 | 0.8055 | −0.0006 | 0.0343 | 60.27 | 0.5337 | |
| 70 (g/L) | 0.5947 | 73.16 | 0.83 | 0.9655 | 0.0004 | 0.0211 | 55.41 | 0.4809 | |
| GT | 10 (g/L) | 0.7168 | 13.62 | 0.84 | 0.9487 | −0.0024 | 0.0142 | 5.87 | 0.7586 |
| 40 (g/L) | 0.2909 | 52.03 | 4.44 | 0.9185 | 0.0005 | 0.0315 | 63.17 | 0.7220 | |
| 70 (g/L) | 0.2503 | 82.03 | 9.18 | 0.9413 | 0.0001 | 0.0394 | 282.80 | 0.8259 | |
| AT | 10 (g/L) | 0.7798 | 15.34 | 1.24 | 0.9636 | −0.0075 | 0.0468 | 6.24 | 0.9228 |
| 40 (g/L) | 0.2757 | 46.12 | 2.46 | 0.9012 | 0.0019 | 0.0863 | 45.75 | 0.8479 | |
| 70 (g/L) | 0.3324 | 71.27 | 5.07 | 0.9563 | 0.0015 | 0.0365 | 23.65 | 0.8251 | |
| RT | 10 (g/L) | 0.7978 | 14.64 | 2.49 | 0.9671 | −0.0030 | 0.0876 | 29.15 | 0.8465 |
| 40 (g/L) | 0.2765 | 51.46 | 9.47 | 0.7731 | −0.0003 | 0.0475 | 145.00 | 0.6153 | |
| 70 (g/L) | 0.2459 | 87.58 | 9.64 | 0.8645 | −0.0006 | 0.0671 | 110.88 | 0.7225 | |
| PT | 10 (g/L) | 1.0969 | 8.94 | 1.39 | 0.9750 | −0.0039 | 0.0554 | 14.30 | 0.9526 |
| 40 (g/L) | 0.2011 | 46.40 | 6.97 | 0.8137 | 0.0008 | 0.0568 | 75.30 | 0.8141 | |
| 70 (g/L) | 0.2589 | 79.37 | 6.13 | 0.9332 | 0.0013 | 0.0493 | 39.13 | 0.8193 | |
| BBT | 10 (g/L) | 1.1422 | 12.71 | 0.52 | 0.9166 | −0.0023 | 0.0371 | 16.34 | 0.7676 |
| 40 (g/L) | 0.1671 | 46.97 | 4.90 | 0.8257 | 0.0010 | 0.1349 | 129.31 | 0.7937 | |
| 70 (g/L) | 0.3043 | 79.67 | 1.49 | 0.9088 | 0.0008 | 0.1122 | 135.44 | 0.6790 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarhan Kuzu, K.; Aykut, G.; Tek, S.; Yatmaz, E.; Germec, M.; Yavuz, I.; Turhan, I. Production and Characterization of Kombucha Tea from Different Sources of Tea and Its Kinetic Modeling. Processes 2023, 11, 2100. https://doi.org/10.3390/pr11072100
Tarhan Kuzu K, Aykut G, Tek S, Yatmaz E, Germec M, Yavuz I, Turhan I. Production and Characterization of Kombucha Tea from Different Sources of Tea and Its Kinetic Modeling. Processes. 2023; 11(7):2100. https://doi.org/10.3390/pr11072100
Chicago/Turabian StyleTarhan Kuzu, Kubra, Gamze Aykut, Serap Tek, Ercan Yatmaz, Mustafa Germec, Ibrahim Yavuz, and Irfan Turhan. 2023. "Production and Characterization of Kombucha Tea from Different Sources of Tea and Its Kinetic Modeling" Processes 11, no. 7: 2100. https://doi.org/10.3390/pr11072100
APA StyleTarhan Kuzu, K., Aykut, G., Tek, S., Yatmaz, E., Germec, M., Yavuz, I., & Turhan, I. (2023). Production and Characterization of Kombucha Tea from Different Sources of Tea and Its Kinetic Modeling. Processes, 11(7), 2100. https://doi.org/10.3390/pr11072100







