Abstract
Coffee is among the most widely consumed beverages worldwide, leading to the annual generation of substantial quantities of spent coffee grounds (SCGs). This study explored the influence of fabrication methods on the properties and potential applications of the resulting biocarbon materials. Dry methods (torrefaction at 270 °C and slow pyrolysis at 500 °C) and wet methods (hydrothermal carbonization HTC at 210 °C and hydrothermal liquefaction HTL at 270 °C) were employed to fabricate SCG-based biochar and hydrochar, respectively. The carbonization degree followed the order of slow pyrolysis > HTL > HTC ≈ torrefaction, yielding significant differences in energy properties, elemental composition, morphology, and surface functionality. Slow pyrolysis biochar was suitable for energy applications due to a similar fuel ratio as and higher heating value than semianthracite coal. For agricultural applications, SCG biochar produced through dry methods could be utilized to mitigate acidic soil conditions, whereas HTC hydrochar, with its elevated surface area and porosity, could enhance soil microbiological diversity and water-holding capacity, as well as benefit environmental applications such as wastewater remediation. In summary, the findings of this study are anticipated to inform decision-making processes concerning sustainable waste management of SCGs and the exploration of carbon-based materials applications across diverse sectors.
1. Introduction
Biomass represents the only abundant source of renewable organic carbon which can be converted to a variety of biochemicals, biomaterials, and biofuels. Among these biomass-based bioproducts, biocarbon materials such as biochar and hydrochar have attracted significant attention due to their green nature, cost-effectiveness, tunable pore size and distribution, presence of varying functional groups on the surface, and chemical and mechanical stability influenced by the selection of appropriate processing conditions [1]. To date, an extensive number of studies have investigated the use of biochar in wastewater treatment [2], as a soil amendment [3], and for gas purification [4], while hydrochar has been applied in fuel pellets [5], the removal of heavy metals [6], and CO2 capture [7].
In general, there are four primary thermochemical conversion methods commonly used for producing chars from biomass, including torrefaction, slow pyrolysis, hydrothermal carbonization (HTC), and hydrothermal liquefaction (HTL). The former two are dry processes for biochar preparation, while the latter two are wet routes and the resulting carbon-based materials are typically referred to as hydrochar due to the use of water as the reaction medium in the process. It is conclusive that the physicochemical properties of carbon-based materials derived from biomass are not only dependent on the characteristics of the parent biomass feedstock but are also influenced by the selection of the biomass conversion methods and the processing parameters [8]. Past studies have commonly focused on understanding the influences of reaction conditions and biomass properties on the biochar/hydrochar yield and quality [5,9,10]. However, only a few studies have compared the differences in physicochemical properties of biochar/hydrochar produced using wet and dry processes. Consequently, this makes the selection of the appropriate production method for certain applications challenging.
Applications of carbon-based materials are highly dependent on their characteristics, which in turn are influenced by the nature of the feedstock and the production method used. Different applications may require different favorable properties. For example, for use as a bioabsorbent in wastewater treatment, specific surface area, pore size, pore volume, and surface functional groups of biochar/hydrochar are the most important properties [11]. Contaminant removal efficacy depends on the nature of the contaminants as well as the physical and chemical adsorption capacity of the biocarbon-based materials. In agriculture, biochar has been extensively used as an organic soil amendment to improve soil properties such as nutrient retention, water-holding capacity (WHC), the profile of microbial communities, and plant growth [12]. However, some past studies have noted that hydrochar can cause N immobilization and plant growth reduction [13,14]. The use of biochar/hydrochar as organic soil amendments also serves to sequester carbon and, thus, reduce greenhouse gas (GHG) emissions, which provides environmental benefits. It is generally known that biochar from pyrolysis is remarkably recalcitrant and could take hundreds of years to decompose, thus minimizing CO2 emissions back to the atmosphere and being carbon emissions negative [15,16]. Hydrochar, however, has a high proportion of labile carbon which decomposes faster than biochar [17,18]. Therefore, it is essential to identify the difference between biochar and hydrochar, and more importantly, the desired morphological and physicochemical properties of biochar/hydrochar for specific applications.
In this study, spent coffee grounds (SCGs) were chosen as the representative biomass to produce biochar and hydrochar using both dry methods and wet methods. Coffee is one of the largest commodities traded globally with around 9.9 million tonnes consumed in 2021 and, according to the International Coffee Organization, consumption is projected to increase well into the future [19]. Accordingly, an enormous amount of SCG waste (residue after coffee brewing) is generated every year. Unfortunately, most of the SCGs are disposed of by landfilling, causing serious environmental concerns. Converting SCGs into beneficial carbon-based materials represents a promising solution to avoid the current land disposal approach and to adopt a waste-to-wealth pathway. In the current study, four different thermochemical processing pathways, including torrefaction (270 °C), slow pyrolysis (500 °C), HTC (210 °C), and HTL (270 °C), were employed to investigate the differences between wet processes and dry processes in terms of the physicochemical properties of biochar/hydrochar as well as the associated end-use of biochar/hydrochar derived from SCGs. Four SCG-based char materials were comprehensively characterized. The study outcomes provide correlations between the resulting biocarbon material properties and processing methods, which are largely missing in the current research literature. The knowledge created is expected to help tailor and optimize the application of SCG-based char materials based on their properties and the processing methods used.
2. Materials and Methods
2.1. Biochar Preparation
Wet SCGs were collected from Tim Hortons, Truro, Canada, and oven dried at 105 °C for 24 h. The dried SCGs were then utilized for biochar preparation through torrefaction and slow pyrolysis. Biochar preparation was carried out in a laboratory-scale pyrolyzer system which consisted of a stainless-steel reactor, external heater, temperature controller, nitrogen gas cylinder, and a water-cooled condenser. For each test, dried SCGs were placed inside the reactor and then heated from room temperature to 270 °C and 500 °C for torrefaction and slow pyrolysis, respectively, at a heating rate of 10 °C/min. The residence time for both torrefaction and slow pyrolysis was 60 min. N2 was continuously purged into the reactor to create inert conditions. After the reaction, the reactor was naturally cooled down to room temperature and the biochar was collected.
2.2. Hydrochar Preparation by HTC and HTL
Dried SCGs and distilled water were mixed in ratios of 1:4 and 1:8 for HTC and HTL, respectively, and loaded into a high-temperature/pressure reactor (Parr 4580). The reactor was tightly sealed, and pure N2 was initially purged into the reactor to replace the air in the reactor. Then, N2 was purged into the reactor to create a pressure of 20 bar to avoid the boiling of water during the heating process. For the HTC experiment, the reactor was heated to 210 °C and held at this temperature for 60 min. For the HTL run, the reactor was heated to 270 °C and then held at this temperature for 20 min. When the reaction was completed, the reactor was cooled to room temperature, and the gaseous products were released into a fume hood, followed by transferring the solid–liquid mixture to a beaker. The solid product was separated from the mixture by vacuum separation, and the solid product retained in the filter paper was collected and placed in an oven at 105 °C overnight. The dried solid product from HTC and HTL was denoted as hydrochar. Three trials were conducted for biochar and hydrochar fabrication. The products from each trial were then mixed together and used for various property tests, and the testing errors were within 5%.
2.3. Biocarbon Materials Characterization
Proximate analysis of the original SCGs and SCG-based biochar/hydrochar was conducted using the thermogravimetric analysis (TGA) method. The moisture content and volatile matter were determined using a PerkinElmer Pyris 1 Thermogravimetric Analyzer (Waltham, MA, USA) by heating the biomass sample in a nitrogen flow at 50 mL/min from 25 °C to 800 °C at a heating rate of 10 °C/min, followed by 20 min in 30 mL/min of air flow for ashing. The mass loss at 100 °C was taken as the moisture content, and the mass loss between 100 °C and 600 °C was taken as the mass loss of volatile matter. The ash content was determined according to ASTM D482 specifications.
Elemental analyses were conducted using a CHNS/O analyzer (PerkinElmer Series II 2400, USA), where the O content was determined by difference. Higher heating value (HHV) of the chars was measured using a Parr 6100 Calorimeter. In terms of the trace metals analysis, samples (~0.5 g) were firstly digested in HNO3 by microwave-assisted digestion (CEM MARS5, USA) based on EPA Method 3051. The trace metals content was then obtained using ICP-MS (Thermo Icap, Waltham, MA, USA) based on EPA Method 200.8. To determine the mercury content, a portion of the trace metals digestion solution was further digested with H2SO4 and K2S2O8 and then analyzed using CVAAS (M-7600, Teledyne Leeman Labs, Hudson, NH, USA) based on EPA 245.1, 245.5, and 245.6. For the polyaromatic hydrocarbon (PAH) analysis, the sample was analyzed on an Agilent 6890 series GC coupled with an Agilent 5973 mass Spectrometer detector. The PAH calibration curve was prepared using 6 levels of standards, 0.01 ppm, 0.05 ppm, 0.10 ppm, 0.50 ppm, 1.0 ppm, and 2.0 ppm. The polychlorinated biphenyl (PCB) analysis was carried out on an Agilent 7890A with an electron capture detector. The standard concentrations in the calibration curve for the PCB analysis were 0.1 ppm, 0.5 ppm, and 1 ppm.
As for the morphology, the sample was initially degassed at 150 °C until a stable static vacuum of less than 10−4 psi was achieved. Then, the surface area and porosity of SCGs and biochar/hydrochar were analyzed by N2 isothermal adsorption (77 K) using a Micrometrics ASAP 2010 instrument. For scanning electron microscopy (SEM), the samples were sprinkled into a carbon paste on an aluminum stub. After drying, the samples were imaged using secondary electrons in a Hitachi S4700 cold field emission instrument. The accelerating voltage was 1 kV, and the emission current was 20 uA. The functional groups were characterized using a Fourier transform infrared (FTIR) spectrometer (PerkinElmer Spectrum Two). The scanning range of 400–4000 cm−1 with a resolution of 4 cm−1 was employed.
The pH and point of zero charge (pHPZC) were determined using a multi-purpose pH meter (EC 500 ExStik II S/N 252957, EXTECH Instrument, Nashua, NH, USA). Briefly, 5 g of dried sample was mixed with 100 mL of distilled water, resulting in a 1:20 biomass/water mass ratio. The mixture was then stirred for 1 h using a DLM1886X1 Isotemp stirring plate (Fisher Scientific Inc., Markham, ON, Canada) and the liquid phase was recovered/obtained by vacuum filtration for pH measurement. Each determination was replicated three times. pHPZC was assessed by the pH drift method [6]. Specifically, gas bubbling was carried out to remove the dissolved carbon dioxide in a 0.01 M NaCl solution, where either HCl or NaOH was utilized to keep the solution pH value between 3 and 11. Around 0.5 g of tested samples was loaded into the-prepared solutions (50 mL) which had different pH values, and the final pH of the sample-containing solutions was measured after 24 h. pHPZC of the sample was identified where the initial and final pH values were equal.
3. Results
3.1. Properties Related to Energy Application
The potential of biochar/hydrochar for heating purposes is commonly evaluated by proximate analysis, ultimate analysis, and HHV. The results of proximate analysis for the original SCGs and obtained biochar/hydrochar are presented in Table 1. Original SCGs contained 75.37% volatile matter and 20.58% fixed carbon along with a limited amount of moisture and ash, which were consistent with the results reported by Setter et al. [20]. The biochar derived from the torrefaction of SCGs comprised 61.54% volatile matter and 36.37% fixed carbon, indicating that the torrefaction process increased the content of fixed carbon at the expense of volatile matter. HTC of SCGs resulted in hydrochar with comparable proximate components as torrefied SCGs. This is understandable, as both torrefaction and HTC were performed at a relatively low temperature and, as a result, a considerable amount of volatile matter was retained in the char. Afolabi et al. utilized SCGs to produce hydrochar through HTC at 220 °C for 1 h and reported the presence of analogous volatile matter and fixed carbon at 66.09% and 32.83%, respectively [21]. The hydrochar derived from HTL of SCGs contained low volatile matter, but more fixed carbon than HTC hydrochar as illustrated in Table 1, suggesting a higher processing temperature reduced hydrochar’s volatile matter content, and thus the percentage of fixed carbon increased. As for slow pyrolysis biochar, it only had 10.91% volatile matter along with a high fixed carbon (85.85%) and ash content (2.27%). Similar results were claimed by Pacioni et al. [22] who conducted slow pyrolysis of SCGs at 500 °C. These results show that temperature significantly impacted the proximate analysis results. To obtain char materials with low volatile matter, a high temperature should be applied.

Table 1.
Proximate analysis of spent coffee grounds (SCGs) and resultant biochar and hydrochar.
The proximate analysis of char materials has significant implications in energy applications, for example, for pelletizing biochar/hydrochar into solid biofuels. A high volatile matter content in biomass usually results in rapid combustion. In addition, a large-volume reactor is needed to prevent the high rate of emissions, such as NOx, and the formation of polycyclic aromatic hydrocarbons (PAHs) during the combustion of biomass with high volatile matter content. Therefore, it is important to reduce the content of volatile matter in biochar/hydrochar when used as solid fuel [23]. The fuel ratio is another important indicator of solid fuel quality which can be determined using Equation (1) [24]. Lower fuel ratios usually result in faster burnout, reduced char combustion, and more flaming combustion.
Fuel ratio = Fixed carbon (%)/volatile matter (%)
As evidenced in Table 1, the fuel ratio of biochar/hydrochar was higher than that for feedstock. The biochar obtained from slow pyrolysis had the highest fuel ratio, which was due to the highest temperature applied in the study. A similar result has been reported by Lu et al. [24], where the mixture of sewage sludge and lignocellulose was used as the feedstock to prepare solid fuel. To compare with differently ranked coals, such as anthracite (fuel ratio > 10), semianthracite (fuel ratio of 6–10), semibituminous coal (fuel ratio of 3–6), and bituminous coal (fuel ratio of ~3) [25], the fuel ratio of slow pyrolysis biochar (7.87, Table 1) is within the range of semianthracite coal, rendering it a promising solid fuel with a property close to coal. However, except for slow pyrolysis biochar, the fuel ratio of other biocarbon materials was lower than coal.
The elemental analysis results and HHV for SCGs and the resultant biochar and hydrochar are presented in Table 2. The original SCGs contained 53.04% C and 37.71% O, and its HHV was 22.23 MJ/kg. Torrefaction and HTC slightly improved the C content to ~66% and lowered O content to ~21% and the HHV was ~29 MJ/kg. Similar observations were noted by Massaya et al. [26] in a study that aimed to produce solid fuel from SCGs. HTL of SCGs further increased the hydrochar’s C content to 72.81% and decreased the O content to 14.44%. An even greater increment in C content and decline in O content was observed for slow pyrolysis biochar as shown in Table 2. However, the HHV of slow pyrolysis biochar was slightly lower than HTL hydrochar (30.44 MJ/kg vs. 32.33 MJ/kg), even though slow pyrolysis had a higher C content. This was presumably because the high carbonization degree of biochar made it harder to fully combust, which subsequently led to a lower HHV. Overall, thermochemical treatment profoundly improved the energy density of SCGs.

Table 2.
The elemental analysis and higher heating values (HHVs) for spent coffee grounds (SCGs) and resultant biochar and hydrochar.
Table 2 also summarizes the O/C and H/C atomic ratios of biomass, biochar, and hydrochar. By plotting O/C and H/C in a van Krevelen diagram, a comparison of biomass, biocarbon materials, and coal at different ranks can be developed [27], as depicted in Figure 1. Clearly, slow pyrolysis biochar falls within the region of coal and can be considered as a solid biofuel with comparable quality to coal. Examining O/C and H/C ratios, it was found that the carbonization degree of biochar/hydrochar was affected by demethanation, dehydration, and decarboxylation. For example, the decreased H/C ratio could be due to the formation of CH4 via demethanation, while a drop in the O/C ratio could be caused by the formation of CO2, carboxylic acids, and carbonyls by decarboxylation. Based on the O/C ratio, the carbonization degree of the four char materials is in the order of slow pyrolysis > HTL > torrefaction ≈ HTC.
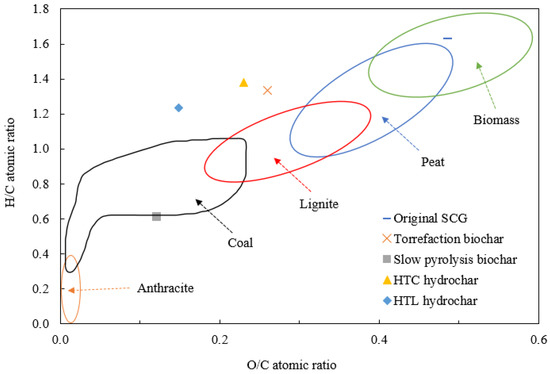
Figure 1.
The van Krevelen diagram for biomass, biochar, and hydrochar.
3.2. Properties Related to Agricultural Application
3.2.1. pH and pHPCZ Values
The pH and pHPCZ of biocarbon materials are important characteristics to assess their suitability for applications in agriculture. The values for the original SCGs, biochar, and hydrochar are presented in Table 3. The details of pH and pHpcz testing are provided in the Supplementary Materials (Figure S1). The pH of the original SCGs was 4.89, indicating the generation of acidic compounds during the coffee brewing process. The pH value of slow pyrolysis biochar was the highest at 7.01 among the four biocarbon materials investigated in this study at the highest temperature of 500 °C. Torrefaction is another dry process that produced biochar with a slightly acidic pH value of 6.5. Chin-Pampillo et al. carried out torrefaction of coffee husks and the pH of the resultant biochar was 6.9, which was comparable to the results in the current study [28]. In another study, Sun et al. observed that the pH values of biochar obtained from pyrolysis of hickory wood (Carya ovata), bagasse, and bamboo (Bambusa vulgaris) were between 7.1 and 9.2 [29].

Table 3.
pH and pHpzc of spent coffee grounds (SCGs) and resultant biochars and hydrochars.
As for hydrochar, the pH value was 3.93 and 3.89, respectively, for HTC and HTL, which was lower than that of the original SCGs (4.89). This observation aligns with the results of the study reported by Xue et al. [30]. The lower pH value of the hydrochar was presumably because cellulose, hemicellulose, and lignin in SCGs started to hydrolyze and decompose during heating, i.e., hemicellulose first at a relatively low temperature, followed by cellulose and lignin. Consequently, HTC of SCGs at 210 °C and HTC of SCGs at 270 °C resulted in a high amount of organic acid and more likely some of these acidic compounds stayed within the solid phase, leading to the low pH of the hydrochar. In general, biochar is known to moderate soil acidity and, thus, enhance soil fertility and crop productivity, and is ideal as most crops perform well at a soil pH range of 6.5 and 7.5 [31]. In the present study, the pH values of the biochar obtained from torrefaction and slow pyrolysis were 6.5 and 7.0, respectively (Table 3), which can be used to ameliorate acidic soils and increase cation exchange capacity (CEC) and plant nutrient availability. In contrast, the pH of the hydrochars obtained from HTC and HTL were relatively low and, thus, can be used for lowering the pH of alkali soils such as clay soils.
pHPZC is the pH at which the surface of a material is neutral. The surface of a material is positively charged when the pH is lower than pHPZC and vice versa [32]. Overall, there was no significant difference between pH and pHPZC of torrefied SCGs, HTC hydrochar, and HTL hydrochar. Meanwhile, the pH and pHPZC of slow pyrolysis biochar were approximately 7.0 and 8.4, respectively. This implies that the surface of the slow pyrolysis biochar was positively charged, likely due to the existence of various metals on the surface of slow pyrolysis biochar. Positively charged biochar might help soils retain essential nutrients that might otherwise leach away [33]. This is particularly valuable in highly weathered soils where nutrient leaching can be a significant problem.
3.2.2. Nutrient Content
In the context of being used as a soil amendment, nutrients such as N, P, and K in biochar/hydrochar are of value for soil fertility. SCGs originally contained 2.1% N, and thermochemical treatment of SCGs resulted in biochar/hydrochar with higher N contents as shown in Table 4. Unlike other lignocellulosic biomasses such as woody biomass, the biochar/hydrochar derived from SCGs contained a fairly high content of N (close to 3%), most probably due to the presence of protein and lipids in the parent biomass [29]. As such, SCG biochar/hydrochar showed promising potential of being used to improve the nutrient status of agricultural soils. The levels of P and K in the biochar/hydrochar were <1%, which were consistent with a previous study of producing biochar from coffee husks, pine bark, and sugarcane bagasse [34]. N, P, and K are primary macronutrients that plants require for their growth and development. When biochar/hydrochar is enriched with these nutrients, it can be particularly beneficial in agricultural applications, such as improved plant nutrients, soil fertility, and microbial activity [35].

Table 4.
Nitrogen (N), phosphorus (P), and potassium (K) content of spent coffee grounds (SCGs) and resultant biochar and hydrochar.
3.2.3. Inorganics and Potentially Toxic Components
For agricultural application, consideration of possible hazardous heavy metal content and contaminant levels in char materials is crucial. The contents of metals in SCGs and the resultant biochars/hydrochars are presented in Table 5. The contents of K (3200 mg/kg), Mg (1340 mg/kg), and Ca (1250 mg/kg) in the original SCGs were much higher compared to other elements, which were well in agreement with the findings by Evaristo et al. [36]. Regarding the four types of biochar/hydrochar produced, the content of K, Mg, and Ca ranged from 604–11,400 mg/kg, 555–4410 mg/kg, and 960–4200 mg/kg, respectively. Specifically, torrefaction of SCGs at 210 °C led to a moderate increment in the content of K (4780 mg/kg), Mg (1950 mg/kg), and Ca (1860 mg/kg), and further increasing temperature (slow pyrolysis at 500 °C) highly increased K, Mg, and Ca among the four chars. Hussain et al. studied biochar from SCGs via pyrolysis at 400 °C and reported that the contents of K, Mg, and Ca were 18,500 mg/kg, 2000 mg/kg, and 1900 mg/kg, respectively [37], which were in the same order of magnitude as in this study.

Table 5.
The trace metal content in spent coffee grounds (SCGs) and resultant biochar and hydrochar.
Unlike biochar, hydrochar contained much lower K and Mg content compared to the original SCGs, indicating that a part of K and Mg was released into the aqueous phase during the hydrothermal process. In terms of Ca, although HTC also decreased its content from 1250 mg/kg (in SCGs) to 960 mg/kg (Table 5), the HTL hydrochar contained 2370 mg/kg Ca, which was higher than HTC hydrochar. Notably, the concentration of heavy metals (e.g., Zn) in SCG-derived chars (<27 mg/kg) was much lower than that from sludge (over 1300 mg/kg) [34]. In the case of metals of environmental and food safety concern such as arsenic, cadmium, chromium, cobalt, copper, lead, mercury, molybdenum, nickel, selenium, and zinc, their content was found to be either not detectable or much lower than the maximum levels regulated by the Canadian Food Inspection Agency (CFIA). In terms of contaminants, polycyclic aromatic hydrocarbon (PAH) content is lower than the reporting limit of 0.01 mg/kg, while polychlorinated biphenyl content is lower than the reporting limit of 0.05 mg/kg in this study. It is notable that the formation of PAH during biochar fabrication is also dependent on the type of feedstock used. For instance, Krzyszczak et al. utilized sewage sludge and plant material to fabricate biochar through pyrolysis and observed a higher PAH content for sewage sludge biochar than that of plant material [38]. Overall, SCGs and the derived chars are environmentally friendly and safe to use as soil amendments.
3.2.4. Surface and Morphological Properties
Scanning electron microscopy (SEM) was carried out for SCGs and the resultant biocarbon materials, as illustrated in Figure 2. This type of analysis can help examine and compare the microstructural changes to different biocarbon materials obtained using different fabrication methods. Raw materials (Figure 2A,B) exhibited an irregular and rough surface morphology, which is in good agreement with the past research literature [21]. SCGs exhibited a dense morphology, mainly due to the presence of large amounts of organic compounds [39]. Figure 2C–F show the 100 µm SEM images for torrefied SCGs, slow pyrolysis biochar, HTC hydrochar, and HTL hydrochar, respectively. The differences in the patterns among biochar and hydrochar samples can be attributed to differences in the fabrication methods and the processing temperature. In comparison, biochar obtained from slow pyrolysis had a more obvious porous and cracked structure compared to that obtained from torrefaction, as indicated in Figure 2C,D. This observation is attributed to volatilization and mass conversion during slow pyrolysis compared to torrefaction. In addition, although the porous structure of obtained hydrochars was less pronounced than slow pyrolysis biochar, hydrochars exhibited better pores and a loose structure. This is attributed to the fact that the dielectric constant of water reduces with increasing temperature which, in turn, makes the water behave like an organic solvent [36]. In the case of hydrothermal treatment, this results in more organic compounds that can be dissolved in the water solvent, thereby promoting the dissolution of organics from feedstock and forming better pores and a more loose structure [40].
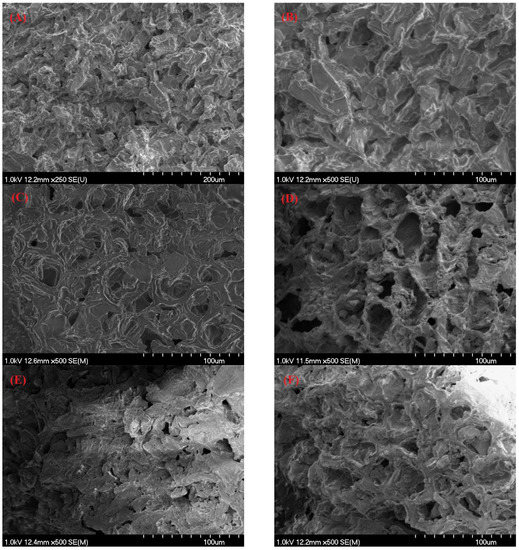
Figure 2.
Scanning electron microscopy (SEM) for (A,B) spent coffee grounds, (C) torrefied SCGs, (D) pyrolysis biochar, (E) HTC hydrochar, and (F) HTL hydrochar.
BET analysis was conducted to further understand the morphological properties of biocarbon materials and support the observations derived from the SEM images. The surface area and porosity (i.e., pore volume/size) of the chars obtained were determined and are presented in Table 6. The adsorption details for obtained biochars/hydrochars are provided in the Supplementary Materials (Figure S2). Original SCGs had a surface area of 8.05 m2/g, which was larger than the values reported in previous studies. Surface areas of 2.5 m2/g and 0.4 m2/g were reported for SCGs by [41,42], respectively. The biochar derived from SCG torrefaction had a slightly larger surface area (8.53 m2/g) and pore size (0.86 nm) compared to the original SCGs. However, the increase in temperature (slow pyrolysis at 500 °C) led to a decline in biochar surface area (4.17 m2/g) but an increase in pore size (2.24 nm) compared to other treatments. Zhang et al. characterized the SCG biochar obtained under different temperatures and observed that a higher temperature (>400 °C) caused the small pores to be destroyed, resulting in a decrease in the surface area [43]. These observations are consistent with the current results.

Table 6.
Surface area and porosity (pore volume/size) of spent coffee grounds (SCGs), biochar obtained from torrefaction and slow pyrolysis, and hydrochar obtained from HTC and HTL.
Surprisingly, the porosities of the hydrochars were different from those of the biochars, with hydrochars having greater pore volume and pore size. In particular, the hydrochar obtained from HTC showed the largest specific surface area of 15.15 m2/g, which was much larger than the HTL biochar of 7.74 m2/g (Table 6). This trend was similar to that of biochar, i.e., a higher temperature was less favorable for specific surface area. A high processing temperature might induce more extensive breakdown of biomass to form highly viscous liquid, which might stay within the pores and lead to a smaller surface area. HTC hydrochar obtained from this study had a similar specific surface area to that obtained from HTC of rice husks (13.09 m2/g) at 175 °C, both of which are much smaller than that of the activated carbon (i.e., 950–2000 m2/g) [44,45]. Amendment of agricultural soils with the highly porous biochar/hydrochar could improve soil structure and increase soil water-holding capacity, nutrient retention and release, and microbial diversity as previously observed by Zhang et al. [46].
3.2.5. Functional Groups
FTIR analysis was carried out to characterize the functional groups on the surface of the original SCGs, biochar, and hydrochar, as shown in Figure 3. The original SCGs were characterized by -OH (3335 cm−1), C-H (2920 cm−1 and 2850 cm−1), C=O (~1700 cm−1), C=C (1605 cm−1), C-O-C (1161 cm−1), C-N (1097 cm−1), and C-O (1027 cm−1) bonds, which were comparable with the SCGs used in previous studies [38]. Torrefied SCGs, HTC hydrochar, and HTL hydrochar shared some functional groups with the original SCGs such as -OH, C-H, C=O, and C-O-C bonds. The functional groups in torrefied SCGs were very comparable with those of the HTC hydrochar. However, HTL hydrochar exhibited more complete carbonization compared to torrefied SCGs and HTC hydrochar, as evidenced by its weaker peak of the -OH group, absence of the C-O bond, and the presence of the C=C bond. As for slow pyrolysis biochar, its characteristic peaks were significantly less abundant than the others, which implied that the carbonization during SCG slow pyrolysis was extremely thorough. Mukherjee et al. pyrolyzed SCGs under different temperatures and found that the characteristic peaks gradually disappeared as the temperature increased from 400 °C to 600 °C [41].
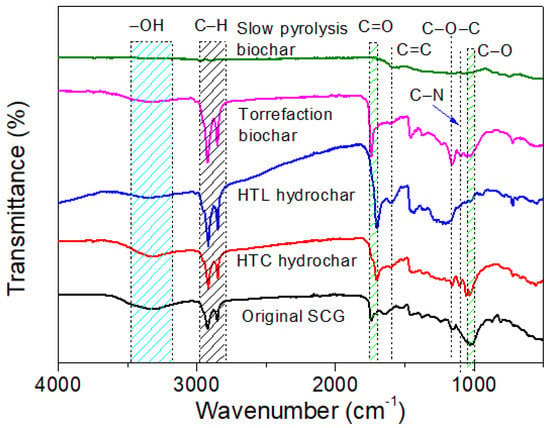
Figure 3.
Fourier transform infrared spectroscopy (FTIR) spectra of spent coffee grounds (SCGs), torrefied SCGs, slow pyrolysis biochar, hydrothermal carbonization (HTC) hydrochar, and hydrothermal liquefaction (HTL) hydrochar.
It can be summarized that surface functionality may have critical impact on nutrient retention and soil microbial communities as reviewed by Zhao et al. [47]. The functional groups of SCG-based char materials generated from wet and dry routes are different as noted above. However, it is challenging to identify what functional groups could best enhance the performance of soil without specifying soil and crop types.
3.3. Discussions on the Applications of SCG Biocarbon Materials
Compared to hydrochar, biochar is a more stable carbon sink for carbon sequestration as the higher fixed carbon content is less susceptible to biological decomposition and more stable in the environment [40]. This can also be evidenced by the lowest O/C and H/C ratios of biochar compared to other char samples as shown in Table 2. Low O/C and H/C ratios would imply a higher level of aromatic compounds [48].
Aside from carbon sequestration, biocarbon materials can be used as adsorbents for wastewater remediation. pH values, porosity, surface morphology, and the functional groups as discussed in Section 3.2 are the most important properties in the context of environmental applications. For wastewater treatment, the removal efficiency of a bioadsorbent is highly related to the characteristics of the contaminants. SCG-derived hydrochars had lower pH values than that of slow pyrolysis char (Table 3), which are thus more suitable for the removal of contaminants that are basic. The pHpzc of adsorbents is an important parameter that can identify the potential of the interaction between the functional groups at the interface of the adsorbent/adsorbate in an aqueous solution. Generally, the surface of char materials is positively charged when pH values are lower than those of pHpzc and vice versa [49]. The adsorption capacity of hydrochar and biochar can be optimized based on the nature of the contaminants in wastewater and the pHpzc of the bioadsorbents.
The surface area and porous structure are key factors that affect the ability of a bioadsorbent to remove contaminants as a large surface area suggests a more porous structure within the material. For instance, the literature has suggested that biocarbon materials with a large specific surface area often show a better adsorption capacity towards aromatics such as toluene [50]. Additionally, the surface area is critical for enhancing the transport and interaction between biocarbon materials with the surrounding medium. Hydrochar derived from HTC has a loose structure as shown in the SEM image (Figure 2E), and a relatively larger specific surface, larger pore volume, and pore sizes compared to the other three chars as evident in Table 6, rendering it the best adsorbent candidate for wastewater treatment. The specific surface areas of the four char materials are in the range of 7.74–15.5 m2/g, which are generally smaller than that of commercial adsorbents, implying that further physical/chemical activation treatment is necessary to achieve efficient treatment of wastewater [51,52]. Past studies have noted that the type of feedstock and activation conditions played a significant role in influencing the specific surface area of the activated hydrochar which, in turn, influences the adsorption capability for contaminants [50]. Based on the standard developed by the International Union of Pure and Applied Chemistry (IUPAC), hydrochar obtained from HTC belongs to the mesopore group (the average pore size of the mesopore group is 2–50 nm) [53]. As recommended in the research literature, the mesopores present in the bioadsorbent are favorable for removing carcinogenic herbicides such as atrazine, which is commonly used to control broadleaf weeds and grasses [54,55].
Hydrophilicity/hydrophobicity of the char materials also has an impact on their adsorption capacity. O/C ratio can partially indicate the surface hydrophilicity of the char materials. Comparing the four char materials, hydrochar obtained from HTC had the highest O/C ratio (Table 2), indicating a high level of polar groups on its surface that can act as water adsorption centers, thereby leading to high surface hydrophilicity. Based on the O/C ratio, the hydrophobicity of the four char materials is in the order of slow pyrolysis > HTL > torrefaction ≈ HTC. In terms of surface functional groups, hydrochars derived from wet methods are rich in functional groups compared to those from slow pyrolysis as shown in Figure 3, which would benefit contaminant removal through surface complexation between the adsorbent and adsorbate. However, this would differ based on the nature of the targeted wastewater characteristics.
4. Conclusions
In this study, a readily available food processing waste, namely, SCGs, was converted to biochar and hydrochar using dry and wet methods, respectively. The influence of the production methods on the properties and the associated applications of various biocarbon materials was evaluated. The results obtained indicated that the biochar generated through slow pyrolysis was preferable to be used for energy applications due to its similar fuel ratio and HHV to semianthracite coal. For agricultural application, biochar obtained from torrefaction or slow pyrolysis was able to ameliorate acidic soils because of its basic nature. HTC hydrochar is the best candidate as a soil amendment because its larger specific surface area, porous structure, and rich surface functional groups are beneficial to water and nutrient retention/release. In terms of environmental application, HTC hydrochar was more favorable for wastewater remediation due to its textural properties and surface functional groups. The study outcomes presented provide baseline knowledge for decision making for the selection of the most suitable production methods to convert waste SCGs into valuable biocarbon materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr11072099/s1, Figure S1: Detailed information for the pH and pHpzc of the obtained biochars and hydrochars; Figure S2: The relation between relative pressure and adsorbed volume for the obtained biochars and hydrochars from spent coffee grounds (SCGs).
Author Contributions
Conceptualization, J.Y. and Z.Z.; methodology, J.Y. and Y.H.; formal analysis, Z.Z. and Y.H.; investigation, J.Y. and Y.H.; resources, L.A., I.C. and A.G.; data curation, Q.H. and Y.H.; writing—original draft preparation, J.Y. and Y.H.; writing—review and editing, L.A., I.C. and A.G.; supervision, Q.H.; funding acquisition, J.Y., Z.Z., Q.H. and Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fujian Provincial Natural Science Foundation (2022J05242, 2022J011134), Minjiang University, National Sciences and Engineering Research Council Discovery, Canada (grant numbers RGPIN-2020-05695, NSERC ALLRP 571708-2, and RGPIN-2022-03203).
Data Availability Statement
Data will be made available on request.
Acknowledgments
We also acknowledge Xiaoyu Lin, Shaorong Du, Quan Zhang, and Dengge Qin for their help in the manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, M.; Xu, Z.; Hou, D.; Gao, B.; Cao, X.; Ok, Y.S.; Rinklebe, J.; Bolan, N.S.; Tsang, D.C.W. Waste-Derived Biochar for Water Pollution Control and Sustainable Development. Nat. Rev. Earth Environ. 2022, 3, 444–460. [Google Scholar] [CrossRef]
- Shi, M.; Wang, X.; Shao, M.; Lu, L.; Ullah, H.; Zheng, H.; Li, F. Resource Utilization of Typical Biomass Wastes as Biochars in Removing Plasticizer Diethyl Phthalate from Water: Characterization and Adsorption Mechanisms. Front. Environ. Sci. Eng. 2022, 17, 5. [Google Scholar] [CrossRef]
- Sun, C.; Sun, J.; Gao, J.; Liu, J.; Yu, X.; Wang, Z.; Yang, X.; Ji, N. Comprehensive Application of Bio-Char and Nitrogen Fertilizer in Dry-Land Maize Cultivation. Sci. Rep. 2022, 12, 13478. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, E.; Mishra, R.; Lo, S.-L.; Kumar, S. A Green Approach towards Sorption of CO2 on Waste Derived Biochar. Environ. Res. 2022, 214, 113954. [Google Scholar] [CrossRef]
- Hu, Y.; Gallant, R.; Salaudeen, S.; Farooque, A.A.; He, S. Hydrothermal Carbonization of Spent Coffee Grounds for Producing Solid Fuel. Sustainability 2022, 14, 8818. [Google Scholar] [CrossRef]
- Nadarajah, K.; Bandala, E.R.; Zhang, Z.; Mundree, S.; Goonetilleke, A. Removal of Heavy Metals from Water Using Engineered Hydrochar: Kinetics and Mechanistic Approach. J. Water Process Eng. 2021, 40, 101929. [Google Scholar] [CrossRef]
- Rehman, A.; Nazir, G.; Rhee, K.Y.; Park, S.-J. Valorization of Orange Peel Waste to Tunable Heteroatom-Doped Hydrochar-Derived Microporous Carbons for Selective CO2 Adsorption and Separation. Sci. Total Environ. 2022, 849, 157805. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into Biochar and Hydrochar Production and Applications: A Review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Chen, Y.; Syed-Hassan, S.S.A.; Li, Q.; Deng, Z.; Hu, X.; Xu, J.; Jiang, L.; Su, S.; Hu, S.; Wang, Y.; et al. Effects of Temperature and Aspect Ratio on Heterogeneity of the Biochar from Pyrolysis of Biomass Pellet. Fuel Process. Technol. 2022, 235, 107366. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, X.; Ke, S.; Shao, J.; Yang, H.; Zhang, S.; Chen, H. Effect of Different Biomass Species and Pyrolysis Temperatures on Heavy Metal Adsorption, Stability and Economy of Biochar. Ind. Crops Prod. 2022, 186, 115238. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar Technology in Wastewater Treatment: A Critical Review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.X.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Ibrahim, M.L.; Ghasemi, M. A Review on Biochar Production from Different Biomass Wastes by Recent Carbonization Technologies and Its Sustainable Applications. J. Environ. Chem. Eng. 2022, 10, 107017. [Google Scholar] [CrossRef]
- Gajić, A.; Koch, H.-J. Sugar Beet (Beta vulgaris L.) Growth Reduction Caused by Hydrochar Is Related to Nitrogen Supply. J. Environ. Qual. 2012, 41, 1067–1075. [Google Scholar] [CrossRef]
- Luutu, H.; Rose, M.T.; McIntosh, S.; Van Zwieten, L.; Rose, T. Plant Growth Responses to Soil-Applied Hydrothermally-Carbonised Waste Amendments: A Meta-Analysis. Plant Soil 2022, 472, 1–15. [Google Scholar] [CrossRef]
- Ameloot, N.; Graber, E.R.; Verheijen, F.G.A.; De Neve, S. Interactions between Biochar Stability and Soil Organisms: Review and Research Needs. Eur. J. Soil Sci. 2013, 64, 379–390. [Google Scholar] [CrossRef]
- Gurwick, N.P.; Moore, L.A.; Kelly, C.; Elias, P. A Systematic Review of Biochar Research, with a Focus on Its Stability in Situ and Its Promise as a Climate Mitigation Strategy. PLoS ONE 2013, 8, e75932. [Google Scholar] [CrossRef]
- Gronwald, M.; Vos, C.; Helfrich, M.; Don, A. Stability of Pyrochar and Hydrochar in Agricultural Soil—A New Field Incubation Method. Geoderma 2016, 284, 85–92. [Google Scholar] [CrossRef]
- Malghani, S.; Jüschke, E.; Baumert, J.; Thuille, A.; Antonietti, M.; Trumbore, S.; Gleixner, G. Carbon Sequestration Potential of Hydrothermal Carbonization Char (Hydrochar) in Two Contrasting Soils; Results of a 1-Year Field Study. Biol. Fertil. Soils 2015, 51, 123–134. [Google Scholar] [CrossRef]
- International Coffee Organization—Trade Statistics Tables. Available online: http://www.ico.org/trade_statistics.asp (accessed on 15 December 2019).
- Setter, C.; Borges, F.A.; Cardoso, C.R.; Mendes, R.F.; Oliveira, T.J.P. Energy Quality of Pellets Produced from Coffee Residue: Characterization of the Products Obtained via Slow Pyrolysis. Ind. Crops Prod. 2020, 154, 112731. [Google Scholar] [CrossRef]
- Afolabi, O.O.D.; Sohail, M.; Cheng, Y.-L. Optimisation and Characterisation of Hydrochar Production from Spent Coffee Grounds by Hydrothermal Carbonisation. Renew. Energy 2020, 147, 1380–1391. [Google Scholar] [CrossRef]
- Pacioni, T.R.; Soares, D.; Di Domenico, M.; Alves, J.L.F.; Virmond, E.; Moreira, R.d.F.P.M.; José, H.J. Kinetic Modeling of CO2 Gasification of Biochars Prepared from Brazilian Agro-Industrial Residues: Effect of Biomass Indigenous Mineral Content. Biomass Convers. Biorefin. 2021, 13, 6675–6688. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Odusote, J.K.; Ikubanni, P.P.; Lasode, O.A.; Malathi, M.; Paswan, D. The Ignitability, Fuel Ratio and Ash Fusion Temperatures of Torrefied Woody Biomass. Heliyon 2020, 6, e03582. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal Carbonization of Renewable Waste Biomass for Solid Biofuel Production: A Discussion on Process Mechanism, the Influence of Process Parameters, Environmental Performance and Fuel Properties of Hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Smart, J.P.; Riley, G.S. On the Effects of Firing Semi-Anthracite and Bituminous Coal under Oxy-Fuel Firing Conditions. Fuel 2011, 90, 2812–2816. [Google Scholar] [CrossRef]
- Massaya, J.; Pickens, G.; Mills-Lamptey, B.; Chuck, C.J. Enhanced Hydrothermal Carbonization of Spent Coffee Grounds for the Efficient Production of Solid Fuel with Lower Nitrogen Content. Energy Fuels 2021, 35, 9462–9473. [Google Scholar] [CrossRef]
- Fakudze, S.; Chen, J. A Critical Review on Co-Hydrothermal Carbonization of Biomass and Fossil-Based Feedstocks for Cleaner Solid Fuel Production: Synergistic Effects and Environmental Benefits. Chem. Eng. J. 2023, 457, 141004. [Google Scholar] [CrossRef]
- Chin-Pampillo, J.S.; Alfaro-Vargas, A.; Rojas, R.; Giacomelli, C.E.; Perez-Villanueva, M.; Chinchilla-Soto, C.; Alcañiz, J.M.; Domene, X. Widespread Tropical Agrowastes as Novel Feedstocks for Biochar Production: Characterization and Priority Environmental Uses. Biomass Convers. Biorefin. 2021, 11, 1775–1785. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of Feedstock Type, Production Method, and Pyrolysis Temperature on Biochar and Hydrochar Properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen Peroxide Modification Enhances the Ability of Biochar (Hydrochar) Produced from Hydrothermal Carbonization of Peanut Hull to Remove Aqueous Heavy Metals: Batch and Column Tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Thomas, J. Soil PH and the Availability of Plant Nutrients 2010. Available online: https://nutrientstewardship.org/implementation/soil-ph-and-the-availability-of-plant-nutrients/ (accessed on 15 November 2022).
- Saha, N.; Xin, D.; Chiu, P.C.; Reza, M.T. Effect of Pyrolysis Temperature on Acidic Oxygen-Containing Functional Groups and Electron Storage Capacities of Pyrolyzed Hydrochars. ACS Sustain. Chem. Eng. 2019, 7, 8387–8396. [Google Scholar] [CrossRef]
- Rens, H.; Bera, T.; Alva, A.K. Effects of Biochar and Biosolid on Adsorption of Nitrogen, Phosphorus, and Potassium in Two Soils. Water. Air. Soil Pollut. 2018, 229, 281. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; de Melo, I.C.N.A.; Melo, L.C.A.; Magriotis, Z.M.; Sánchez-Monedero, M.A. Properties of Biochar Derived from Wood and High-Nutrient Biomasses with the Aim of Agronomic and Environmental Benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef]
- Tahery, S.; Munroe, P.; Marjo, C.E.; Rawal, A.; Horvat, J.; Mohammed, M.; Webber, J.B.W.; Arns, J.-Y.; Arns, C.H.; Pan, G.; et al. A Comparison between the Characteristics of a Biochar-NPK Granule and a Commercial NPK Granule for Application in the Soil. Sci. Total Environ. 2022, 832, 155021. [Google Scholar] [CrossRef] [PubMed]
- Evaristo, R.B.W.; Ferreira, R.; Petrocchi Rodrigues, J.; Sabino Rodrigues, J.; Ghesti, G.F.; Silveira, E.A.; Costa, M. Multiparameter-Analysis of CO2/Steam-Enhanced Gasification and Pyrolysis for Syngas and Biochar Production from Low-Cost Feedstock. Energy Convers. Manag. X 2021, 12, 100138. [Google Scholar] [CrossRef]
- Hussain, N.; Chantrapromma, S.; Suwunwong, T.; Phoungthong, K. Cadmium (II) Removal from Aqueous Solution Using Magnetic Spent Coffee Ground Biochar: Kinetics, Isotherm and Thermodynamic Adsorption. Mater. Res. Express 2020, 7, 085503. [Google Scholar] [CrossRef]
- Krzyszczak, A.; Dybowski, M.P.; Czech, B. Formation of Polycyclic Aromatic Hydrocarbons and Their Derivatives in Biochars: The Effect of Feedstock and Pyrolysis Conditions. J. Anal. Appl. Pyrolysis 2021, 160, 105339. [Google Scholar] [CrossRef]
- Lee, K.-T.; Cheng, C.-L.; Lee, D.-S.; Chen, W.-H.; Vo, D.-V.N.; Ding, L.; Lam, S.S. Spent Coffee Grounds Biochar from Torrefaction as a Potential Adsorbent for Spilled Diesel Oil Recovery and as an Alternative Fuel. Energy 2022, 239, 122467. [Google Scholar] [CrossRef]
- Fu, M.-M.; Mo, C.-H.; Li, H.; Zhang, Y.-N.; Huang, W.-X.; Wong, M.H. Comparison of Physicochemical Properties of Biochars and Hydrochars Produced from Food Wastes. J. Clean. Prod. 2019, 236, 117637. [Google Scholar] [CrossRef]
- Mukherjee, A.; Borugadda, V.B.; Dynes, J.J.; Niu, C.; Dalai, A.K. Carbon Dioxide Capture from Flue Gas in Biochar Produced from Spent Coffee Grounds: Effect of Surface Chemistry and Porous Structure. J. Environ. Chem. Eng. 2021, 9, 106049. [Google Scholar] [CrossRef]
- Tongcumpou, C.; Usapein, P.; Tuntiwiwattanapun, N. Complete Utilization of Wet Spent Coffee Grounds Waste as a Novel Feedstock for Antioxidant, Biodiesel, and Bio-Char Production. Ind. Crops Prod. 2019, 138, 111484. [Google Scholar] [CrossRef]
- Zhang, X. Characterization and Sulfonamide Antibiotics Adsorption Capacity of Spent Coffee Grounds Based Biochar and Hydrochar. Sci. Total Environ. 2020, 10, 137015. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Hu, X.; Wang, X.; Liu, S.; Jiang, L. Biochar as Potential Sustainable Precursors for Activated Carbon Production: Multiple Applications in Environmental Protection and Energy Storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An Overview on Engineering the Surface Area and Porosity of Biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dijkstra, F.A.; Liu, X.; Wang, Y.; Huang, J.; Lu, N. Effects of Biochar on Soil Microbial Biomass after Four Years of Consecutive Application in the North China Plain. PLoS ONE 2014, 9, e102062. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Yao, G.; Lin, Z.; Xu, L.; Jiang, Y.; Jin, Z.; Shan, S.; Ping, L. Advances in the Effects of Biochar on Microbial Ecological Function in Soil and Crop Quality. Sustainability 2022, 14, 10411. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, J.; Yi, Y. Biochar and Hydrochar Derived from Freshwater Sludge: Characterization and Possible Applications. Sci. Total Environ. 2021, 763, 144550. [Google Scholar] [CrossRef] [PubMed]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy Metals Removal from Aqueous Environments by Electrocoagulation Process– a Systematic Review. J. Environ. Health Sci. Eng. 2015, 13, 74. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of Biochar Surface Characteristics in the Adsorption of Aromatic Compounds: Pore Structure and Functional Groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Congsomjit, D.; Areeprasert, C. Hydrochar-Derived Activated Carbon from Sugar Cane Bagasse Employing Hydrothermal Carbonization and Steam Activation for Syrup Decolorization. Biomass Convers. Biorefin. 2021, 11, 2569–2584. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Qian, F.; Zhou, C.; Zhang, S.; Chen, J. Role of Hydrochar Properties on the Porosity of Hydrochar-Based Porous Carbon for Their Sustainable Application. ACS Sustain. Chem. Eng. 2015, 3, 833–840. [Google Scholar] [CrossRef]
- Zdravkov, B.; Čermák, J.; Šefara, M.; Janků, J. Pore Classification in the Characterization of Porous Materials: A Perspective. Open Chem. 2007, 5, 385–395. [Google Scholar] [CrossRef]
- Phan, K.A.; Phihusut, D.; Tuntiwiwattanapun, N. Preparation of Rice Husk Hydrochar as an Atrazine Adsorbent: Optimization, Characterization, and Adsorption Mechanisms. J. Environ. Chem. Eng. 2022, 10, 107575. [Google Scholar] [CrossRef]
- Tan, G.; Sun, W.; Xu, Y.; Wang, H.; Xu, N. Sorption of Mercury (II) and Atrazine by Biochar, Modified Biochars and Biochar Based Activated Carbon in Aqueous Solution. Bioresour. Technol. 2016, 211, 727–735. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).