An Assessment of the Chemical Compatibility of Viton Fluoropolymers and Some Harsh Organic Liquid Mixtures
Abstract
1. Introduction
2. Materials and Methods
3. Results
- -
- For sorption < 55%, also known as the short time model [17]:
- -
- After steady sorption is attained:
3.1. Sorption Kinetics
3.2. Diffusion Coefficients
3.3. Permeability and Solubility Coefficients
3.4. Permeation Fluxes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gardiner, J. Fluoropolymers: Origin, Production, and Industrial and Commercial Applications. Aust. J. Chem. 2015, 68, 13. [Google Scholar] [CrossRef]
- Drobny, J.G. Technology of Fluoropolymers; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Ebnesajjad, S. Fluoroplastics: Melt Processible Fluoropolymers. The Definitive Users Guide and Data Book; Plastics Design Library; William Andrew: Norwich, NY, USA, 2016; Volume 2. [Google Scholar]
- Moore, A.L. Fluoroelastomers Handbook: The Definitive User’s Guide and Databook; Taylor & Francis: Abingdon, UK, 2006. [Google Scholar]
- Worm, A.T.; Grootaert, W. Fluorocarbon Elastomers in Encyclopedia of Polymer Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2001; Volume 2. [Google Scholar]
- Améduri, B.; Boutevin, B.; Kostov, G. Fluoroelastomers: Synthesis, Properties and Applications. Prog. Polym. Sci. 2001, 26, 105–187. [Google Scholar] [CrossRef]
- Ellis, B.; Smith, R. Polymers: A Property Database; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Smart, B.E. Fluorocarbons. In Supplement D: The Chemistry of Halides, Pseudo-Halides and Azides; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 1. [Google Scholar]
- Crank, J.; Park, G.S. (Eds.) Diffusion in Polymers; Academic Press: London, UK, 1968. [Google Scholar]
- Yang, D.; Pacheco, R.; Henderson, K.; Hubbard, K.; Devlin, D. Diffusion and Sorption of Nitroplasticizers in Vinyl Copolymer Elastomer and Its Composites. J. Appl. Polym. Sci. 2014, 131, 131. [Google Scholar] [CrossRef]
- de Angelis, M.G.; Sarti, G.C.; Sanguineti, A.; Maccone, P. Permeation, Diffusion, and Sorption of Dimethyl Ether in Fluoroelastomers. J. Polym. Sci. B Polym. Phys. 2004, 42, 1987–2006. [Google Scholar] [CrossRef]
- Khinnavar, R.S.; Aminabhavi, T.M. Resistance of Barrier Elastomers to Hazardous Organic Liquids. J. Appl. Polym. Sci. 1992, 45, 1107–1125. [Google Scholar] [CrossRef]
- Wijmans, J.G.H.; Baker, R.W. The Solution-Diffusion Model: A Unified Approach to Membrane Permeation. In Materials Science of Membranes for Gas and Vapor Separation; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Westwater, J.W.; Drickamer, H.G. The Mathematics of Diffusion. J. Am. Chem. Soc. 1957, 79, 1267–1268. [Google Scholar] [CrossRef]
- Santos, C.I.A.V.; Mialdun, A.; Legros, J.C.; Shevtsova, V. Sorption Equilibria and Diffusion of Toluene, Methanol, and Cyclohexane in/through Elastomers. J. Appl. Polym. Sci. 2016, 133, 43449–43457. [Google Scholar] [CrossRef]
- Legros, J.C.; Mialdun, A.; Strizhak, P.; Shevtsova, V. Permeation of Supercritical CO2 through Perfluoroelastomers. J. Supercrit. Fluids 2017, 126, 1–13. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Harlapur, S.F.; Aralaguppi, M.I. A Study on Molecular Transport of Organic Esters and Aromatics into Viton Fluoropolymers. J. Appl. Polym. Sci. 1997, 66, 717–723. [Google Scholar] [CrossRef]
- Vergnaud, J.M. Liquid Transport Processes in Polymeric Materials: Modeling and Industrial Applications; Prentice Hall International Ltd.: London, UK, 1991. [Google Scholar]
- McKeen, L. Elastomers and Rubbers. In Permeability Properties of Plastics and Elastomers; Elsevier: Edinburgh, UK, 2017; pp. 209–247. [Google Scholar]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1593. [Google Scholar]
- Danner, R.P.; High, M.S. Handbook of Polymer Solution Thermodynamics; John Wiley & Sons: Hoboken, NJ, USA, 1993. [Google Scholar]
- Aminabhavi, T.M.; Munk, P. Preferential Adsorption onto Polystyrene in Mixed Solvent Systems. Macromolecules 1979, 12, 607–613. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Khinnavar, R.S. Diffusion and Sorption of Organic Liquids through Polymer Membranes: 10. Polyurethane, Nitrile-Butadiene Rubber and Epichlorohydrin versus Aliphatic Alcohols (C1-C5). Polymer 1993, 34, 1006–1018. [Google Scholar] [CrossRef]
- Arimoto, A.; Ogawa, H.; Murakami, S. Temperature Dependence of Molar Excess Volumes and Excess Thermal Expansion Coefficients for Binary Mixtures of Cyclohexane with Some Hydrocabons between 298.15 and 313.15 K. Thermochim. Acta 1990, 163, 191–202. [Google Scholar] [CrossRef]
- Narayan, J. Excess Properties of (Methanol + Toluene or p-Xylene) Binary Liquid Mixture. Phys. Chem. Liq. 1992, 25, 15–26. [Google Scholar] [CrossRef]
- Miyano, Y.; Hayduk, W. Solubilities of Butane, Vapor Pressures, and Densities for Benzene + Cyclohexane, Benzene + Methanol, and Methanol + Cyclohexane Solutions at 298 K. J. Chem. Eng. Data 1993, 38, 277–281. [Google Scholar] [CrossRef]
- Hammond, L.W.; Howard, K.S.; McAllister, R.A. Viscosities and Densities of Methanol-Toluene Solutions up to Their Normal Boiling Points. J. Phys. Chem. 1958, 62, 637–639. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Aralaguppi, M.I.; Gopalakrishna, B.; Khinnavar, R.S. Densities, Shear Viscosities, Refractive Indices, and Speeds of Sound of Bis(2-Methoxyethyl) Ether with Hexane, Heptane, Octane, and 2,2,4-Trimethylpentane in the Temperature Interval 298.15–318.15 K. J. Chem. Eng. Data 1994, 39, 522–528. [Google Scholar] [CrossRef]
- Aralaguppi, M.I.; Aminabhavi, T.M.; Balundgi, R.H.; Joshi, S.S. Thermodynamic Interactions in Mixtures of Bromoform with Hydrocarbons. J. Phys. Chem. 1991, 95, 5299–5308. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. A Simple Predictive Treatment of the Permeation Process in Pervaporation. J. Memb. Sci. 1993, 79, 101–113. [Google Scholar] [CrossRef]

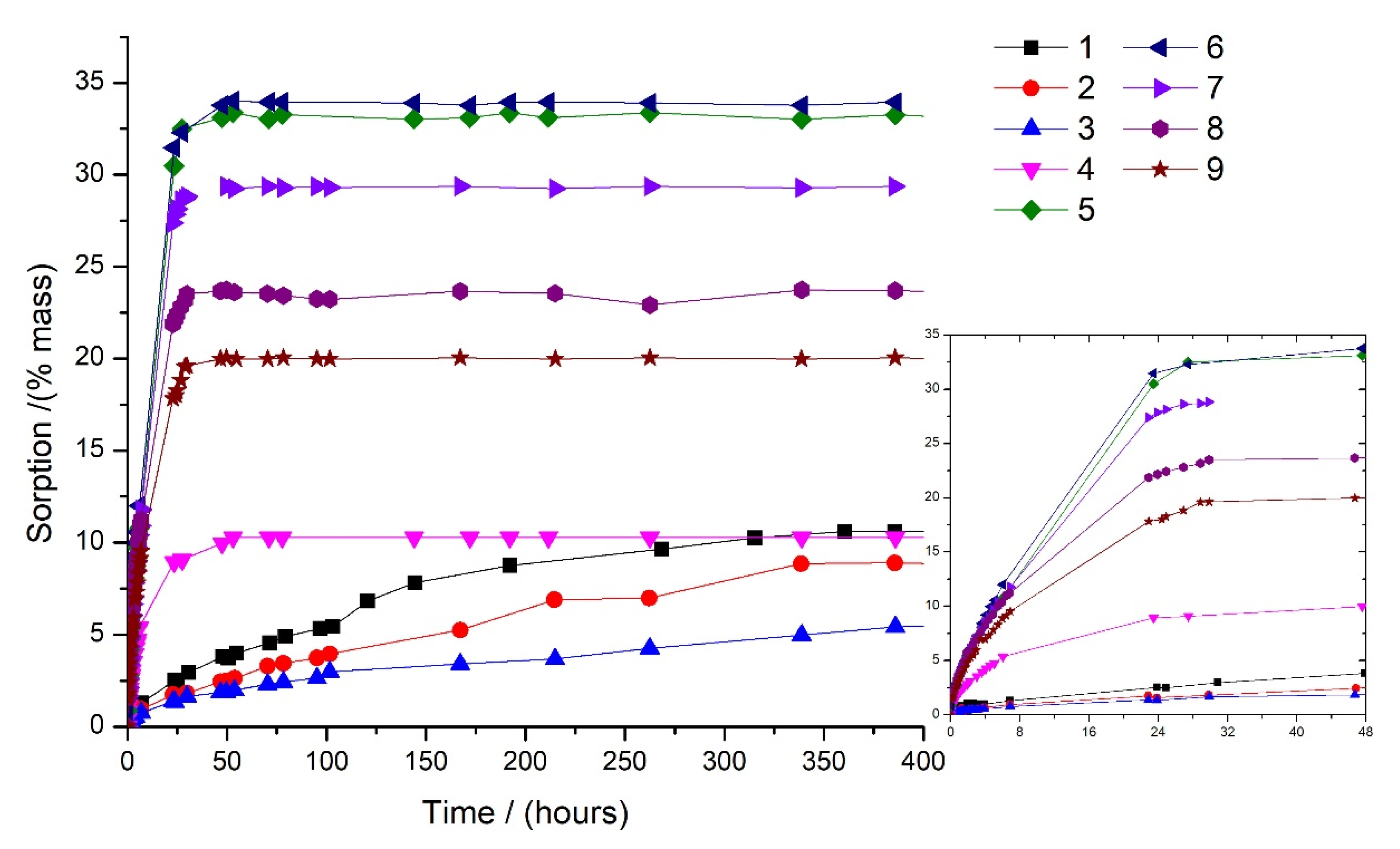
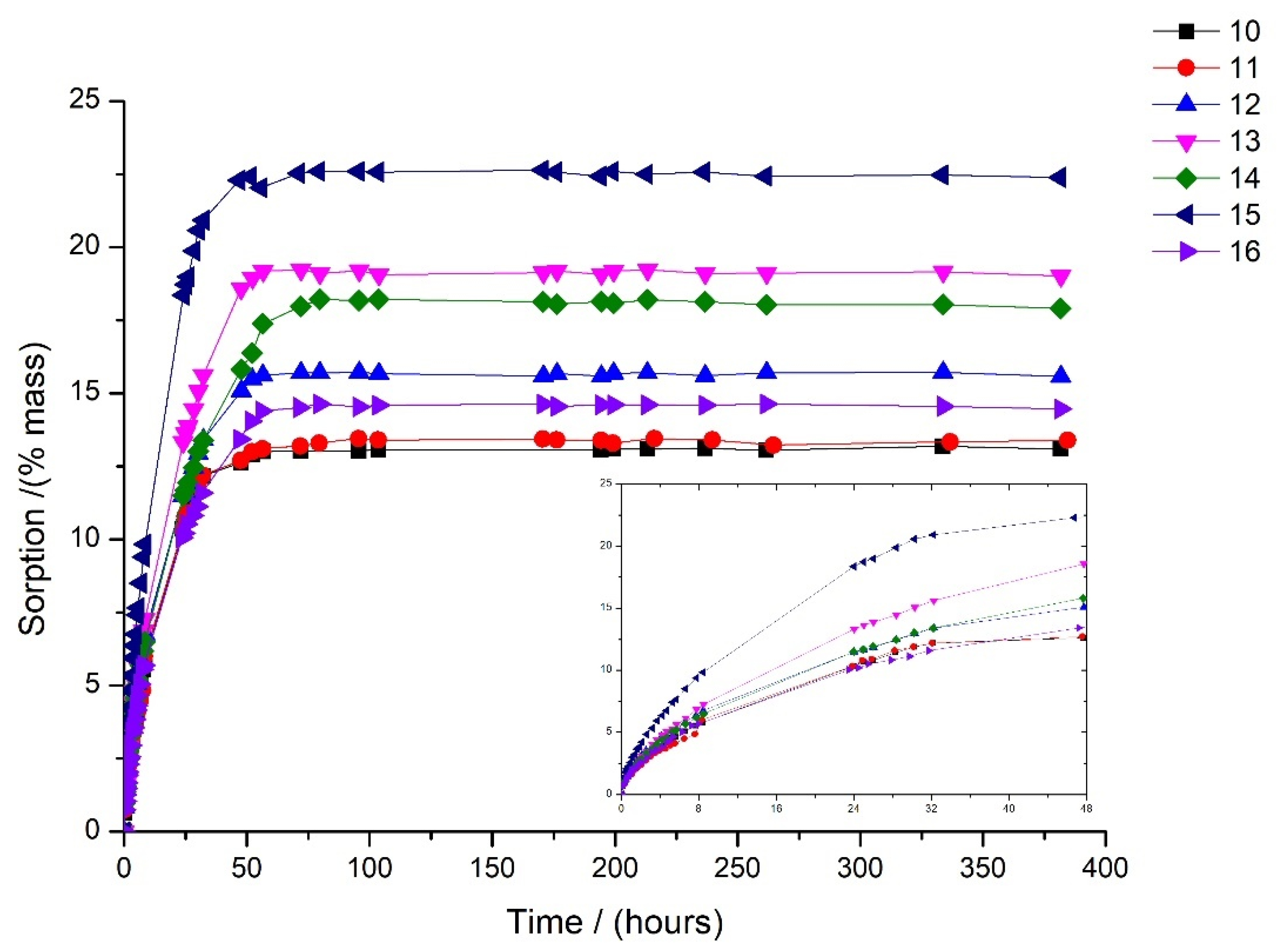
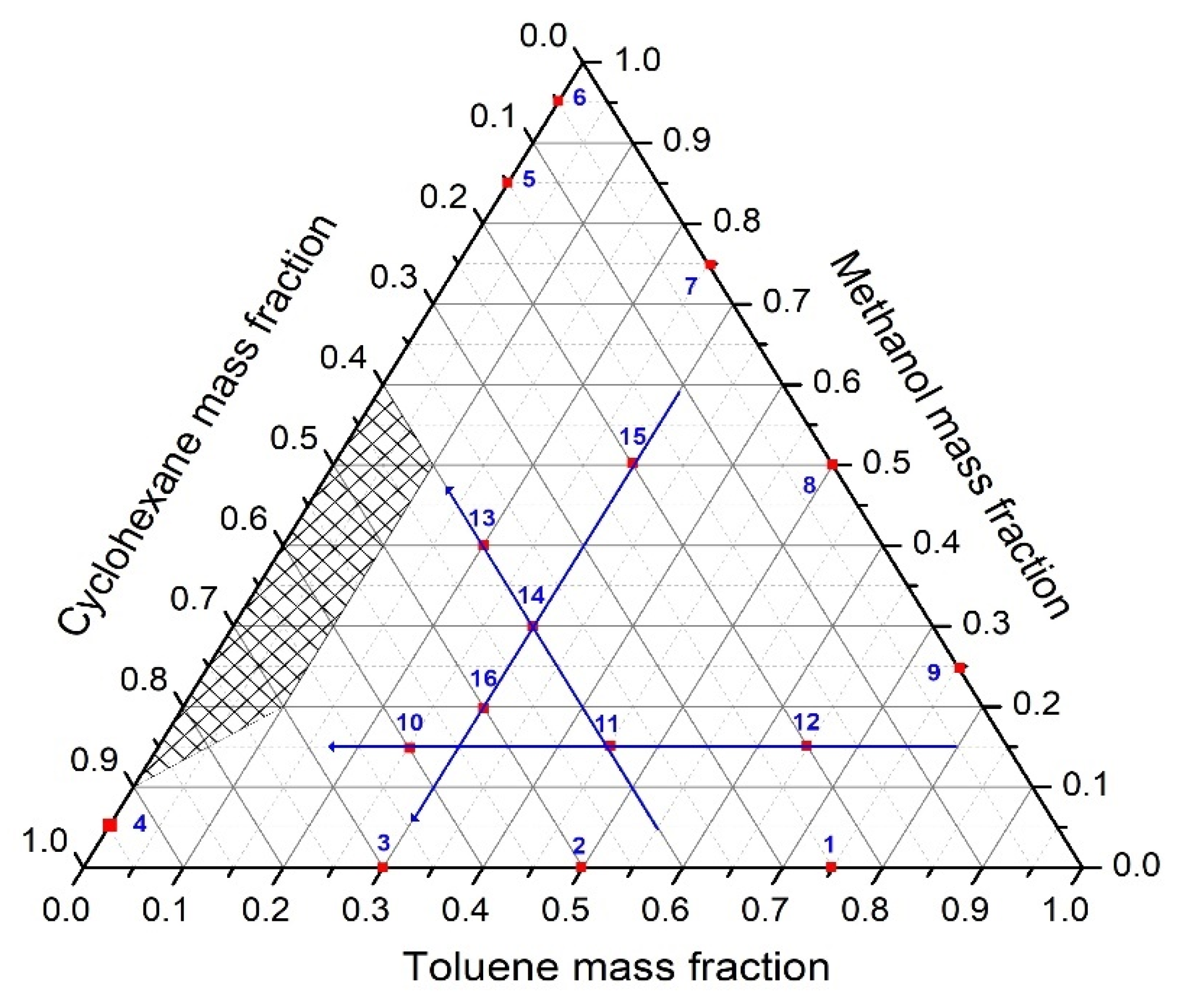
| Liquid Mixtures | % Toluene | % Methanol | % Cyclohexane |
|---|---|---|---|
| 1 | 75 | 0 | 25 |
| 2 | 50 | 0 | 50 |
| 3 | 25 | 0 | 75 |
| 4 | 0 | 5 | 95 |
| 5 | 0 | 85 | 15 |
| 6 | 0 | 95 | 5 |
| 7 | 25 | 75 | 0 |
| 8 | 50 | 50 | 0 |
| 9 | 75 | 25 | 0 |
| 10 | 25 | 15 | 60 |
| 11 | 45 | 15 | 40 |
| 12 | 65 | 15 | 20 |
| 13 | 20 | 40 | 40 |
| 14 | 30 | 30 | 40 |
| 15 | 30 | 50 | 20 |
| 16 | 30 | 20 | 50 |
| Solvent | Structure | Vm/(cm3 mol−1) |
|---|---|---|
| Toluene | 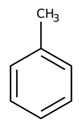 | 106.9 |
| Methanol | 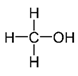 | 40.73 |
| Cyclohexane | 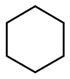 | 108.9 |
| Sorption (Mass %) | n | 103 K | ||
|---|---|---|---|---|
| Liquids | Toluene | 10.51 | 0.49 | 1.72 |
| Methanol | 31.62 | 0.59 | 0.95 | |
| Cyclohexane | 1.17 | 0.50 | 0.55 | |
| Liquid mixtures | 1 | 10.60 | 0.49 | 1.19 |
| 2 | 8.80 | 0.49 | 1.13 | |
| 3 | 5.56 | 0.45 | 1.03 | |
| 4 | 10.28 | 0.50 | 0.91 | |
| 5 | 33.37 | 0.60 | 0.85 | |
| 6 | 34.01 | 0.62 | 0.72 | |
| 7 | 29.37 | 0.54 | 1.60 | |
| 8 | 23.73 | 0.54 | 2.06 | |
| 9 | 20.04 | 0.51 | 2.67 | |
| 10 | 13.19 | 0.52 | 1.24 | |
| 11 | 13.45 | 0.50 | 1.61 | |
| 12 | 15.21 | 0.51 | 1.65 | |
| 13 | 19.22 | 0.54 | 1.79 | |
| 14 | 18.22 | 0.53 | 1.38 | |
| 15 | 22.59 | 0.55 | 1.39 | |
| 16 | 14.63 | 0.53 | 1.39 |
| Liquid | D /(10−20 m2 s−1) | Dd /(10−12 m2 s−1) | D∞ /(10−12 m2 s−1) |
|---|---|---|---|
| Toluene | 1.33 | 0.79 | 0.95 |
| Methanol | 4.94 | 0.42 | 4.60 |
| Cyclohexane | 0.20 | 0.50 | 0.91 |
| 1 | 2.64 | 1.10 | 0.83 |
| 2 | 1.31 | 1.55 | 0.69 |
| 3 | 0.94 | 0.83 | 0.80 |
| 4 | 0.26 | 0.26 | 5.50 |
| 5 | 4.78 | 0.45 | 4.58 |
| 6 | 4.94 | 0.16 | 4.37 |
| 7 | 6.38 | 0.33 | 5.98 |
| 8 | 9.63 | 2.19 | 5.69 |
| 9 | 8.43 | 0.37 | 3.94 |
| 10 | 6.12 | 0.16 | 2.25 |
| 11 | 6.56 | 0.02 | 2.39 |
| 12 | 7.18 | 0.49 | 2.51 |
| 13 | 4.32 | 0.01 | 3.95 |
| 14 | 4.86 | 0.18 | 3.92 |
| 15 | 5.26 | 0.20 | 4.13 |
| 16 | 4.90 | 0.14 | 3.20 |
| Liquid | D∞ /(10−12 m2 s−1) | S /(10−2 kg m−3 Pa−1) | P /(10−14 kg m−1 s−1 Pa−1) |
|---|---|---|---|
| Toluene | 0.95 | 5.16 | 4.89 |
| Methanol | 4.60 | 3.56 | 16.4 |
| Cyclohexane | 0.91 | 1.65 | 1.51 |
| 1 | 0.83 | 4.03 | 3.34 |
| 2 | 0.69 | 2.34 | 1.61 |
| 3 | 0.80 | 1.72 | 1.38 |
| 4 | 5.50 | 1.36 | 7.47 |
| 5 | 4.58 | 3.59 | 16.44 |
| 6 | 4.37 | 3.89 | 17.01 |
| 7 | 5.98 | 4.16 | 24.86 |
| 8 | 5.69 | 4.23 | 24.05 |
| 9 | 3.94 | 5.62 | 22.16 |
| 10 | 2.25 | 2.02 | 4.54 |
| 11 | 2.39 | 2.38 | 5.70 |
| 12 | 2.51 | 2.77 | 6.95 |
| 13 | 3.95 | 2.88 | 11.38 |
| 14 | 3.92 | 2.86 | 11.22 |
| 15 | 4.13 | 2.95 | 12.19 |
| 16 | 3.20 | 2.41 | 7.70 |
| Liquid | χ | Vm /cm3 mol−1 | VE /cm3 mol−1 |
|---|---|---|---|
| Toluene | 1.27 | 106.90 | |
| Methanol | 0.83 | 40.73 | |
| Cyclohexane | 2.71 | 108.90 | |
| 1 | 1.26 | 128.59 | 0.43 |
| 2 | 1.06 | 132.76 | 0.58 |
| 3 | 1.58 | 137.13 | 0.44 |
| 4 | 1.23 | 130.42 | 3.60 |
| 5 | 0.81 | 57.72 | 7.30 |
| 6 | 0.81 | 53.65 | 2.76 |
| 7 | 0.85 | 47.54 | 0.10 |
| 8 | 0.92 | 57.68 | −0.05 |
| 9 | 0.99 | 74.03 | −0.39 |
| 10 | 1.07 | 87.10 | 0.54 |
| 11 | 0.87 | 86.39 | 0.45 |
| 12 | 1.07 | 85.51 | 0.21 |
| 13 | 0.98 | 65.29 | 0.45 |
| 14 | 1.00 | 72.24 | 0.45 |
| 15 | 0.93 | 58.75 | 0.18 |
| 16 | 1.09 | 81.47 | 0.50 |
| Liquid | JToluene /(10−8 kg m−2 s−1) | JMethanol /(10−8 kg m−2 s−1) | JCyclohexane /(10−8 kg m−2 s−1) | J∑ /(10−8 kg m−2 s−1) |
|---|---|---|---|---|
| 1 | 4.78 | 1.79 | 6.56 | |
| 2 | 3.11 | 3.50 | 6.61 | |
| 3 | 1.52 | 5.13 | 6.65 | |
| 4 | 10.6 | 5.88 | 16.5 | |
| 5 | 81.8 | 0.42 | 82.2 | |
| 6 | 85.5 | 0.13 | 85.7 | |
| 7 | 0.68 | 78.2 | 78.9 | |
| 8 | 1.68 | 64.8 | 66.5 | |
| 9 | 3.33 | 42.7 | 46.1 | |
| 10 | 1.22 | 28.1 | 3.28 | 32.6 |
| 11 | 2.22 | 28.5 | 2.22 | 33.0 |
| 12 | 3.26 | 28.9 | 1.13 | 33.3 |
| 13 | 0.73 | 56.1 | 1.64 | 58.5 |
| 14 | 1.22 | 47.0 | 1.83 | 50.1 |
| 15 | 1.00 | 64.0 | 0.76 | 65.8 |
| 16 | 1.38 | 35.2 | 2.58 | 39.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, M.C.F.; Ribeiro, A.C.F.; Santos, C.I.A.V. An Assessment of the Chemical Compatibility of Viton Fluoropolymers and Some Harsh Organic Liquid Mixtures. Processes 2023, 11, 2088. https://doi.org/10.3390/pr11072088
Barros MCF, Ribeiro ACF, Santos CIAV. An Assessment of the Chemical Compatibility of Viton Fluoropolymers and Some Harsh Organic Liquid Mixtures. Processes. 2023; 11(7):2088. https://doi.org/10.3390/pr11072088
Chicago/Turabian StyleBarros, Marisa C. F., Ana C. F. Ribeiro, and Cecília I. A. V. Santos. 2023. "An Assessment of the Chemical Compatibility of Viton Fluoropolymers and Some Harsh Organic Liquid Mixtures" Processes 11, no. 7: 2088. https://doi.org/10.3390/pr11072088
APA StyleBarros, M. C. F., Ribeiro, A. C. F., & Santos, C. I. A. V. (2023). An Assessment of the Chemical Compatibility of Viton Fluoropolymers and Some Harsh Organic Liquid Mixtures. Processes, 11(7), 2088. https://doi.org/10.3390/pr11072088







