Evaluation of a Resorcinarene-Based Sorbent as a Solid-Phase Extraction Material for the Enrichment of L-Carnitine from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of calix[4]resorcinarenes
2.2. Studies of Molecular Interaction between Resorcinarenes and L Carnitine
2.2.1. NMR Studies
2.2.2. ESI-MS Studies
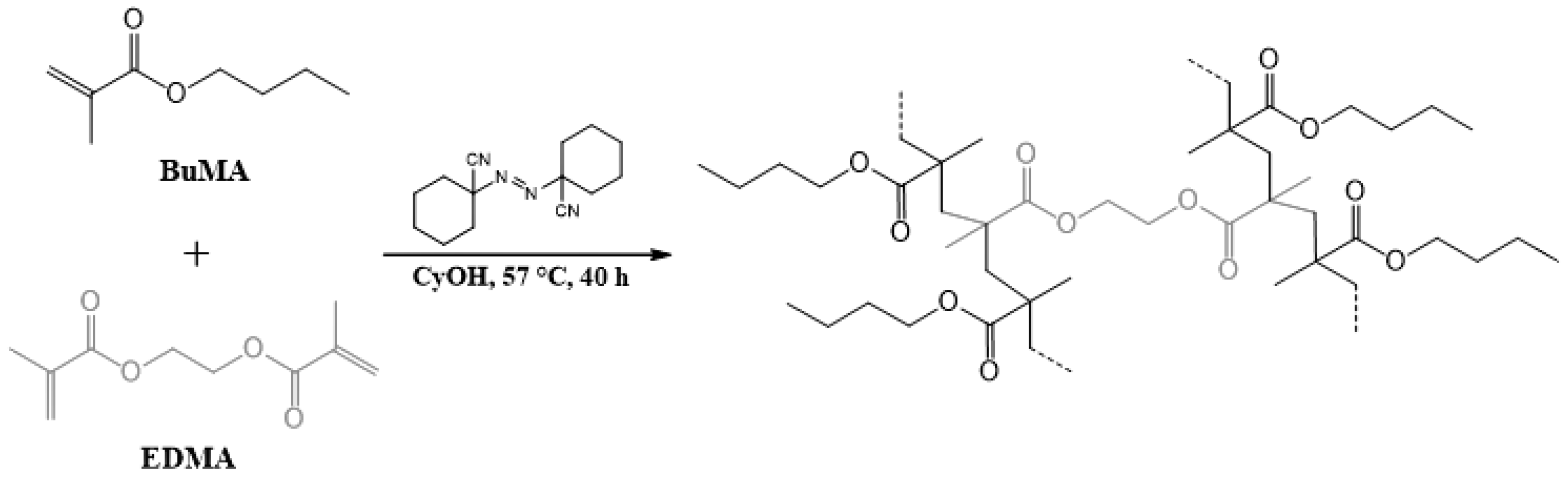
2.3. Obtention of Poly(BuMA-Co-EDMA)
2.4. Physical Modification of Polymers with Resorcinarenes
2.5. SPE Protocol
3. Results and Discussion
3.1. Synthesis of Resorcinarenes
3.2. Molecular Interaction Studies between Resorcinarenes and L-Carnitine
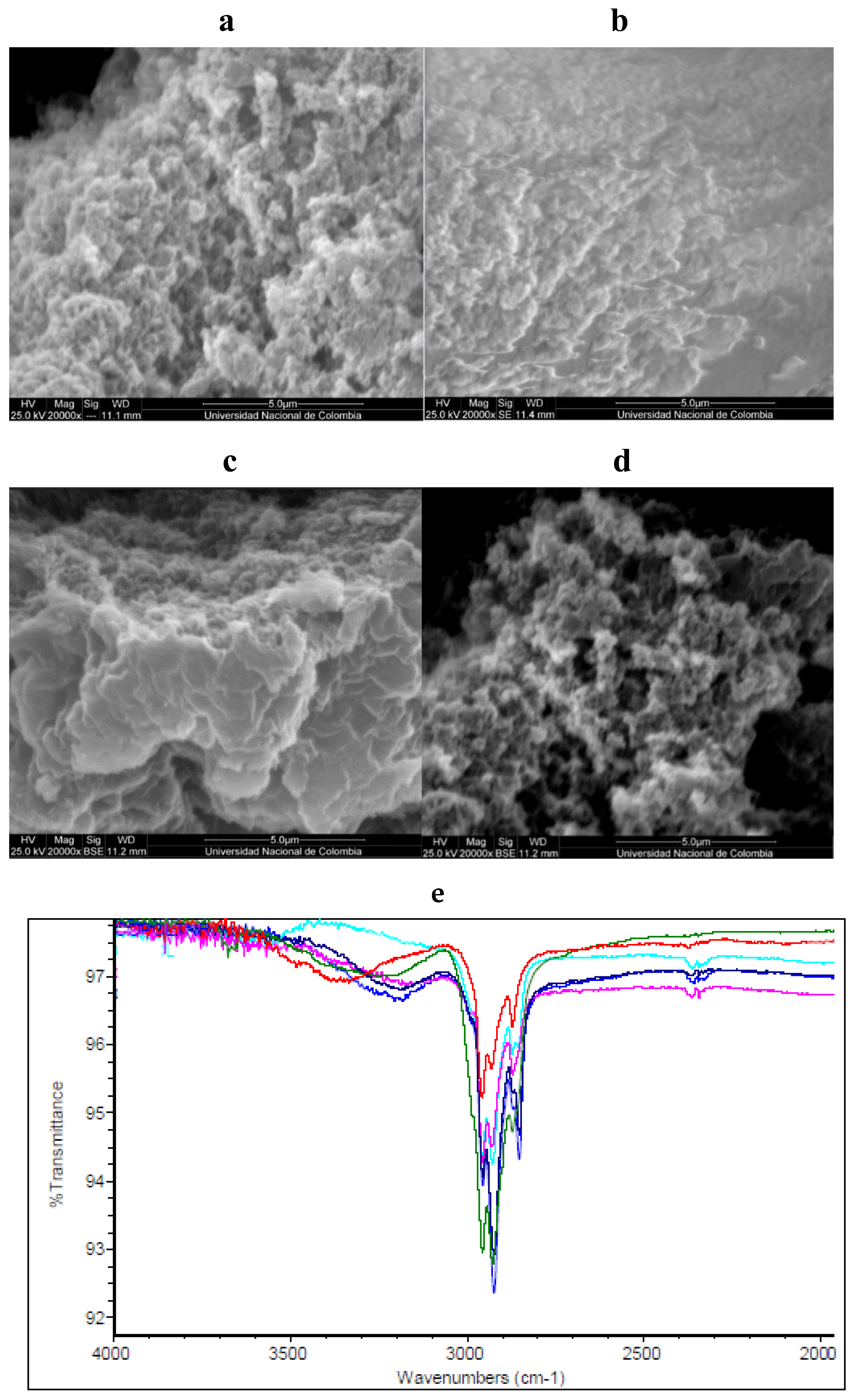
3.3. Obtention and Characterization of Sorbents
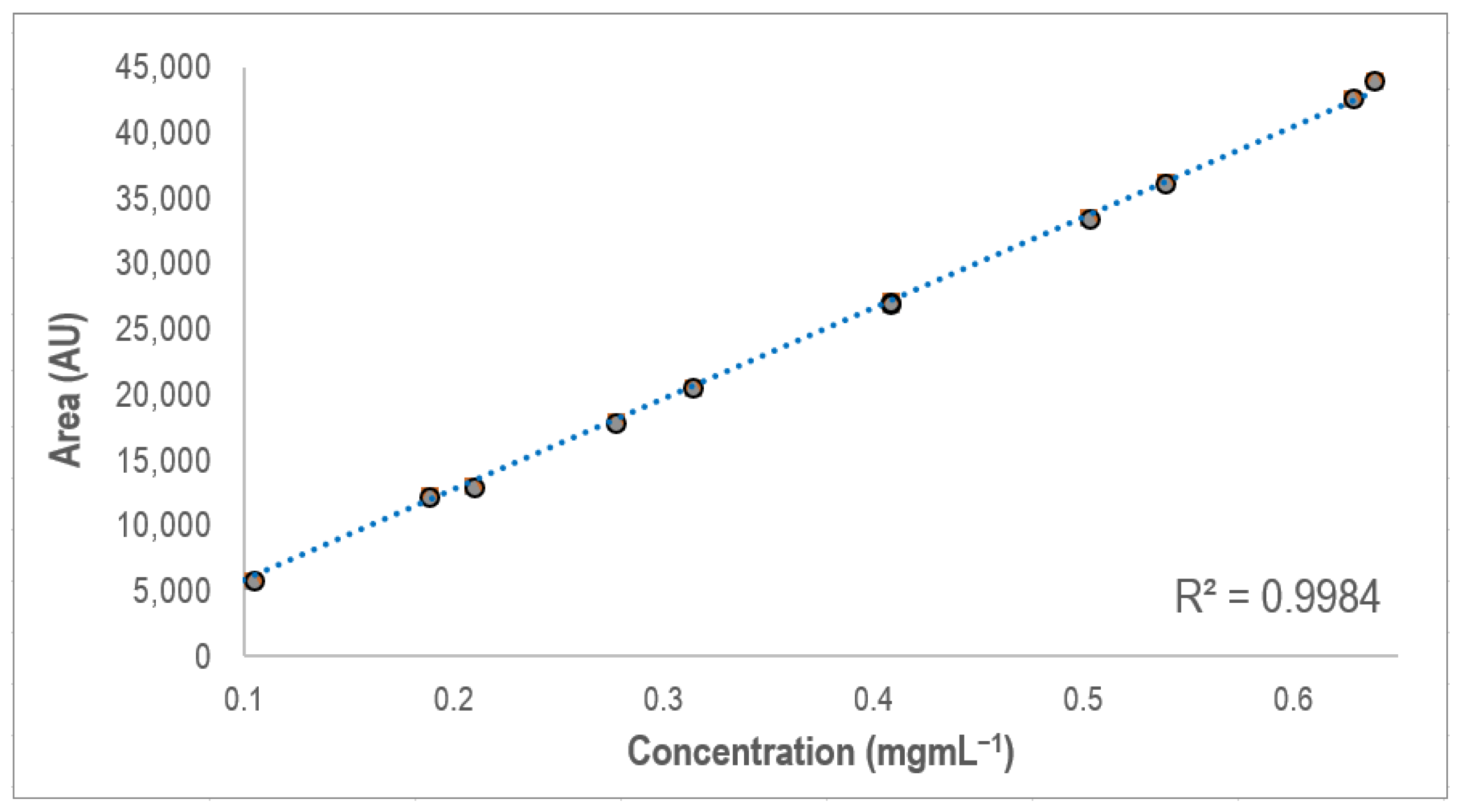
3.4. Optimization of the SPE Procedure
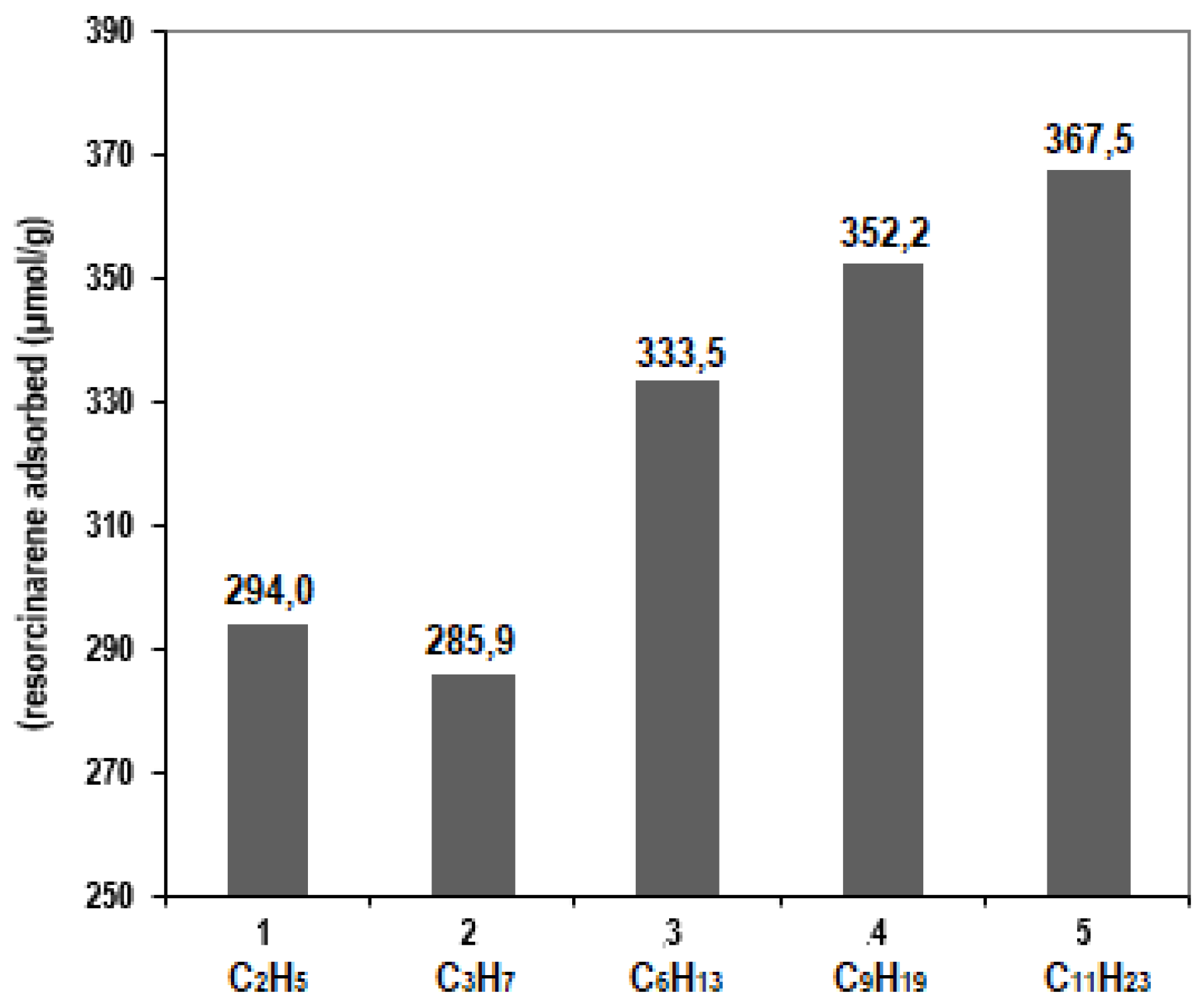
3.5. Analyses of the Extraction of L-Carnitine with the Modified Sorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wrona, M.Z.; Dryhurst, G. Low Molecular Weight Neurotransmitters. Bioelectrochemistry 1994, 267, 117–169. [Google Scholar] [CrossRef]
- Bartfai, T.; Iverfeldt, K.; Fisone, G.; Serfozo, P. Regulation of the Release of Coexisting Neurotransmitters. Annu. Rev. Pharmacol. Toxicol. 1988, 28, 285–310. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, M.; Starek, M. Analytical approaches to determination of carnitine in biological materials, foods and dietary supplements. Food Chem. 2014, 142, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Möder, M.; Kießling, A.; Löster, H. Current Methods for Determination of L-Carnitine and Acylcarnitines. Mon. Fur Chem. 2005, 136, 1279–1291. [Google Scholar] [CrossRef]
- Johnson, D.W. An acid hydrolysis method for quantification of plasma free and total carnitine by flow injection tandem mass spectrometry. Clin. Biochem. 2010, 43, 1362–1367. [Google Scholar] [CrossRef]
- Zhang, Z.; Niwa, O.; Shiba, S.; Tokito, S.; Nagamine, K.; Ishikawa, S.; Sugimoto, M. Electrochemical enzyme biosensor for carnitine detection based on cathodic stripping voltammetry. Sens. Actuators B Chem. 2020, 321, 128473. [Google Scholar] [CrossRef]
- Wang, M.; Yang, R.; Mu, H.; Zeng, J.; Zhang, T.; Zhou, W.; Wang, S.; Tang, Y.; Li, H.; Zhang, C.; et al. A simple and precise method for measurement of serum free carnitine and acylcarnitines by isotope dilution HILIC-ESI-MS/MS. Int. J. Mass Spectrom. 2019, 446, 116208. [Google Scholar] [CrossRef]
- Seline, K.-G.; Johein, H. The determination of l-carnitine in several food samples. Food Chem. 2007, 105, 793–804. [Google Scholar] [CrossRef]
- Lu, W.-H.; Chiu, H.-H.; Kuo, H.-C.; Chen, G.-Y.; Chepyala, D.; Kuo, C.-H. Using matrix-induced ion suppression combined with LC-MS/MS for quantification of trimethylamine-N-oxide, choline, carnitine and acetylcarnitine in dried blood spot samples. Anal. Chim. Acta 2021, 1149, 338214. [Google Scholar] [CrossRef]
- Rudolph, W.; Remane, D.; Wissenbach, D.K.; Peters, F.T. Liquid chromatography-mass spectrometry-based determination of ergocristine, ergocryptine, ergotamine, ergovaline, hypoglycin A, lolitrem B, methylene cyclopropyl acetic acid carnitine, N-acetylloline, N-formylloline, paxilline, and peramine in equine hair. J. Chromatogr. B 2019, 1117, 127–135. [Google Scholar] [CrossRef]
- Minkler, P.E.; Stoll, M.S.K.; Ingalls, S.T.; Kerner, J.; Hoppel, C.L. Validated Method for the Quantification of Free and Total Carnitine, Butyrobetaine, and Acylcarnitines in Biological Samples. Anal. Chem. 2015, 87, 8994–9001. [Google Scholar] [CrossRef]
- Morand, R.; Donzelli, M.; Haschke, M.; Krähenbühl, S. Quantification of plasma carnitine and acylcarnitines by high-performance liquid chromatography-tandem mass spectrometry using online solid-phase extraction. Anal. Bioanal. Chem. 2013, 405, 8829–8836. [Google Scholar] [CrossRef]
- Prokorátová, V.; Kvasnička, F.; Ševčík, R.; Voldřich, M. Capillary electrophoresis determination of carnitine in food supplements. J. Chromatogr. A 2005, 1081, 60–64. [Google Scholar] [CrossRef]
- Tan, X.; Yang, Y.; Luo, S.; Zhang, Z.; Zeng, W.; Zhang, T.; Su, F.; Zhou, L. Novel Competitive Fluorescence Sensing Platform for L-carnitine Based on Cationic Pillar[5]Arene Modified Gold Nanoparticles. Sensors 2018, 18, 3927. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Tsai, C.-J.; Feng, C.-H. Fluorescent derivatization combined with aqueous solvent-based dispersive liquid-liquid microextraction for determination of butyrobetaine, l-carnitine and acetyl-l-carnitine in human plasma. J. Chromatogr. A 2016, 1464, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Manjón, A.; Obón, J.; Iborra, J. Determination of -Carnitine by Flow Injection Analysis with NADH Fluorescence Detection. Anal. Biochem. 2000, 281, 176–181. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Vargas-Zúñiga, G.I.; Kim, S.H.; Kim, S.K.; Sessler, J.L. Macrocycles as Ion Pair Receptors. Chem. Rev. 2019, 119, 9753–9835. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Botella, S.; Vidossich, P.; Ujaque, G.; Vicent, C.; Peris, E. A Tetraferrocenyl-Resorcinarene Cavitand as a Redox-Switchable Host of Ammonium Salts. Chem. A Eur. J. 2015, 21, 10558–10565. [Google Scholar] [CrossRef]
- Taylor, P.J. Matrix effects: The Achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry. Clin. Biochem. 2005, 38, 328–334. [Google Scholar] [CrossRef]
- Huang, Z.; Richards, M.A.; Zha, Y.; Francis, R.; Lozano, R.; Ruan, J. Determination of inorganic pharmaceutical counterions using hydrophilic interaction chromatography coupled with a Corona® CAD detector. J. Pharm. Biomed. Anal. 2009, 50, 809–814. [Google Scholar] [CrossRef]
- Johnson, W.M.; Kido Soule, M.C.; Kujawinski, E.B. Extraction efficiency and quantification of dissolved metabolites in targeted marine metabolomics. Limnol. Oceanogr. Methods 2017, 15, 417–428. [Google Scholar] [CrossRef]
- Lee, M.; Oh, S.Y.; Pathak, T.S.; Paeng, I.R.; Cho, B.Y.; Paeng, K.J. Selective solid-phase extraction of catecholamines by the chemically modified polymeric adsorbents with crown ether. J. Chromatogr. A 2007, 1160, 340–344. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Xu, Z.; Zhang, Q.; Liu, J.; Shen, J.; Zhang, W. High-throughput and selective solid-phase extraction of urinary catecholamines by crown ether-modified resin composite fiber. J. Chromatogr. A 2018, 1561, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Aguirre, A.; Maldonado, M. Preparation of methacrylate-based polymers modified with chiral resorcinarenes and their evaluation as sorbents in norepinephrine microextraction. Polymers 2019, 11, 1428. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Silva, B.A.; Castillo-Aguirre, A.; Rivera-Monroy, Z.J.; Maldonado, M. Aminomethylated calix[4]resorcinarenes as modifying agents for glycidyl methacrylate (GMA) rigid copolymers surface. Polymers 2019, 11, 1147. [Google Scholar] [CrossRef]
- Castillo, A.; Esteso, M.A.; Maldonado, M. Resorcin[4]arenes: Generalities and their role in the modification and detection of amino acids. Curr. Org. Chem. 2020, 24, 2412–2425. [Google Scholar] [CrossRef]
- Li, N.; Harrison, R.G.; Lamb, J.D. Application of resorcinarene derivatives in chemical separations. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 39–60. [Google Scholar] [CrossRef]
- Davis, F.; Faul, C.F.J.; Higson, S.P.J. Calix[4]resorcinarene-surfactant complexes: Formulation, structure and potential sensor applications. Soft Matter. 2009, 5, 2746–2751. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, Y.; Han, Y.; Yan, C. Preparation of resorcinarene-functionalized gold nanoparticles and their catalytic activities for reduction of aromatic nitro compounds. Chin. J. Chem. 2010, 28, 705–712. [Google Scholar] [CrossRef]
- Natarajan, N.; Brenner, E.; Sémeril, D.; Matt, D.; Harrowfield, J. The Use of Resorcinarene Cavitands in Metal-Based Catalysis. Eur. J. Org. Chem. 2017, 2017, 6100–6113. [Google Scholar] [CrossRef]
- Pinalli, R.; Pedrini, A.; Dalcanale, E. Biochemical sensing with macrocyclic receptors. Chem. Soc. Rev. 2018, 47, 7006–7026. [Google Scholar] [CrossRef]
- Hong, M.; Zhang, Y.M.; Liu, Y. Selective binding affinity between quaternary ammonium cations and water-soluble calix[4]resorcinarene. J. Org. Chem. 2015, 80, 1849–1855. [Google Scholar] [CrossRef]
- Torvinen, M.; Kalenius, E.; Sansone, F.; Casnati, A.; Jänis, J. Noncovalent complexation of monoamine neurotransmitters and related ammonium ions by tetramethoxy tetraglucosylcalix[4]arene. J. Am. Soc. Mass Spectrom. 2012, 23, 359–365. [Google Scholar] [CrossRef]
- Lledó, A.; Hooley, R.J.; Rebek, J. Recognition of guests by water-stabilized cavitand hosts. Org. Lett. 2008, 10, 3669–3671. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.S.; Uzunova, V.D.; Su, X.; Liu, Y.; Nau, W.M. Operational calixarene-based fluorescent sensing systems for choline and acetylcholine and their application to enzymatic reactions. Chem. Sci. 2011, 2, 1722–1734. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Petropoulou, S.S.E.; Theoharis, G. Biosensors for the Rapid Repetitive Detection of Adrenaline Using Stabilized Bilayer Lipid Membranes (BLMs) with Incorporated Calix[4]resorcinarene Receptor. Electroanalysis 2003, 15, 1616–1624. [Google Scholar] [CrossRef]
- Ballester, P.; Shivanyuk, A.; Far, A.R.; Rebek, J. A synthetic receptor for choline and carnitine. J. Am. Chem. Soc. 2002, 124, 14014–14016. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Sanabria, E.; Velasquez-Silva, A.; Casas-Hinestroza, J.L.; Esteso, M.A. Comparative study of the volumetric properties of three regioisomers of diazoted C-tetra(propyl)resorcin[4]arene in DMSO at various temperatures. J. Mol. Liq. 2021, 325, 115252. [Google Scholar] [CrossRef]
- Castillo-Aguirre, A.; Rivera-Monroy, Z.; Maldonado, M. Selective O-Alkylation of the Crown Conformer of Tetra(4-hydroxyphenyl)calix[4]resorcinarene to the Corresponding Tetraalkyl Ether. Molecules 2017, 22, 1660. [Google Scholar] [CrossRef]
- Castillo-Aguirre, A.A.; Velásquez-Silva, B.A.; Palacio, C.; Baez, F.; Rivera-Monroy, Z.J.; Maldonado, M. Surface modification of poly(GMA-co-EDMA-co-MMA) with resorcarenes. J. Braz. Chem. Soc. 2018, 29, 1965–1972. [Google Scholar] [CrossRef]
- Castillo-Aguirre, A.; Maldonado, M.; Esteso, M.A. Removal of Toxic Metal Ions Using Poly(BuMA–co–EDMA) Modified with C-Tetra(nonyl)calix[4]resorcinarene. Toxics 2022, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Casas-Hinestroza, J.L.; Bueno, M.; Ibáñez, E.; Cifuentes, A. Recent advances in mass spectrometry studies of non-covalent complexes of macrocycles—A review. Anal. Chim. Acta 2019, 1081, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Casas-Hinestroza, J.L.; Cifuentes, A.; Ibáñez, E.; Maldonado, M. Effect of the formation of capsules of tetra(propyl) pyrogallol[4]arene on the host-guest interaction with neurotransmitters. J. Mol. Struct. 2020, 1210, 128063. [Google Scholar] [CrossRef]
- Casas-Hinestroza, J.L.; Pérez-Redondo, A.; Maldonado, M. Inclusion complexation between neurotransmitters with polyacetylated calix[4]pyrogallolarenes: 1H-NMR and crystallographic analysis. Res. Chem. Intermed. 2022, 48, 3091–3107. [Google Scholar] [CrossRef]
- Velásquez-Silva, A.; Forero, R.S.; Sanabria, E.; Pérez-Redondo, A.; Maldonado, M. Host-guest inclusion systems of tetra(alkyl)resorcin [4]arenes with choline in DMSO: Dynamic NMR studies and X-ray structural characterization of the 1:1 inclusion complex. J. Mol. Struct. 2019, 1198, 126846. [Google Scholar] [CrossRef]
- Velásquez-Silva, A.; Cortés, B.; Rivera-Monroy, Z.J.; Pérez-Redondo, A.; Maldonado, M. Crystal structure and dynamic NMR studies of octaacetyl-tetra(propyl)calix[4]resorcinarene. J. Mol. Struct. 2017, 1137, 380–386. [Google Scholar] [CrossRef]


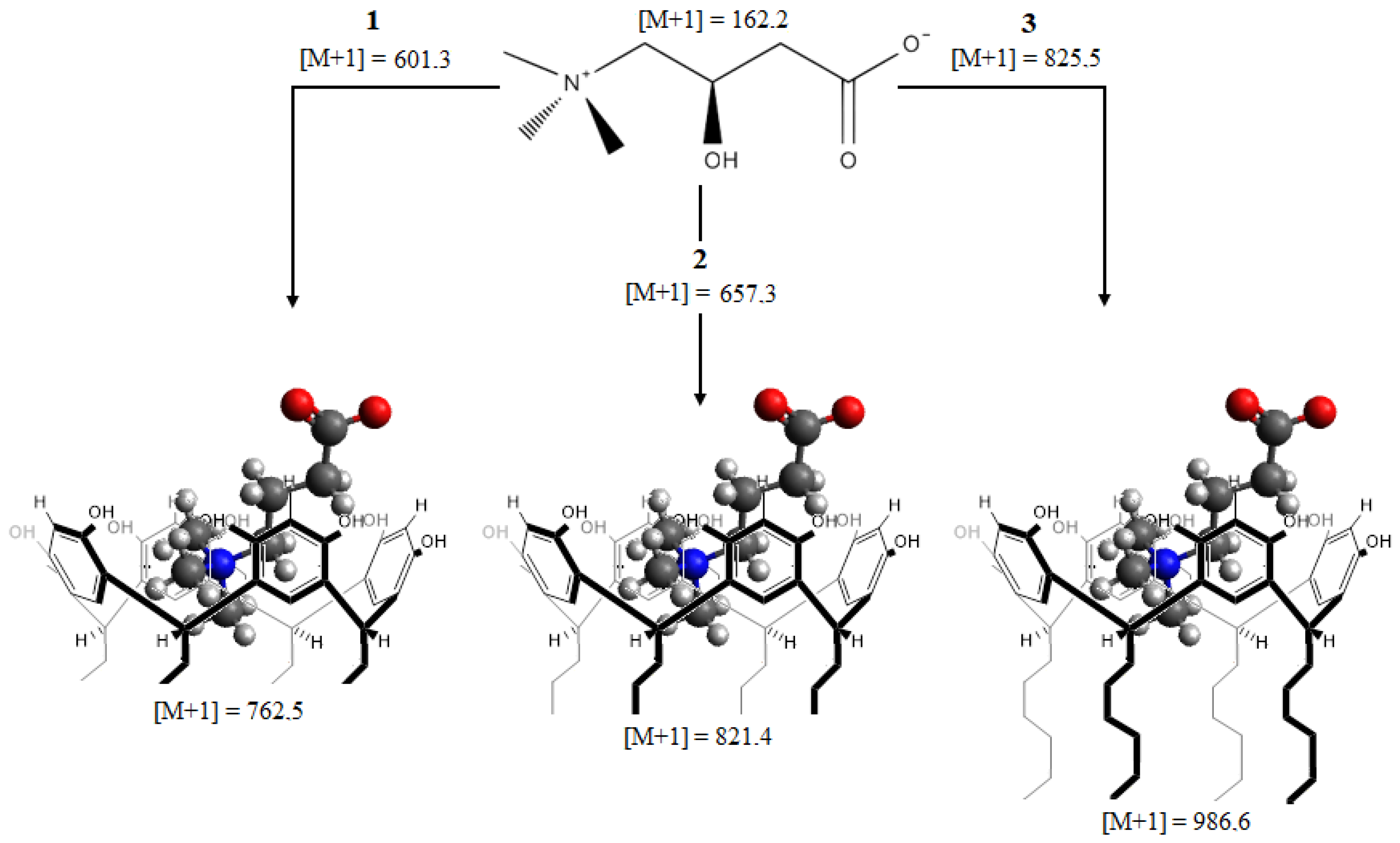
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| ESI–TOF/MS (m/z) [M + H]+ | 601.32 | 657.33 | 847.51 | 993.71 | 1105.84 | |
| IR (KBr/cm−1) | (O–H) | 3400 | 3280 | 3300 | 3201 | 3235 |
| (ArC-H), | 2980 | 2953 | 2950 | 2920 | 2918 | |
| (aliphatic C-H) | 2920 | 2926 | 2860 | 2851 | 2849 | |
| (C=C) | 1610 | 1606 | 1610 | 1617 | 1617 | |
| (ArC-O) | 1290 | 1283 | 1280 | 1218 | 1211 | |
| 13C and 1H NMR DMSO-d6 (1, 2, 3) CHCl3-d1 (4, 5) δ (ppm) | (C–OH) | (s, 8H) 8.93 (C) 151.6 | (s, 8H) 8.94 (C) 152.0 | (s, 8H) 8.87 (C) 151.6 | (s, 8H) 9.45 (C) 150.6 | (s, 8H) 9.63 (C) 150.4 |
| (C–H), meta to OH | (s, 4H) 7.23 (C) 102.3 | (s, 4H) 7.24 (C) 102.8 | (s, 4H) 7.13 (C) 102.3 | (s, 4H) 7.23 (C) | (s, 4H) 7.23 (C) | |
| (C–H), ortho to OH | (s, 4H) 6.15 (C) 125.0 | (s, 4H) 6.15 (C) 125.5 | (s, 4H) 6.14 (C) 124.8 | (s, 4H) 6.14 (C) 124.9 | (s, 4H) 6.16 (C) 124.8 | |
| (C–H), CH | (t, 4H) 4.09 (C) 35.1 | (t, 4H) 4.23 (C) 36.15 | (t, 4H) 4.21 (C) 34.0 | (t, 4H) 4.32 (C) 33.24 | (t, 4H) 4.32 (C) 58.5 | |
| (C–H), CH2 | (m, 8H) 2.11 (C) 26.6 | (m, 16H) 2.07–1.19 (C) 33.0–21.1 | (m, 40H) 2.00–1.21 (C) 31.4–22.1 | (m, 64H) 4.15–1.30 (C) 32.0–22.7 | (m, 80H) 4.14–1.25 (C) 33.3–18.3 | |
| (C–H), CH3 | (t, 12H) 0.79 (C) 12.6 | (t, 12H) 0.90 (C) 14.4 | (t, 12H) 0.83 (C) 13.8 | (t, 12H) 0.91 (C) 14.1 | (t, 12H) 0.90 (C) 14.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez, G.; Cadavid-Montoya, N.A.; Maldonado, M. Evaluation of a Resorcinarene-Based Sorbent as a Solid-Phase Extraction Material for the Enrichment of L-Carnitine from Aqueous Solutions. Processes 2023, 11, 1705. https://doi.org/10.3390/pr11061705
Ramirez G, Cadavid-Montoya NA, Maldonado M. Evaluation of a Resorcinarene-Based Sorbent as a Solid-Phase Extraction Material for the Enrichment of L-Carnitine from Aqueous Solutions. Processes. 2023; 11(6):1705. https://doi.org/10.3390/pr11061705
Chicago/Turabian StyleRamirez, Gabriel, Nicolas Alejandro Cadavid-Montoya, and Mauricio Maldonado. 2023. "Evaluation of a Resorcinarene-Based Sorbent as a Solid-Phase Extraction Material for the Enrichment of L-Carnitine from Aqueous Solutions" Processes 11, no. 6: 1705. https://doi.org/10.3390/pr11061705
APA StyleRamirez, G., Cadavid-Montoya, N. A., & Maldonado, M. (2023). Evaluation of a Resorcinarene-Based Sorbent as a Solid-Phase Extraction Material for the Enrichment of L-Carnitine from Aqueous Solutions. Processes, 11(6), 1705. https://doi.org/10.3390/pr11061705








