The Efficient Utilization of Carbon Dioxide in a Power-to-Liquid Process: An Overview
Abstract
1. Introduction
2. Power-to-Liquid Technology
2.1. CO2 Capture Technologies
2.1.1. Chemical Absorption Method
2.1.2. Physical Absorption Method
- (a)
- A high solubility and selectivity for CO2 and H2S;
- (b)
- A low saturated vapor pressure;
- (c)
- A high chemical stability;
- (d)
- A low cost;
- (e)
- A low toxicity and minimal environmental impact.
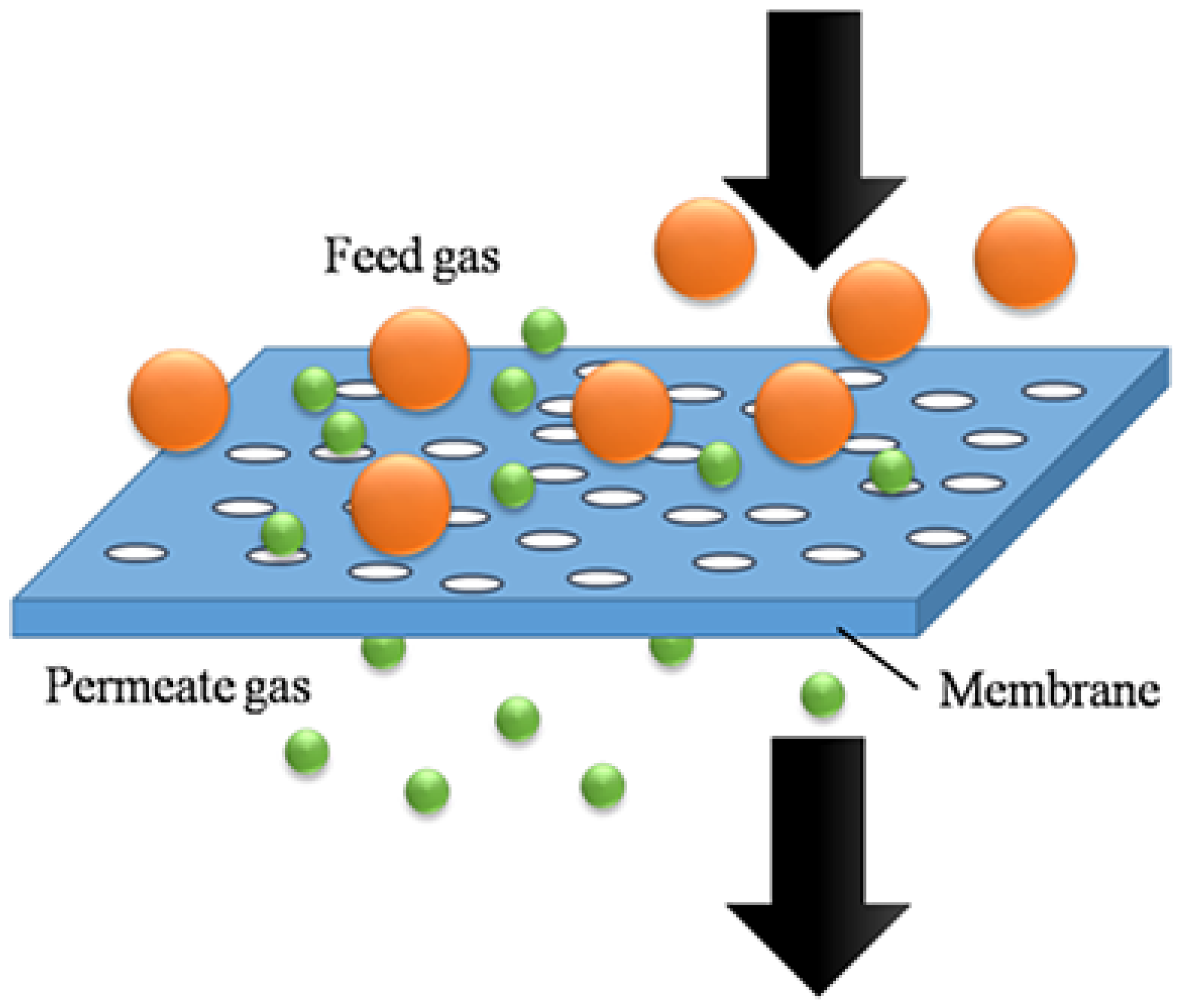
2.1.3. Membrane Separation Method
2.1.4. Pressure Swing Adsorption
| Capture Method | Chemical Absorption Method | Physical Absorption Method | Membrane Separation Method | Pressure Swing Adsorption Method |
|---|---|---|---|---|
| Absorbent/Adsorbent | MEA/MDEA | Methanol | Inorganic/organic membrane | Zeolite/molecular sieve/activated carbon/alumina/MOF |
| Inlet CO2 concentration | <20% | >20% | >28% | 20–60% |
| Outlet CO2 concentration | ≥99 | ≥95 | ≥99.9 | Single-stage: 76 |
| Multiple-stage: >95 | ||||
| Capture rate | 90% | 90% | 90% | 90% |
| Technology maturity | High | High | Pilot stage | High |
| Processing capacity | High | High | Low | Medium |
| Operational difficulty | Low | Low | High | Medium |
| Cost ($/t CO2) | 50–65 | 40–60 | 23–47 | 32–34 |
| Advantages |
|
|
|
|
|
|
|
| |
|
|
|
| |
| Disadvantages |
|
|
|
|
|
|
|
| |
|
|
2.1.5. Comparison of Four CO2 Capture Methods
2.2. Water Electrolysis Technologies
2.2.1. Alkaline Water Electrolysis
2.2.2. Proton Exchange Membrane Electrolysis
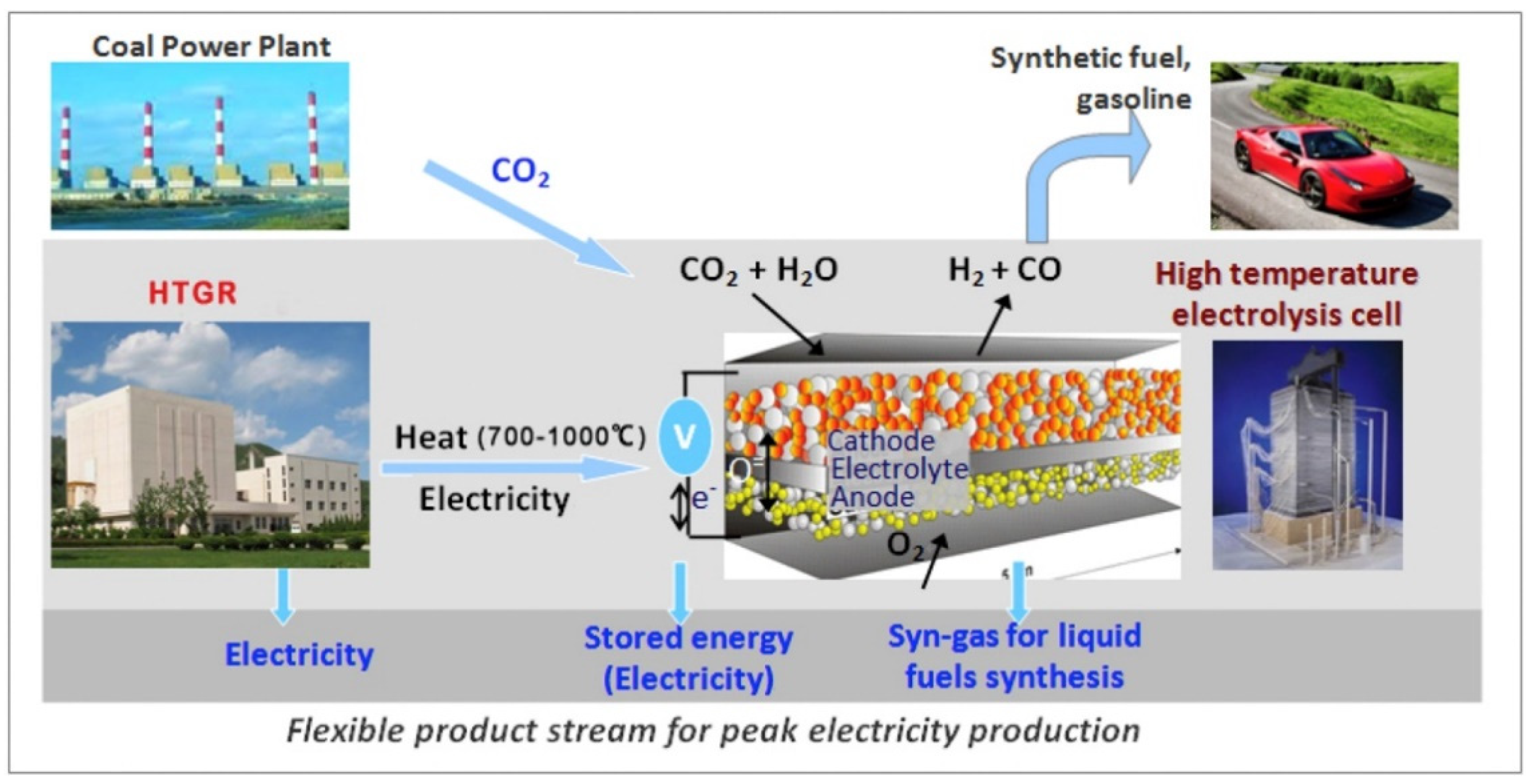
2.2.3. Solid Oxide Water Electrolysis
2.2.4. Anion Exchange Membrane Electrolysis
2.2.5. Comparison of Four Electrolysis Technologies
| AWE | PEM | SOEC | AEM | |
|---|---|---|---|---|
| Operation conditions | ||||
| Charge carrier | OH− | H+ | O2− | OH− |
| Cathode materials | Ni | Ni | Ni-YSZ | Ni-Fe-Co or NiMo-NH3/H2 |
| Cathode catalyst | Pt | Pt, Ir, Ru | Ni | CeO2-La2O3 |
| Anode materials | Ni | Ni or C | LSM-YSZ or LSF-YSZ | Ni-Fe-Ox or Fe-NiMo-NH3/H2 |
| Anode catalyst | Pt | Pt | LSM-YSZ or LSF-YSZ | Co3O4 |
| Electrolyte | KOH | H2SO4 | YSZ | 1 wt% K2CO3 |
| Isolation medium | PPS/PES a | PFSA b | N.A. | QAPS c |
| Operation parameters | ||||
| Pressure of work (bar) | 10–30 | 20–50 | 1–15 | 30 |
| Electrolytic temperature (°C) | 60–80 | 50–80 | 700–900 | 50–80 |
| Current density (A/cm2) | 0.25–0.45 | 1–2 | 0.3–1.0 | 0.2–0.5 |
| Flexibility | ||||
| Start-up time | 5 min | <10 s | 15 min | N.A. |
| Efficiency | ||||
| Commercial system electrolysis efficiency (LHV) | 51–60 | 60–65 | 76–81 | 64 |
| Specific energy consumption (kWh/Nm3) | 5.0–5.9 | 4.6–5.0 | 3.7–3.9 | 4.7 |
| H2 purity (vol%) | 99.5 | 99.99 | 99.9 | 99.99 |
| Durability | ||||
| Life time (kh) | 55–120 | 60–100 | 8–20 | N.A. |
| Economic parameters | ||||
| Investment cost (EUR/kW) | 800–1500 | 1400–2100 | >2000 | N.A. |
| Maintenance costs (% of annual investment cost) | 2–3 | 3–5 | N.A. | N.A. |
| Anode reaction | 4OH− → 2H2O + O2 + 4e− | 2H2O → 4H+ + O2 + 4e− | H2O + 2e− → H2 + O2− | 4OH− → O2 + 2H2O + 4e− |
| Cathodic reaction | 4H2O + 4e− → 2H2 + 4OH− | 2H+ + 2e− → H2 | 2O2− → 4e− + O2 | 4H2O + 4e− → 2H2 + 4OH− |
| Total reaction | 2H2O → 2H2 + O2 | |||
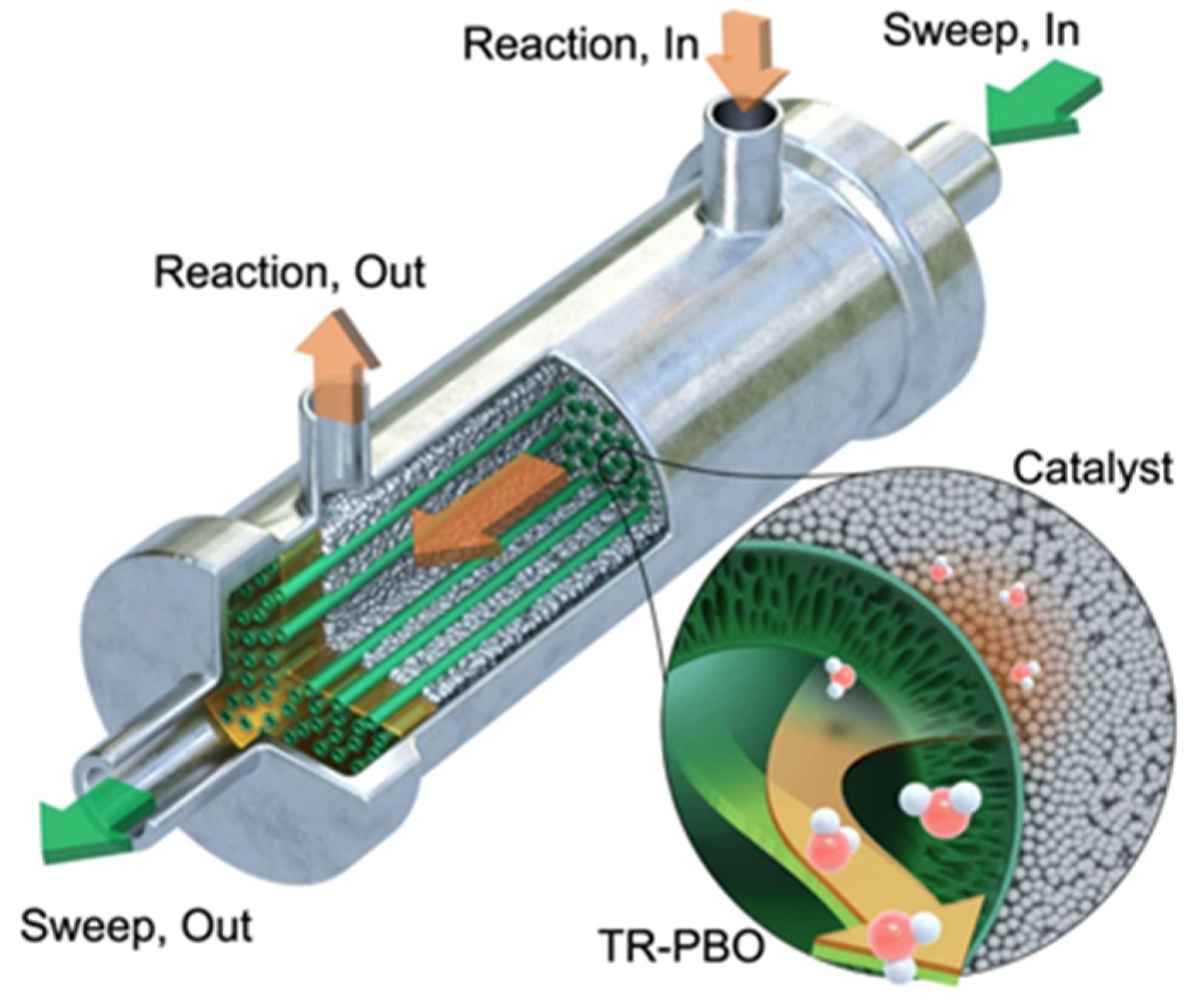
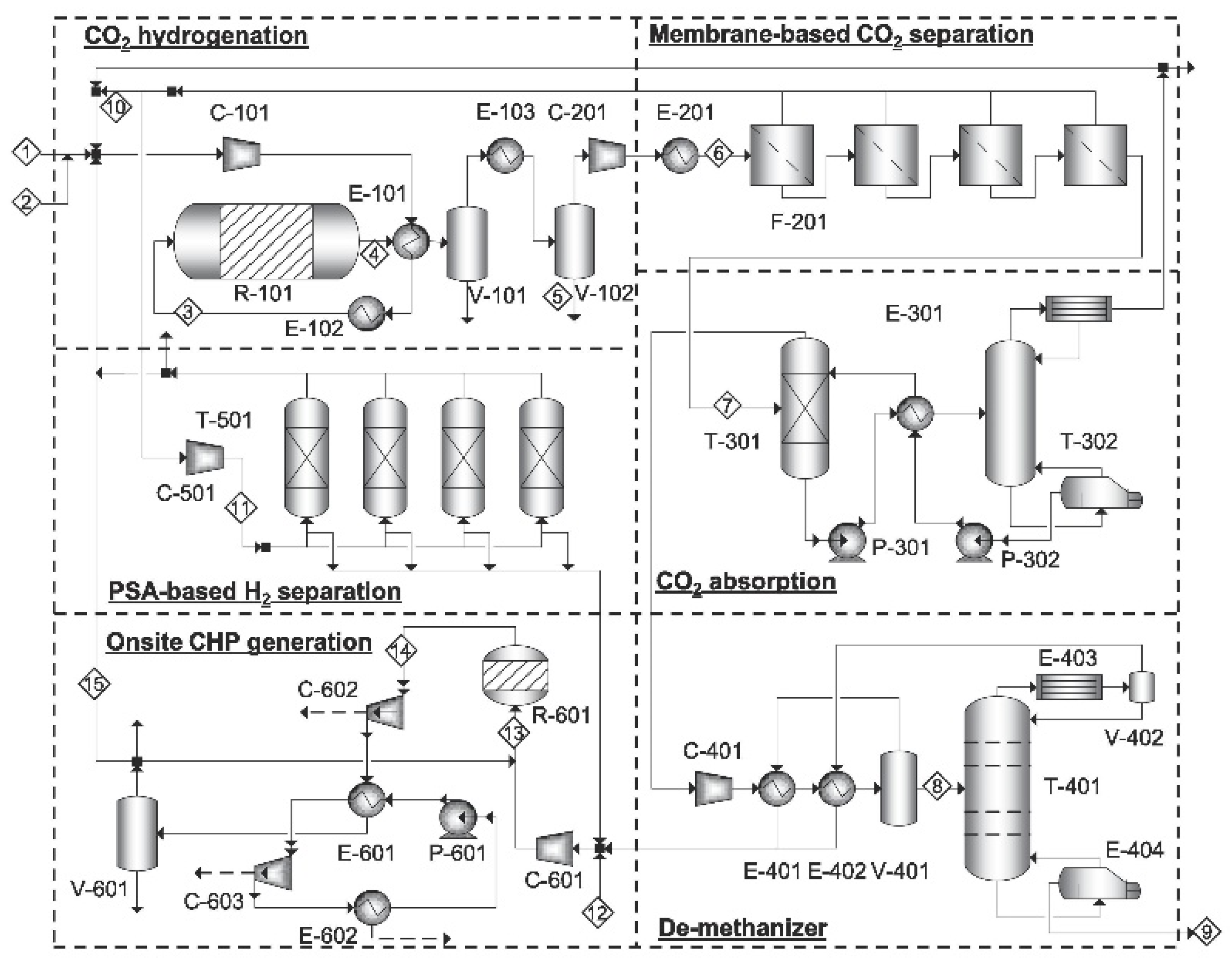
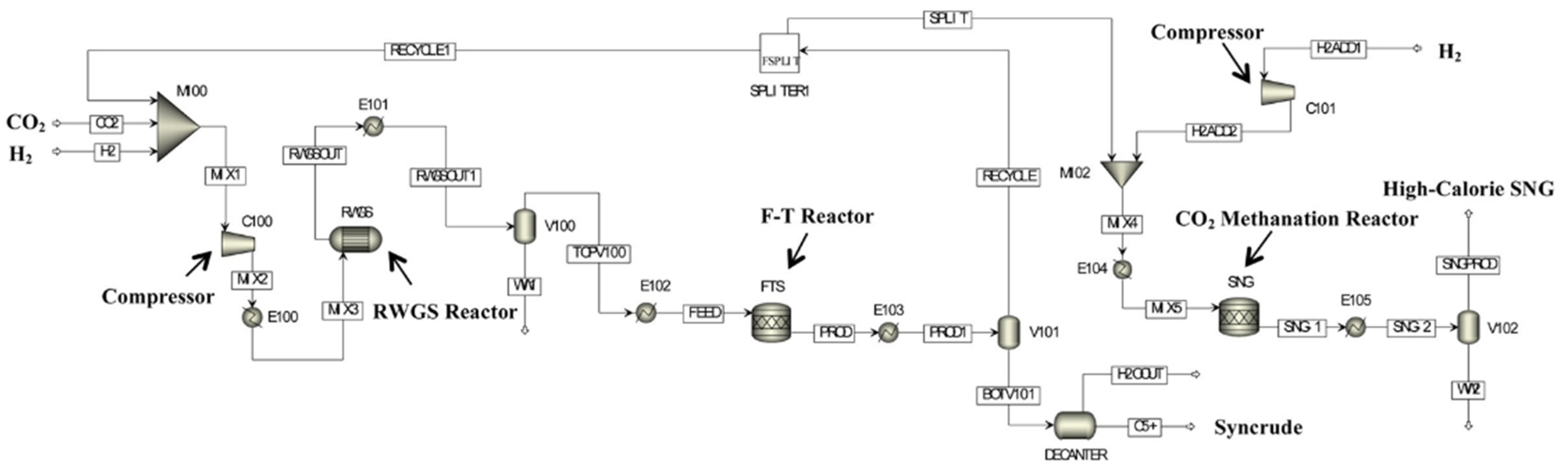
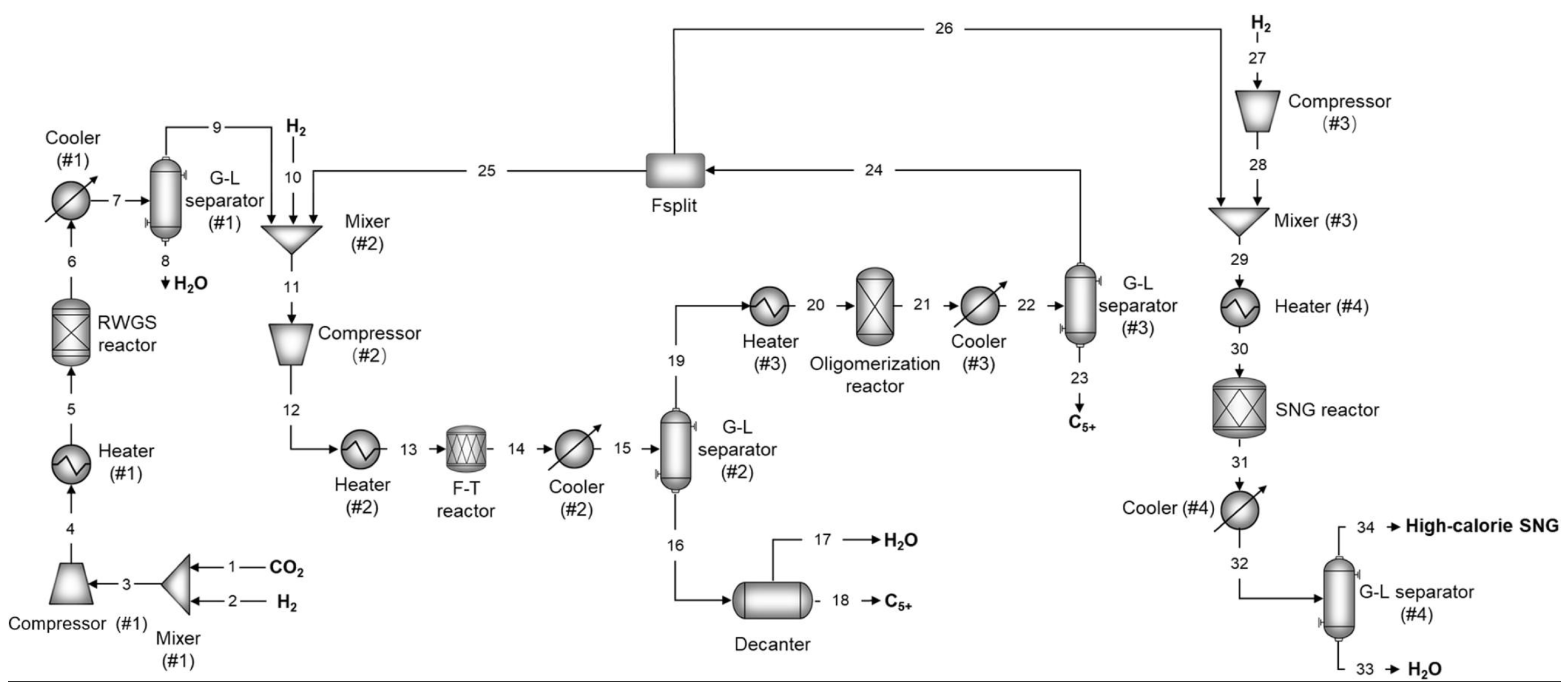
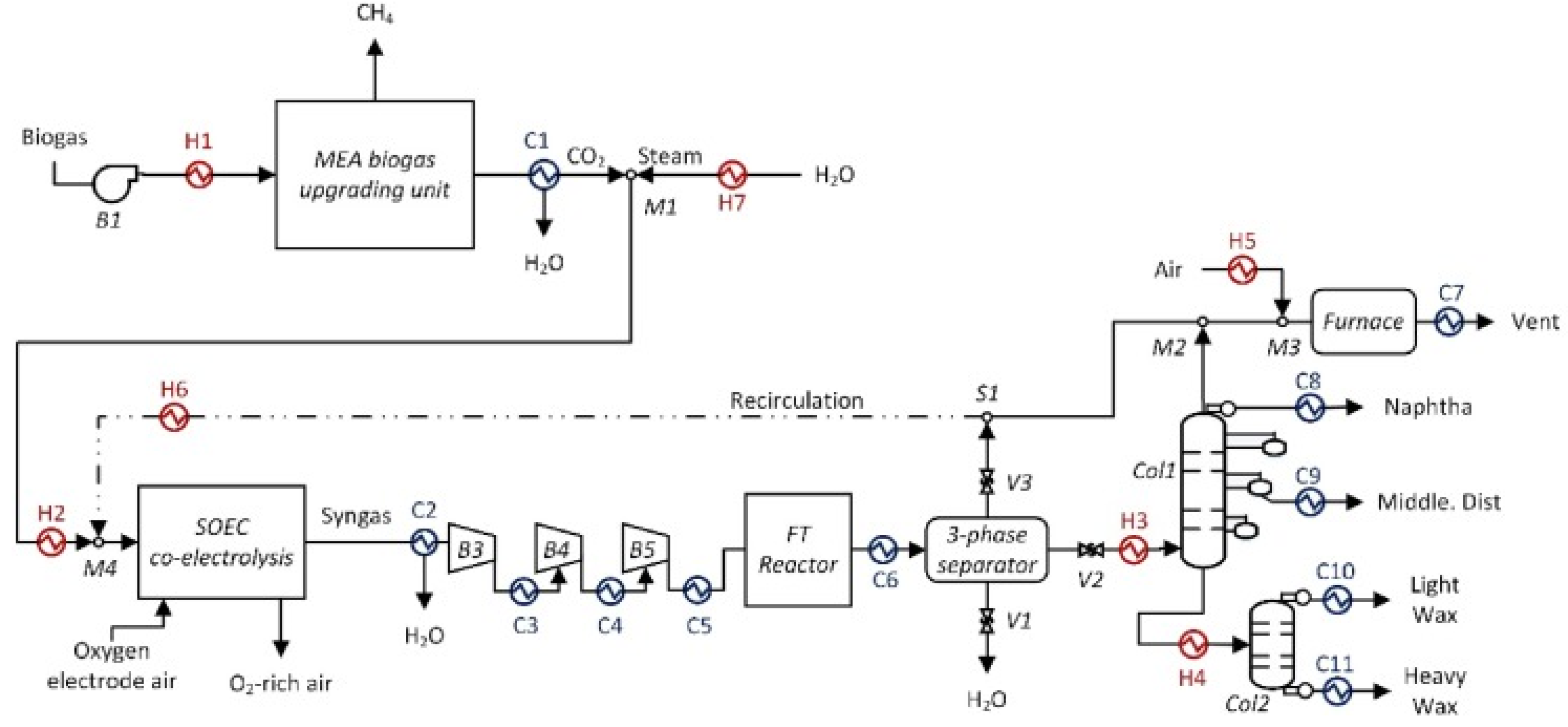
2.3. CO2 Hydrogenation to Liquid Fuels Technologies
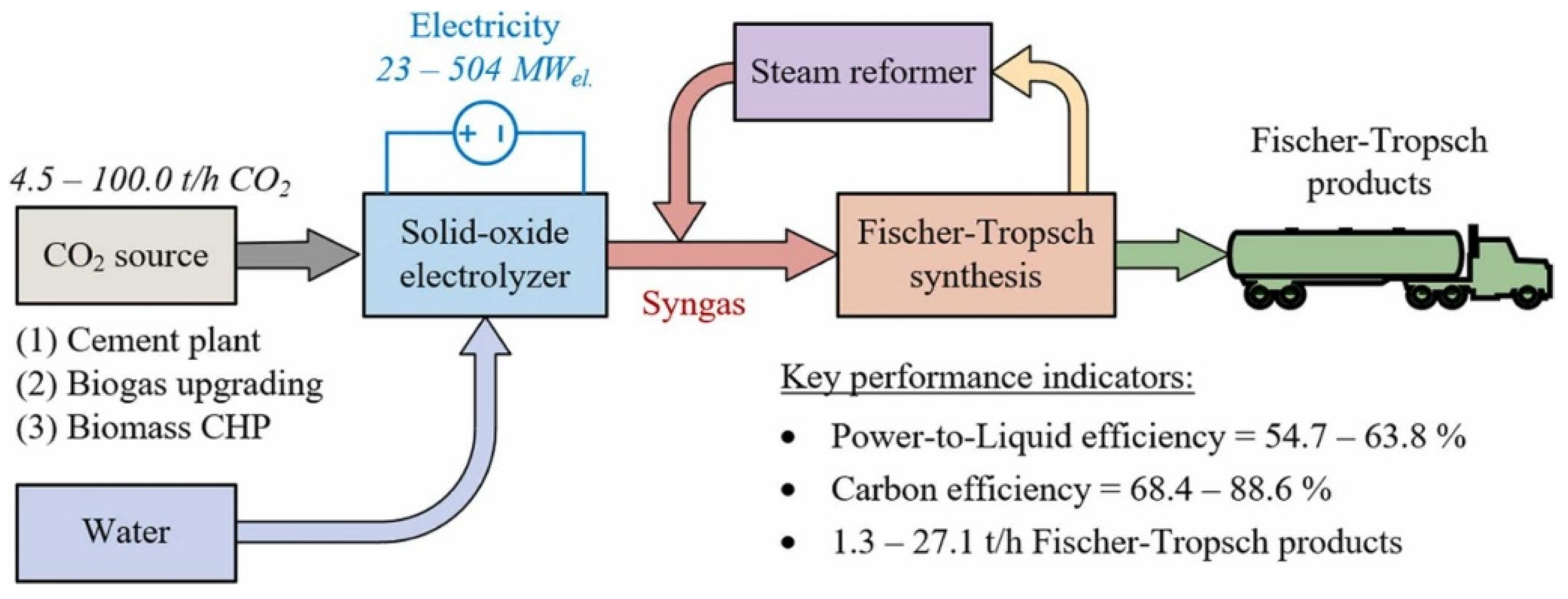
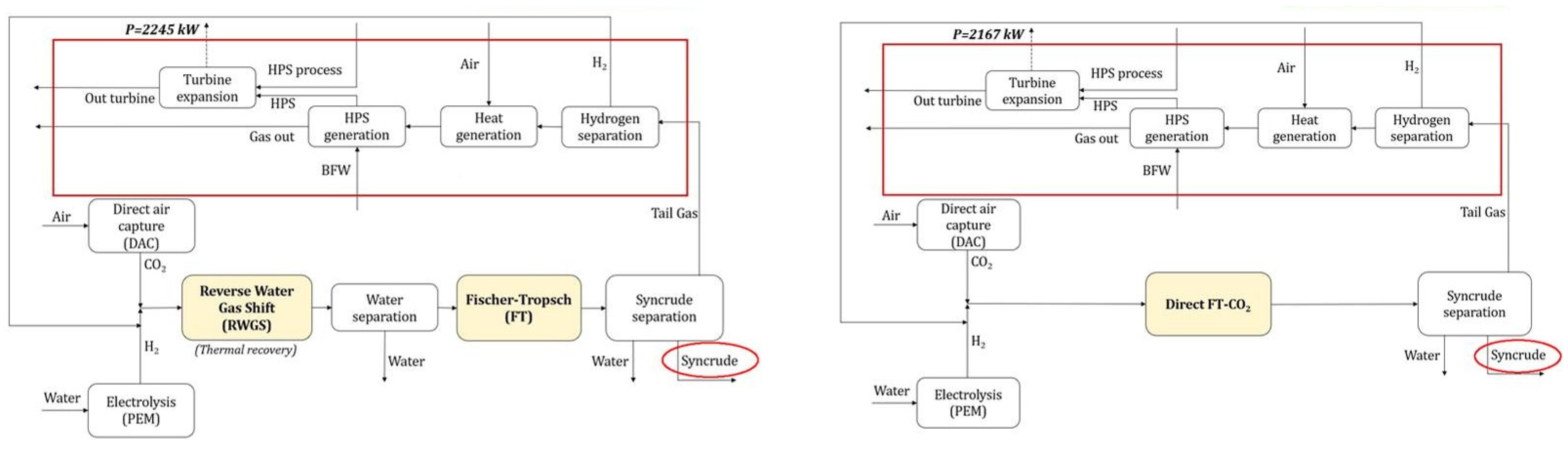
3. Technical and Economic Analysis of PTL Processes
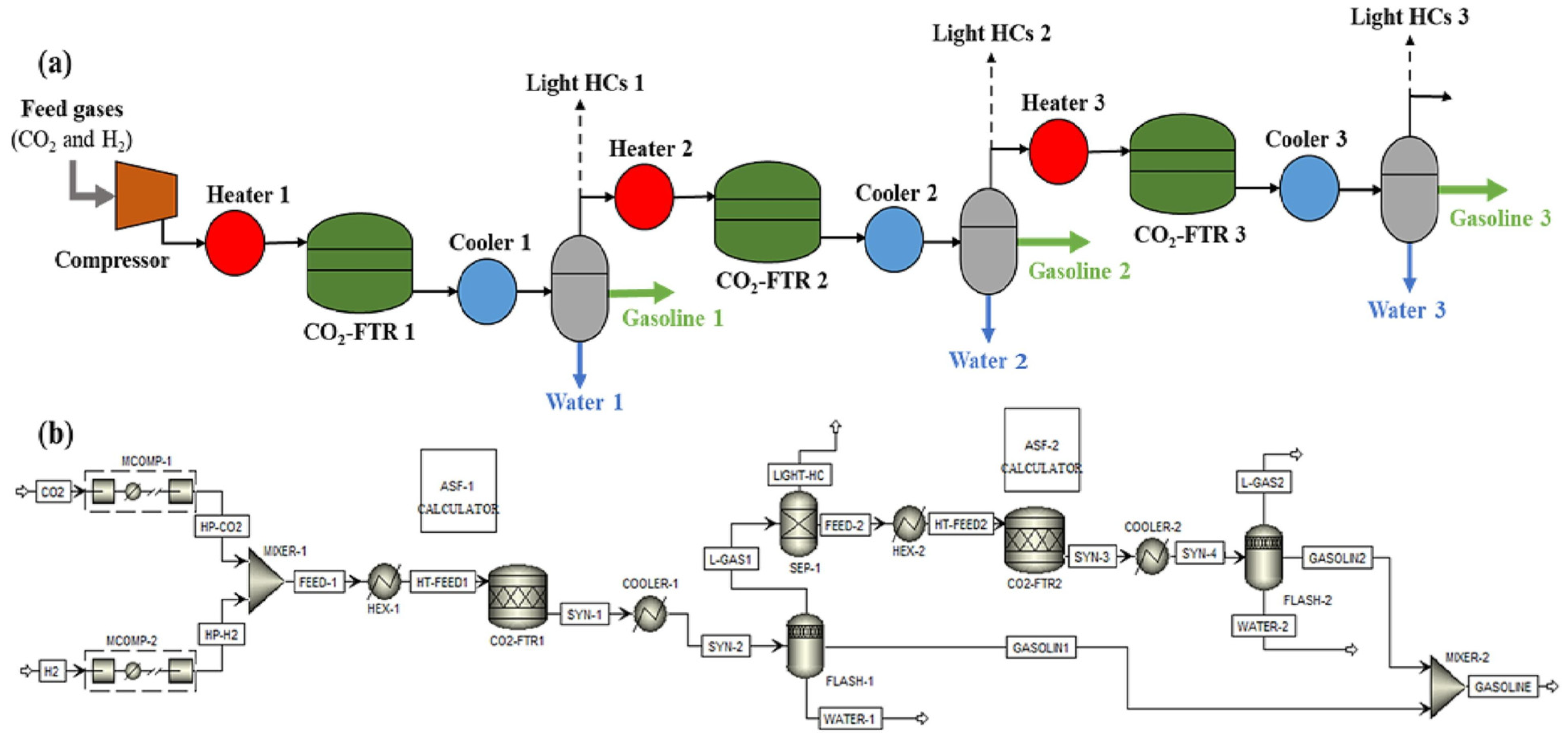
3.1. Technical Analysis of PTL Processes
3.2. Economic Analysis of PTL Processes
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IEA. Global Energy Review: CO2 Emissions in 2021-Global Emissions Rebound Sharply to Highest Ever Level; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Lee, B.; Lee, H.; Lim, D.; Brigljević, B.; Cho, W.; Cho, H.-S.; Kim, C.-H.; Lim, H. Renewable methanol synthesis from renewable H2 and captured CO2: How can power-to-liquid technology be economically feasible? Appl. Energy 2020, 279, 115827. [Google Scholar] [CrossRef]
- Pires, J.C.M.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Feng, C.; Lin, T.; Zhu, R.; Wei, G.; Dong, K. Key technologies for CO2 capture and recycling after combustion: CO2 enrichment in oxygen enriched combustion of converter gas. J. Clean. Prod. 2022, 380, 135128. [Google Scholar] [CrossRef]
- Chowdhury, S.; Parshetti, G.K.; Balasubramanian, R. Post-combustion CO2 capture using mesoporous TiO2/graphene oxide nanocomposites. Chem. Eng. J. 2015, 263, 374–384. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen production from biomasses and wastes: A technological review. Int. J. Hydrog. Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Velazquez Abad, A.; Dodds, P.E. Green hydrogen characterisation initiatives: Definitions, standards, guarantees of origin, and challenges. Energy Policy 2020, 138, 111300. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, L.; Wang, L.; Zhang, X.; Zhang, C.; Jun, K.-W.; Ki Kim, S.; Park, H.-G.; Gao, Y.; Zhu, Y.; et al. A comparative study on hybrid power-to-liquids/power-to-gas processes coupled with different water electrolysis technologies. Energy Convers. Manag. 2022, 263, 115671. [Google Scholar] [CrossRef]
- Lee, B.; Heo, J.; Kim, S.; Sung, C.; Moon, C.; Moon, S.; Lim, H. Economic feasibility studies of high pressure PEM water electrolysis for distributed H2 refueling stations. Energy Convers. Manag. 2018, 162, 139–144. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer-Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Saeidi, S.; Najari, S.; Hessel, V.; Wilson, K.; Keil, F.J.; Concepción, P.; Suib, S.L.; Rodrigues, A.E. Recent advances in CO2 hydrogenation to value-added products—Current challenges and future directions. Prog. Energy Combust. Sci. 2021, 85, 100905. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, K.; Wang, Y.; Liu, Z.; Gao, X.; Zhang, J.; Ma, Q.; Fan, S.; Zhao, T.-S.; Yao, M. Recent advances in thermocatalytic hydrogenation of carbon dioxide to light olefins and liquid fuels via modified Fischer-Tropsch pathway. J. CO2 Util. 2023, 67, 102321. [Google Scholar] [CrossRef]
- Atsonios, K.; Li, J.; Inglezakis, V.J. Process analysis and comparative assessment of advanced thermochemical pathways for e-kerosene production. Energy 2023, 278, 127868. [Google Scholar] [CrossRef]
- Ye, R.P.; Ding, J.; Gong, W.B.; Argyle, M.D.; Zhong, Q.; Wang, Y.J.; Russell, C.K.; Xu, Z.H.; Russell, A.G.; Li, Q.H.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 15. [Google Scholar] [CrossRef]
- White, C.M.; Strazisar, B.R.; Granite, E.J.; Hoffman, J.S.; Pennline, H.W. Separation and capture of CO2 from large stationary sources and sequestration in geological formations—Coalbeds and deep saline aquifers. J. Air Waste Manag. Assoc. 2003, 53, 645–715. [Google Scholar] [CrossRef]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment. Fuel 2023, 342, 127776. [Google Scholar] [CrossRef]
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Mun, J.-H.; Shin, B.-J.; Kim, S.-M.; You, J.K.; Park, Y.C.; Chun, D.-H.; Lee, J.-S.; Min, B.-M.; Lee, U.; Kim, K.-M. Optimal MEA/DIPA/water blending ratio for minimizing regeneration energy in absorption-based carbon capture process: Experimental CO2 solubility and thermodynamic modeling. Chem. Eng. J. 2022, 444, 136523. [Google Scholar] [CrossRef]
- Chu, F.; Yang, L.; Du, X.; Yang, Y. CO2 capture using MEA (monoethanolamine) aqueous solution in coal-fired power plants: Modeling and optimization of the absorbing columns. Energy 2016, 109, 495–505. [Google Scholar] [CrossRef]
- Patel, H.A.; Byun, J.; Yavuz, C.T. Carbon dioxide capture adsorbents: Chemistry and methods. ChemSusChem 2017, 10, 1303–1317. [Google Scholar] [CrossRef]
- Li, K.; Leigh, W.; Feron, P.; Yu, H.; Tade, M. Systematic study of aqueous monoethanolamine (MEA)-based CO2 capture process: Techno-economic assessment of the MEA process and its improvements. Appl. Energy 2016, 165, 648–659. [Google Scholar] [CrossRef]
- Jaffar, M.; Brandoni, C.; Martinez, J.; Snape, C.; Kaldis, S.; Rolfe, A.; Santos, A.; Lysiak, B.; Lappas, A.; Hewitt, N.; et al. Comparative techno-economic analysis of the integration of MEA-based scrubbing and silica PEI adsorbent-based CO2 capture processes into cement plants. J. Clean. Prod. 2023, 414, 137666. [Google Scholar] [CrossRef]
- Rodriguez-Flores, H.A.; Mello, L.C.; Salvagnini, W.M.; de Paiva, J.L. Absorption of CO2 into aqueous solutions of MEA and AMP in a wetted wall column with film promoter. Chem. Eng. Process. Process Intensif. 2013, 73, 1–6. [Google Scholar] [CrossRef]
- Ma’mun, S.; Jakobsen, J.P.; Svendsen, H.F.; Juliussen, O. Experimental and modeling study of the solubility of carbon dioxide in aqueous 30 mass% 2-((2-aminoethyl) amino) ethanol solution. Ind. Eng. Chem. Res. 2006, 45, 2505–2512. [Google Scholar] [CrossRef]
- Ma’mun, S.; Dindore, V.Y.; Svendsen, H.F. Kinetics of the reaction of carbon dioxide with aqueous solutions of 2-((2-aminoethyl) amino) ethanol. Ind. Eng. Chem. Res. 2007, 46, 385–394. [Google Scholar] [CrossRef]
- Rochelle, G.; Chen, E.; Freeman, S.; Van Wagener, D.; Xu, Q.; Voice, A. Aqueous piperazine as the new standard for CO2 capture technology. Chem. Eng. J. 2011, 171, 725–733. [Google Scholar] [CrossRef]
- Freeman, S.A.; Dugas, R.; Van Wagener, D.H.; Nguyen, T.; Rochelle, G.T. Carbon dioxide capture with concentrated, aqueous piperazine. Int. J. Greenh. Gas Control. 2010, 4, 119–124. [Google Scholar] [CrossRef]
- Bougie, F.; Iliuta, M.C. CO2 absorption in aqueous piperazine solutions: Experimental study and modeling. J. Chem. Eng. Data 2011, 56, 1547–1554. [Google Scholar] [CrossRef]
- Idem, R.; Wilson, M.; Tontiwachwuthikul, P.; Chakma, A.; Veawab, A.; Aroonwilas, A.; Gelowitz, D. Pilot plant studies of the CO2 capture performance of aqueous MEA and mixed MEA/MDEA solvents at the University of Regina CO2 capture technology development plant and the boundary dam CO2 capture demonstration plant. Ind. Eng. Chem. Res. 2006, 45, 2414–2420. [Google Scholar] [CrossRef]
- Ramazani, R.; Mazinani, S.; Jahanmiri, A.; Darvishmanesh, S.; Van der Bruggen, B. Investigation of different additives to monoethanolamine (MEA) as a solvent for CO2 capture. J. Taiwan Inst. Chem. Eng. 2016, 65, 341–349. [Google Scholar] [CrossRef]
- Mondal, B.K.; Bandyopadhyay, S.S.; Samanta, A.N. Equilibrium solubility and enthalpy of CO2 absorption in aqueous bis(3-aminopropyl) amine and its mixture with MEA, MDEA, AMP and K2CO3. Chem. Eng. Sci. 2017, 170, 58–67. [Google Scholar] [CrossRef]
- Apaiyakul, R.; Nimmanterdwong, P.; Kanchanakungvalkul, T.; Puapan, P.; Gao, H.; Liang, Z.; Tontiwachwuthikul, P.; Sema, T. Precipitation behavior, density, viscosity, and CO2 absorption capacity of highly concentrated ternary AMP-PZ-MEA solvents. Int. J. Greenh. Gas Control 2022, 120, 103775. [Google Scholar] [CrossRef]
- Ban, Z.H.; Keong, L.K.; Mohd Shariff, A. Physical absorption of CO2 capture: A review. Adv. Mater. Res. 2014, 917, 134–143. [Google Scholar] [CrossRef]
- Nazir, S.M.; Cloete, J.H.; Cloete, S.; Amini, S. Efficient hydrogen production with CO2 capture using gas switching reforming. Energy 2019, 185, 372–385. [Google Scholar] [CrossRef]
- Nexant, I. Survey and Down-Selection of Acid Gas Removal Systems for the Thermochemical Conversion of Biomass to Ethanol with a Detailed Analysis of an MDEA System; National Renewable Energy Laboratory: Golden, CO, USA, 2011.
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Maniarasu, R.; Rathore, S.K.; Murugan, S. A review on materials and processes for carbon dioxide separation and capture. Energy Environ. 2023, 34, 3–57. [Google Scholar] [CrossRef]
- Folger, P. Carbon Capture: A Technology Assessment; Library of Congress Washington DC Congressional Research Service: Washington DC, USA, 2013.
- Mumford, K.A.; Wu, Y.; Smith, K.H.; Stevens, G.W. Review of solvent based carbon-dioxide capture technologies. Front. Chem. Sci. Eng. 2015, 9, 125–141. [Google Scholar] [CrossRef]
- Bucklin, R.; Schendel, R. Comparison of Fluor Solvent and Selexol Processes; U.S. Department of Energy Office of Scientic and Technical Information: Washington, DC, USA, 1984; Volume 4.
- Burr, B.; Lyddon, L. A Comparison of Physical Solvents for Acid Gas Removal; Gas Processors’ Association Convention: Grapevine, TX, USA, 2008. [Google Scholar]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Ansaloni, L.; Salas-Gay, J.; Ligi, S.; Baschetti, M.G. Nanocellulose-based membranes for CO2 capture. J. Membr. Sci. 2017, 522, 216–225. [Google Scholar] [CrossRef]
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-combustion carbon capture by membrane separation, Review. Sep. Purif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon capture and utilization update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Khaisri, S.; deMontigny, D.; Tontiwachwuthikul, P.; Jiraratananon, R. Comparing membrane resistance and absorption performance of three different membranes in a gas absorption membrane contactor. Sep. Purif. Technol. 2009, 65, 290–297. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Chuah, C.Y.; Kim, K.; Lee, J.; Koh, D.-Y.; Bae, T.-H. CO2 absorption using membrane contactors: Recent progress and future perspective. Ind. Eng. Chem. Res. 2019, 59, 6773–6794. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, Z.; Zhang, Z.; Zhang, W.; Zhang, L.; Baena-Moreno, F.M.; Lichtfouse, E. CO2 capture from coalbed methane using membranes: A review. Environ. Chem. Lett. 2020, 18, 79–96. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [PubMed]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zhao, M. Membrane separation technology in carbon capture. In Recent Advances in Carbon Capture and Storage; Intech Open: Hampshire, UK, 2017; pp. 59–90. [Google Scholar]
- Baker, R.W.; Freeman, B.; Kniep, J.; Huang, Y.I.; Merkel, T.C. CO2 capture from cement plants and steel mills using membranes. Ind. Eng. Chem. Res. 2018, 57, 15963–15970. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Z.; Wang, J.; Wang, S. Potential of two-stage membrane system with recycle stream for CO2 capture from postcombustion gas. Energy Fuels 2009, 23, 4755–4762. [Google Scholar] [CrossRef]
- Glier, J.C.; Rubin, E.S. Assessment of solid sorbents as a competitive post-combustion CO2 capture technology. Energy Procedia 2013, 37, 65–72. [Google Scholar] [CrossRef]
- Nikolaidis, G.N.; Kikkinides, E.S.; Georgiadis, M.C. Modelling and Simulation of Pressure Swing Adsorption (PSA) Processes for post-combustion Carbon Dioxide (CO2) capture from flue gas. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 287–292. [Google Scholar]
- Abd, A.A.; Shabbani, H.J.K.; Helwani, Z.; Othman, M.R. Novel design of pressure swing adsorption using compact plates for adsorption heat recuperation of CO2 capture in biomethane upgrading process. Energy Convers. Manag. 2022, 268, 115999. [Google Scholar] [CrossRef]
- Pirngruber, G.D.; Leinekugel-le-Cocq, D. Design of a pressure swing adsorption process for postcombustion CO2 capture. Ind. Eng. Chem. Res. 2013, 52, 5985–5996. [Google Scholar] [CrossRef]
- Zhang, J.; Webley, P.A.; Xiao, P. Effect of process parameters on power requirements of vacuum swing adsorption technology for CO2 capture from flue gas. Energy Convers. Manag. 2008, 49, 346–356. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Leperi, K.T.; Snurr, R.Q.; You, F. Optimization of two-stage pressure/vacuum swing adsorption with variable dehydration level for postcombustion carbon capture. Ind. Eng. Chem. Res. 2016, 55, 3338–3350. [Google Scholar] [CrossRef]
- Riboldi, L.; Bolland, O. Overview on pressure swing adsorption (PSA) as CO2 capture technology: State-of-the-art, limits and potentials. Energy Procedia 2017, 114, 2390–2400. [Google Scholar] [CrossRef]
- Yu, X.; Liu, B.; Shen, Y.; Zhang, D. Design and experiment of high-productivity two-stage vacuum pressure swing adsorption process for carbon capturing from dry flue gas. Chin. J. Chem. Eng. 2022, 43, 378–391. [Google Scholar] [CrossRef]
- Mondino, G.; Grande, C.A.; Blom, R.; Nord, L.O. Moving bed temperature swing adsorption for CO2 capture from a natural gas combined cycle power plant. Int. J. Greenh. Gas Control. 2019, 85, 58–70. [Google Scholar] [CrossRef]
- Li, G.; Xiao, P.; Webley, P.; Zhang, J.; Singh, R.; Marshall, M. Capture of CO2 from high humidity flue gas by vacuum swing adsorption with zeolite 13X. Adsorption 2008, 14, 415–422. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, S.; Zhao, L.; Yuan, X.; Du, Z.; Li, S.; Chen, L.; Wu, K. Understanding the effect of H2O on CO2 adsorption capture: Mechanism explanation, quantitative approach and application. Sustain. Energy Fuels 2020, 4, 5970–5986. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Haghpanah, R.; Rajendran, A.; Farooq, S. Simulation and optimization of a dual-adsorbent, two-bed vacuum swing adsorption process for CO2 capture from wet flue gas. Ind. Eng. Chem. Res. 2014, 53, 14462–14473. [Google Scholar] [CrossRef]
- Gilassi, S.; Taghavi, S.M.; Rodrigue, D.; Kaliaguine, S. Techno-economic evaluation of membrane and enzymatic-absorption processes for CO2 capture from flue-gas. Sep. Purif. Technol. 2020, 248, 116941. [Google Scholar] [CrossRef]
- Tao, M.; Xu, N.; Gao, J.; Zhang, W.; Li, Y.; Bernards, M.T.; Shi, Y.; He, Y.; Pan, H. Phase-change mechanism for capturing CO2 into an environmentally benign nonaqueous solution: A combined nmr and molecular dynamics simulation study. Energy Fuels 2018, 33, 474–483. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Wang, Z.; Qiao, Z.; Zhao, S.; Wang, J. Recent advances on the membrane processes for CO2 separation. Chin. J. Chem. Eng. 2018, 26, 2280–2291. [Google Scholar] [CrossRef]
- Yun, S.; Oh, S.-Y.; Kim, J.-K. Techno-economic assessment of absorption-based CO2 capture process based on novel solvent for coal-fired power plant. Appl. Energy 2020, 268, 114933. [Google Scholar] [CrossRef]
- Zhao, S.; Feron, P.H.; Deng, L.; Favre, E.; Chabanon, E.; Yan, S.; Hou, J.; Chen, V.; Qi, H. Status and progress of membrane contactors in post-combustion carbon capture: A state-of-the-art review of new developments. J. Membr. Sci. 2016, 511, 180–206. [Google Scholar] [CrossRef]
- David, M.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef]
- Fortin, P.; Khoza, T.; Cao, X.; Martinsen, S.Y.; Barnett, A.O.; Holdcroft, S. High-performance alkaline water electrolysis using Aemion™ anion exchange membranes. J. Power Sources 2020, 451, 227814. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Haug, P.; Koj, M.; Turek, T. Influence of process conditions on gas purity in alkaline water electrolysis. Int. J. Hydrog. Energy 2017, 42, 9406–9418. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, B.; Wang, P.; Liu, S. Hydrogen generation with acid/alkaline amphoteric water electrolysis. J. Energy Chem. 2019, 38, 162–169. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, J.; He, W.; Sun, L.; Li, Y. Alkaline zero gap bipolar water electrolyzer for hydrogen production with independent fluid path. Energy Rep. 2023, 9, 352–360. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrog. Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Babic, U.; Suermann, M.; Büchi, F.N.; Gubler, L.; Schmidt, T.J. Critical review—Identifying critical gaps for polymer electrolyte water electrolysis development. J. Electrochem. Soc. 2017, 164, F387. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Advances in the high performance polymer electrolyte membranes for fuel cells. Chem. Soc. Rev. 2012, 41, 2382–2394. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-López, M.; Darras, C.; Poggi, P.; Glises, R.; Baucour, P.; Rakotondrainibe, A.; Besse, S.; Serre-Combe, P. Modelling and experimental validation of a 46 kW PEM high pressure water electrolyzer. Renew. Energy 2018, 119, 160–173. [Google Scholar] [CrossRef]
- Toghyani, S.; Fakhradini, S.; Afshari, E.; Baniasadi, E.; Jamalabadi, M.Y.A.; Shadloo, M.S. Optimization of operating parameters of a polymer exchange membrane electrolyzer. Int. J. Hydrog. Energy 2019, 44, 6403–6414. [Google Scholar] [CrossRef]
- Parnian, M.J.; Rowshanzamir, S.; Prasad, A.K.; Advani, S.G. Effect of ceria loading on performance and durability of sulfonated poly(ether ether ketone) nanocomposite membranes for proton exchange membrane fuel cell applications. J. Membr. Sci. 2018, 565, 342–357. [Google Scholar] [CrossRef]
- Ren, P.; Pei, P.; Li, Y.; Wu, Z.; Chen, D.; Huang, S. Degradation mechanisms of proton exchange membrane fuel cell under typical automotive operating conditions. Prog. Energy Combust. Sci. 2020, 80, 100859. [Google Scholar] [CrossRef]
- Fouda-Onana, F.; Chandesris, M.; Médeau, V.; Chelghoum, S.; Thoby, D.; Guillet, N. Investigation on the degradation of MEAs for PEM water electrolysers part I: Effects of testing conditions on MEA performances and membrane properties. Int. J. Hydrog. Energy 2016, 41, 16627–16636. [Google Scholar] [CrossRef]
- Gómez, S.Y.; Hotza, D. Current developments in reversible solid oxide fuel cells. Renew. Sustain. Energy Rev. 2016, 61, 155–174. [Google Scholar] [CrossRef]
- Jiang, S.P. Challenges in the development of reversible solid oxide cell technologies: A mini review. Asia-Pac. J. Chem. Eng. 2016, 16, 386–391. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef]
- Graves, C.; Ebbesen, S.D.; Mogensen, M. Co-electrolysis of CO2 and H2O in solid oxide cells: Performance and durability. Solid State Ion. 2011, 192, 398–403. [Google Scholar] [CrossRef]
- Yue, X.; Irvine, J.T. (La, Sr)(Cr, Mn) O3/GDC cathode for high temperature steam electrolysis and steam-carbon dioxide co-electrolysis. Solid State Ion. 2012, 225, 131–135. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Wang, G.; Bao, X. Co-electrolysis of CO2 and H2O in high-temperature solid oxide electrolysis cells: Recent advance in cathodes. J. Energy Chem. 2017, 26, 839–853. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Y.; Yu, B.; Wang, J.; Chen, J. Electrochemical characterization and mechanism analysis of high temperature Co-electrolysis of CO2 and H2O in a solid oxide electrolysis cell. Int. J. Hydrog. Energy 2017, 42, 29911–29920. [Google Scholar] [CrossRef]
- Leng, Y.; Chen, G.; Mendoza, A.J.; Tighe, T.B.; Hickner, M.A.; Wang, C.-Y. Solid-state water electrolysis with an alkaline membrane. J. Am. Chem. Soc. 2012, 134, 9054–9057. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Najibah, M.; Harms, C.; Žitka, J.; Hnát, J.; Bouzek, K. Overview: State-of-the art commercial membranes for anion exchange membrane water electrolysis. J. Electrochem. Energy Convers. Storage 2021, 18, 024001. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Bareiß, K.; de la Rua, C.; Möckl, M.; Hamacher, T. Life cycle assessment of hydrogen from proton exchange membrane water electrolysis in future energy systems. Appl. Energy 2019, 237, 862–872. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.; Shao, Z.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Michailos, S.; McCord, S.; Sick, V.; Stokes, G.; Styring, P. Dimethyl ether synthesis via captured CO2 hydrogenation within the power to liquids concept: A techno-economic assessment. Energy Convers. Manag. 2019, 184, 262–276. [Google Scholar] [CrossRef]
- Tenhumberg, N.; Büker, K. Ecological and Economic Evaluation of Hydrogen Production by Different Water Electrolysis Technologies. Chem. Ing. Tech. 2020, 92, 1586–1595. [Google Scholar] [CrossRef]
- Thema, M.; Bauer, F.; Sterner, M. Power-to-Gas: Electrolysis and methanation status review. Renew. Sustain. Energy Rev. 2019, 112, 775–787. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef] [PubMed]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Chen, P.; Hu, X. High-efficiency anion exchange membrane water electrolysis employing non-noble metal catalysts. Adv. Energy Mater. 2020, 10, 2002285. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- Lu, S.; Pan, J.; Huang, A.; Zhuang, L.; Lu, J. Alkaline polymer electrolyte fuel cells completely free from noble metal catalysts. Proc. Natl. Acad. Sci. USA 2008, 105, 20611–20614. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y. Technological development of hydrogen production by solid oxide electrolyzer cell (SOEC). Int. J. Hydrog. Energy 2008, 33, 2337–2354. [Google Scholar] [CrossRef]
- Marques Mota, F.; Kim, D.H. From CO2 methanation to ambitious long-chain hydrocarbons: Alternative fuels paving the path to sustainability. Chem. Soc. Rev. 2019, 48, 205–259. [Google Scholar] [CrossRef]
- Panahi, M.; Rafiee, A.; Skogestad, S.; Hillestad, M. A natural gas to liquids process model for optimal operation. Ind. Eng. Chem. Res. 2012, 51, 425–433. [Google Scholar] [CrossRef]
- Cheng, K.; Ordomsky, V.V.; Virginie, M.; Legras, B.; Chernavskii, P.A.; Kazak, V.; Cordier, C.; Paul, S.; Wang, Y.; Khodakov, A.Y. Support effects in high temperature Fischer-Tropsch synthesis on iron catalysts. Appl. Catal. A Gen. 2014, 488, 66–77. [Google Scholar] [CrossRef]
- Huang, Y.; Yi, Q.; Wei, G.-Q.; Kang, J.-X.; Li, W.-Y.; Feng, J.; Xie, K.-C. Energy use, greenhouse gases emission and cost effectiveness of an integrated high–and low–temperature Fisher–Tropsch synthesis plant from a lifecycle viewpoint. Appl. Energy 2018, 228, 1009–1019. [Google Scholar] [CrossRef]
- Mahouri, S.; Catalan, L.J.J.; Rezaei, E. Process design and techno-economic analysis of CO2 reformation to synthetic crude oil using H2 produced by decomposition of CH4 in a molten media. Energy Convers. Manag. 2023, 276, 116548. [Google Scholar] [CrossRef]
- Scarfiello, C.; Pham Minh, D.; Soulantica, K.; Serp, P. Oxide Supported Cobalt Catalysts for CO2 Hydrogenation to Hydrocarbons: Recent Progress. Adv. Mater. Interfaces 2023, 10, 2202516. [Google Scholar] [CrossRef]
- Arora, A.; Iyer, S.S.; Bajaj, I.; Hasan, M.M.F. Optimal Methanol Production via Sorption-Enhanced Reaction Process. Ind. Eng. Chem. Res. 2018, 57, 14143–14161. [Google Scholar] [CrossRef]
- Atashi, H.; Torang, H.Z. Fischer-Tropsch synthesis in a bed reactor using Co catalyst over silica supported: Process optimization and selectivite modeling. J. Environ. Chem. Eng. 2018, 6, 5520–5529. [Google Scholar] [CrossRef]
- Qin, K.; Men, Y.; Liu, S.; Wang, J.; Li, Z.; Tian, D.; Shi, T.; An, W.; Pan, X.; Li, L. Direct conversion of carbon dioxide to liquid hydrocarbons over K-modified CoFeOx/zeolite multifunctional catalysts. J. CO2 Util. 2022, 65, 102208. [Google Scholar] [CrossRef]
- Kang, S.C.; Jun, K.-W.; Lee, Y.-J. Effects of the CO/CO2 Ratio in Synthesis Gas on the Catalytic Behavior in Fischer–Tropsch Synthesis Using K/Fe–Cu–Al Catalysts. Energy Fuels 2013, 27, 6377–6387. [Google Scholar] [CrossRef]
- Li, Z.; Deng, Y.; Dewangan, N.; Hu, J.; Wang, Z.; Tan, X.; Liu, S.; Kawi, S. High Temperature Water Permeable Membrane Reactors for CO2 Utilization. Chem. Eng. J. 2021, 420, 129834. [Google Scholar] [CrossRef]
- Hyeon, M.-H.; Park, H.-G.; Lee, J.; Kong, C.-I.; Kim, E.-Y.; Kim, J.H.; Moon, S.-Y.; Kim, S.K. Equilibrium shift, poisoning prevention, and selectivity enhancement in catalysis via dehydration of polymeric membranes. Nat. Commun. 2023, 14, 1673. [Google Scholar] [CrossRef] [PubMed]
- Meiri, N.; Radus, R.; Herskowitz, M. Simulation of novel process of CO2 conversion to liquid fuels. J. CO2 Util. 2017, 17, 284–289. [Google Scholar] [CrossRef]
- Kamkeng, A.D.N.; Wang, M. Technical analysis of the modified Fischer-Tropsch synthesis process for direct CO2 conversion into gasoline fuel: Performance improvement via ex-situ water removal. Chem. Eng. J. 2023, 462, 142048. [Google Scholar] [CrossRef]
- van Bavel, S.; Verma, S.; Negro, E.; Bracht, M. Integrating CO2 Electrolysis into the Gas-to-Liquids–Power-to-Liquids Process. ACS Energy Lett. 2020, 5, 2597–2601. [Google Scholar] [CrossRef]
- Fakhroleslam, M.; Sadrameli, S.M. Thermal/catalytic cracking of hydrocarbons for the production of olefins; a state-of-the-art review III: Process modeling and simulation. Fuel 2019, 252, 553–566. [Google Scholar] [CrossRef]
- Do, T.N.; Kim, J. Green C2-C4 hydrocarbon production through direct CO2 hydrogenation with renewable hydrogen: Process development and techno-economic analysis. Energy Convers. Manag. 2020, 214, 112866. [Google Scholar] [CrossRef]
- Adelung, S.; Maier, S.; Dietrich, R.-U. Impact of the reverse water-gas shift operating conditions on the Power-to-Liquid process efficiency. Sustain. Energy Technol. Assess 2021, 43, 100897. [Google Scholar] [CrossRef]
- Cinti, G.; Baldinelli, A.; Di Michele, A.; Desideri, U. Integration of Solid Oxide Electrolyzer and Fischer-Tropsch: A sustainable pathway for synthetic fuel. Appl. Energy 2016, 162, 308–320. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, C.; Jun, K.-W.; Kim, S.K.; Park, H.-G.; Zhao, T.; Wang, L.; Wan, H.; Guan, G. Green liquid fuel and synthetic natural gas production via CO2 hydrogenation combined with reverse water-gas-shift and co-based Fischer-Tropsch synthesis. J. CO2 Util. 2021, 51, 101619. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, C.; Jun, K.-W.; Kim, S.K.; Park, H.-G.; Zhao, T.; Wang, L.; Wan, H.; Guan, G. Transformation of CO2 into liquid fuels and synthetic natural gas using green hydrogen: A comparative analysis. Fuel 2021, 291, 120111. [Google Scholar] [CrossRef]
- Gao, R.; Wang, L.; Zhang, L.; Zhang, C.; Jun, K.-W.; Ki Kim, S.; Park, H.-G.; Zhao, T.; Gao, Y.; Zhu, Y.; et al. Upcycling of CO2 into sustainable hydrocarbon fuels via the integration of Fe-based Fischer-Tropsch synthesis and olefin oligomerization: A comparative case study. Fuel 2022, 325, 124855. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, L.; Wang, L.; Zhang, C.; Jun, K.-W.; Kim, S.K.; Park, H.-G.; Gao, Y.; Zhu, Y.; Wan, H.; et al. Efficient production of renewable hydrocarbon fuels using waste CO2 and green H2 by integrating Fe-based Fischer-Tropsch synthesis and olefin oligomerization. Energy 2022, 248, 123616. [Google Scholar] [CrossRef]
- Dahiru, A.R.; Vuokila, A.; Huuhtanen, M. Recent development in Power-to-X: Part I-A review on techno-economic analysis. J. Energy Storage 2022, 56, 105861. [Google Scholar] [CrossRef]
- Herz, G.; Reichelt, E.; Jahn, M. Techno-economic analysis of a co-electrolysis-based synthesis process for the production of hydrocarbons. Appl. Energy 2018, 215, 309–320. [Google Scholar] [CrossRef]
- Markowitsch, C.; Lehner, M.; Maly, M. Evaluation of process structures and reactor technologies of an integrated power-to-liquid plant at a cement factory. J. CO2 Util. 2023, 70, 102449. [Google Scholar] [CrossRef]
- Pratschner, S.; Hammerschmid, M.; Müller, F.J.; Müller, S.; Winter, F. Simulation of a Pilot Scale Power-to-Liquid Plant Producing Synthetic Fuel and Wax by Combining Fischer–Tropsch Synthesis and SOEC. Energies 2022, 15, 4134. [Google Scholar] [CrossRef]
- Marchese, M.; Giglio, E.; Santarelli, M.; Lanzini, A. Energy performance of Power-to-Liquid applications integrating biogas upgrading, reverse water gas shift, solid oxide electrolysis and Fischer-Tropsch technologies. Energy Convers. Manag. X 2020, 6, 100041. [Google Scholar] [CrossRef]
- Pratschner, S.; Hammerschmid, M.; Müller, S.; Winter, F. Evaluation of CO2 sources for Power-to-Liquid plants producing Fischer-Tropsch products. J. CO2 Util. 2023, 72, 102508. [Google Scholar] [CrossRef]
- GmbH, S. Breakthrough for Power-to-X: Sunfire Putsfirst Co-Electolysis into Operation and Starts Scaling. Available online: https://www.sunfire.de/en/news/detail/breakthrough-for-power-to-x-sunfire-puts-first-co-electrolysis-into-operation-and-starts-scaling (accessed on 29 May 2023).
- Colelli, L.; Segneri, V.; Bassano, C.; Vilardi, G. E-fuels, technical and economic analysis of the production of synthetic kerosene precursor as sustainable aviation fuel. Energy Convers. Manag. 2023, 288, 117165. [Google Scholar] [CrossRef]
- Schemme, S.; Breuer, J.L.; Köller, M.; Meschede, S.; Walman, F.; Samsun, R.C.; Peters, R.; Stolten, D. H2-based synthetic fuels: A techno-economic comparison of alcohol, ether and hydrocarbon production. Int. J. Hydrog. Energy 2020, 45, 5395–5414. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, Y.; Ren, H.; Makowski, M.; Granat, J.; Nahorski, Z.; Ma, T. How the power-to-liquid technology can contribute to reaching carbon neutrality of the China’s transportation sector? Energy 2022, 261, 125058. [Google Scholar] [CrossRef]
- Adelung, S.; Dietrich, R.-U. Impact of the reverse water-gas shift operating conditions on the Power-to-Liquid fuel production cost. Fuel 2022, 317, 123440. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, L.; Wang, L.; Zhang, C.; Jun, K.-W.; Kim, S.K.; Park, H.-G.; Zhao, T.; Wan, H.; Guan, G. Efficient utilization of CO2 in power-to-liquids/power-to-gas hybrid processes: An economic-environmental assessment. J. CO2 Util. 2023, 68, 102376. [Google Scholar] [CrossRef]
- Adelung, S. Global sensitivity and uncertainty analysis of a Fischer-Tropsch based Power-to-Liquid process. J. CO2 Util. 2022, 65, 102171. [Google Scholar] [CrossRef]
- Fasihi, M.; Bogdanov, D.; Breyer, C. Techno-economic assessment of power-to-liquids (PtL) fuels production and global trading based on hybrid PV-wind power plants. Energy Procedia 2016, 99, 243–268. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, R.; Jun, K.-W.; Kim, S.K.; Hwang, S.-M.; Park, H.-G.; Guan, G. Direct conversion of carbon dioxide to liquid fuels and synthetic natural gas using renewable power: Techno-economic analysis. J. CO2 Util. 2019, 34, 293–302. [Google Scholar] [CrossRef]

| Physical Absorption Method | Major Solvent | Operating Requirements, Advantages, and Limitations: |
|---|---|---|
| Rectisol | Methanol |
|
| Limitations: Higher selectivity for H2S over CO2. | ||
| Selexol | Polyethylene glycol dimethyl ether |
|
| Limitations: Higher viscosity than most physical solvents. | ||
| Fluor | Propylene Carbonate |
|
| Limitations: Poor sulfur resistance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, L.; Zhang, C.; Wang, L.; Tang, Z.; Gao, R. The Efficient Utilization of Carbon Dioxide in a Power-to-Liquid Process: An Overview. Processes 2023, 11, 2089. https://doi.org/10.3390/pr11072089
Li X, Zhang L, Zhang C, Wang L, Tang Z, Gao R. The Efficient Utilization of Carbon Dioxide in a Power-to-Liquid Process: An Overview. Processes. 2023; 11(7):2089. https://doi.org/10.3390/pr11072089
Chicago/Turabian StyleLi, Xianqiang, Leiyu Zhang, Chundong Zhang, Lei Wang, Zongyue Tang, and Ruxing Gao. 2023. "The Efficient Utilization of Carbon Dioxide in a Power-to-Liquid Process: An Overview" Processes 11, no. 7: 2089. https://doi.org/10.3390/pr11072089
APA StyleLi, X., Zhang, L., Zhang, C., Wang, L., Tang, Z., & Gao, R. (2023). The Efficient Utilization of Carbon Dioxide in a Power-to-Liquid Process: An Overview. Processes, 11(7), 2089. https://doi.org/10.3390/pr11072089






