To Promote the Catalytic Ozonation of Typical VOCs by Modifying NiO with Cetyltrimethylammonium Bromide
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of Catalyst
2.3. Characterization
2.4. Performance Evaluation of Catalytic Ozonation
3. Results and Discussion
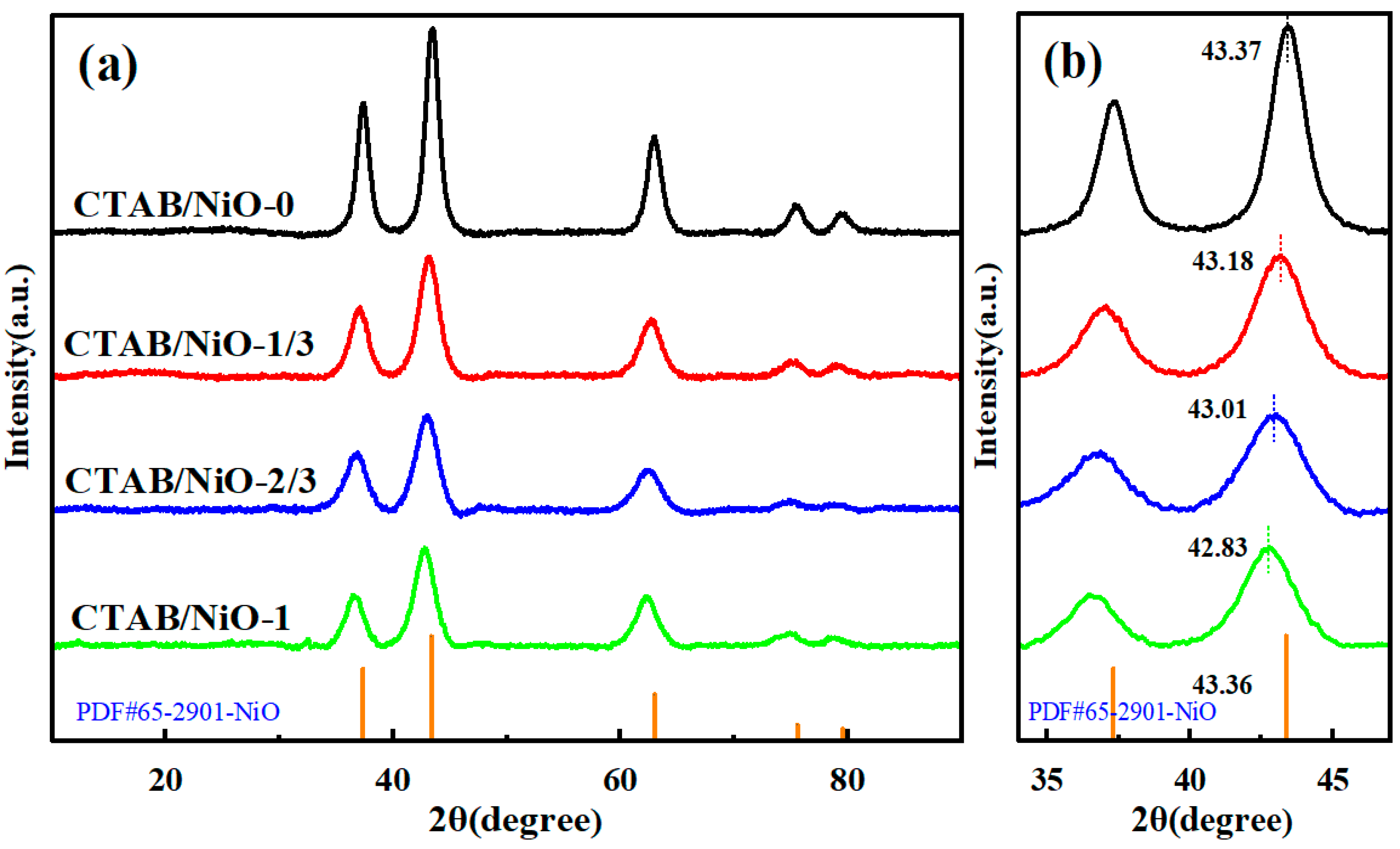
3.1. Structure and Morphology
3.2. Surface Area and Pore Structure
3.3. Redox Properties
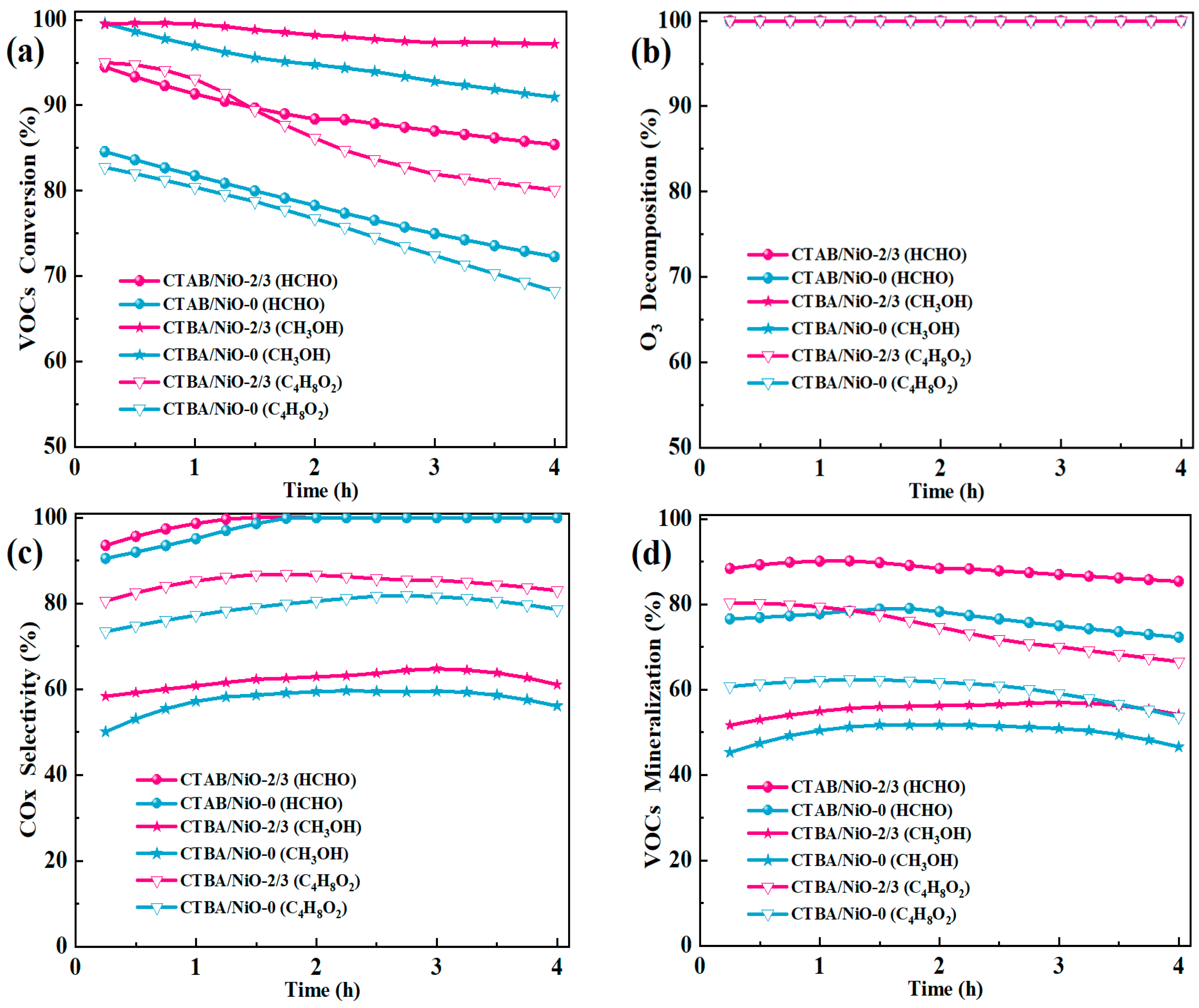
3.4. Catalytic Performance
3.5. Reaction Path
3.6. Catalytic Ozonation of Other Typical VOCs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, R.J.; Zhang, Y.L.; Bozzetti, C.; Ho, K.F.; Cao, J.J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High Secondary Aerosol Contribution toParticulate Pollution During Haze Events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef]
- Suzuki, N.; Nakaoka, H.; Nakayama, Y.; Tsumura, K.; Takaguchi, K.; Takaya, K.; Eguchi, A.; Hanazato, M.; Todaka, E.; Mori, C. Association Between Sum of Volatile Organic Compounds and Occurrence of Building-Related Symptoms in Humans: A Study in Real Full-scale Laboratory Houses. Sci. Total Environ. 2021, 750, 141635. [Google Scholar] [CrossRef]
- Peel, A.M.; Wilkinson, M.; Sinha, A.; Loke, Y.K.; Fowler, S.J.; Wilson, A.M. Volatile Organic Compounds Associated with Diagnosis and Disease Characteristics in Asthma-A Systematic Review. Res. Med. 2020, 169, 105984. [Google Scholar] [CrossRef]
- Mesquita, A.S.; Zamora-Obando, H.R.; Santos, F.N.; Schmidt-Filho, J. Volatile organic compounds analysis optimization and biomarker discovery in urine of Non-Hodgkin lymphoma patients before and during chemotherapy. Microchem. J. 2020, 159, 105479. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Li, C.L. A Review and Perspective of Recent Research in Biological Treatment Applied in Removal of Chlorinated Volatile Organic Compounds from Waste Air. Chemosphere 2020, 250, 126338. [Google Scholar] [CrossRef] [PubMed]
- Blanch, A.; Bianchi, A.C.; Leach, J. Volatile organic compounds in an urban airborne environment adjacent to a municipal incinerator, waste collection centre and sewage treatment plant. Atmos. Environ. 1999, 23, 4309–4325. [Google Scholar]
- Dimosthenis, A.; Sarigiannis, S.P.; Karakitsios, A.G. Exposure to Major Volatile Organic Compounds and Carbonyls in European Indoor Environments and Associated Health Risk. Environ. Int. 2011, 37, 743–765. [Google Scholar]
- Lu, Y.Q.; Deng, H.; Pan, T.T.; Zhang, C.B.; He, H. Thermal Annealing Induced Surface Oxygen Vacancy Clusters in α-MnO2 Nanowires for Catalytic Ozonation of VOCs at Ambient Temperature. ACS Appl. Mater. Interfaces 2023, 15, 9362–9372. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.P.; Ma, Z.R.; Ma, J.; Wang, H.Y.; Ren, K.; Wu, X.D.; Wang, B.D. In Situ DRIFTS Study of Single-Atom, 2D, and 3D Pt on γ-Al2O3 Nanoflakes and Nanowires for C2H4 Oxidation. Processes 2022, 10, 1773. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Jiang, Z.; Shangguan, W.F. Low-temperature Catalysis for VOCs Removal in Technology and Application: A state-of-the-art Review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Mo, S.P.; Li, Y.X.; Zhang, Y.C.; Wu, J.L.; Fu, M.L.; Niu, X.J.; Hu, Y.; Ye, D.Q. Pt/MnOx for Toluene Mineralization via Ozonation Catalysis at Low Temperature: SMSI Optimization of Surface Oxygen Species. Chemosphere 2022, 286, 131754. [Google Scholar] [CrossRef]
- An, C.G.; Jiang, X.X.; Hong, W.; Zhu, T.L.; Sun, Y.; Li, X.; Shen, F.X. Synergistic Promotion Effects of Surface Hydroxyl Groups (-OH) and Nitrate Groups (-NO3) on Catalytic Ozonation of Toluene over MnFe Catalyst. Appl. Catal. A-Gen. 2023, 654, 119078. [Google Scholar] [CrossRef]
- Tian, S.H.; Zhan, S.J.; Lou, Z.C.; Zhu, J.Z.; Feng, J.X.; Xiong, Y. Electrodeposition Synthesis of 3D-NiO1-δ Flowers Grown on Ni Foam Monolithic Catalysts for Efficient Catalytic Ozonation of VOCs. J. Catal. 2021, 398, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, K.Y.; Zheng, J.; Zuo, S.F. Studies of Sulfur Poisoning Process via Ammonium Sulfate on MnO2/γ-Al2O3 Catalyst for Catalytic Combustion of Toluene. Appl. Catal. B-Environ. 2021, 298, 120595. [Google Scholar] [CrossRef]
- Zhang, W.X.; Xue, M.; Fan, J.; Qiu, L.L.; Zheng, W.X.; Liu, Y.Y.; Meng, Z.H. Flory-Huggins VOC Photonics Sensor Made of Cellulose Derivatives. ACS Appl. Mater. Interfaces 2022, 14, 10701–10711. [Google Scholar] [CrossRef]
- Lou, B.Z.; Shakoor, N.; Adeel, M.; Zhang, P.; Huang, L.L.; Zhao, Y.W.; Zhao, W.C.; Jiang, Y.Q.; Rui, Y.K. Catalytic Oxidation of Volatile Organic Compounds by Non-noble Metal Catalyst: Current Advancement and Future Prospectives. J. Clean. Prod. 2022, 363, 132523. [Google Scholar] [CrossRef]
- Ádám, A.A.; Ziegenheim, S.; Papp, Á.; Szabados, M.; Kónya, Z.; Kukovecz, Á.; Varga, G. Nickel Nanoparticles for Liquid Phase Toluene Oxidation-Phenomenon, Opportunities and Challenges. ChemCatChem 2022, 14, e20220070. [Google Scholar] [CrossRef]
- Peng, J.L.; Lai, L.D.; Jiang, X.; Jiang, W.J.; Lai, B. Catalytic Ozonation of Succinic acid in Aqueous Solution using the Catalyst of Ni/Al2O3 Prepared by Electroless Plating-calcination Method. Sep. Purif. Technol. 2018, 195, 138–148. [Google Scholar] [CrossRef]
- Zhan, S.J.; Hu, X.N.; Lou, Z.C.; Zhu, J.Z.; Xiong, Y.; Tian, S.H. In-situ Growth of Defect-enriched NiO Film on nickel Foam (NF@NiO) Monolithic Catalysts for Ozonation of Gaseous Toluene. J. Alloy. Compd. 2022, 893, 162160. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Valenzuela, M.A.; Poznyak, T.; Lartundo, L.; Chairez, I. Reactivity of NiO for 2,4-D Degradation with Ozone: XPS Studies. J. Hazard. Mater. 2013, 262, 472–481. [Google Scholar] [CrossRef]
- Stoyanova, M.; Konova, P.; Nikolov, P.; Naydenov, A.; Christoskova, S.; Mehandjiev, D. Alumina-supported Nickel Oxide for Ozone Decomposition and Catalytic Ozonation of CO and VOCs. Chem. Eng. J. 2006, 122, 41–46. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, J.; Kaur, G. CTAB Assisted Co-precipitation Synthesis of NiO Nanoparticles and their Efficient Potential towards the Removal of Industrial Dyes. Micro Nano Lett. 2019, 14, 856–859. [Google Scholar] [CrossRef]
- Lu, W.T.; Zhang, G.; Wei, F.; Li, W.H.; Cheng, K.; Ding, F.; Zhang, J.Y.; Zheng, W.Q. Shape-Controlled Synthesis of Pd Nanocrystals in Aqueous Solutions. Adv. Funct. Mater. 2009, 19, 2–3. [Google Scholar]
- Tong, J.X.; Wang, J.; Huang, H.X. Surfactant-Assisted Synthesis of LiNi0.8Co0.1Mn0.1O2 Cathode Material. Chin. J. Inorg. Chem. 2021, 37, 835–843. [Google Scholar]
- Tawfik, S.M.; Negm, N.A.; Bekheit, M.; El-Rahman, N.R.A.; Abd-Elaal, A.A. Synergistic Interaction in Cationic Antipyrine/CTAB Mixed Systems at Different Phases. J. Disper. Sci. Technol. 2021, 35, 835–843. [Google Scholar] [CrossRef]
- Li, X.T.; Ma, J.Z.; Yang, L.; He, G.Z.; Zhang, C.B.; Zhang, R.D.; He, H. Oxygen Vacancies Induced by Transition Metal Doping in γ-MnO2 for Highly Efficient Ozone Decomposition. Environ. Sci. Technol. 2018, 52, 12685–12696. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Wang, J.; Xia, C.J.; Yan, X.A.; Cheng, P.F.; Liu, H.C.; Wang, C.L.; Duan, B.R. Preparation and Photocatalytic Characterization of Flower-like ZnO via CTAB Assisted Hydrothermal Synthesis. Basic Sci. J. Text. Univ. 2017, 30, 541–546. [Google Scholar]
- Yang, X.Q.; Yu, X.L.; Jing, M.Z.; Song, W.Y.; Liu, J.; Ge, M.F. Defective MnxZr1-xO2 Solid Solution for the Catalytic Oxidation of Toluene: Insights into the Oxygen Vacancy Contribution. ACS Appl. Mater. Interfaces 2018, 11, 730–739. [Google Scholar] [CrossRef]
- Mo, S.P.; Zhang, Q.; Li, J.Q.; Sun, Y.H.; Ren, Q.M.; Zou, S.B.; Zhang, Q.; Lu, J.H.; Fu, M.L.; Mo, D.Q.; et al. Highly Efficient Mesoporous MnO2 Catalysts for the Total Toluene Oxidation: Oxygen-Vacancy Defect Engineering and Involved Intermediates using in situ DRIFTS. Appl. Catal. B-Environ. 2020, 264, 118464. [Google Scholar] [CrossRef]
- Zhao, S.; Hu, F.Y.; Li, J.H. Hierarchical Core-Shell Al2O3@Pd-CoAlO Microspheres for Low-Temperature Toluene Combustion. ACS Catal. 2016, 6, 3433–3441. [Google Scholar] [CrossRef]
- Liu, X.L.; Zeng, J.L.; Shi, W.B.; Wang, J.; Zhu, T.Y.; Chen, Y.F. Catalytic Oxidation of Benzene over Ruthenium-cobalt Bimetallic Catalysts and Study of its Mechanism. Catal. Sci. Technol. 2017, 7, 213–221. [Google Scholar] [CrossRef]
- Zhong, J.P.; Zeng, Y.K.; Chen, D.D.; Mo, S.P.; Zhang, M.Y.; Fu, M.L.; Wu, J.L.; Su, Z.X.; Chen, P.R.; Ye, D.Q. Toluene Oxidation over Co3+-rich Spinel Co3O4: Evaluation of Chemical and by-product Species Identified by in situ DRIFTS Combined with PTR-TOF-MS. J. Hazard. Mater. 2020, 386, 121957. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Na, H.B.; Zeng, X.L.; Zhu, T.L.; Liu, Z.M. In situ DRIFTS Investigation for the Oxidation of Toluene by Ozone over Mn/HZSM-5, Ag/HZSM-5 and Mn-Ag/HZSM-5 Catalysts. Appl. Surf. Sci. 2014, 311, 690–696. [Google Scholar] [CrossRef]
- Chen, X.; Cai, S.C.; Chen, J.; Xu, W.J.; Jia, H.P.; Chen, J. Catalytic Combustion of Toluene over Mesoporous Cr2O3-supported Platinum Catalysts Prepared by in situ Pyrolysis of MOFs. Chem. Eng. J. 2018, 334, 768–779. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Z.G.; Chen, S.; Quan, X. The Role of Lattice Oxygen on the Activity and Selectivity of the OMS-2 Catalyst for the Total Oxidation of Toluene. Chem. Eng. J. 2015, 270, 58–65. [Google Scholar] [CrossRef]
- Shao, Q.; Wei, S.S.; Hu, X.Y.; Dong, H.; Wen, T.C.; Gao, L.; Long, C. Tuning the Micro-coordination Environment of Al in Dealumination Y Zeolite to Enhance Electron Transfer at the Cu-Mn Oxides Interface for Highly Efficient Catalytic Ozonation of Toluene at Low Temperatures. Environ. Sci. Technol. 2022, 56, 15449–15459. [Google Scholar] [CrossRef]
- Tang, H.R.; He, Y.; Lin, F.W.; Zhu, Y.Q.; Duan, Y.X.; Wang, Z.H. Simultaneous Catalytic Ozonation of NO and Dichloromethane on Mn/H-ZSM-5 Catalysts: Interaction effect and mechanism. Proc. Combust. Inst. 2022, 39, 4387–4397. [Google Scholar] [CrossRef]
- Zhang, T.; Lang, X.Y.; Dong, A.Q.; Wan, X.; Gao, S.; Wang, L.; Wang, L.X.; Wang, W.C. Difference of Oxidation Mechanism between Light C3-C4 Alkaneand Alkene over Mullite YMn2O5 Oxides’ Catalyst. ACS Catal. 2020, 10, 7269–7282. [Google Scholar] [CrossRef]
- Wang, L.C.; Huang, Z.W.; Jiang, Z.; Jiang, Z.; Zhang, Y.; Zhang, Z.X.; Shangguan, W.F. Trifunctional C@MnO Catalyst for Enhanced Stable Simultaneously Catalytic Removal of Formaldehyde and Ozone. ACS Catal. 2018, 8, 3164–3318. [Google Scholar] [CrossRef]
- Tian, M.Z.; Liu, S.J.; Wang, L.L.; Ding, H.; Zhao, D.; Wang, Y.Q.; Cui, J.H.; Fu, J.F.; Shang, J.; Li, G.K. Complete Degradation of Gaseous Methanol over Pt/FeOx Catalysts by Normal Temperature Catalytic Ozonation. Environ. Sci. Technol. 2020, 54, 1938–1945. [Google Scholar] [CrossRef]
- Li, J.; Mo, S.P.; Ding, X.G.; Huang, L.L.; Zhou, X.B.; Fan, Y.M.; Zhang, Y.N.; Fu, M.M.; Xie, Q.L.; Ye, D.Q. Hollow Cavity Engineering of MOFs-derived Hierarchical MnOx Structure for Highly Efficient Photothermal Degradation of Ethyl Acetate under Light Irradiation. Chem. Eng. J. 2023, 464, 142412. [Google Scholar] [CrossRef]
- Shen, Z.D.; Gao, E.G.; Meng, X.Y.; Xu, J.C.; Sun, Y.; Zhu, J.L.; Li, J.; Wu, Z.L.; Wang, W.; Yao, S.L.; et al. Mechanistic Insight into Catalytic Combustion of Ethyl Acetate on Modified CeO2 Nanobelts: Hydrolysis-Oxidation Process and Shielding Effect of Acetates/Alcoholates. Environ. Sci. Technol. 2023, 57, 3864–3874. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.T.; Miao, G.; Pi, Y.H.; Xia, Q.B.; Wu, J.L.; Li, Z.; Xiao, J. Abatement of Various Types of VOCs by Adsorption/catalytic Oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]





| Catalyst | SBET (m2/g) | Pore Size (nm) | Pore Volume (cm³/g) |
|---|---|---|---|
| CTAB/NiO-0 | 141.16 | 5.14 | 0.1542 |
| CTAB/NiO-1/3 | 163.18 | 4.85 | 0.2317 |
| CTAB/NiO-2/3 | 202.33 | 4.22 | 0.2606 |
| CTAB/NiO-1 | 172.18 | 4.21 | 0.2494 |
| Position (cm−1) | Assignment | Characteristic of |
|---|---|---|
| 3600~3000 | O-H stretching vibration | metal-OH |
| 1741 | C=O symmetrical stretching vibration | quinone |
| 1450~1650 | C=C stretching vibrations | aromatic ring |
| 1540 | C=O stretching vibrations | benzoate |
| 1382 | COO- symmetrical stretching oscillation | carboxylic acid |
| 1315 | C-C-C (O) in-plane bending vibration | maleic anhydride |
| 1176 | C-O symmetric stretching vibration | benzaldehyde |
| 1050 | C-O symmetric stretching vibration | alcohols |
| 976 | C-H torsional vibration | olefin |
| 891 | C-H stretching vibration | alkyne |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, C.; Jiang, X.; Hong, W.; Sun, Y.; Zhu, T. To Promote the Catalytic Ozonation of Typical VOCs by Modifying NiO with Cetyltrimethylammonium Bromide. Processes 2023, 11, 1893. https://doi.org/10.3390/pr11071893
An C, Jiang X, Hong W, Sun Y, Zhu T. To Promote the Catalytic Ozonation of Typical VOCs by Modifying NiO with Cetyltrimethylammonium Bromide. Processes. 2023; 11(7):1893. https://doi.org/10.3390/pr11071893
Chicago/Turabian StyleAn, Chenguang, Xinxin Jiang, Wei Hong, Ye Sun, and Tianle Zhu. 2023. "To Promote the Catalytic Ozonation of Typical VOCs by Modifying NiO with Cetyltrimethylammonium Bromide" Processes 11, no. 7: 1893. https://doi.org/10.3390/pr11071893
APA StyleAn, C., Jiang, X., Hong, W., Sun, Y., & Zhu, T. (2023). To Promote the Catalytic Ozonation of Typical VOCs by Modifying NiO with Cetyltrimethylammonium Bromide. Processes, 11(7), 1893. https://doi.org/10.3390/pr11071893





