Abstract
Nitrate is an important component of PM2.5, and its dry deposition and wet deposition can have an impact on ecosystems. Nitrate in the atmosphere is mainly transformed by nitrogen oxides (NOX = NO + NO2) through a number of photochemical processes. For effective management of the atmosphere’s environment, it is crucial to understand the sources of atmospheric NOX and the processes that produce atmospheric nitrate. The stable isotope method is an effective analytical method for exploring the sources of NO3− in the atmosphere. This study discusses the range and causes of δ15N data from various sources of NOX emissions, provides the concepts of stable isotope techniques applied to NOX traceability, and introduces the use of Bayesian mixture models for the investigation of NOX sources. The combined application of δ15N and δ18O to determine the pathways of nitrate formation is summarized, and the contribution of Δ17O to the atmospheric nitrate formation pathway and the progress of combining Δ17O simulations to reveal the atmospheric oxidation characteristics of different regions are discussed, respectively. This paper highlights the application results and development trend of stable isotope techniques in nitrate traceability, discusses the advantages and disadvantages of stable isotope techniques in atmospheric NOX traceability, and looks forward to its future application in atmospheric nitrate pollution. The research results could provide data support for regional air pollution control measures.
1. Introduction
Human health and daily life have been negatively impacted by PM2.5 [1,2,3]. In view of air pollution, China has taken many measures, and the anthropogenic emission of pollutants has been reduced [4,5,6]. However, the amount and duration of haze have grown [7]. The composition of atmospheric particulate matter has also changed with the management of the environment. In recent years, the proportion of nitrate in PM2.5 has increased significantly, surpassing sulfate and becoming the most abundant component [8]. Winter haze is primarily caused by nitrate, as evidenced by the presence of up to 60% NO3− in severe winter haze with PM2.5 concentrations above 100 µg m−3 [9].
Numerous academic studies have been conducted on NOX measurement and conversion pathways in order to better understand the issue of NOX pollution. Nitric acid, a key component of the nitrogen biogeochemical cycle, is formed when atmospheric NOX (NOX = NO + NO2) experiences a variety of oxidation reactions and is then deposited to the surface by dry and wet deposition [10]. This process modifies the atmosphere’s acid–base balance, favoring the development of acid rain and reducing biodiversity. The nitrates created either adsorb on the alkaline particulate matter to form nitrate or interact with NH3 in the air to create particulate matter.
1.1. Formation Pathways of Nitrate in Atmospheric Particulate Matter
The gas-phase interaction of NO2 and OH and the non-homogeneous reaction of N2O5 hydrolysis are the two main pathways identified for the formation of nitrate from ambient NOX, as shown in Figure 1 [11,12].
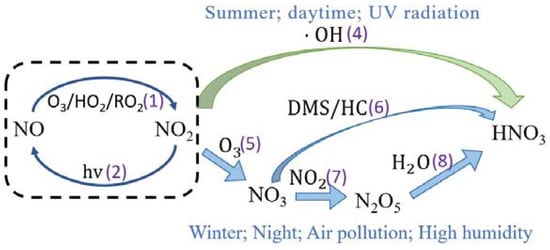

Figure 1.
Two reaction pathways for the formation of nitrate from NOX in the air [10,11]. In this figure, (1) to (6) correspond to the corresponding reactions (1) to (6) below.
During the day, NO reacts rapidly with O3 to produce NO2, as shown in Reaction (1), NO2 is photolyzed to produce NO and O atoms as in Reaction (2), and the generated O atoms combine with O2 to produce ozone. Reaction (3) is the most frequent route for tropospheric O3 generation.
In Reactions (1)(2), the cyclic reaction between NO and NO2 reaches a steady state in a short time [13,14], and the concentration of O3 and the photolysis rate of NO2 affects the reaction rate in Reaction (1) and (2); the relative concentrations of NO and NO2 in the atmosphere are affected. NO2 in the atmosphere also oxidizes to form nitric acid. The formation of nitric acid can be divided into two types: daytime and nighttime. During the day, the reaction in Reaction (4) occurs, and NO2 is oxidized by OH to form nitric acid:
At night, the concentration of OH is extremely low and NO2 cannot be oxidized directly to nitric acid. NO2 is oxidized by ozone to NO3 (Reaction (5)), which then reacts with dimethyl sulfide (DMS) or hydrocarbons (HC) to produce nitric acid (Reaction (6)), or NO3 reacts with NO2 to make N2O5 (Reaction (7)), which then hydrolyzes to produce nitric acid (Reaction (8)).
Particulate nitrate is formed when nitric acid combines with alkaline chemicals in the environment, as shown in Reaction (9).
O3 and OH are the major oxidants in the atmosphere, which are closely related to pollutants, such as NOX, SO2, CO, CH4, and volatile organic compounds (VOCs), in the atmosphere. Studies have indicated that NO2 + OH and N2O5 + H2O are the two most important pathways of tropospheric nitrate generation below 1 km, contributing 41~42% and 28~41% to nitrate generation at the global scale, respectively [15]. As a result of other mechanisms, 6% of nitrate synthesis occurs close to the surface, though they may also take over in some areas [15,16]. In Beijing, the OH route contributed to NO3 production by about 45.3%, N2O5 hydrolysis contributed by about 46.5%, and the NO3 + HC pathway contributed by about 8.2‰ [17]. Additionally, in Nanchang, the OH route, N2O5 hydrolysis, and NO3 + HC pathway contributed 37.1‰, 33.4‰, 60.3‰, and 2.6‰ of the NO3−, respectively [18].
1.2. Major Sources of NOX and Available Analytical Methods
The majority of NOX’s sources come from anthropogenic and natural sources. Natural sources typically refer to biogenic soil emission, lightning production, and stratospheric transfer. The primary anthropogenic sources include biomass burning, fossil fuel burning, vehicle emissions, and other sources. NOX emissions in the troposphere have been determined to be mostly caused by human activity, with the burning of fossil fuels responsible for up to 60% of all NOX emissions globally. Therefore, quantifying the contribution of different emission sources to NOX emissions is conducive to a better understanding of the atmospheric nitrogen cycle so as to develop effective emission reduction strategies. The reduction of the number of high-polluting sources and the reduction of emissions from low-polluting sources are the two main focuses of the treatment of NOX sources in the atmosphere. How to determine the source of NOX in a certain area at a certain time has always been the goal of researchers.
Current work by researchers has focused on four sources of emissions: coal combustion, vehicle emission, biomass burning combustion, and biogenic soil emissions. The main methods for monitoring NOX in the atmosphere are emission inventory methods, satellite observations, and stable isotope methods. The assessment of emission flux can be achieved with the emission inventory method [19] and the satellite observation method. The emission inventory method has uncertainties in the types of emission sources and emission factors, and even the emission factors of the same emission source under different conditions are quite different [20]. It has been demonstrated that tropospheric NOX concentrations can be monitored using satellite remote sensing techniques [21]. However, the satellite observation method cannot properly determine the precise sources of NOX because there are frequently many sources of NOX emissions in the same area [22]. Both methods can provide a large amount of data and achieve very effective results, but neither method can accurately assess the contributions from different sources.
Stable isotope techniques have the advantages of high measurement accuracy, accurate source resolution, and small measurement error, and their application in various fields has promoted the progress of scientific research. They have steadily developed into a key instrument for researching the source tracing of NOX emissions in the atmosphere and the mechanism of nitrate creation in the atmosphere, along with the continual advancement of isotope technology, detecting equipment, and analytical methodologies [23,24,25].
1.3. Analysis of Stable Isotope Techniques and Its NOX Traceability
The abundance of stable isotopes in a species depends not only on its natural abundance but also on the processes that occurred during the generation of the species. Isotope fractionation is the process whereby an element’s isotopes are dispersed among several compounds during a reaction in varying ratios, resulting in slight weight variations amongst the same species. Thus, isotope fractionation can be used to trace information on the origin of species, the processes that produced them, etc. δ is used to indicate isotopic composition values, and the calculation is shown in Equation (10):
where R represents the proportion of heavy to light isotopes. The international standard for nitrogen is atmospheric N2, and for oxygen is the Vienna Standard Mean Ocean Water (VSMOW).
Different metabolic processes and migratory transformations of atmospheric nitrogen molecules result in nitrogen isotope fractionation, which changes the values of 15N. The value of the δ is positive when the sample is rich in heavy isotopes and negative when the sample is deficient in heavy isotopes.
Combining stable nitrogen isotopes with isotope mixing models has become one of the most reliable tools for quantifying NOX sources. Since nitrogen isotope fractionation rarely occurs in the process of NOX conversion to nitrate, δ15N from different sources is different. According to this concept, we can use δ15N-NO3− to determine the source of nitrate [26,27]. For example, changes in δ15N-NO3− in ice cores by Hastings et al. can clearly indicate a shift in the source of NOx from pre-industrial to modern times. [27]. By measuring δ15N-NO3− and Δ17O-NO3− in shallow ice cores drilled in Antarctica, Cao et al. studied the ability of nitrate isotopes in ice cores to reflect the variation of the ozone column [28]. Zeng et al. determined the proportion of nitrate sources in rainwater in Beijing by studying the δ values of dual isotopes combined with a Bayesian mixing model [29].
Two methods are commonly used to determine the NO3− nitrogen to oxygen isotope ratio using the stable isotope method: the bacterial denitrifier method and the chemical reduction method. Both methods measure N2O gas with less interference, require less sample volume, and allow the simultaneous determination of δ15N and δ18O.
The bacterial denitrifier method is based on the quantitative production of nitrous oxide gas (N2O) from nitrate by denitrifying bacteria. The classical denitrification pathway involves the gradual reduction of nitrate (NO3−) to nitrite (NO2−), nitric oxide (NO), nitrous oxide (N2O), and dinitrogen (N2). In this method, NO3− in the sample is converted to nitrous oxide (N2O) using denitrifying bacteria (Pseudomonas aureofaciens) lacking N2O reductase activity and then fed into a stable isotope ratio mass spectrometer for analysis [30]. The nitrator method for bacterial denitrification was updated by McIlvin et al. to improve the precision and yield of isotope analysis, and the updated analytical system is shown in Figure 2 and includes a GC-PAL autosampler (CTC Analytics, LEAP Technologies), a custom capture system for the separation and purification of N2O, and an isotope ratio mass spectrometer (DeltaPLUS XP, Finnigan, Germany) [31].
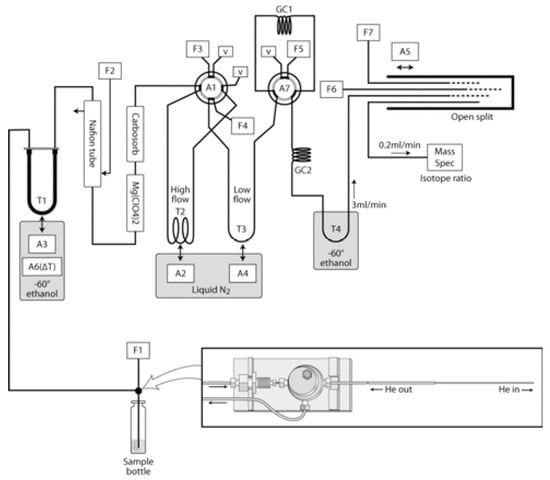
Figure 2.
Purge and trap system: T = trap, A = automation controlled by Isodat, F = flow controller, GC = gas chromatogram, V = vent to atmosphere. Inset: custom concentric needle design using the CombiPAL 1 mL syringe holder. Gas flow paths are indicated by arrows. Please refer to text for additional details. Reprinted with permission from Ref. [31]. Copyright 2011 ACS.
The chemical reduction method consists of a two-step reduction for the extracted samples, first to NO3− to NO2− using Cd and then to NO2− to N2O using an azide-acetate-buffered solution [32,33]. Finally, the reaction is terminated using sodium hydroxide solution (NaOH). With the Precon + Gas Bench II system, the upper layer of N2O gas in the headspace bottle was blown into MAT253 by an automatic sampler to measure δ15N and δ18O, and the sample measurement process is shown in Figure 3. Zhao et al. optimized the scheme as follows: NO3− is reduced to NO2− by HCl-Cd at pH = 8 under the condition of Cl− concentration over 5 M, NO2− is reduced to N2O at pH = 4.5–4.6 in a sodium azide acetate buffer solution, and δ15N and δ18O are analyzed by Stable isotope ratio mass spectrometer (MAT253) [34].
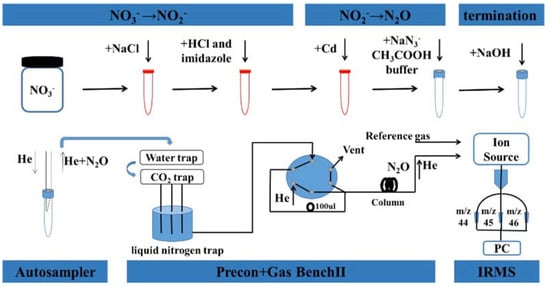
Figure 3.
Schematic diagram of nitrate nitrogen and oxygen isotope analysis in atmospheric samples. Reprinted with permission from Ref. [34]. Copyright 2019 Elsevier.
An effective method for examining potential NO3− sources in the atmosphere is the stable isotope. Here, we list the δ15N values for several emission sources and discuss how to locate the source of NOX emissions using the Bayesian isotope mixing model. The paths of nitrate synthesis were then determined using two techniques that combined δ15N and δ18O and Δ17O. Finally, shortcomings of the currently employed techniques are discussed, and recommendations for future isotopic studies of the origins and oxidation processes of atmospheric nitrate are made.
2. Stable Isotope Techniques Determine the δ15N Values of NOX in Various Emission Sources
Nitrogen isotopes’ accuracy in resolving the source of atmospheric NOX is dependent on two major aspects. The first relates to the impact of isotope fractionation on the chemical oxidation, transport, and atmospheric N deposition processes, while the second deals with the properties of δ15N-NOX from various emission sources. δ15N-NOX is calculated as the following calculation formula in Equation (11), using the calculation of Equation (10):
Figure 4 summarized the δ15N characteristic values of NOX emissions from different sources in the available literature, using the δ15N-NOX values as the X-axis and the different NOX sources as the Y-axis. The δ15N values for the different emission sources have been summarized in the figure so far, and it can be seen that the δ15N for NOX emissions from petrol vehicles ranges from −19.4‰ to 17‰ and from −23.3‰ to 8.5‰ for diesel vehicles. The characteristic δ15N values for the coal combustion process are −5.3‰ to 25.6‰, for natural gas 2.9‰ to 15.4‰, for biomass combustion −11.9‰ to 5‰, and for the flash process less data is obtained, −0.5‰ to 1.4‰. The data showed that the δ15N values of NOX emitted from all sources overlapped with each other in the range, except for the δ15N values of NOX emitted from soil, which were in the more negative range. The δ15N values of NOX by the same emission source vary considerably due to different N chemical conversion processes. The following section describes the four aspects of vehicle emission, coal and natural gas combustion, biomass burning combustion, and biogenic soil emissions, respectively.
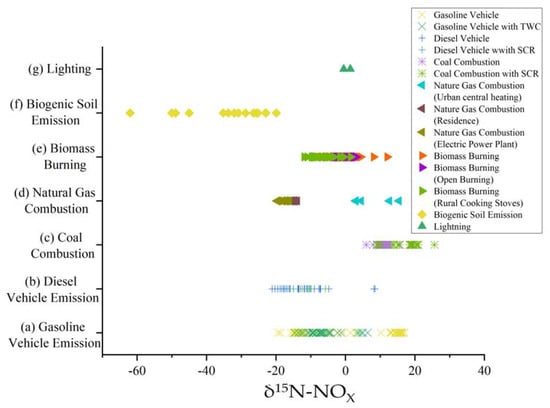
Figure 4.
δ15N values for NOX emissions from different sources. The Y-axis indicates different sources of NOX emissions and the X-axis indicates the δ15N-NOX value, with one point representing a measured value; different colors indicate different NOX generation processes for this source. (a) Walters et al. (2015) [27], Heaton (1990) [35], Moore (1977) [36], Walters et al. (2015) [37], Li et al. (2022) [38]; (b) Chang et al. (2018) [22], Heaton (1990) [35], Walters et al. (2015) [37], Li et al. (2022) [38], Su et al. (2020) [39], Felix et al. (2012) [40], Zong et al. (2020) [41], Widory (2007) [42]; (c) Heaton (1990) [35], Felix et al. (2012) [40], Widory (2007) [42]; (d) Walters et al. (2015) [27], Widory (2007) [42]; (e) Zong et al. (2022) [25], Fibiger (2016) [43], Shi (2021) [44]; (f) Fibiger (2016) [43], Li et al. (2008) [45], Felix et al. (2014) [46], Miller et al. (2018) [47], Su et al. (2020) [48]; (g) Hoering (1957) [49].
2.1. δ15N of Fossil Fuel Combustion
With a wide range of δ15N values, fossil fuels, particularly car exhaust and coal combustion exhaust, predominate the NOX emission inventory. Estimates at the global level show that soils and fossil fuels both contribute about the same amount of NOX. Additionally, the primary cause of nitrate pollution in cities is NOX emissions from the combustion of fossil fuels [50]. Different structures of combustion devices will lead to different proportions of NOX emitted from fossil fuel combustion.
There are two primary sources of NOX that result from the combustion of fossil fuels: (i) NOX generated from NOX inside the fuel (fuel NOX) and (ii) NOX generated from atmospheric N2 oxidation. Different combustion unit configurations can result in different proportions of NOX sources emitted from fossil fuel combustion (thermal cracking NOX). For example, NOX emissions from coal-fired power plants mainly originate from fuel NOX because their combustion units are at temperatures of 1277~1402 °C, which are too low to oxidize atmospheric N2, while car engines are at temperatures >1727 °C and can oxidize atmospheric N2, so NOX emissions from car exhaust are mainly thermal cracking NOX [35].
Although the mechanism of NOX generation is similar for different automotive engines, which originate from the conversion of atmospheric N2, the δ15N-NOX values are also influenced by fuel type, operating conditions, engine type, air-fuel ratio, etc. [51]. In 1977, Moore reported that vehicles equipped with three-way catalytic converters (TWC) emitted NOX with some δ15N values of 3.4‰ to 3.9‰ [36]. Later, Walters et al. found by comparison that the NOX concentrations emitted from vehicles equipped with TWC units had a good non-correlation with the δ15N values because the TWC reacted preferentially with 14NOX, giving the remaining emitted NOX a higher δ15N value [27].
Heaton reported δ15N values for the emitted exhaust of cars under load and no load, ranging from −11‰ to −2‰ and −13‰ to −7‰, respectively [35]. Walters et al. measured δ15N-NOX values for the exhaust of cars as −12.2‰ to −9.8‰ for gasoline cars and −23.3‰ to −2‰ for diesel cars, respectively [27]. Zong et al. also found the same trend but with different values; they measured the highest δ15N-NOX values for LPG vehicles (−0.1 ± 1.8‰), followed by gasoline vehicles (−7.0 ± 4.8‰) and diesel vehicles (−12.7 ± 3.4‰). In addition, they found a significant correlation between δ15N-NOX values and NOX concentrations in vehicle exhausts (p < 0.01). Compared with gasoline engines, the combustion gases of diesel engines mix with air more rapidly, so that only a small fraction of NOX is decomposed in diesel engines compared with gasoline engines. Additionally, Zong et al. found that the δ15N values of NOX emitted from cars in different operating conditions differed, following the trend of warm start (−5.9 ± 5.0‰) > driving (−7.3 ± 5.9‰) > cold start (−9.2 ± 2.7‰) [24]. In summary, the δ15N characteristic values of NOX from vehicle exhaust emissions ranged from −23.3‰ to 17‰.
The δ15N value of NOX emitted from coal-fired power plants is generally positive because organic nitrogen in coal combustion decomposes into nitrogen-containing reactive substances after combustion to produce NO in the atmosphere, while NO continues to react with nitrogen (NO + N → N2 + O), and in this reaction process, 14NO is preferentially involved in the reaction, making the remaining NO more enriched 15N, resulting in a δ15N value of NOX emitted from coal-fired power plants positive values [35].
Felix et al. measured NOX emissions from coal-fired power plants installed with SCR installations [40]. The SCR device is a selective catalytic reduction device. Ammonia is injected into the smoke stream, and the gas reacts with NH3 through the catalyst in the presence of oxygen to form N2 and water vapor, which can reduce the emission of nitrogen oxides by 80–90‰ [52]. NOX emissions from power plants installed with SCR units have higher δ15N values, probably due to the kinetic fractionation that occurs during the selective catalysis process, where the catalytic unit prioritizes 14NOX, enriching 15NOX resulting in higher δ15N values in the emitted NOX [27,40]. Zong et al. measured the δ15N values of NOX emissions from residential coal combustion in China and found that δ15N-NOX from residential coal combustion was lower than industrial emissions at 17.9 ± 3.1‰ [25]. Widory measured a negative δ15N value of −5.3‰ for NOX emissions from coal combustion for heating purposes in Paris, which is somewhat different from the δ15N value for coal-fired power plants [42]. The distribution of soil NO emissions varies considerably with the region and is dominated by land use type and nitrogen fertilizer use.
Li et al. determined δ15N values of −48.9‰ to −19.9‰ for NO produced by soils under fertilizer application [45]. Later, Felix and Elliott measured values of −30.8‰ and −26.5‰ in the same range [46]. Miller et al. found a significant difference in δ15N-NOX values between injected (−32.2 ± 12.1‰) and untilled sown manure (−23.4 ± 2.1‰) [47]. Su et al. found that soil in aerobic conditions had δ15N values in NO emitted under aerobic and anaerobic conditions were different, ranging from −62‰ to −50‰ under aerobic conditions, much lower than −45‰ to −23‰ under anaerobic conditions, due to the predominance of nitrification and denitrification in soil under aerobic and anaerobic conditions, respectively [48]. The δ15N values for NOX emitted from soil sources ranged from −62‰ to −19.9‰, and the low δ15N values for NOX emitted from the soil are due to the fact that NOX emitted from the soil is an intermediate product of microbial nitrification and denitrification reactions, both of which preferentially utilize 14N.
Previous researchers have focused mainly on NOX emissions from fossil fuel combustion, ignoring the contribution of soil sources to atmospheric NOX. Subsequent studies will continue to focus on NOX emissions from soils.
2.2. δ15N of Other Sources
Ikegami et al. analyzed the composition of aerosol particles in haze weather and found that the haze particles were most likely caused by the growth of particles emitted during biomass combustion through heterogeneous reactions [53]. Furthermore, the analysis of the chemical composition of the particulate matter revealed that the oxidation coefficient of the nitrogen oxide in the atmosphere increased significantly during the combustion phase, which shows that biomass combustion has a significant contribution to NOX in the atmosphere [54].
Fibiger et al. measured δ15N values for NOX emissions from the combustion of forest samples ranging from −7‰ to 5‰ [43]. Shi et al. measured δ15N values for NOX emissions from biomass combustion in China and compared δ15N-NOX from open burning and rural cookstoves with −3.7‰ to 3.1‰ and −11.9‰ to 1.5‰, respectively [44]. The measured δ15N values ranged from 0.1‰ to 4.1‰ for nine biomass fuel sources, and this variation was influenced by the biomass fuel source [44]. Zong et al. measured δ15N-NOX from biomass combustion ranging from −5.6‰ to 3.2‰ (−0.4‰ ± 2.4‰), which showed a significant linear relationship with δ15N-biomass [25]. The interval of δ15N values for NOX emissions from biomass combustion was −11.9‰ to 5‰.
For lightning sources, it is more difficult to collect actual samples and measure them. δ15N values of −0.5‰ to 1.4‰ for NOX were collected by Hoering in a laboratory simulation of lightning [49].
In summary, there is a significant overlap in δ15N values from different sources, and even for the same source, differences in N conversion mechanisms, sampling methods, and regional effects can lead to differences in δ15N values; e.g., SCR devices during coal combustion, TWC devices during vehicle emissions, etc., can affect the N conversion mechanisms. In addition, inconsistencies in NOX collection methods in the above literature, such as different collection devices, different absorption solutions, and different collection objects, may lead to some differences in NOX collection efficiency, which should be avoided in later studies.
3. Bayesian Isotope Mixing Model
3.1. Principle of Bayesian Mixture Model
Mathematical models are frequently used to assess and discuss the origins of NOX in the collected air particulate matter for the δ15N data received after sampling and analysis. Phillips et al. created the IsoSoure model in order to assess the contribution of various emission sources using the mass balance concept. The model cannot identify the sources of NOX because it does not account for the fractionation effect in the NOX to the nitric acid synthesis process, which limits its applicability to the case when the sources are not more than three [55].
The Bayesian isotope mixing model (SIAR model) was later introduced for the traceability analysis of δ15N data. As an important branch of stable isotope mass balance models, the Bayesian isotope mixing model can use stable isotope signatures to determine the probability distribution of each source’s contribution to the mixture and explicitly account for the uncertainties associated with multiple sources, fractionation, and isotopic signatures, and has been widely used in recent years [56]. The SIAR model sums the total contribution to 100‰ based on the isotope mass balance law, which can quantitatively resolve the contribution of different sources to the mixture and thus assess the respective contributions of different sources in the mixture, overcoming to some extent the overlap in the δ15N range of different NOX sources [57]. The Bayesian isotope mixing model can be expressed as Equations (12) and (13):
Equation (12) is an equation based on the mass balance principle, Xij is the δ value of isotope j in mixture i (I = 1, 2, 3,…, N; j = 1, 2, 3,…, J); Pk is the proportion of source k to be calculated, Sjk is the δ value of isotope j in source k (k = 1, 2, 3,…, K); Cjk is the fractionation factor (mean λ, standard deviation τ) of isotope j in source k; and εij is the residual error. Equation (13) indicates that Sjk, Cjk, and εij all obey normal distribution.
There are still considerable ambiguities in SIAR models, despite the fact that they can predict emission sources accurately. Based on earlier comments, Fan et al. recommended the following changes to increase the SIAR model’s accuracy [16]: In order to reduce uncertainty caused by differing qualities between the input data, the environmental samples used as inputs first need to share the same natural properties. For instance, the sample data need to come from the same season. Second, all sources should be included in the SIAR model; otherwise, the proportions of other sources will be biased. However, developing and employing a conforming local composition of emission sources can also lessen the model’s uncertainty.
Additionally, the Bayesian isotope mixing model is being optimized. Zong et al. improved the Bayesian isotope mixing model by introducing isotopic fractionation of the equilibrium/Leighton reaction using the improved model to apportion the annual NOX sources in North China, and the improved process is shown in Figure 5 [57]. Later, a method was proposed to calculate the isotopic fractionation coefficients by combining oxygen isotopes and the Bayesian isotope mixing model, which can lead to the nitrogen isotopic fractionation coefficients for the conversion of atmospheric NOX(g) to HNO3(g), and thus to the resolution of the sources of atmospheric nitrate from different sources [25].
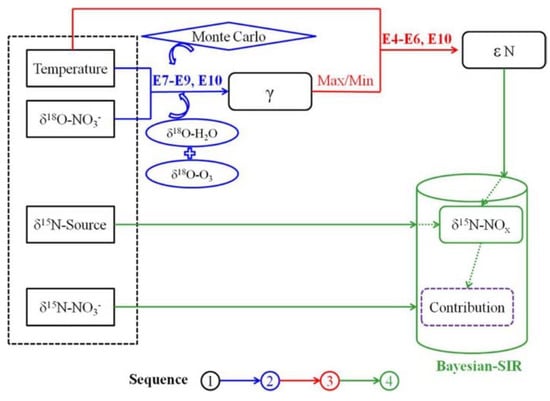
Figure 5.
Principle and process of the improved Bayesian model by Zong et al.; the “E” represents equation in the following section, “εN” refers to N fractionation, and “SIR” is “sampling-importance-resampling”. Reprinted with permission from Ref. [57]. Copyright 2017 ACS.
3.2. The Practical Application of Bayesian Mixture Model
The Bayesian isotope mixing model was used to assign sources for several different regions and the results obtained are shown in Table 1.

Table 1.
Sources of NOX in different regions.
The importance of coal combustion sources rises noticeably in the majority of northern Chinese towns during the winter [15,38,61]. This is because coal is frequently used for heating in the winter, and even if denitrification technology is utilized to reduce NOX emissions during coal combustion, the amount of NOX in the atmosphere is still increased. Fan et al. showed that the contribution of coal combustion to nitrate aerosols has decreased in recent years, possibly due to the replacement of coal combustion by natural gas as a crop heating fuel [15]. Jiaozuo contributes less to emissions from coal-fired power plants because its industry is less than that of significant cities like Beijing and Tianjin [38]. For Guangdong, there is no need to burn coal for heating, and the significant contribution of coal combustion may be attributed to power plants [39]. The contribution of coal-fired emissions to Ganzhou’s energy mix is minimal because clean energy sources like photovoltaic electricity, hydroelectric power, wind power, and biomass power make up a sizeable share of Ganzhou’s energy mix [63].
The total number of vehicles in all regions is rapidly increasing due to rapid economic development and rising living standards of the population [58,59], leading to a significant contribution of mobile sources to atmospheric NOX, especially in Ganzhou [63]. Whereas Tianjin, the largest industrial city in northern China, has more frequent diesel vehicle transport activities, the contribution of diesel vehicle emissions in winter is much lower, probably due to traffic regulation that significantly reduces the number of diesel vehicles during winter days when pollution is more frequent [59]. Additionally, the enhanced contribution of vehicle exhaust to particulate matter NO3− under severe pollution in Beijing was discovered by Fan et al. [15]. The contribution of vehicle emissions to NOX is higher in large cities. Ship emissions are an important source of atmospheric NOX in coastal areas such as Beihuangcheng Island [57]. In summary, vehicle emissions are relatively more important in urban environments, but coal combustion is more important in non-urban areas for NOX in PM2.5.
The large-scale burning of crop residues occurs in spring and autumn, especially in small cities, generally without the use of pollution control equipment, leading to a sharp increase in NOX emission values, like in Jiaozuo [38] and northeast China [61]. Agricultural waste, including wood and crop waste, is still utilized for cooking and heating in rural areas of northeast China [61]. In addition, 30‰ of the power and industrial fuels used in Guangdong come from biomass sources [39]. It is reported that NOX emissions from biomass burning in China increased more than sixfold from 1990 to 2013 [64].
Soil emissions of NOX are mainly generated by nitrification and denitrification reactions occurring during the cultivation of crops [46] and are influenced by temperature and humidity [15]. Emissions from soil sources are higher in agricultural land in northeast China [61], as well as in Jiaozhou [38] and Ganzhou [63]. More fertilizer is given to crops during the warm season, which leads to an increased contribution of soil to NOX [60]. It can be inferred that soil sources are more important for atmospheric NOX in non-urban areas. Growing urbanization and shrinking agricultural land area in Hangzhou have resulted in a low contribution to atmospheric NOX from soil sources [60].
At present, our means of combating air pollution is mainly to limit fossil fuel combustion, but it can be seen from Table 1 that non-fossil fuels (biomass burning emissions, biogenic soil emissions) should be equally important for atmospheric NOX emissions, which is consistent with the study of Song et al. [65].
4. Pathways of Nitrate Production
The oxidation process of NOX conversion to nitrate is an important reaction mechanism in atmospheric chemistry. Analyzing the formation pathway and relative contribution of nitrate in the atmosphere is of great significance to the study of atmospheric chemistry, such as the assessment of regional air quality and atmospheric oxidation capacity.
4.1. δ18O and δ15N for the Atmospheric Nitrate Pathway
Nitrogen and oxygen isotopic fractionation is assumed to be the same as the temperature and the starting δ18O and δ15N of NOX for the various oxidation pathways. For these pathways, Walters and Michalski calculated relatively different values for δ18O-δ15N. For each of the many oxidation processes, HNO3 generally has different δ18O and δ15N values. In a hybrid model that may be used to explain the observed δ18O and δ15N values in atmospheric NO3, the δ18O-δ15N arrays for various HNO3 production pathways can therefore be specified as isotopic end elements [66]. δ18O is calculated in Equation (14), using the calculation in Equation (10).
We mentioned earlier in the Introduction section that the main pathways in the conversion of atmospheric NOX to NO3−, after going through the cycle of Reactions (1)–(3), are divided into the gas-phase interaction of NO2 and OH, the non-homogeneous reaction of N2O5 hydrolysis NO2 (Reaction (4)), and OH and the non-homogeneous reaction of N2O5 hydrolysis (Reactions (5), (7), (8)). At the same time, NO3 also reacts with DMS or HC to form nitric acid (Reaction (6)).
The identification of pollution sources by δ15N-NO3− alone has some limitations, and the high value of δ18O-NO3− is usually used to reveal the oxidation pathway of NO3− generation from NOX and to resolve the uncertainty of source analysis using δ15N alone. Combining δ15N and δ18O dual isotopes can further clarify the source, formation, and transport transformation processes of NO3− in PM2.5 by means of backward trajectories and the Bayesian isotope mixing model [67].
In Guha’s study, it was shown that the δ18O range of O3 is much higher than that of ⋅OH, (90–122‰) and (−25–0‰), respectively, and that five-sixths of the O atoms in the N2O5 pathway come from O3, whereas two-thirds of the O atoms in the ⋅OH pathway come from O3, leading to a higher expected δ18O of nitrate formed in the N2O5 pathway than in the ⋅OH pathway [68].
Li et al. sampled and analyzed the atmosphere of Jiaozuo, China, and found that δ18O-HNO3− values for winter samples (82.7‰ to 103.9‰) were close to δ18O-HNO3− values calculated via the N2O5 pathway (103‰ ± 0.8‰), and values for summer samples (67.8‰ to 85.7‰) were close to δ18O-HNO3− values calculated by the ⋅OH oxidation pathway (61‰ ± 0.8‰), indicating that winter nitrate is mainly produced by the N2O5 pathway, while summer samples are mainly from the ⋅OH pathway [38]. Hastings et al. also showed that the ⋅OH pathway dominates in summer, while the N2O5 pathway dominates in winter [11].
According to Xiao et al., more than 60‰ of the nitrate production in Nanchang was caused by the hydrolysis of N2O5. Due to long days and high temperatures, ⋅OH radical oxidation of NOX predominated in the summer, whereas N2O5 hydrolysis oxidation predominated in the winter due to long nights and low temperatures. While lower ⋅OH radical concentrations with higher O3 to ⋅OH ratios result in higher N2O5 hydrolysis contributions and higher NO3− concentrations generated in winter, higher ⋅OH radical concentrations with higher ⋅OH to O3 ratios result in lower NO3− concentrations. The O3 to NOX ratio rises with low ⋅OH, boosting the NO3 + HC pathway’s contribution [17]. Luo et al. found that δ18O-NO3− values were higher in colder months (63.5–103‰) than in warmer months (50.3–85.4‰), also indicating a shift in the nitrate formation pathway from NO2 +⋅OH in the warmer months to the N2O5 + H2O and NO3 + HC pathways in the colder months [69].
Additionally, N2O5 hydrolysis and NO3 radical reactions with hydrocarbons occur in Nanchang throughout the winter. On average, NO3 + HCs account for 60‰ of the chemical conversion of NOX to NO3−. With a 33‰ average and a potential of 45‰ on days of exceptionally high nitrate aerosol pollution, NO3 + HCs channels play a significant role in NOX oxidation [58]. In Walters’ study, it was shown that the daily cycle of the variation of δ18O is due to the NOX photochemical cycle driven by the O3 pathway, resulting in higher daytime δ18O values [70].
Additionally, Luo et al. discovered that PM2.5 had an impact on the nitrate generation pathway. When PM2.5 climbed from ~25 to >100 μg/m3 during the winter months, δ18O-NO3− levels increased from 65.2–79.9‰ to 80.7–96.2‰, although the change in δ18O-NO3− was only marginal when PM2.5 was over 100 μg/m3. It demonstrates that when PM2.5 was below 100 μg/m3, the nitrate synthesis route switched from the NO2 +⋅OH pathway to the NO3 + HC and N2O5 + H2O pathways, but when PM2.5 was over 100 μg/m3, N2O5 + H2O and NO3 + HC predominated [69]. According to Cheng et al., NO3 + HC pathway and N2O5 + H2O pathway both contributed more when the concentration of NO3− was high, indicating the significance of these two pathways for nitrate production in PM2.5, particularly in the winter [63].
Li et al. demonstrate the nonlinear relationships between particle nitrate and the emission controls of nitrogen oxides (NOX) in the Chinese megalopolises using extensive observations and a regional meteorology chemistry model with optimized mechanisms [71]. They discovered that in the Beijing–Tianjin–Hebei (BTH) and Yangtze River Delta (YRD), decreases in NOX emissions would result in an approximately linear drop in PM2.5 over the summer and fall. Whereas NOX causes a rather complex response in PM composition in winter, for every 10‰ reduction in NOX emissions, nitrate increases by 4.1‰ in BTH and 5.1‰ in YTD. It is only at a 30–50‰ reduction in NOX emissions that a reduction in ammonia oxides leads to a reduction in nitrates.
In the report of Liu et al., using δ15N as an indicator, the δ15N value of NO3 was 6.9 ± 4.6‰ higher than that of NO2, indicating that the formation of HNO3 in the atmosphere is dominated by the hydroxyl radical pathway. The δ15N value of NO3−in the atmospheric particulate matter was 4.9 ± 4.2‰ higher than that of HNO3, indicating that HNO3 is the main precursor of particulate matter NO3− [72]. Significant isotopic fractionation during oxidation uses significant seasonal variation in nitrate δ15N concentrations (lower in summer and greater in winter), with seasonal variation in the NO2 oxidation pathway serving as the primary driver [73].
The proportional contribution of the OH oxidation pathway to nitrate was found to be significantly correlated with latitude (p < 0.01) in Zong et al.’s field measurements of δ15N-NO3− and δ18O-NO3− in five Chinese cities, with longer sunlight hours and longer atmospheric action of radicals like OH being more significant for NOX oxidation at low latitudes than at high latitudes. For the first time, the relationship between the NOX conversion pathway and δ18O and dimensionality was discovered. They also discovered a substantial correlation between latitude and UV intensity, and they demonstrated and confirmed how UV intensity affects the pathway for NOX oxidation. [37].
Previous investigations have all shown that heterogeneous hydrolysis of N2O5 was the primary source of explosive nitrate accumulation and that photochemistry is typically thought to be secondary in secondary nitrate generation due to lower solar radiation in winter and at night. However, a field study by Zhang et al. in North China highlighted the role of the OH oxidation pathway in the synthesis of nitrate during the winter season, with the daytime OH oxidation pathway playing an equal role to the nocturnal channel (46.4‰ vs. 53.6‰) [74]. In a study, Chen et al. also discovered the significance of the OH channel in Beijing during pollution events, with percentages of 74‰ in urban regions and 76‰ in suburban areas [75]. According to Zhang et al.’s analysis of the atmospheric nitrate in Jinan, the accumulation of nitrate during the summer is sensitive to the formation of HNO3, and during the night, when heterogeneous nitrogen chemistry is most favorable, the N2O5 pathway dominates the oxidation of NOX due to increased O3 levels and high aerosol water content during the summer [76]. These results are different from those that had previously been obtained and require more investigation
4.2. Δ17O in the Atmosphere
The wide distribution of δ15N and δ18O from different sources and a certain degree of overlap increase the difficulty of the source resolution. Since the reactions leading to 17O value excess occur only in photochemical reactions, they can further quantify the proportional contribution of different oxidation pathways in nitrate formation and become a powerful tool for the in-depth study of atmospheric chemistry, which has been applied to the study of oxidation pathways of nitrate in aerosols [77,78,79].
In the process of oxygen isotope mass fractionation, a linear relationship is maintained between δ17O and δ18O: δ17O = 0.52 × δ18O. Due to the symmetric structure of O3, 18O and 17O are uniformly distributed during the formation of O3, independent of their atomic masses, and the above linear relationship does not exist, so this process is called non-mass isotope fractionation (MIF), and the formation of O3 is the only non-mass isotopic fractionation process that has been discovered so far [80]. In the NOX photochemical cycle and the HNO3 production process, the value of Δ17O is equal to or approximately equal to 0 for all the reactants that provide oxygen atoms for the reaction products (HO2, RO2, OH, H2O, etc.), except for O3. The degree of non-mass isotopic fractionation is measured by Δ17O in Equation (15) [81], δ17O using the calculation of Equation (10):
Abnormal Δ17O values due to MIF is also present in reaction products when non-mass isotope fractionation occurs or during a reaction process in which Δ17O ≠ 0 provides oxygen atoms to the reaction products. Therefore, Δ17O can be used to study the pathway of atmospheric nitrate production [82,83].
Changes in external factors such as seasonal variations, human activity effects, geographical variations, different PM2.5 concentrations, and relative humidity all have an impact on the value of Δ17O.
The value of Δ17O can alter due to fluctuations in external factors such as relative humidity, seasonal variations, anthropogenic influences, regional variations, differing PM2.5 concentrations, and seasonal changes. Numerous studies have discovered that the seasonal variation of nitrate, which is low in summer and high in winter, is caused by the relatively low nitrate Δ17O-NO3− formed via the OH pathway in summer and the high nitrate formed via the N2O5 and NO3 pathways in winter, which is related to the seasonal variation characteristics of the concentrations of oxidants O3 and OH [80,84]. Additionally, there is a distinct diurnal variation in Δ17O, which is associated with the production pathway and is lower during the day and greater at night [82,85].
Human activities also have an influence on Δ17O values. Wang et al. measured isotopes in PM2.5 in Beijing in 2014 and combined them with stable isotope analysis in the R model and found that air pollution (PM2.5, NO2, and NO3−) reduced the contribution of the OH pathway to nitrate, while atmospheric ozone pollution increased the importance of the OH pathway. They concluded that the influence of Δ17O values is mainly due to the availability of OH [84]. The study by Li et al. found higher oxygen isotopes at rural sites than at urban sites, suggesting that human activities reduce the contribution of the O3 pathway to atmospheric nitrate [79].
At the same time, different geographical environments also lead to differences in nitrate formation pathways. Li et al. found in Guiyang, China that the strong ultraviolet radiation in the plateau region may make the OH pathway more important [73]. Zhao et al. sampled and analyzed atmospheric aerosols from different regions in northeast China and found that tropospheric O3 concentrations in one region were significantly smaller than in other regions, leading to more difficult N2O5 production. This may lead to a high contribution of ⋅OH to the formation of nitrate in this region [86].
In addition, several studies have shown that high PM2.5 concentrations and relative humidity during haze are more favorable for nitrate formation via the N2O5 non-homogeneous pathway, which may be due to the wetter atmosphere during the haze, which is more favorable for non-homogeneous reactions [86,87]. In the study of Fan et al., the contribution of the N2O5 pathway to nitrate during haze was elevated to 64‰, which was significantly higher than during clean atmosphere (39‰) [15].
Because the formation process of atmospheric nitrate is complex and the formation pathways are different under different conditions, it is difficult to analyze the oxidation results only based on the observation results. In addition to simulating Δ17O in various places to better understand the peculiarities of atmospheric oxidation in various regions and seasons, atmospheric chemistry models are coupled with previous studies to achieve a quantitative analysis of atmospheric nitrate oxidation routes.
Box simulations are simulations of atmospheric photochemical reactions in an enclosed space. All nitrates in the simulation are oxidized by NOX, which can be used to simulate the Δ17O of atmospheric nitrates and thus calculate the number of nitrates produced by each reaction pathway in the atmosphere. However, the box simulation does not take into account atmospheric transport, and the calculation results have some bias. In the first simulation of atmospheric nitrate Δ17O in the contaminated marine boundary layer in California, USA, using the box model, the simulation results can be in good agreement with the test results of the actual samples by Michalski et al. However, the box simulation ignored the regional transport in the atmosphere, resulting in a large difference (0~5‰) between the simulated and tested results in spring [85].
Subsequently, Michalski et al. developed an isotope mass balance model ISO-RACM that uses regional atmospheric chemistry mechanisms to predict Δ17O values of atmospheric nitrate, which can be used to explain Δ17O in ice cores, aerosols, soils, and precipitation, breaking the limits of the atmospheric medium [88]. Morin et al. further developed a daily integrated isotopic signature model that takes into account the influence of transport processes on the diurnal variation of Δ17O and helps to quantify the effect of different environments [89]. The application of this model to secondary species with a longer life than one day allows the seasonal variation of Δ17O to be studied.
In 2009, Alexander et al. presented the first global three-dimensional chemical transport model, GEOS-Chem, incorporating horizontal and vertical transport as well as spatial variation in surface fluxes of some important major pollutants; however, Δ17O values were overestimated for mid- and high-latitudes in winter and underestimated for polar regions in spring and summer [13].
Later, in 2020, Alexander et al. updated the GEOS-Chem global chemical transport model and re-evaluated the model’s nitrate formation pathway. The results obtained from the simulations are generally consistent with previous observations, with annual mean Δ17O variability ranging from 7‰ to 41‰ and higher Δ17O values in the cold season. It was also found that Δ17O-NO3− is lower near the equator and higher in polar regions on a global scale. However, the model underestimates the Δ17O in the polar region in spring and summer and in the middle and high dimensional regions to some extent, 0‰~5‰ and 1‰~7‰, respectively. The underestimation of Δ17O for polar regions is mainly due to the GEOS-Chem model overestimating the contribution of the N2O5 hydrolysis pathway by ignoring the importance of the hydrolysis reactions of halogenated elements (XNO3, X for halogenated elements such as Cl, Br, I) to generate nitrate [15].
In summary, it is concluded that there are differences in the observed values of ∆17O-O3 in different regions and differences between observed and simulated values, which may be due to the often large scales of model studies and possible discrepancies with observations in the real atmosphere at local sites. Therefore, observations of ∆17O-O3 values on a global scale are important for model validation and for understanding the real atmospheric nitrate formation mechanisms. In future studies, modelling data can be combined with actual observations. The modelling results provide research directions for the actual observations, while the results obtained from the actual observations can provide favorable evidence for the modelling predictions.
5. Conclusions
Nitrate is now a significant component of haze particles as a result of changes in the composition of atmospheric particulate matter during the past several years. One of the hotspots of research in this direction is the process of nitrate synthesis and the origin of its precursor NOX. In order to analyze NOX sources in atmospheric particulate matter, stable isotope techniques have made significant strides in recent years.
This paper summarizes the benefits and drawbacks of emission inventories, satellite observations, and stable isotope methods. It also highlights the main developments of stable isotope techniques in the study of NOX sources and nitrate formation pathways. First, a summary of the stable isotope techniques used for NOX traceability is presented. The range of δ15N-NOX values for various NOX sources is then noted, while the Bayesian mixture model’s calculation approach and its use in the investigation of NOX sources are discussed. After that, a discussion of how to utilize δ15N and δ18O in conjunction to figure out the quantities and paths of nitrate production follows. Finally, the study of routes using Δ17O is provided in order to learn how different conditions affect the pathways of nitrate generation and to simulate Δ17O in various places in order to better understand the peculiarities of atmospheric oxidation in various regions and seasons.
The analogous analysis of the literature in this paper presents the continuous progress and application of traceability analysis methods in NOX in recent decades. The work of current researchers has demonstrated that stable isotope techniques are a useful method for the traceability analysis of NOX in the atmosphere, and the research findings are helpful for the development of effective emission reduction plans and the implementation of targeted measures, which are crucial for the accurate understanding of atmospheric oxidation activity and atmospheric pollutants.
Although the findings of the current research effort are remarkable, there are still some issues with the stable isotope analysis of NOX, and we think that the following areas can be further improved.
The lack of source δ15N data for NOX tracing using nitrogen isotopes and the large overlap in the data of δ15N values of NOX emitted from different sources lead to large uncertainty. More actual measurements of NOX sources are needed to enrich the δ15N value data and establish a perfect δ15N value system to quantify NOX sources.
The atmospheric NOX sources and nitrate formation mechanisms in different regions also have great uncertainties. The results of Chen et al. showed that the drivers of nitrate pollution and mitigation strategies for particulate nitrate differed between Beijing and the United States [75]. Therefore, it is necessary to determine a normative way of collecting NOX to determine precisely the δ15N values of emission sources in each location and to obtain a more reliable indication of NO3− sources. In addition, the contribution of non-major oxidation pathways for NOX in specific regions (coastal areas, highland areas, etc.) remains to be evaluated.
For the study of the atmospheric nitrate production pathway, Δ17O is only related to the nitrate production pathway and should be combined with δ15N, δ18O isotope analysis to exclude the possible fractionation process during nitrate production to better distinguish and reveal the source and production process of nitrate.
Table 2 summarizes and explains the acronyms used in this paper.

Table 2.
List of abbreviations.
Author Contributions
S.Z. was responsible for the concept of the review and had a leading role in the preparation of the manuscript. M.L. and L.M. participated in the preparation as well as revision of the manuscript. Y.S. and D.X. were responsible for the revision and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, (Grant/Award Number: 12075261, 12105311, U1832212), Beijing Natural Science Foundation (Grant/Award Number: 7191008).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The Contribution of Outdoor Air Pollution Sources to Premature Mortality on a Global Scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Pozzer, A.; Cao, C.X.; Lelieveld, J. Long-term (2001–2012) concentrations of fine particulate matter (PM 2.5) and the impact on human health in Beijing, China. Atmos. Chem. Phys. 2015, 15, 5715–5725. [Google Scholar] [CrossRef]
- Lelieveld, J.; Klingmüller, K.; Pozzer, A.; Burnett, R.T.; Haines, A.; Ramanathan, V. Effects of fossil fuel and total anthropogenic emission removal on public health and climate. Proc. Natl. Acad. Sci. USA 2019, 116, 7192–7197. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Xie, Z.; Yu, X.; Wang, L.; Kang, H.; Yue, F. The observation of isotopic compositions of atmospheric nitrate in Shanghai China and its implication for reactive nitrogen chemistry. Sci. Total Environ. 2020, 714, 136727. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Sun, Y.; Wang, Q.; Zhao, J.; Wang, J.; Ge, X.; Xie, C.; Zhou, W.; Du, W.; Li, J.; et al. Changes in Aerosol Chemistry From 2014 to 2016 in Winter in Beijing: Insights From High-Resolution Aerosol Mass Spectrometry. J. Geophys. Res. Atmos. 2019, 124, 1132–1147. [Google Scholar] [CrossRef]
- Cheng, J.; Su, J.; Cui, T.; Li, X.; Dong, X.; Sun, F.; Yang, Y.; Ting, D.; Zheng, Y.; Li, Y.; et al. Dominant role of emission reduction in PM 2.5 air quality improvement in Beijing during 2013–2017: A model-based decomposition analysis. Atmos. Chem. Phys. 2019, 19, 6125–6146. [Google Scholar] [CrossRef]
- Lim, S.; Yang, X.; Lee, M.; Li, G.; Gao, Y.; Shang, X.; Zhang, K.; Czimczik, C.I.; Xu, X.; Bae, M.-S.; et al. Fossil-driven secondary inorganic PM2.5 enhancement in the North China Plain: Evidence from carbon and nitrogen isotopes. Environ. Pollut. 2020, 266, 115163. [Google Scholar] [CrossRef]
- Shao, P.; Tian, H.; Sun, Y.; Liu, H.; Wu, B.; Liu, S.; Liu, X.; Wu, Y.; Liang, W.; Wang, Y.; et al. Characterizing remarkable changes of severe haze events and chemical compositions in multi-size airborne particles (PM1, PM2.5 and PM10) from January 2013 to 2016–2017 winter in Beijing, China. Atmos. Environ. 2018, 189, 133–144. [Google Scholar] [CrossRef]
- Lim, S.; Lee, M.; Savarino, J.; Laj, P. Oxidation pathways and emission sources of atmospheric particulate nitrate in Seoul: Based on δ15N and Δ17O measurements. Atmos. Chem. Phys. 2022, 22, 5099–5115. [Google Scholar] [CrossRef]
- Klimasmith, I.M.; Kent, A.D. Micromanaging the nitrogen cycle in agroecosystems. Trends Microbiol. 2022, 30, 1045–1055. [Google Scholar] [CrossRef]
- Hastings, M.; Steig, E.J.; Sigman, D.M. Seasonal variations in N and O isotopes of nitrate in snow at Summit, Greenland: Implications for the study of nitrate in snow and ice cores. J. Geophys. Res. Earth Surf. 2004, 109, D20. [Google Scholar] [CrossRef]
- Khoder, M.I. Atmospheric conversion of sulfur dioxide to particulate sulfate and nitrogen dioxide to particulate nitrate and gaseous nitric acid in an urban area. Chemosphere 2002, 49, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.; Hastings, M.G.; Allman, D.J.; Dachs, J.; Thornton, J.A.; Kunasek, S.A. Quantifying atmospheric nitrate formation pathways based on a global model of the oxygen isotopic composition (Δ17O) of atmospheric nitrate. Atmos. Chem. Phys. 2009, 9, 5043–5056. [Google Scholar] [CrossRef]
- Freyer, H.D.; Kley, D.; Volz-Thomas, A.; Kobel, K. On the interaction of isotopic exchange processes with photochemical reactions in atmospheric oxides of nitrogen. J. Geophys. Res. Atmos. 1993, 98, 14791–14796. [Google Scholar] [CrossRef]
- Alexander, B.; Sherwen, T.; Holmes, C.D.; Fisher, J.A.; Chen, Q.; Evans, M.J.; Kasibhatla, P. Global inorganic nitrate production mechanisms: Comparison of a global model with nitrate isotope observations. Atmos. Meas. Technol. 2020, 20, 3859–3877. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, Y.; Lin, Y.; Cao, F.; Zhao, Z.; Sun, Y.; Qiu, Y.; Fu, P.; Wang, Y. Changes of Emission Sources to Nitrate Aerosols in Beijing After the Clean Air Actions: Evidence from Dual Isotope Compositions. J. Geophys. Res. Atmos. 2020, 125, e2019JD031998. [Google Scholar] [CrossRef]
- Xiao, H.; Zhu, R.; Pan, Y.; Guo, W.; Zheng, N.; Liu, Y.; Liu, C.; Zhang, Z.; Wu, J.; Kang, C.; et al. Differentiation Between Nitrate Aerosol Formation Pathways in a Southeast Chinese City by Dual Isotope and Modeling Studies. J. Geophys. Res. Atmos. 2020, 125, e2020JD032604. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, R.G.; Song, C.B.; Peng, J.-F.; Guo, W.; Liu, Y.; Zheng, N.; Xiao, H.; Xiao, H.-Y. Changes in nitrate accumulation mechanisms as PM(2.5) levels increase on the North China Plain: A perspective from the dual isotopic compositions of nitrate. Chemosphere 2021, 263, 127915. [Google Scholar] [CrossRef]
- Battye, W.; Aneja, V.P.; Roelle, P.A. Evaluation and improvement of ammonia emissions inventories. Atmos. Environ. 2003, 37, 3873–3883. [Google Scholar] [CrossRef]
- Lamarque, J.-F.; Dentener, F.; McConnell, J.; Ro, C.-U.; Shaw, M.; Vet, R.; Bergmann, D.; Cameron-Smith, P.; Dalsoren, S.; Doherty, R.; et al. Multi-model mean nitrogen and sulfur deposition from the Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP): Evaluation of historical and projected future changes. Atmos. Chem. Phys. 2013, 13, 7997–8018. [Google Scholar] [CrossRef]
- Streets, D.G.; Canty, T.; Carmichael, G.R.; De Foy, B.; Dickerson, R.R.; Duncan, B.N.; Edwards, D.P.; Haynes, J.A.; Henze, D.K.; Houyoux, M.R.; et al. Emissions estimation from satellite retrievals: A review of current capability. Atmos. Environ. 2013, 77, 1011–1042. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, Y.; Tian, C.; Zhang, S.; Ma, X.; Cao, F.; Liu, X.; Zhang, W.; Kuhn, T.; Lehmann, M.F. Nitrogen isotope fractionation during gas-to-particle conversion of NOX to NO3− in the atmosphere–implications for isotope-based NOX source apportionment. Atmos. Chem. Phys. 2018, 18, 11647–11661. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.-L.; Yang, W.; Sun, X.-C.; Tong, Y.-D.; Wang, X.-M.; Liu, C.-Q.; Bai, Z.-P.; Liu, X.-Y. Isotopic evaluation on relative contributions of major NOX sources to nitrate of PM2.5 in Beijing. Environ. Pollut. 2019, 248, 183–190. [Google Scholar] [CrossRef]
- Zong, Z.; Sun, Z.; Xiao, L.; Tian, C.; Liu, J.; Sha, Q.; Li, J.; Fang, Y.; Zheng, J.; Zhang, G. Insight into the Variability of the Nitrogen Isotope Composition of Vehicular NOX in China. Environ. Sci. Technol. 2020, 54, 14246–14253. [Google Scholar] [CrossRef]
- Zong, Z.; Shi, X.; Sun, Z.; Tian, C.; Li, J.; Fang, Y.; Gao, H.; Zhang, G. Nitrogen isotopic composition of NOX from residential biomass burning and coal combustion in North China. Environ. Pollut. 2022, 304, 119238. [Google Scholar] [CrossRef]
- Hastings, M.G.; Jarvis, J.C.; Steig, E.J. Anthropogenic Impacts on Nitrogen Isotopes of Ice-Core Nitrate. Science 2009, 324, 1288. [Google Scholar] [CrossRef]
- Walters, W.W.; Tharp, B.D.; Fang, H.; Kozak, B.J.; Michalski, G. Nitrogen Isotope Composition of Thermally Produced NOX from Various Fossil-Fuel Combustion Sources. Environ. Sci. Technol. 2015, 49, 11363–11371. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, Z.; Alexander, B.; Cole-Dai, J.; Savarino, J.; Erbland, J.; Geng, L. On the potential fingerprint of the Antarctic ozone hole in ice-core nitrate isotopes: A case study based on a South Pole ice core. Atmos. Chem. Phys. 2022, 22, 13407–13422. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Zhang, S.; Qu, R. Nitrate dynamics and source identification of rainwater in Beijing during rainy season: Insight from dual isotopes and Bayesian model. Sci. Total Environ. 2022, 856, 159234. [Google Scholar] [CrossRef]
- Sigman, D.M.; Casciotti, K.L.; Andreani, M.; Barford, C.; Galanter, M.; Böhlke, J.K. A Bacterial Method for the Nitrogen Isotopic Analysis of Nitrate in Seawater and Freshwater. Anal. Chem. 2001, 73, 4145–4153. [Google Scholar] [CrossRef]
- McIlvin, M.R.; Casciotti, K.L. Technical Updates to the Bacterial Method for Nitrate Isotopic Analyses. Anal. Chem. 2011, 83, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Fang, Y.; Liu, D.; Pan, Y. Modifications to the azide method for nitrate isotope analysis. Rapid Commun. Mass Spectrom. 2016, 30, 1213–1222. [Google Scholar] [CrossRef]
- Liu, D.W.; Tu, Y.; Fang, Y.T. Isotope analysis of ammonium and nitrate: A review on measured methods and their application. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2017, 28, 2353–2360. [Google Scholar]
- Zhao, Z.-Y.; Cao, F.; Zhang, W.-Q.; Zhai, X.-Y.; Fang, Y.; Fan, M.-Y.; Zhang, Y.-L. Determination of Stable Nitrogen and Oxygen Isotope Ratios in Atmospheric Aerosol Nitrates. Chin. J. Anal. Chem. 2019, 47, 907–915. [Google Scholar] [CrossRef]
- Heaton, T.H.E. 15N/14N ratios of NOx from vehicle engines and coal-fired power stations. Tellus B Chem. Phys. Meteorol. 1990, 42, 304–307. [Google Scholar] [CrossRef]
- Moore, H. The isotopic composition of ammonia, nitrogen dioxide and nitrate in the atmosphere. Atmos. Environ. 1977, 11, 1239–1243. [Google Scholar] [CrossRef]
- Walters, W.W.; Goodwin, S.R.; Michalski, G. Nitrogen stable isotope composition (δ15N) of vehicle-emitted NOX. Environ. Sci. Technol. 2015, 49, 2278–2285. [Google Scholar] [CrossRef]
- Li, Y.; Geng, Y.; Hu, X.; Yin, X. Seasonal differences in sources and formation processes of PM2.5 nitrate in an urban environment of North China. J. Environ. Sci. 2022, 120, 94–104. [Google Scholar] [CrossRef]
- Su, T.; Li, J.; Tian, C.; Zong, Z.; Chen, D.; Zhang, G. Source and formation of fine particulate nitrate in South China: Constrained by isotopic modeling and online trace gas analysis. Atmos. Environ. 2020, 231, 117563. [Google Scholar] [CrossRef]
- Felix, J.D.; Elliott, E.M.; Shaw, S.L. Nitrogen Isotopic Composition of Coal-Fired Power Plant NOX: Influence of Emission Controls and Implications for Global Emission Inventories. Environ. Sci. Technol. 2012, 46, 3528–3535. [Google Scholar] [CrossRef]
- Zong, Z.; Tan, Y.; Wang, X.; Tian, C.; Li, J.; Fang, Y.; Chen, Y.; Cui, S.; Zhang, G. Dual-modelling-based source apportionment of NOX in five Chinese megacities: Providing the isotopic footprint from 2013 to 2014. Environ. Int. 2020, 137, 105592. [Google Scholar] [CrossRef] [PubMed]
- Widory, D. Nitrogen isotopes: Tracers of origin and processes affecting PM10 in the atmosphere of Paris. Atmos. Environ. 2007, 41, 2382–2390. [Google Scholar] [CrossRef]
- Fibiger, D.L.; Hastings, M.G. First Measurements of the Nitrogen Isotopic Composition of NOX from Biomass Burning. Environ. Sci. Technol. 2016, 50, 11569–11574. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, P.; Jin, Z.; Hu, Y.; Zhang, Y.; Li, F. Stable nitrogen isotope composition of NOX of biomass burning in China. Sci. Total Environ. 2021, 803, 149857. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, X. Nitrogen isotopic signature of soil-released nitric oxide (NO) after fertilizer application. Atmos. Environ. 2008, 42, 4747–4754. [Google Scholar] [CrossRef]
- Felix, J.D.; Elliott, E.M. Isotopic composition of passively collected nitrogen dioxide emissions: Vehicle, soil and livestock source signatures. Atmos. Environ. 2014, 92, 359–366. [Google Scholar] [CrossRef]
- Miller, D.J.; Chai, J.; Guo, F.; Dell, C.J.; Karsten, H.; Hastings, M.G. Isotopic composition of in situ soil NOX emissions in manure-fertilized cropland. Geophys. Res. Lett. 2018, 45, 12–058. [Google Scholar] [CrossRef]
- Su, C.; Kang, R.; Zhu, W.; Huang, W.; Song, L.; Wang, A.; Liu, D.; Quan, Z.; Zhu, F.; Fu, P.; et al. δ15N of Nitric Oxide Produced Under Aerobic or Anaerobic Conditions From Seven Soils and Their Associated N Isotope Fractionations. J. Geophys. Res. Biogeosciences 2020, 125, e2020JG005705. [Google Scholar] [CrossRef]
- Hoering, T. The isotopic composition of the ammonia and the nitrate ion in rain. Geochim. Cosmochim. Acta 1957, 12, 97–102. [Google Scholar] [CrossRef]
- Ding, J.; van der, A.R.J.; Eskes, H.J.; Mijling, B.; Stavrakou, T.; Van Geffen, J.H.G.M.; Veefkind, J.P. NOX emissions reduction and rebound in China due to the COVID-19 crisis. Geophys. Res. Lett. 2020, 47, e2020GL089912. [Google Scholar] [CrossRef]
- Twigg, M.V. Progress and future challenges in controlling automotive exhaust gas emissions. Appl. Catal. B Environ. 2007, 70, 2–15. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Hall, R.E.; Khan, S.; Culligan, K.; Lani, B.W. Nitrogen Oxides Emission Control Options for Coal-Fired Electric Utility Boilers. J. Air Waste Manag. Assoc. 2005, 55, 1367–1388. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, M.; Okada, K.; Zaizen, Y.; Makino, Y.; Jensen, J.B.; Gras, J.L.; Harjanto, H. Very high weight ratios of S/K in individual haze particles over Kalimantan during the 1997 Indonesian forest fires. Atmos. Environ. 2001, 35, 4237–4243. [Google Scholar] [CrossRef]
- Cheng, M.-T.; Horng, C.-L.; Su, Y.-R.; Lin, L.-K.; Lin, Y.-C.; Chou, C.C.-K. Particulate matter characteristics during agricultural waste burning in Taichung City, Taiwan. J. Hazard. Mater. 2009, 165, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Suarez, R.K.; Herrera M, L.G.; Welch, K.C. The sugar oxidation cascade: Aerial refueling in hummingbirds and nectar bats. J. Exp. Biol. 2011, 214, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.L.; Gregg, J.W. Source partitioning using stable isotopes: Coping with too many sources. Oecologia 2003, 136, 261–269. [Google Scholar] [CrossRef]
- Zong, Z.; Wang, X.; Tian, C.; Chen, Y.; Fang, Y.; Zhang, F.; Li, C.; Sun, J.; Li, J.; Zhang, G. First Assessment of NOX Sources at a Regional Background Site in North China Using Isotopic Analysis Linked with Modeling. Environ. Sci. Technol. 2017, 51, 5923–5931. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, N.; Zhang, D.; Xiao, H.; Cao, Y.; Xiao, H. Rayleigh based concept to track NOX emission sources in urban areas of China. Sci. Total Environ. 2020, 704, 135362. [Google Scholar] [CrossRef]
- Zhang, W.; Bi, X.; Zhang, Y.; Wu, J.; Feng, Y. Diesel vehicle emission accounts for the dominate NOX source to atmospheric particulate nitrate in a coastal city: Insights from nitrate dual isotopes of PM2.5. Atmos. Res. 2022, 278, 106328. [Google Scholar] [CrossRef]
- Jin, Z.; Qian, L.; Shi, Y.; Fu, G.; Li, G.; Li, F. Quantifying major NOX sources of aerosol nitrate in Hangzhou, China, by using stable isotopes and a Bayesian isotope mixing model. Atmos. Environ. 2021, 244, 117979. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Cao, F.; Fan, M.-Y.; Zhang, W.-Q.; Zhai, X.-Y.; Wang, Q.; Zhang, Y.-L. Coal and biomass burning as major emissions of NOX in Northeast China: Implication from dual isotopes analysis of fine nitrate aerosols. Atmos. Environ. 2020, 242, 117762. [Google Scholar] [CrossRef]
- Yin, M.; Guan, H.; Luo, L.; Xiao, H.; Zhang, Z. Using nitrogen and oxygen stable isotopes to analyze the major NOX sources to nitrate of PM2.5 in Lanzhou, northwest China, in winter-spring periods. Atmos. Environ. 2022, 276, 119036. [Google Scholar] [CrossRef]
- Cheng, C.; Yu, R.; Chen, Y.; Chen, Y.; Yan, Y.; Hu, G.; Wang, S. Quantifying the source and formation of nitrate in PM(2.5) using dual isotopes combined with Bayesian mixing model: A case study in an inland city of southeast China. Chemosphere 2022, 308, 136097. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Bo, Y.; Xie, S. High-resolution historical emission inventories of crop residue burning in fields in China for the period 1990–2013. Atmos. Environ. 2016, 138, 152–161. [Google Scholar] [CrossRef]
- Song, W.; Liu, X.-Y.; Hu, C.-C.; Chen, G.-Y.; Walters, W.W.; Michalski, G.; Liu, C.-Q. Important contributions of non-fossil fuel nitrogen oxides emissions. Nat. Commun. 2021, 12, 243. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, N.; Liang, Y.; Luo, L.; Xiao, H.; Xiao, H. Dominance of Heterogeneous Chemistry in Summertime Nitrate Accumulation: Insights from Oxygen Isotope of Nitrate (δ18O–NO3−). ACS Earth Space Chem. 2020, 4, 818–824. [Google Scholar] [CrossRef]
- Walters, W.W.; Michalski, G. Theoretical calculation of oxygen equilibrium isotope fractionation factors involving various NOy molecules, OH, and H2O and its implications for isotope variations in atmospheric nitrate. Geochim. Cosmochim. Acta 2016, 191, 89–101. [Google Scholar] [CrossRef]
- Zong, Z.; Tan, Y.; Wang, X.; Tian, C.; Fang, Y.; Chen, Y.; Fang, Y.; Han, G.; Li, J.; Zhang, G. Assessment and quantification of NOX sources at a regional background site in North China: Comparative results from a Bayesian isotopic mixing model and a positive matrix factorization model. Environ. Pollut. 2018, 242, 1379–1386. [Google Scholar] [CrossRef]
- Guha, T.; Lin, C.; Bhattacharya, S.; Mahajan, A.; Ou-Yang, C.-F.; Lan, Y.-P.; Hsu, S.; Liang, M.-C. Isotopic ratios of nitrate in aerosol samples from Mt. Lulin, a high-altitude station in Central Taiwan. Atmos. Environ. 2017, 154, 53–69. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, L.; Liang, Y.; Guo, W.; Guan, H.; Zheng, N. Importance of NO3 radical in particulate nitrate formation in a southeast Chinese urban city: New constraints by δ15N-δ18O space of NO3−. Atmos. Environ. 2021, 253, 118387. [Google Scholar] [CrossRef]
- Walters, W.W.; Fang, H.; Michalski, G. Summertime diurnal variations in the isotopic composition of atmospheric nitrogen dioxide at a small midwestern United States city. Atmos. Environ. 2018, 179, 1–11. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Yao, Q.; Wang, T.; Xie, M.; Li, S.; Zhuang, B.; Han, Y. Nonlinear responses of particulate nitrate to NOX emission controls in the megalopolises of China. Atmos. Chem. Phys. 2021, 21, 15135–15152. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Yin, Y.-M.; Song, W. Nitrogen Isotope Differences between Major Atmospheric NOy Species: Implications for Transformation and Deposition Processes. Environ. Sci. Technol. Lett. 2020, 7, 227–233. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.-D.; Yang, Z.; Cui, G.; Ding, S. Diurnal and seasonal variations in water-soluble inorganic ions and nitrate dual isotopes of PM2.5: Implications for source apportionment and formation processes of urban aerosol nitrate. Atmos. Res. 2020, 248, 105197. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Guan, H.; Liang, Y.; Zheng, N.; Guo, W. Isotopic Evidence for the High Contribution of Wintertime Photochemistry to Particulate Nitrate Formation in Northern China. J. Geophys. Res. Atmos. 2021, 126, e2021JD035324. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Lu, K.; Li, C.; Zhai, T.; Tan, Z.; Ma, X.; Yang, X.; Liu, Y.; Chen, S.; et al. Field Determination of Nitrate Formation Pathway in Winter Beijing. Environ. Sci. Technol. 2020, 54, 9243–9253. [Google Scholar] [CrossRef]
- Ishino, S.; Hattori, S.; Savarino, J.; Jourdain, B.; Preunkert, S.; Legrand, M.; Caillon, N.; Barbero, A.; Kuribayashi, K.; Yoshida, N. Seasonal variations of triple oxygen isotopic compositions of atmospheric sulfate, nitrate, and ozone at Dumont d’Urville, coastal Antarctica. Atmos. Chem. Phys. 2017, 17, 3713–3727. [Google Scholar] [CrossRef]
- He, P.; Xie, Z.; Chi, X.; Yu, X.; Fan, S.; Kang, H.; Liu, C. Atmospheric Δ17O (NO3−) reveals nocturnal chemistry dominates nitrate production in Beijing haze. Atmos. Chem. Phys. 2018, 18, 14465–14476. [Google Scholar] [CrossRef]
- Li, Z.; Walters, W.W.; Hastings, M.G.; Song, L.; Huang, S.; Zhu, F.; Liu, D.; Shi, G.; Li, Y.; Fang, Y. Atmospheric nitrate formation pathways in urban and rural atmosphere of Northeast China: Implications for complicated anthropogenic effects. Environ. Pollut. 2021, 296, 118752. [Google Scholar] [CrossRef]
- Hathorn, B.C.; Marcus, R.A. An intramolecular theory of the mass-independent isotope effect for ozone. I. J. Chem. Phys. 1999, 111, 4087–4100. [Google Scholar] [CrossRef]
- Miller, M.F. Isotopic fractionation and the quantification of 17O anomalies in the oxygen three-isotope system: An appraisal and geochemical significance. Geochim. Cosmochim. Acta 2002, 66, 1881–1889. [Google Scholar] [CrossRef]
- Savarino, J.; Kaiser, J.; Morin, S.; Sigman, D.M.; Thiemens, M.H. Nitrogen and oxygen isotopic constraints on the origin of atmospheric nitrate in coastal Antarctica. Atmos. Chem. Phys. 2007, 7, 1925–1945. [Google Scholar] [CrossRef]
- Savarino, J.; Morin, S.; Erbland, J.; Grannec, F.; Patey, M.D.; Vicars, W.; Alexander, B.; Achterberg, E.P. Isotopic composition of atmospheric nitrate in a tropical marine boundary layer. Proc. Natl. Acad. Sci. USA 2013, 110, 17668–17673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Song, W.; Yang, W.; Sun, X.-C.; Tong, Y.-D.; Wang, X.-M.; Liu, C.-Q.; Bai, Z.-P.; Liu, X.-Y. Influences of atmospheric pollution on the contributions of major oxidation pathways to PM2. 5 nitrate formation in Beijing. J. Geophys. Res. Atmos. 2019, 124, 4174–4185. [Google Scholar] [CrossRef]
- Michalski, G.; Scott, Z.; Kabiling, M.; Thiemens, M.H. First measurements and modeling of Δ17O in atmospheric nitrate. Geophys. Res. Lett. 2003, 30. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Cao, F.; Fan, M.-Y.; Zhai, X.-Y.; Yu, H.-R.; Hong, Y.; Ma, Y.-J.; Zhang, Y.-L. Nitrate aerosol formation and source assessment in winter at different regions in Northeast China. Atmos. Environ. 2021, 267, 118767. [Google Scholar] [CrossRef]
- Wang, H.; Lu, K.; Chen, X.; Zhu, Q.; Chen, Q.; Guo, S.; Jiang, M.; Li, X.; Shang, D.; Tan, Z.; et al. High N2O5 Concentrations Observed in Urban Beijing: Implications of a Large Nitrate Formation Pathway. Environ. Sci. Technol. Lett. 2017, 4, 416–420. [Google Scholar] [CrossRef]
- Michalski, G.; Xu, F. Isotope modeling of nitric acid formation in the atmosphere using ISO-RACM: Testing the importance of NO oxidation, heterogeneous reactions, and trace gas chemistry. Atmos. Chem. Phys. Discuss. 2010, 10, 6829–6869. [Google Scholar]
- Morin, S.; Sander, R.; Savarino, J. Simulation of the diurnal variations of the oxygen isotope anomaly (Δ17O) of reactive atmospheric species. Atmos. Chem. Phys. 2011, 11, 3653–3671. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).