Ataulfo Mango (Mangifera indica L.) Peel Extract as a Potential Natural Antioxidant in Ground Beef
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mango Peel Extracts
2.2. Analysis of Extracts
2.2.1. Total Phenolic and Flavonoid Content and In Vitro Antioxidant Capacity
2.2.2. Antibacterial Activity
2.3. Preparation of Ground Beef
2.4. Analysis of Meat Samples
2.4.1. Instrumental and Chemical Analysis of Ground Beef
2.4.2. Microbiological Analysis
2.4.3. Sensory Analysis
2.5. Statistical Analysis
3. Results
3.1. Analysis of Extracts
3.1.1. Total Phenolic Content (TPC), Total Flavonoid Content (TFC) and In Vitro Antioxidant Activity
3.1.2. In Vitro Antimicrobial Activity of EE and HE
3.2. Analysis of Ground Beef with EE
3.2.1. Colour Characteristics
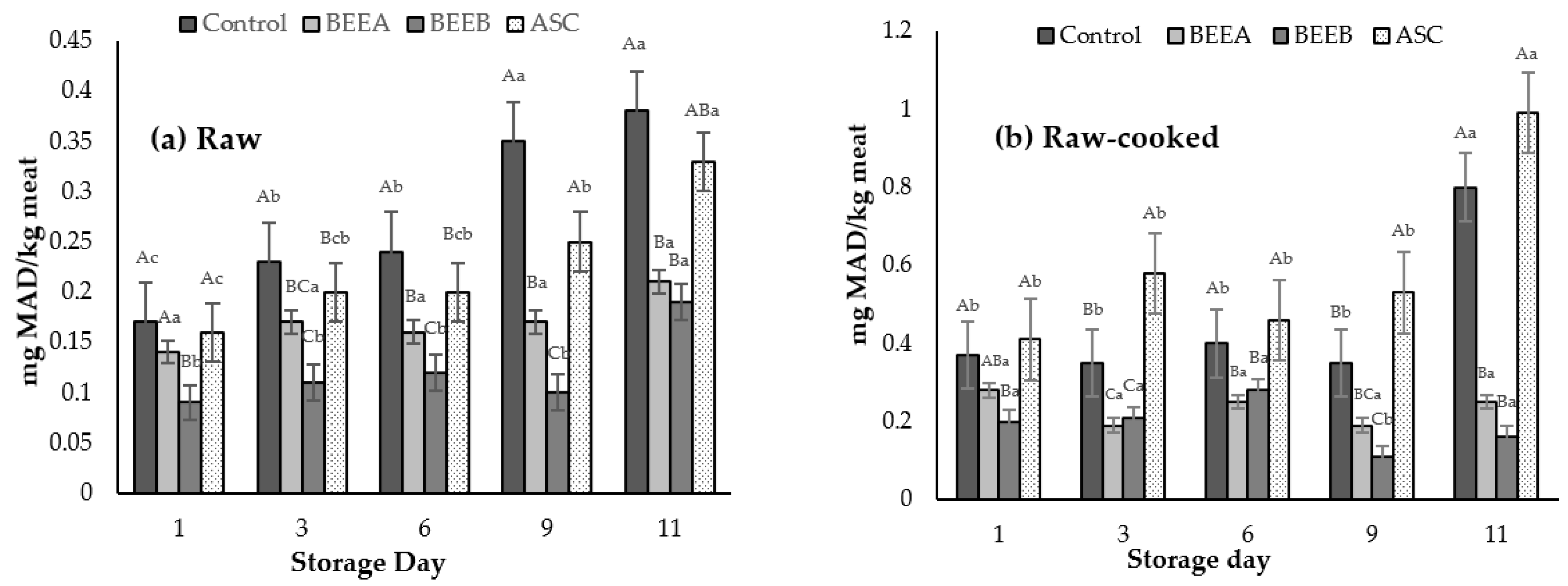
3.2.2. Lipid Oxidation
3.2.3. pH and Microbiological Analysis
3.2.4. Sensorial Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.; Ahmedna, M.; Goktepe, I. Potential of Peanut Skin Phenolic Extract as Antioxidative and Antibacterial Agent in Cooked and Raw Ground Beef. Int. J. Food Sci. Technol. 2010, 45, 1337–1344. [Google Scholar] [CrossRef]
- Mariem, C.; Sameh, M.; Nadhem, S.; Soumaya, Z.; Najiba, Z.; Raoudha, E.G. Antioxidant and Antimicrobial Properties of the Extracts from Nitraria retusa Fruits and Their Applications to Meat Product Preservation. Ind. Crops Prod. 2014, 55, 295–303. [Google Scholar] [CrossRef]
- de Oliveira Ferreira, N.S.; Rosset, M.; Lima, G.; Stuelp Campelo, P.M.; de Macedo, R.E.F. Effect of Adding Brosimum gaudichaudii and Pyrostegia venusta Hydroalcoholic Extracts on the Oxidative Stability of Beef Burgers. LWT 2019, 108, 145–152. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of Natural Extracts on the Shelf Life of Modified Atmosphere-Packaged Pork Patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M.; Chabani, Z.; Farag, M.A.; Domínguez, R. Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications. Molecules 2021, 26, 3880. [Google Scholar] [CrossRef]
- Villalobos-Delgado, L.H.; Mateo, J.; Caro, I.; Leal Ramos, M.-Y.; Mendez, N.G.; Cansino, R.G.; González Mondragón, E.G. Natural Antioxidants in Fresh and Processed Meat. In Sustainable Meat Production and Processing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 207–236. [Google Scholar]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of Oxidative Processes in Meat and Toxicity Induced by Postprandial Degradation Products: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Parafati, L.; Palmeri, R.; Trippa, D.; Restuccia, C.; Fallico, B. Quality Maintenance of Beef Burger Patties by Direct Addiction or Encapsulation of a Prickly Pear Fruit Extract. Front. Microbiol. 2019, 10, 1760. [Google Scholar] [CrossRef]
- Bañón, S.; Díaz, P.; Rodríguez, M.; Garrido, M.D.; Price, A. Ascorbate, Green Tea and Grape Seed Extracts Increase the Shelf Life of Low Sulphite Beef Patties. Meat Sci. 2007, 77, 626–633. [Google Scholar] [CrossRef]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana Seed Extracts as a Useful Strategy to Extend the Shelf Life of Pork Patties: UHPLC-ESI/QTOF Phenolic Profile and Impact on Microbial Inactivation, Lipid and Protein Oxidation and Antioxidant Capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef]
- Andrés, A.I.; Petrón, M.J.; Adámez, J.D.; López, M.; Timón, M.L. Food By-Products as Potential Antioxidant and Antimicrobial Additives in Chill Stored Raw Lamb Patties. Meat Sci. 2017, 129, 62–70. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the Chemistry, Food Applications, Legislation and Role as Preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Estévez, M.; Lorenzo, J.M. Impact of Antioxidants on Oxidized Proteins and Lipids in Processed Meat. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 600–608. [Google Scholar]
- Rodríguez-Carpena, J.; Morcuende, D.; Estévez, M. Avocado By-Products as Inhibitors of Color Deterioration and Lipid and Protein Oxidation in Raw Porcine Patties Subjected to Chilled Storage. Meat Sci. 2011, 89, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Pimentel-Moral, S.; Arráez-Román, D.; Segura-Carretero, A. Profiling Phenolic Compounds in Underutilized Mango Peel By-Products from Cultivars Grown in Spanish Subtropical Climate over Maturation Course. Food Res. Int. 2021, 140, 109852. [Google Scholar] [CrossRef] [PubMed]
- Marçal, S.; Pintado, M. Mango Peels as Food Ingredient/Additive: Nutritional Value, Processing, Safety and Applications. Trends Food Sci. Technol. 2021, 114, 472–489. [Google Scholar] [CrossRef]
- Okino-Delgado, C.H.; Prado, D.Z.; Pereira, M.S.; Camargo, D.A.; Koike, M.A.; Fleuri, L.F. Mango. In Valorization of Fruit Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2016, pp. 167–181. [Google Scholar]
- Patiño-Rodríguez, O.; Bello-Pérez, L.A.; Agama-Acevedo, E.; Pacheco-Vargas, G. Pulp and Peel of Unripe Stenospermocarpic Mango (Mangifera indica L. Cv Ataulfo) as an Alternative Source of Starch, Polyphenols and Dietary Fibre. Food Res. Int. 2020, 138, 109719. [Google Scholar] [CrossRef] [PubMed]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.-L.; Norulaini, N.A.N.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-Products and Their Valuable Components: A Review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef]
- de Ancos, B.; Sánchez-Moreno, C.; Zacarías, L.; Rodrigo, M.J.; Sáyago Ayerdí, S.; Blancas Benítez, F.J.; Domínguez Avila, J.A.; González-Aguilar, G.A. Effects of Two Different Drying Methods (Freeze-Drying and Hot Air-Drying) on the Phenolic and Carotenoid Profile of ‘Ataulfo’ Mango by-Products. J. Food Meas. Charact. 2018, 12, 2145–2157. [Google Scholar] [CrossRef]
- Matheyambath, A.C.; Subramanian, J.; Paliyath, G. Mangoes. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 641–645. [Google Scholar]
- Pacheco-Ordaz, R.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.; González-Aguilar, G. Intestinal Permeability and Cellular Antioxidant Activity of Phenolic Compounds from Mango (Mangifera indica Cv. Ataulfo) Peels. Int. J. Mol. Sci. 2018, 19, 514. [Google Scholar] [CrossRef]
- Castro-Vargas, H.; Ballesteros Vivas, D.; Ortega Barbosa, J.; Morantes Medina, S.; Aristizabal Gutiérrez, F.; Parada-Alfonso, F. Bioactive Phenolic Compounds from the Agroindustrial Waste of Colombian Mango Cultivars ‘Sugar Mango’ and ‘Tommy Atkins’—An Alternative for Their Use and Valorization. Antioxidants 2019, 8, 41. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, S.; Yousuf, B. Effect of Bioactive-Rich Mango Peel Extract on Physicochemical, Antioxidant and Functional Characteristics of Chicken Sausage. Appl. Food Res. 2022, 2, 100183. [Google Scholar] [CrossRef]
- García-Magaña, M.d.L.; García, H.S.; Bello-Pérez, L.A.; Sáyago-Ayerdi, S.G.; de Oca, M.M.-M. Functional Properties and Dietary Fiber Characterization of Mango Processing By-Products (Mangifera indica L., Cv Ataulfo and Tommy Atkins). Plant Foods Hum. Nutr. 2013, 68, 254–258. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.-J.; Ye, W.; Korivi, M. Nutritional Composition and Bioactive Compounds in Three Different Parts of Mango Fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef]
- Rojas, R.; Alvarez-Pérez, O.B.; Contreras-Esquivel, J.C.; Vicente, A.; Flores, A.; Sandoval, J.; Aguilar, C.N. Valorisation of Mango Peels: Extraction of Pectin and Antioxidant and Antifungal Polyphenols. Waste Biomass Valorization 2020, 11, 89–98. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Barrow, C.J.; Dunshea, F.R. Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Vicenssuto, G.M.; de Castro, R.J.S. Development of a Novel Probiotic Milk Product with Enhanced Antioxidant Properties Using Mango Peel as a Fermentation Substrate. Biocatal. Agric. Biotechnol. 2020, 24, 101564. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart Advanced Solvents for Bioactive Compounds Recovery from Agri-Food by-Products: A Review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Rizou, M.; Aldawoud, T.M.S.; Ucak, I.; Rowan, N.J. Innovations and Technology Disruptions in the Food Sector within the COVID-19 Pandemic and Post-Lockdown Era. Trends Food Sci. Technol. 2021, 110, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Ramos, L.; Moreno, C.; Zúñiga-Paredes, J.C.; Carlosama-Yepez, M.; Ruales, P. Antimicrobial Activity of Plant-Food by-Products: A Review Focusing on the Tropics. Livest. Sci. 2016, 189, 32–49. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana NOM-188-SCFI-2012 Mango Ataulfo del Soconusco, Chiapas (Mangifera caesia Jack Ex Wall)-Especificaciones y Métodos de Prueba; Diario Oficial de la Federación: Mexico City, Mexico, 2012; p. 7.
- Adilah, A.N.; Jamilah, B.; Noranizan, M.A.; Hanani, Z.A.N. Utilization of Mango Peel Extracts on the Biodegradable Films for Active Packaging. Food Packag. Shelf Life 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Villalobos-Delgado, L.H.; González-Mondragón, E.G.; Salazar Govea, A.Y.; Andrade, J.R.; Santiago-Castro, J.T. Potential Application of Epazote (Chenopodium ambrosioides L.) as Natural Antioxidant in Raw Ground Pork. LWT 2017, 84, 306–313. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Cid-Pérez, T.; Ávila-Sosa, R.; Ochoa-Velasco, C.; Rivera-Chavira, B.; Nevárez-Moorillón, G. Antioxidant and Antimicrobial Activity of Mexican Oregano (Poliomintha longiflora) Essential Oil, Hydrosol and Extracts from Waste Solid Residues. Plants 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- AMSA American Meat Science Association Meat Color Measurement Guidelines: AMSA; AMSA: Champaign, IL, USA, 2012.
- Trujillo-Santiago, E.; Villalobos-Delgado, L.H.; Guzmán-Pantoja, L.E.; López, M.G.; Zafra-Ciprián, D.I.; Nevárez-Moorillón, G.V.; Santiago-Castro, J.T. The Effects of Hierba Santa (Piper auritum Kunth) on the Inhibition of Lipid Oxidation in Beef Burgers. LWT 2021, 146, 111428. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana NOM-092-SSA1-1994 Bienes y Servicios. Método Para la Cuenta de Bacterias Aerobias en Placa; Diario Oficial de la Federación: Mexico City, Mexico, 1994; p. 6.
- do Prado, M.E.A.; Queiroz, V.A.V.; Correia, V.T.d.V.; Neves, E.O.; Roncheti, E.F.S.; Gonçalves, A.C.A.; de Menezes, C.B.; de Oliveira, F.C.E. Physicochemical and Sensorial Characteristics of Beef Burgers with Added Tannin and Tannin-Free Whole Sorghum Flours as Isolated Soy Protein Replacer. Meat Sci. 2019, 150, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Vega-Vega, V.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Bernal-Mercado, A.T.; González-Aguilar, G.A.; Ruíz-Cruz, S.; Moctezuma, E.; Siddiqui, M.W.; Ayala-Zavala, J.F. Antimicrobial and Antioxidant Properties of Byproduct Extracts of Mango Fruit. J. Appl. Bot. Food Qual. 2013, 86, 205–211. [Google Scholar]
- Rodríguez, C.R.P.; Durán, V.H.Z.; Muriel, J.L.F.; Franco, D.T. Physico-Chemical Quality Parameters of Mango (Mangifera indica L.) Fruits Grown in a Mediterranean Subtropical Climate (SE Spain). J. Agric. Sci. Technol. 2012, 14, 365–374. [Google Scholar]
- Gómez-Maldonado, D.; Lobato-Calleros, C.; Aguirre-Mandujano, E.; Leyva-Mir, S.G.; Robles-Yerena, L.; Vernon-Carter, E.J. Antifungal Activity of Mango Kernel Polyphenols on Mango Fruit Infected by Anthracnose. LWT 2020, 126, 109337. [Google Scholar] [CrossRef]
- Martínez-Ramos, T.; Benedito-Fort, J.; Watson, N.J.; Ruiz-López, I.I.; Che-Galicia, G.; Corona-Jiménez, E. Effect of Solvent Composition and Its Interaction with Ultrasonic Energy on the Ultrasound-Assisted Extraction of Phenolic Compounds from Mango Peels (Mangifera indica L.). Food Bioprod. Process. 2020, 122, 41–54. [Google Scholar] [CrossRef]
- Masibo, M.; He, Q. Major Mango Polyphenols and Their Potential Significance to Human Health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Heredia, J.B. Effect of Hydrophilic and Lipophilic Antioxidants from Mango Peel (Mangifera indica L. Cv. Ataulfo) on Lipid Peroxidation in Fish Oil. CyTA-J. Food. 2018, 16, 1095–1101. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Anaguano, M.; Molina, M.; Tupuna-Yerovi, D.S.; Ruales, J. Characterization and Quantification of Bioactive Compounds and Antioxidant Activity in Three Different Varieties of Mango (Mangifera indica L.) Peel from the Ecuadorian Region Using HPLC-UV/VIS and UPLC-PDA. NFS J. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.d.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids from Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Rodríguez De Luna, S.L.; Ramírez-Garza, R.E.; Serna Saldívar, S.O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar] [CrossRef]
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of Current Extraction Techniques for Flavonoids from Plant Materials. Processes 2020, 8, 1222. [Google Scholar] [CrossRef]
- Kothalawala, S.G.; Yatiwella, L.N.S.B. Analysis of Antioxidant Activities in Mango Peel among Different Sri Lankan Cultivars. J. Pharmacogn. Phytochem. 2018, 7, 1668–1671. [Google Scholar]
- Blancas-Benitez, F.J.; Mercado-Mercado, G.; Quirós-Sauceda, A.E.; Montalvo-González, E.; González-Aguilar, G.A.; Sáyago-Ayerdi, S.G. Bioaccessibility of Polyphenols Associated with Dietary Fiber and in Vitro Kinetics Release of Polyphenols in Mexican ‘Ataulfo’ Mango (Mangifera indica L.) by-Products. Food Funct. 2015, 6, 859–868. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Moreno-Hernández, C.L.; Montalvo-González, E.; García-Magaña, M.L.; Mata-Montes de Oca, M.; Torres, J.L.; Pérez-Jiménez, J. Mexican ‘Ataulfo’ Mango (Mangifera indica L) as a Source of Hydrolyzable Tannins. Analysis by MALDI-TOF/TOF MS. Food Res. Int. 2013, 51, 188–194. [Google Scholar] [CrossRef]
- Nikmaram, N.; Budaraju, S.; Barba, F.J.; Lorenzo, J.M.; Cox, R.B.; Mallikarjunan, K.; Roohinejad, S. Application of Plant Extracts to Improve the Shelf-Life, Nutritional and Health-Related Properties of Ready-to-Eat Meat Products. Meat Sci. 2018, 145, 245–255. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant Extracts as Natural Antioxidants in Meat and Meat Products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Wasim Siddiqui, M.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-Industrial Potential of Exotic Fruit Byproducts as a Source of Food Additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural Products as Antimicrobial Agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Hayrapetyan, H.; Hazeleger, W.C.; Beumer, R.R. Inhibition of Listeria Monocytogenes by Pomegranate (Punica granatum) Peel Extract in Meat Paté at Different Temperatures. Food Control 2012, 23, 66–72. [Google Scholar] [CrossRef]
- Tirado-Kulieva, V.; Atoche-Dioses, S.; Hernández-Martínez, E. Phenolic Compounds of Mango (Mangifera indica) by-Products: Antioxidant and Antimicrobial Potential, Use in Disease Prevention and Food Industry, Methods of Extraction and Microencapsulation. Sci. Agropecu. 2021, 12, 283–293. [Google Scholar] [CrossRef]
- Warner, R. Measurement of Meat Quality|Measurements of Water-Holding Capacity and Color: Objective and Subjective. In Encyclopedia of Meat Sciences; Elsevier: Amsterdam, The Netherlands, 2014; pp. 164–171. [Google Scholar]
- MacDougall, D.B. Colour in Food. Improving Quality; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Motoyama, M.; Kobayashi, M.; Sasaki, K.; Nomura, M.; Mitsumoto, M. Pseudomonas Spp. Convert Metmyoglobin into Deoxymyoglobin. Meat Sci. 2010, 84, 202–207. [Google Scholar] [CrossRef]
- Balentine, C.W.; Crandall, P.G.; O’Bryan, C.A.; Duong, D.Q.; Pohlman, F.W. The Pre- and Post-Grinding Application of Rosemary and Its Effects on Lipid Oxidation and Color during Storage of Ground Beef. Meat Sci. 2006, 73, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Escalante, A.; Djenane, D.; Torrescano, G.; Beltran, J.A.; Roncales, P. Antioxidant Action of Borage, Rosemary, Oregano, and Ascorbic Acid in Beef Patties Packaged in Modified Atmosphere. J. Food Sci. 2003, 68, 339–344. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Yang, J.-Y.; Lu, H.-B.; Wang, S.-S.; Yang, J.; Yang, X.-C.; Chai, M.; Li, L.; Cao, J.-X. Effect of Chitosan Film Incorporated with Tea Polyphenol on Quality and Shelf Life of Pork Meat Patties. Int. J. Biol. Macromol. 2013, 61, 312–316. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kelly, A.L.; Kerry, J.P. Effects of High-Pressure and Heat Treatments on Physical and Biochemical Characteristics of Oysters (Crassostrea gigas). Innov. Food Sci. Emerg. Technol. 2007, 8, 30–38. [Google Scholar] [CrossRef]
- Bastida, S.; Sánchez-Muniz, F.J.; Olivero, R.; Pérez-Olleros, L.; Ruiz-Roso, B.; Jiménez-Colmenero, F. Antioxidant Activity of Carob Fruit Extracts in Cooked Pork Meat Systems during Chilled and Frozen Storage. Food Chem. 2009, 116, 748–754. [Google Scholar] [CrossRef]
- Bekhit, A.E.; Geesink, G.; Ilian, M.; Morton, J.; Bickerstaffe, R. The Effects of Natural Antioxidants on Oxidative Processes and Metmyoglobin Reducing Activity in Beef Patties. Food Chem. 2003, 81, 175–187. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Vargas, F.C.; Strozzi, I.; Pateiro, M.; Furtado, M.M.; Sant’Ana, A.S.; Rocchetti, G.; Barba, F.J.; Dominguez, R.; Lucini, L.; et al. Influence of Pitanga Leaf Extracts on Lipid and Protein Oxidation of Pork Burger during Shelf-Life. Food Res. Int. 2018, 114, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Vithana, M.D.K.; Singh, Z.; Johnson, S.K. Regulation of the Levels of Health Promoting Compounds: Lupeol, Mangiferin and Phenolic Acids in the Pulp and Peel of Mango Fruit: A Review. J. Sci. Food Agric. 2019, 99, 3740–3751. [Google Scholar] [CrossRef]
- Heck, R.T.; Ferreira, D.F.; Fagundes, M.B.; Dos Santos, B.A.; Cichoski, A.J.; Saldaña, E.; Lorenzo, J.M.; de Menezes, C.R.; Wagner, R.; Barin, J.S.; et al. Jabuticaba Peel Extract Obtained by Microwave Hydrodiffusion and Gravity Extraction: A Green Strategy to Improve the Oxidative and Sensory Stability of Beef Burgers Produced with Healthier Oils. Meat Sci. 2020, 170, 108230. [Google Scholar] [CrossRef]
- Turgut, S.S.; Soyer, A.; Işıkçı, F. Effect of Pomegranate Peel Extract on Lipid and Protein Oxidation in Beef Meatballs during Refrigerated Storage. Meat Sci. 2016, 116, 126–132. [Google Scholar] [CrossRef]
- Gill, A.; Gill, C. Packaging and the Shelf Life of Fresh Red and Poultry Meats. In Food Packaging and Shelf Life; Robertson, G.L., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 259–277. [Google Scholar]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total Volatile Basic Nitrogen (TVB-N) and Its Role in Meat Spoilage: A Review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana NOM-034-SSA1-1993 Bienes y Servicios. Productos de la Carne. Carne Molida y Carne Molida Moldeada. Envasadas. Especificaciones Sanitarias; Diario Oficial de la Federación: Mexico City, Mexico, 1994; p. 5.
- ICMSF International Commission on Microbiological Specifications for Foods Sampling Plans for Raw Meats. Microorganisms in Foods 2: Sampling for Microbiological Analysis: Principles and Specific Applications; Blackwell Scientific Publications: Toronto, ON, Canada, 1986; p. 278. [Google Scholar]
- Villalobos-Delgado, L.H.; Nevárez-Moorillon, G.V.; Caro, I.; Quinto, E.J.; Mateo, J. Natural Antimicrobial Agents to Improve Foods Shelf Life. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 125–157. [Google Scholar]
- He, M.; Tian, H.; Luo, X.; Qi, X.; Chen, X. Molecular Progress in Research on Fruit Astringency. Molecules 2015, 20, 1434–1451. [Google Scholar] [CrossRef]
| Treatments | TPC (mg GAE/g DW) | TFC (mg EQ/g DW) | DPPH• (% Inhibition) | ABTS•+ (µmol TE/g DW) | FRAP (µmol TE/g DW) |
|---|---|---|---|---|---|
| EE | 344.0 a | 153.7 a | 77.6 a | 1.3 a | 11.6 a |
| HE | 132.5 b | 59.7 b | 59.4 b | 0.9 b | 5.6 b |
| SEM | 5.69 | 4.11 | 2.25 | 0.13 | 1.34 |
| P-level | *** | *** | *** | ** | *** |
| Microbial Strains | Minimal Inhibitory Concentration (mg/mL) | Minimal Bactericidal Concentration (mg/mL) | ||
|---|---|---|---|---|
| EE | HE | EE | HE | |
| S. Typhimurium | 50 | 100 | 100 | 100 |
| E. coli | >100 | >100 | >100 | >100 |
| Ps. fluorescens | >100 | >100 | >100 | 25 |
| S. aureus | 25 | 12.5 | 25 | 100 |
| L. monocytogenes | 50 | 25 | 100 | 50 |
| Attribute | Storage Day | Treatments | SEM | P-Level | |||
|---|---|---|---|---|---|---|---|
| L* | Control | BEEA | BEEB | ASC | |||
| 1 | 52.1 a,A | 51.1 a,A | 51.3 a,A | 52.1 a,A | 0.43 | n.s | |
| 3 | 51.0 a,A | 50.7 a,A | 51.5 a,A | 51.2 a,A | 0.36 | n.s | |
| 6 | 48.1 b,B | 50.2 a,A | 50.7 a,A | 51.2 a,A | 0.39 | *** | |
| 9 | 45.4 b,C | 49.7 a,A | 49.6 a,A | 50.8 a,A | 0.62 | *** | |
| 11 | 51.2 a,A | 49.1 a,A | 49.7 a,A | 50.5 a,A | 0.60 | n.s. | |
| SEM | 0.50 | 0.44 | 0.47 | 0.33 | |||
| P-level | *** | n.s | n.s | n.s | |||
| a* | 1 | 7.0 b,A | 8.8 a,A | 9.6 a,A | 8.1 a,b,A | 0.30 | * |
| 3 | 6.6 b,A | 8.4 a,B | 9.0 a,A | 8.0 a,A | 0.32 | ** | |
| 6 | 5.0 b,A | 6.1 a,BC | 6.4 a,B | 7.8 a,A | 0.32 | ** | |
| 9 | 5.0 b,A | 5.9 a,C | 6.0 a,B | 6.9 a,A,B | 0.20 | ** | |
| 11 | 3.2 b,B | 5.2 a,C | 5.3 a,B | 5.4 a,B | 0.24 | *** | |
| SEM | 0.27 | 0.31 | 0.28 | 0.32 | |||
| P-level | *** | *** | *** | * | |||
| b* | 1 | 12.5 a,A | 12.8 a,A | 13.7 a,A | 12.6 a,A | 0.29 | n.s. |
| 3 | 11.4 b,A,B | 11.7 b,A,B | 13.0 a,A,B | 12.4 a,b,A | 0.24 | * | |
| 6 | 10.4 b,B,C | 11.1 a,b,B | 11.2 a,b,C | 12.7 a,A | 0.30 | * | |
| 9 | 9.5 b,C | 9.9 b,C | 11.7 a,C | 12.1 a,A | 0.27 | *** | |
| 11 | 11.8 a,A,B | 10.0 a,C | 11.0 a,C | 11.0 a,A | 0.30 | n.s. | |
| SEM | 0.30 | 0.23 | 0.26 | 0.26 | |||
| P-level | *** | *** | *** | n.s. | |||
| a/b* | 1 | 0.6 c,A | 0.8 a,A | 0.7 b,A | 0.7 b,A | 0.02 | *** |
| 3 | 0.5 b,A | 0.7 a,A | 0.7 a,A | 0.6 a,A | 0.02 | *** | |
| 6 | 0.6 a,A | 0.6 a,B | 0.6 a,B | 0.6 a,A | 0.02 | n.s. | |
| 9 | 0.7 a,A | 0.5 b,B | 0.5 b,B | 0.6 a,b,A | 0.02 | * | |
| 11 | 0.3 b,B | 0.5 a,B | 0.5 a,B | 0.5 a,B | 0.03 | *** | |
| SEM | 0.03 | 0.02 | 0.02 | 0.02 | |||
| P-level | *** | *** | *** | * | |||
| Chroma | 1 | 14.3 a,A | 16.0 a,A | 16.4 a,A | 15.0 a,A | 0.35 | n.s. |
| 3 | 12.9 b,A,B | 14.4 a,b,B | 15.9 a,A | 14.9 a,A | 0.35 | * | |
| 6 | 12.4 b,A,B | 12.7 b,C | 12.9 a,b,B | 15.0 a,A | 0.39 | * | |
| 9 | 11.3 b,B | 11.6 b,D | 12.9 a,B | 13.9 a,A,B | 0.29 | *** | |
| 11 | 11.1 b,B | 11.5 b,D | 12.3 a,B | 12.3 a,B | 0.29 | ** | |
| SEM | 0.32 | 0.33 | 0.34 | 0.35 | |||
| P-level | * | *** | *** | * | |||
| Hue | 1 | 61.1 a,B | 53.2 c,B | 57.2 b,B | 57.2 b,B | 0.71 | *** |
| 3 | 62.2 a,B | 54.4 c,B | 55.3 b,c,B | 58.3 b,A,B | 0.78 | *** | |
| 6 | 57.9 b,B | 61.5 a,A | 60.7 a,A | 59.3 b,A,B | 0.85 | ** | |
| 9 | 57.7 b,B | 63.4 a,A | 62.4 a,b,A | 60.2 a,b,A,B | 0.86 | * | |
| 11 | 74.3 a,A | 62.5 b,A | 63.8 b,A | 63.8 b,A | 1.18 | *** | |
| SEM | 1.12 | 0.85 | 0.73 | 0.84 | |||
| P-level | *** | *** | *** | * | |||
| MetMb (%) | 1 | 25.5 a,B | 20.2 a,C | 23.1 a,A | 21.0 a,B | 0.97 | n.s |
| 3 | 35.1 a,B | 19.3 b,C | 14.2 b,B | 26.1 ab,AB | 2.94 | ** | |
| 6 | 38.3 a,B | 24.1 bc,B | 19.0 c,A,B | 26.2 ab,AB | 1.81 | ** | |
| 9 | 43.6 a,A | 26.3 bc,B | 21.5 c,A,B | 31.7 b,A | 3.15 | ** | |
| 11 | 45.3 a,A | 30.9 bc,A | 20.6 c,A,B | 35.4 b,A | 3.20 | ** | |
| SEM | 2.83 | 1.14 | 1.13 | 1.71 | |||
| P-level | ** | *** | ** | * | |||
| Treatments | ΔE1–11 | SEM |
|---|---|---|
| Control | 7.8 a | 0.90 |
| BEEA | 5.2 b | 0.65 |
| BEEB | 4.4 b | 0.78 |
| ASC | 4.5 b | 0.56 |
| Storage Day | Treatments | SEM | P-Level | ||||
|---|---|---|---|---|---|---|---|
| Control | BEEA | BEEB | ASC | ||||
| pH | 1 | 5.63 a,D | 5.55 a,C | 5.22 b,C | 5.26 b,C | 0.15 | ** |
| 3 | 5.43 ab,D | 5.39 b,C | 5.25 c,C | 5.68 a,C | 0.16 | *** | |
| 6 | 6.23 a,C | 6.01 b,B | 6.02 b,B | 6.05 b,B | 0.16 | ** | |
| 9 | 6.65 a,B | 6.38 b,B | 6.10 b,AB | 6.27 b,A | 0.17 | *** | |
| 11 | 7.10 a,A | 6.44 b,A | 6.35 b,A | 6.49 b,A | 0.17 | ** | |
| SEM | 0.16 | 0.15 | 0.15 | 0.17 | |||
| P-level | *** | *** | *** | *** | |||
| TBC | 1 | 3.2 a,C | 2.9 a,C | 3.1 a,C | 3.1 a,C | 0.25 | n.s. |
| 3 | 4.0 a,C | 3.2 a,C | 3.6 a,C | 3.4 a,C | 0.23 | n.s. | |
| 6 | 6.7 a,B | 5.1 b,B | 5.6 b,B | 6.0 a,b,B | 0.25 | * | |
| 9 | 7.5 a,A,B | 7.6 a,A | 7.4 a,A | 7.7 a,A | 0.13 | n.s. | |
| 11 | 8.2 a,A | 8.0 a,A | 7.6 a,A | 8.0 a,A | 0.15 | n.s. | |
| SEM | 0.34 | 0.40 | 0.35 | 0.37 | |||
| P-level | *** | *** | *** | *** | |||
| Treatments | Odour | Colour | Flavour | Overall Acceptability |
|---|---|---|---|---|
| Control | 5.2 b | 6.2 a | 5.1 b | 5.6 b |
| BEEA | 5.3 b | 6.2 a | 5.7 b | 5.7 b |
| BEEB | 6.0 a | 6.0 a | 6.3 a | 6.3 a |
| ASC | 6.1 a | 6.2 a | 6.4 a | 6.4 a |
| SEM | 0.10 | 0.09 | 0.10 | 0.10 |
| P-level | ** | n.s | ** | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafra Ciprián, D.I.; Nevárez Moorillón, G.V.; Soto Simental, S.; Guzmán Pantoja, L.E.; López Hernández, L.H.; Santiago Castro, J.T.; Villalobos Delgado, L.H. Ataulfo Mango (Mangifera indica L.) Peel Extract as a Potential Natural Antioxidant in Ground Beef. Processes 2023, 11, 1772. https://doi.org/10.3390/pr11061772
Zafra Ciprián DI, Nevárez Moorillón GV, Soto Simental S, Guzmán Pantoja LE, López Hernández LH, Santiago Castro JT, Villalobos Delgado LH. Ataulfo Mango (Mangifera indica L.) Peel Extract as a Potential Natural Antioxidant in Ground Beef. Processes. 2023; 11(6):1772. https://doi.org/10.3390/pr11061772
Chicago/Turabian StyleZafra Ciprián, Dalia I., Guadalupe V. Nevárez Moorillón, Sergio Soto Simental, Ludmila E. Guzmán Pantoja, Luis H. López Hernández, Joaquín T. Santiago Castro, and Luz H. Villalobos Delgado. 2023. "Ataulfo Mango (Mangifera indica L.) Peel Extract as a Potential Natural Antioxidant in Ground Beef" Processes 11, no. 6: 1772. https://doi.org/10.3390/pr11061772
APA StyleZafra Ciprián, D. I., Nevárez Moorillón, G. V., Soto Simental, S., Guzmán Pantoja, L. E., López Hernández, L. H., Santiago Castro, J. T., & Villalobos Delgado, L. H. (2023). Ataulfo Mango (Mangifera indica L.) Peel Extract as a Potential Natural Antioxidant in Ground Beef. Processes, 11(6), 1772. https://doi.org/10.3390/pr11061772









