Abstract
The influence of nanoparticles of hydrated C60 fullerene and its N-monoamino acid derivatives on the oxidative metabolism and growth of the mycelial biomass of basidiomycetes during their submerged cultivation was studied. It was found that the supplementation of culture media with nanoparticles of the studied compounds at their final concentration range of 10−7 to 10−11 M significantly increased the resulting biomass, while the severity of the effect in this concentration range changed slightly. That prompted the use of nanomolar concentrations of compounds as reasonable. The most pronounced stimulating effect (an increase in biomass of about 240% with respect to control) was observed when culturing Laetiporus sulphureus, the intrinsically high level of oxidative metabolism of which was significantly lowered by the presence of the studied additives. It was shown that the growth-enhancing action of nanoparticles of fullerene C60 and its derivatives could not be attributed to photochemical reactions, particularly fullerene photoexcitation. Fullerene and its derivatives manifest a growth regulatory effect on bio-objects from different kingdoms of the living world (plants and fungi), which is indicative of these compounds’ mechanism of action based on a direct impact on fundamental, universal for all living beings, biophysical processes, primarily chain free-radical oxidation.
1. Introduction
Mushrooms’ medicinal properties include antitumor, immunomodulating, antioxidant, antihypercholesterolemic, antiviral, antibacterial, detoxifying, hepatoprotective, and antidiabetic effects [1,2,3]. The most studied objects have laid the foundation for new drug creation, mainly based on the isolated and purified polysaccharides, especially β-glucans. Some of the latter have been introduced into clinical practice, e.g., crestin (polysaccharide K, PSK) derived from a multicolored polypore mushroom Trametes versicolor (formerly Coriolus versicolor) [4,5]; lentinan isolated from a shiitake mushroom Lentinula edodes [6]; schizophillan purified from the spit-gill mushroom Schizophyllum commune [7,8]; befungin obtained from the chaga mushroom Inonotus obliquus [9].
In addition to the above mushrooms, the possibilities of applying others are widely examined. It is shown that the water-soluble polysaccharide Flammulina velutipes I-A shows inhibitory activity in relation to hydroxyl radical and superoxide anion radical, as well as stimulates NO production and enhances the secretion of tumor necrosis factor-α, interleukin-1β and interleukin-6 by macrophages RAW264.7 [10]. A large number of studies are devoted to the antiviral and antibacterial activity of mushrooms from Ganoderma genus. Extracts from G. applanatum, G. atrum, and G. capense inhibited DNA polymerase of the hepatitis B virus by 80, 70, and 60%, respectively, in the cells of the PLC/PRF/5 line, with a decrease in DNA content of the hepatitis B virus of 28–41% [2]. The in vivo study on ducklings showed that oral treatment with G. appanatum extract at a dose of 50 mg/kg inhibited the DNA polymerase of the hepatitis B virus [11]. Polysaccharides with the oncostatic effects isolated from mushrooms are either water-soluble β-D-glucans, β-D-glycans with heterosaccharide chains of xylose, mannose, galactose, and uronic acid, or β-D-glucan-protein complexes, proteoglycans. Branching is very common in polysaccharides [12]. Most immunomodulating and antitumor polysaccharides are glucans with β-(1→3)-glycosidic bonds in the backbone chain and β-(1→6) in branches [13,14].
G. appanatum mushroom serves as a source of several triterpenoids with potential therapeutic implications. For instance, 5-alfa-ergost-7-en-3-beta-ol isolated from G. applanatum fruit body showed inhibitory activity against Gram-positive and Gram-negative bacteria, such as Bacillus cereus, Corynebacterium diphtheriae, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus saprophyticus, and Streptococcus pyogenes [15]. Antiadipogenic benefits of G. applanatum triterpenoids have been demonstrated repeatedly [16,17]. G. applanatum extracts exhibit significant antioxidative and cytoprotective effects [18]. Anxiolytic and analgesic action make this mushroom a prominent source of antidepressant, anxiety, and pain management [19].
Laetiporus sulphureus is also known as sulfur polypore, sulfur shelf, or “chicken of the woods” [20]. L. sulphureus species are considered a source of both sustainable food and abundant chemical compounds, including polysaccharides, triterpenes, phenolics [21] with cytotoxic, antimicrobial, anticancer, anti-inflammatory, hypoglycemic, and antioxidant activity [22].
Thus, the edible and medicinal basidiomycetes biomass represents one of the best sources of functional food and a rich reservoir of bioactive compounds used in the production of medications against many diseases. Therefore, the urgent task is to find ways to enhance the mushrooms’ yield resulting from their cultivation under artificial conditions. In order to solve this problem, various supplements to the nutrient media have been implemented. Recently, the attention of researchers has been attracted to biotechnologically applied nanostructured materials capable of exhibiting explicit positive effects on biological objects.
The possibilities of using nanoparticles of various materials in such areas as anticancer therapy, nanopharmacology, drug delivery, therapy of neurodegenerative diseases, etc., have been demonstrated [23]. In the field of agricultural technology, the use of nanostructured objects has also shown its effectiveness [24]. One of the promising nanosized materials is carbon nanomaterials. Among such nanocompounds, a special place is given to fullerene C60 and its various derivatives. The special interest in fullerenes is due to their physicochemical properties, in particular their spherical shape, high symmetry, optical properties, membranotropic, and antioxidant activity [25,26]. The use of fullerenes in biological systems is difficult due to their insolubility in water. However, due to their high chemical activity, fullerenes can easily enter reactions of nucleophilic, radical, cycloaddition, and it is possible to create amphiphilic, water-soluble compounds [27]. Moreover, it is better to create monosubstituted derivatives in order to save the unique structure of the fullerene “core”. In this study, we used C60 fullerene derivatives with some proteinogenic amino acids due to the high biocompatibility of this type of substituent.
There are few examples of data on the influence of fullerene derivatives on plant development and stress resistance. For example, it was shown that the addition to the nutrient solution of water-soluble derivatives of fullerene C60, such as L-lysine, L-threonine, L-arginine, and L-hydroxyproline, contributed to an increase in the dry mass of spring wheat [28], whereas the compounds from this group have never been tested on mushroom cultures. For this reason, we decided to study the effect of C60 fullerene amino acid derivatives on the growth and oxidative metabolism of three commonly used types of medicinal basidiomycetes.
Most of the researchers agree that the growth-stimulating effect of fullerene derivatives should be attributed to their ability to prevent oxidative stress development by trapping reactive oxygen species (ROS) [28,29]. ROS are universal chemical agents mediating the adverse effects of different types of stress (temperature, osmotic, acid-base, excessive lighting, ultraviolet radiation). For instance, in the course of exploring stress-induced transcription in the cyanobacterium Synechocystis sp., a number of genes have been identified as being induced in response to numerous abiotic stresses [30]. It was found that most of the genes encoding various protection factors are expressed not only in response to the corresponding adverse environmental factor but also in response to exogenous administration of hydrogen peroxide [30]. Thus, by regulating free radical processes, it seems possible to improve the adaptogenic and growth characteristics of cultivated organisms.
It is known that fullerene C60 and its various derivatives are able to participate in the regulation of free radical processes [31]. The most extensively studied is the antiradical activity of hydroxylated derivatives of fullerene C60 (fullerenols) and carboxylated derivatives of fullerene C60 (carboxyfullelenes). Thus, it is shown that fullerenols C60 (OH)18–20 inhibit 59 and 70% of radical anions generated by the xanthine/xanthine oxidase system at their concentrations of 50 and 100 μM, respectively [32]. The inhibitory effect of hydroxy and other derivatives of fullerene C60 exerted in relation to hydroxyl radical (•OH) [33], nitroxyl radical (NO•) [34], solvated electron (eaq-), and singlet oxygen (1O2) is also described [35].
I.M. Andreev et al. have found [36] that fullerene C60 derivatized with such amino acids as proline, ε-aminocaproic acid, and arginine, displays membranotropic activity and penetrates cell membranes in the ionized form, thereby causing their depolarization. Evidence from a single study [37] indicates that basidiomycetes are capable of metabolizing the fullerene derivatives. In the present work, nanoparticles of fullerene C60 and its amino acid derivatives are considered from the viewpoint of their prospects for being mycelial growth stimulants in mushrooms.
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of Fullerene C60 Water Dispersion
Water dispersion of fullerene C60 was obtained in accordance with the methodology described by Andrievsky G.V. et al. [38] by obtaining a saturated solution in toluene, to which water was poured and treated in the dark in an ultrasound bath (42 kHz, 50 W) for several hours with interruptions for cooling.
2.1.2. Amino Acid Fullerene Derivatives (AAFD) Synthesis
N-(monohydrofullerenyl)-D-alanine potassium salt (H-C60-D-Ala-OK), N-(monohydrofullerenyl)-L-alanine potassium salt (H-C60-L-Ala-OK), N-(monohydrofullerenyl)-D-valine potassium salt (H-C60-D-Val-OK), N-(monohydrofullerenyl)-ε-aminocaproic acid potassium salt (H-C60-ε-ACA-OK) (Figure 1) were obtained by means of one-stage synthesis with a direct attachment of the amino acid residue to the fullerene core according to the method described previously by Romanova et al. [39].
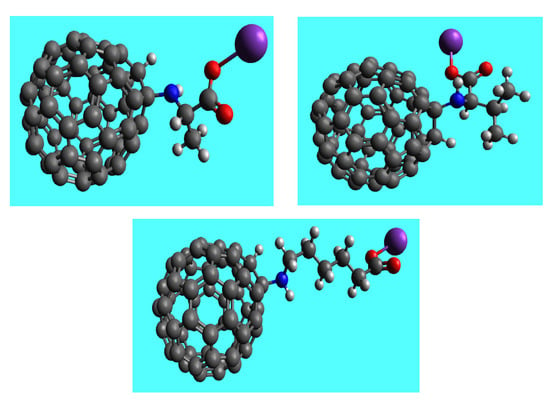
Figure 1.
Structural formulas: H-C60-L-Ala-OK (left), H-C60-D-Val-C60-OK (right), H-C60-ε-ACA-OK (bottom). Black spheres—C; grey spheres—H; blue spheres—N; red spheres—O; purple spheres—K+.
2.1.3. Strains and Cultivation Conditions
The following strains of the basidiomycetes were used: Flammulina velutipes (Curtis) Singer (strain 0535); Ganoderma applanatum (Persoon) Patouillan (strain 0154), both received from the Collection of the Department of Mycology and Algology of the M.V. Lomonosov Moscow State University (Moscow, Russia); and Laetiporus sulphureus (Bull.) Murrill (strain 120707) was obtained from the Collection of the Department of Botany of the Irkutsk State University (Irkutsk, Russia). Mushroom cultures were maintained on wort agar (4 degrees Balling) at 4 °C in the dark, pH 6–6.5.
After the complete overgrowth of Petri dishes, the mycelium-covered blocks from the agar medium were used as an inoculum of a liquid nutrient medium. The latter was composed of an aqueous solution of the dissolved nutritive agents (carbon and nitrogen sources), supplemented by the substances under investigation, i.e., water dispersion of nanoparticles of fullerene C60 or its amino acid derivatives. The following compositions of media (g/L of water) were used for culture: medium A–D-glucose 10; yeast extract 1; medium B–D-glucose 20; yeast extract—2; peptone 0.1.
The resulting concentrations in the nutrient medium of each compound (C60, H-C60-L-Ala-OK, H-C60-D-Ala-OK, H-C60-D-Val-OK, H-C60-ε-ACA-OK) formed a series of dilutions: 1.0 × 10−6 M, 1.0 × 10−7 M, 1.0 × 10−8 M, 1.0 × 10−9 M, 1.0 × 10−10 M, 1.0 × 10−11 M. For that, solutions of these compounds were prepared in a 50% (v/v) water-ethanol mixture to obtain concentrations of 1.0 × 10−4 M, 1.0 × 10−5 M, 1.0 × 10−6 M, 1.0 × 10−7 M, 1.0 × 10−8 M, 1.0 × 10−9 M, and then transferred to a nutrient medium under aseptic conditions at a volume ratio of 1:99. In the control experiments (in the absence of functionalized fullerene supplements), a corresponding portion of aqueous ethanol was introduced. The ethanol solutions implemented made it possible to avoid autoclaving the added compounds and their destruction under the influence of high temperatures and to exclude contamination by foreign microorganisms. Ultimately, the ethanol level in nutrient media ranged from 0.26 to 0.88%, i.e., at concentrations non-toxic for mushrooms.
The concentration dependence studies of the growth-stimulating effect of fullerene C60 and its amino acid derivatives under the conditions of a submerged culture of winter mushroom (F. velutipes), flat polypore (G. applanatum), and sulfur-yellow polypore (L. sulphureus) were carried out in the dark at a temperature of 25 °C for 18 days.
Since fullerene C60 is known as a compound capable of transferring photoexcitation energy to oxygen [40,41], the experiments were conducted to elucidate the role of light in the biostimulating properties of fullerene C60 and its amino acid derivatives when growing the basidiomycetes. These experiments were carried out at the following parameters of submerged cultivation: mode D—for 15 days at 25 °C in the dark; mode L—for 15 days at 25 °C under the natural 11-hour lighting per day in the laboratory room.
2.2. Methods
2.2.1. Mushroom Mycelium Biomass
Mushroom mycelium grown in submerged culture was separated from nutrient liquid, washed with distilled water, and then, dried to a constant mass in a laboratory oven at a temperature of 40 °C to collect dry mycelium [42]. Both fresh and dry mass values were obtained accordingly after weighing on analytical scales.
2.2.2. Hydrodynamic Radius of Particles
The hydrodynamic radius of nanoparticles formed by the studied compounds in aqueous solutions was determined by dynamic light scattering (DLS) with a Zetasizer Nano ZS (Malvern Instruments, GB) device. This method allows one to calculate the hydrodynamic radius (R) of spheres that would move in liquids at the same speed as the studied particles. Measurements of R were carried out by assessing the correlation function of intensity fluctuations of laser radiation at λ = 633 nm scattered on the particles of fullerene C60 or its N-monosubstituted amino acid derivatives at the angle of 173°. Measurements were carried out at a compound concentration of 1 × 10−4 M and a temperature of 25 °C. For each sample, 5 intensity distributions by particle size were recorded; each of them included an average of 10 measurements.
2.2.3. DPPH Scavenging Activity
To measure the content of antiradical antioxidants (AO) in the grown mycelium of basidiomycetes, this dry-ground raw material was extracted with 70% ethanol at a temperature of 24 °C for 24 h at a mass ratio of raw material to extracting agent of 1:300. The extracts were clarified by centrifugation at 4000 rpm for 15 min.
The absorption spectra were recorded using an Agilent Cary 60 spectrophotometer (USA).
Quantitative analysis of AO was performed based on a spectrometric observation of their interaction with the stable chromogen radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) [43,44]. 2.7 mL of 8.1 × 10−5 M DPPH solution in ethanol was added to the test sample, and the reaction system volume was brought to 3.6 mL with ethanol. The optical density was measured at the wavelength of 517 nm at 1 cm optical path length for the first 30 min of the experiment at 5 min intervals, and then every hour during the experiment. The last measurement was made 24 h after the reaction started, and this value was used for calculating the AO concentration. By varying the test sample volume, the radical DPPH conversion degree at experiment termination was controlled to be from 15 to 70% as measured by its optical absorption. The DPPH conversion degree was calculated by the formula:
where Dexp is the optical density of the reaction system 24 h after the reaction started; Dcontr is the optical density of the control solution; ζ is the DPPH conversion degree 24 h after mixing the reagents.
ζ = 1 − Dexp/Dcontr
In the control experiment, 70% ethanol was introduced into the reaction system instead of the extract solution.
Here is the formula for calculating the AO concentration (M) in the units of the reference compound gallic acid:
where [DPPH]0 is the initial concentration of DPPH in the reaction system, mol/L; Vsyst is the volume of the reaction system, mL; Val is the volume of the extract introduced into the reaction system, mL; 5 is the stoichiometric coefficient of the DPPH inhibition by gallic acid. The results were presented as the CAO quantities normalized to a weighed mass (mM/g).
CAO = [DPPH]0Vsystζ/(5Val)
2.2.4. Lipid Peroxidation
For determining the level of lipid peroxidation (LP) in the mycelial extracts under study, the content of malonic dialdehyde (MDA) was measured by a reaction with thio-barbituric acid (TBA). Extraction of the LP products from a dry-ground raw material was performed using 70% ethanol at a temperature of 24 °C for 24 h, with the mass ratio of raw material and extracting agent being 1:40. After extraction, the extracts were diluted with 1 mL of distilled water and clarified by centrifugation at 4000 rpm for 15 min.
MDA concentration was determined according to a widespread method [45]. Briefly, 1 mL of extract was mixed with 1 mL of a 0.65% solution of TBA in a 20% solution of trichloroacetic acid, heated in a water bath at a temperature of 96 °C, then cooled and centrifuged twice at 4000 rpm for 15 min. The resulting solution’s optical density value was measured at 440, 532, and 600 nm, and the MDA concentration was calculated by the formula:
where D440, D532, and D600 are optical densities at 440, 532, and 600 nm, respectively; 0.0571 is a ratio of molar extinction coefficients of the sucrose-TBA complex at 532 nm and 440 nm, 157,000 is a molar extinction coefficient of MDA. The results were presented as the quantities normalized to a weighed mass (microM/g).
[MDA] = (D532 − D600) − ((D440 − D600) × 0.0571)/157000
2.2.5. Statistical Processing of Results
The results were statistically processed using Microsoft Excel software. The data were presented as an average mean with a standard deviation or as a percentage. The values of the parametric Student criterion were found for a 95% level of significance.
3. Results and Discussion
Fullerene possesses hydrophobic properties, which is why in the aqueous medium, the hydrated fullerene C60 and its N-monosubstituted amino acid derivatives tend to form not true but colloidal solutions. In this case, the chemical and biological properties of fullerene C60 and its derivatives should be considered in conjunction with data on the sizes of nanoparticles that they form in water. The DLS data on the average size of particles formed by the compounds used in this study are presented in Table 1.

Table 1.
The average hydrodynamic radius of particles of fullerene C60 and its derivatives in studied water solutions.
Previously, we discovered a significant stimulating effect on the growth and development of pea (Pisum sativum L.) seeds resulting from their treatment with 10−9 M AAFD solutions [46]. In the present work, we have explored the AAFD impact on the biological productivity of some medicinal basidiomycetes during their submerged cultivation and the parameters associated with the level of lipid peroxidation (Table 2).

Table 2.
The influence of hydrated fullerene C60 and its amino acid derivatives at a concentration of 10−9 M on biomass and parameters of the oxidative metabolism of mycelium grown in the dark for 18 days on medium A at 25 °C.
For most samples, the introduction of the studied compounds into the submerged growth medium led to an increase in the mycelial mass harvested on the 18th day of cultivation. Simultaneously with the increase in biomass, in most experiments, a decrease in the level of secondary products of lipid peroxidation reacting with TBA (TBA-reactive species, TBARS) was observed. Furthermore, the most pronounced decrease in the LP level, as well as an increase in biomass yield, was observed in the Laetiporus sulphureus mushroom, which has an intrinsically high level of oxidative metabolism and LP products. The LP processes regulation is the key component of the growth-stimulating mechanism of solubilized forms of fullerene C60. A high level of oxidative processes is characteristic of the oxidative stress state.
The key role played by plant and mushroom extracts in scavenging the free radicals is played by phenolic compounds [47,48]. It is known that fullerene and its derivatives can affect the phenolic composition of plants. The authors of the work [49] showed that a presowing soaking of wheat seeds in 40 and 80 nM fullerenol solutions led to a decrease in the concentration of phenolic compounds in the 30-day seedlings grown under salt stress conditions. The addition of fullerene derivatives to the submerged cultivation medium was not followed by a noticeable tendency to increase or decrease the antioxidant content (CAO) of water–ethanol mycelial extracts from the grown mushrooms. Since the DPPH radical reacts mainly with the antioxidants acting via the hydrogen atom transfer mechanism, data on CAO could be interpreted as the total content of low-molecular-weight non-enzymatic antioxidants expressed in standard antioxidant units. The dynamics of the fungal low-molecular-weight antioxidants interaction with the DPPH radical obey an exponential function for a prolonged period before entering a plateau (Figure 2). Most of the basidiomycetes’ phenolic compounds are represented by phenolic acids [50,51]. The antioxidant capacity of phenolic acids, as well as other phenolic compounds, is known to be determined by hydroxyl groups, while their reactivity increases sharply with two hydroxyl substituents in ortho- or para-positions. Therefore, for example, it was shown that caffeic acid is a stronger antioxidant than ferulic acid and p-coumaric acid owing to the presence of the second OH group in an ortho position in relation to the first one [52].
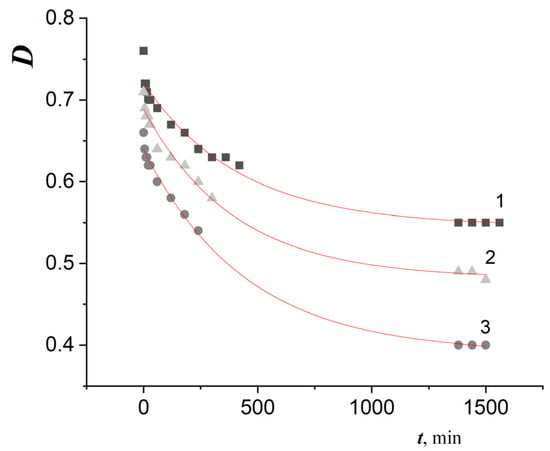
Figure 2.
The dynamics of DPPH optical density decrease at a wavelength of 517 nm in the course of adding water–ethanol extracts of mushroom mycelium grown in the dark on medium A for 15 days, at a temperature of 25 °C. 1—with 0.8 mL of F. velutipes extract, 2—with 0.5 mL of L. sulphureus extract, 3—with 0.3 mL of G. applanatum extract. [DPPH]0 = 6.1 × 10−5 M, the reaction system volume is 3.6 mL.
Data on the component composition of the antioxidants of the studied fungi indicate the presence in them of monophenols and polyphenols with meta-substitution, along with the structures described above. Their presence, along with the formation of C-C-dimers by the oxidized AO forms, could explain the prolonged period before entering the plateau of the radical DPPH interaction kinetic curves. In the extracts from the edible mushroom Flammulina velutipes fruit bodies, the presence of pyrogallol, homogentisic acid, sulfosalicylic acid, gallic acid, 5-caffeoylquinic acid, quercetin, and ferulic acid were found [53]. HPLC analysis of ethyl acetate and ethanol fractions of the L. sulphureus fruit bodies showed the presence of para-coumaric acid, quercetin, kaempferol, caffeic acid, (+)-catechin, gallic acid, 5-caffeoylquinic acid [54]. In the Ganoderma applanatum mushroom mycelium, by means of the HPLC-MS/MS method, para-hydroxybenzoic acid, protocatechuic acid, para-coumaric acid, vanillic acid, gallic acid, esculetin, caffeic acid, and syringic acid were found [55].
Earlier, we demonstrated that AAFDs have the ability to inhibit free-radical oxidation in various model systems, which seems to be one of the apparent mechanisms of their influence on oxidative homeostasis [56].
In the scientific literature, there is some information on the interaction of other fullerene-containing compounds with free radicals. By the EPR method implementing a spin trap 13C, benzyl radical addition to the fullerene C60 molecule in a toluene medium was shown [57]. The pulse radiolysis technique confirmed the efficient interaction of different carboxylated fullerenes with solvated electrons, hydroxyl radicals and singlet oxygen. When interacting with solvated electrons, the carboxylated derivatives of fullerene, according to the authors, form the monoanion radical [35]. Regarding the binding of the hydroxyl radical •OH by the fullerene hydroxylated derivatives, the proposed mechanisms are divisible into two types: (1) •OH captures H from a hydroxyl group with the formation of an etheric bond on the surface of the fullerene nucleus; (2) •OH attaches to the double bond of the fullerene skeleton [33]. Thus, fullerene C60 and its various derivatives are able to effectively bind various free radicals. However, on the other hand, there is evidence that fullerene is C60 and its derivatives are capable of transmitting excitation energy to oxygen with the formation of its reactive species, in particular, singlet oxygen [40]. In order to find out whether photochemical processes are involved in the mechanism of the growth-regulatory effect of fullerene C60 amino acid derivatives, an additional series of experiments on growing the basidiomycetes G. applanatum and L. sulphureus in the absence of light and under the conditions of 11-hour daylight periods at a temperature of 28 °C (Figure 3) was carried out.
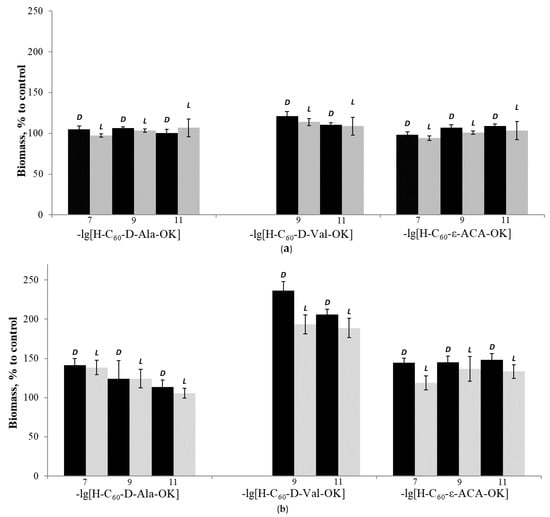
Figure 3.
Biomass of mushroom mycelium, % to control: (a)—G. applanatum; (b)—L. sulphureus after cultivation in the dark (D) and at the 11-hour daylight periods (L) for 15 days on a nutrient medium A, at 28 °C in the presence of different AAFD concentrations.
The results of these experiments demonstrated that within the concentration range of 10−7–10−11 M, despite a slight decrease in the biomass accumulation in a few modes of the “light” experiment compared to the “dark” experiment, the growth-regulatory activity of fullerene C60 and its amino acid derivatives is not associated with the potential ability of the studied compounds to generate singlet oxygen in the presence of light with the subsequent activation of the endogenous antioxidant protection system. Moreover, the capability of the fullerene C60 solubilized forms for growth stimulation in the absence of light is also indicative of the irrelevance of this activity in our earlier experiments with plants [46] to the fullerene’s impact on the photosynthetic apparatus of the plant cell.
The fullerene C60 solubilized forms being the growth stimulants for taxonomically distant groups representing the distinct kingdoms of the living world (plants and mushrooms) is indicative of these compounds’ activity mechanism based on the direct effect on fundamental, universal for all living organisms’ biophysical processes, primarily chain free radical oxidation, as well as effects on the physicochemical properties of biological membranes.
The observed growth-stimulating effects of hydrated fullerene C60 and its amino acid derivatives were not downregulated appreciably by varying their concentration in culture medium from 10−7 to 10−11 M (Figure 4, Figure 5 and Figure 6).
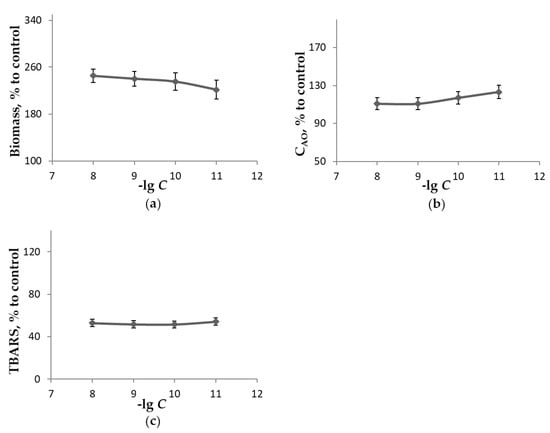
Figure 4.
The influence of H-C60-D-Ala-OK concentration when growing L. sulphureus on medium A in the absence of light for 18 days at 25 °C upon the measured parameters as a percentage of control: (a)—biomass of mycelium; (b)—concentration of low-molecular-weight antioxidants extractable with 70% ethanol; (c)—concentration of TBARS extractable with 70% ethanol.
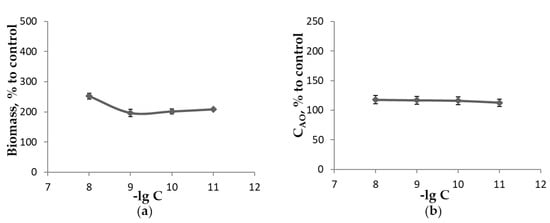
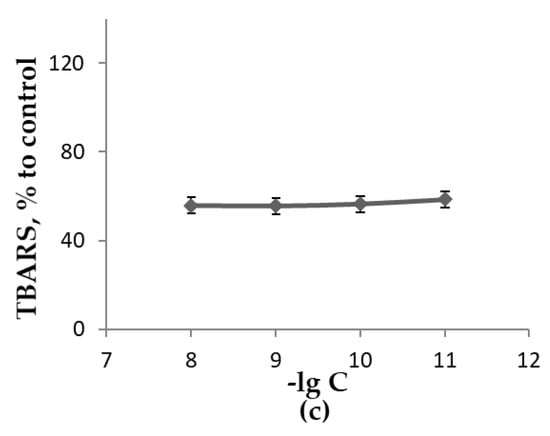
Figure 5.
The influence of H-C60-L-Ala-OK concentration when growing L. sulphureus on medium A in the absence of light for 18 days at 25 °C upon the measured parameters as a percentage of control: (a)—biomass of mycelium; (b)—concentration of low-molecular-weight antioxidants extractable with 70% ethanol; (c)—concentration of TBARS extractable with 70% ethanol.
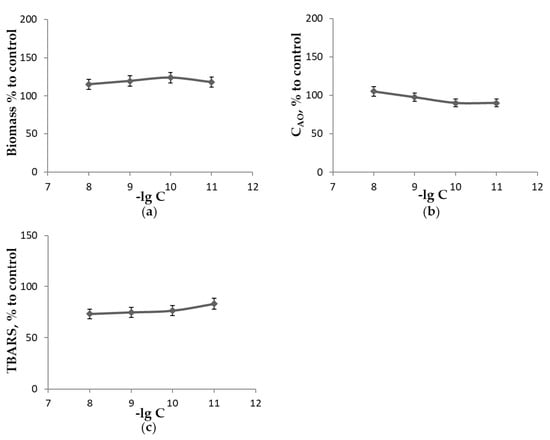
Figure 6.
The influence of unsubstituted fullerene C60 concentration when growing F. velutipes on medium A in the absence of light for 18 days at 25 °C upon the measured parameters as a percentage of control: (a)—biomass of mycelium; (b)—concentration of low-molecular-weight antioxidants extractable with 70% ethanol; (c)—concentration of TBARS extractable with 70% ethanol.
In the work [58], it was found by the radioactive labeling method that the carboxylated derivative of C61(CO2H)2, despite its solubility in water, was located in the membrane, as well as in the membranous cell organelles, including mitochondria, the main primary source of free radicals. The possibility of amino acid derivatives of fullerene C60-Ala, C60-Ala-Ala to penetrate through model phosphatidylcholine membranes was demonstrated [59].
We experimentally confirmed the assumption that the reason for the observed stimulating effect of the studied fullerene C60 water-soluble forms lies in their ability to participate in the regulation of oxidative metabolism. In line with this supposition are the results of F. velutipes culturing on nutrient media A and B, with the different content of nutrients at other conditions being equal. Fullerene C60 amino acid derivatives at high concentrations exerted a profound effect of an increase in biomass on nutrient medium A with a relatively low content of carbon and nitrogen sources (Figure 7), i.e., under the stress conditions induced by poor nutrition. When using the AAFD on medium B enriched with nutritive components, the biomass increment parameters turned out to be lower than the control values at the experiment modes with AAFD concentrations of 10−6–10−8 M. Therefore, it is reasonable to implement AAFD at their nanomolar concentrations.
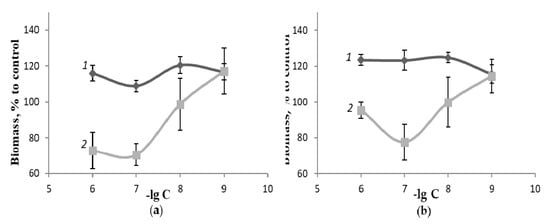
Figure 7.
Biomass of mycelium as a percentage of control when growing F. velutipes in the absence of light for 15 days at 25 °C: 1—on medium A, 2—on medium B, supplemented with different concentrations of H-C60-D-Ala-OK (a) and H-C60-ε-ACA (b).
4. Conclusions
Thus, the studied amino acid derivatives of fullerene C60 could serve as efficient stimulants of medicinal xylotrophic basidial fungi growth, with the highest effects found at the AAFD nanomolar concentrations. The observed growth-stimulating effects are associated with the regulation of oxidative metabolism by the studied water-soluble forms of fullerene C60, since in almost all cases, the positive AAFD action on fungal growth is accompanied by a decrease in the level of LP secondary products. Furthermore, the greatest increase in the biomass accumulation parameter caused by AAFD application takes place in mushrooms with a high level of oxidative metabolism. The fact that the AAFD growth-stimulating effect is manifested in representatives of both the higher plants and the kingdom of fungi is indicative of the relationship of this effect’s mechanism with the fundamental biophysical processes, such as free radical chain peroxidation. The AAFD under study can be of interest as additives to the nutrient medium when growing medicinal basidiomycetes aimed at enhanced mycelial biomass yield. The results of this research provide new information that supports the key role of free radical processes in regulating the positive influence of the fullerene C60 water-soluble derivatives on the organism’s growth and development.
5. Patents
RU 2,789,886 C1 Method for stimulating the growth of mycelium of basidiomycete fungi. Published 14 February 2023.
Author Contributions
Conceptualization, V.V. and V.M.; methodology, M.V. and O.T.; software, M.V.; validation, O.T. and M.V.; investigation, O.T. and M.V.; resources, V.R. and O.T.; data curation, V.V. and V.M.; writing—original draft preparation, M.V. and V.R.; writing—review and editing, O.T. and V.V.; supervision, V.V.; project administration, V.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the financial support of the Ministry of Science and Higher Education of the Russian Federation.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflict of interests.
References
- Dai, Y.C.; Yang, Z.L.; Ui, B.K.; Yu, C.J.; Zhou, L.W. Species diversity and utilization of medicinal mushrooms and fungi in China (review). Int. J. Med. Mushr. 2009, 11, 287–302. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, S.; Huang, M.; Xu, A. Antibacterial and antiviral value of the genus Ganoderma P. Karst. species (Aphyllophoromycetideae):A review. Int. J. Med. Mushrooms 2003, 5, 235–246. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Hayakawa, K.; Mitsuhashi, N.; Saito, Y.; Takahashi, M.; Katano, S.; Shiojima, K.; Furuta, M.; Niibe, H. Effect of Krestin (PSK) as adjuvant treatment on the prognosis after radical radiotherapy in patients with non-small cell lung cancer. Anticancer Res. 1993, 13, 1815–1820. [Google Scholar] [PubMed]
- He, Z.; Lin, J.; He, Y.; Liu, S. Polysaccharide-Peptide from Trametes versicolor: The Potential Medicine for Colorectal Cancer Treatment. Biomedicines. 2022, 10, 2841. [Google Scholar] [CrossRef]
- Fernandes, A.; Nair, A.; Kulkarni, N.; Todewale, N.; Jobby, R. Exploring Mushroom Polysaccharides for the Development of Novel Prebiotics: A Review. Int. J. Med. Mushrooms. 2023, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nakao, I.; Uchino, H.; Orita, K.; Kaido, I.; Kimura, T.; Goto, Y.; Kondo, T.; Takino, T.; Taguchi, T.; Nakajima, T.; et al. Clinical evaluation of schizophyllan (SPG) in advanced gastric cancer—A randomized comparative study by an envelope method. GanTo Kagaku Ryoho. 1983, 10, 1146–1159. [Google Scholar]
- Garcia, J.; Rodrigues, F.; Saavedra, M.J.; Nunes, F.M.; Marques, G. Bioactive polysaccharides from medicinal mushrooms: A review on their isolation, structural characteristics and antitumor activity. Food Biosci. 2022, 49, 101955. [Google Scholar] [CrossRef]
- Teplyakova, T.V.; Pyankov, O.V.; Safatov, A.S.; Ovchinnikova, A.S.; Kosogova, T.A.; Skarnovich, M.O.; Filippova, E.I.; Poteshkina, A.L. Water Extract of the Chaga Medicinal Mushroom, Inonotus obliquus (Agaricomycetes), Inhibits SARS-CoV-2 Replication in Vero E6 and Vero Cell Culture Experiments. Int. J. Med. Mushrooms 2022, 24, 23–30. [Google Scholar] [CrossRef]
- Wu, M.; Luo, X.; Xu, X.; Wei, W.; Yu, M.; Jiang, N.; Ye, L.; Yang, Z.; Fei, X. Antioxidant and immunomodulatory activities of a polysaccharide from Flammulina velutipes. J. Tradit. Chines Med. 2014, 34, 733–740. [Google Scholar] [CrossRef]
- Zhang, Z. Inhibitory effect of medicinal fungi on hepatitis B virus in vitro and in vivo. Bull. Beijing Med. Univ. 1989, 21, 455–459. [Google Scholar]
- Liu, J.; Zhorabek, F.; Dai, X.; Huang, J.; Chau, Y. Minimalist design of an intrinsically disordered protein-mimicking scaffold for an artificial membraneless organelle. ACS Cent. Sci. 2022, 8, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Ooi, V.E.; Liu, F. A review of pharmacological activities of mushroom polysaccharides. Int. J. Med. Mushrooms 1999, 1, 196–206. [Google Scholar] [CrossRef]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Oh, J.W. Mushroom polysaccharide-assisted anticarcinogenic mycotherapy: Reviewing its clinical trials. Molecules 2022, 27, 4090. [Google Scholar] [CrossRef]
- Smania, A.; Monache, F.D.; Smania, E.F.; Cuneo, R.S. Antibacterial activity of steroidal compounds isolated from Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) fruit body. Int. J. Med. Mushr. 1999, 1, 325–330. [Google Scholar] [CrossRef]
- Su, H.G.; Wang, Q.; Zhou, L.; Peng, X.R.; Xiong, W.Y.; Qiu, M.H. Functional triterpenoids from medicinal fungi Ganoderma applanatum: A continuous search for antiadipogenic agents. Bioorganic. Chem. 2021, 112, 104977. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.R.; Wang, Q.; Su, H.G.; Zhou, L.; Xiong, W.Y.; Qiu, M.H. Anti-adipogenic lanostane-type triterpenoids from the edible and medicinal mushroom Ganoderma applanatum. J. Fungi. 2022, 8, 331. [Google Scholar] [CrossRef]
- Li, X.-Y.; Li, S.-Y.; Yin, F.; Chen, H.-M.; Yang, D.-F.; Liu, X.-Q.; Jin, Q.-H.; Lv, X.-M.; Mans, D.; Zhang, X.-D.; et al. Antioxidative and Cytoprotective Effects of Ganoderma applanatum and Fomitopsis pinicola in PC12 Adrenal Phaeochromocytoma Cells. Int. J. Med. Mushrooms 2022, 24, 15–29. [Google Scholar] [CrossRef]
- Hossen, S.M.; Islam, M.J.; Hossain, M.R.; Barua, A.; Uddin, M.G.; Emon, N.U. CNS anti-depressant, anxiolytic and analgesic effects of Ganoderma applanatum (mushroom) along with ligand-receptor binding screening provide new insights: Multi-disciplinary approaches. Biochem. Biophys. Rep. 2021, 27, 101062. [Google Scholar] [CrossRef]
- Wang, J.; Sun, W.; Luo, H.; He, H.; Deng, W.Q.; Zou, K.; Liu, C.; Song, J.; Huang, W. Protective effect of eburicoic acid of the chicken of the woods mushroom, Laetiporus sulphureus (higher Basidiomycetes), against gastric ulcers in mice. Int. J. Med. Mushrooms 2015, 17, 619–626. [Google Scholar] [CrossRef]
- Khatua, S.; Ghosh, S.; Acharya, K. Laetiporus sulphureus (Bull.: Fr.) Murr. as food as medicine. Pharmacogn. J. 2017, 9, s1–s15. [Google Scholar] [CrossRef]
- Patocka, J. Will the sulphur polypore (Laetiporus sulphureus) become a new functional food. Glob. J. Med. Clin. Case Rep. 2019, 6, 006–009. [Google Scholar] [CrossRef]
- Zhang, Y.; Poon, K.; Masonsong, G.S.P.; Ramaswamy, Y.; Singh, G. Sustainable Nanomaterials for Bio-medical Applications. Pharmaceutics 2023, 15, 922. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.; Imran Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2022, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Maragani, S.; Huang, L.; Jeon, S.; Canteenwala, T.; Hamblin, M.R.; Chiang, L.Y. Synthesis of decacationic [60] fullerene decaiodides giving photoinduced production of superoxide radicals and effective PDT-mediation on antimicrobial photoinactivation EUR. J. Med. Chem. 2013, 63, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Vani, J.R.; Mohammadi, M.T.; Foroshani, M.S.; Jafari, M. Polyhydroxylated fullerene nanoparticles attenuate brain infarction and oxidative stress in rat model of ischemic stroke. EXCLI J. 2016, 15, 378–390. [Google Scholar] [CrossRef]
- Castro, E.; Garcia, A.H.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef] [PubMed]
- Panova, G.G.; Kanash, E.V.; Semenov, K.N.; Charykov, N.A.; Khomyakov, Y.V.; Anokona, L.M.; Artem’eva, A.M.; Kornyukhin, D.L.; Vertebnyi, V.E.; Sinyavina, N.G.; et al. Fullerene derivatives influence production process, growth and resistance to oxidative stress in barley and wheat plants. Agric. Biol. 2018, 53, 38–49. [Google Scholar] [CrossRef]
- Yin, J.-J.; Lao, F.; Fu, P.P.; Wamer, W.G.; Zhao, Y.; Wang, P.C.; Qiu, Y.; Sun, B.; Xing, G.; Dong, J.; et al. The scavenging of reactive oxygen species and potential for cell protection by functionalized fullerene materials. Biomaterials 2009, 30, 611–621. [Google Scholar] [CrossRef]
- Mironov, K.S.; Sinetova, M.A.; Shumskaya, M.; Los, D.A. Universal molecular triggers of stress responses in cyanobacterium Synechocystis. Life 2019, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Beuerle, F.; Lebovitz, R.; Hirsch, A. Antioxidant Properties of Water-Soluble Fullerene Derivatives. In Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes; Cataldo, F., Da Ros, T., Eds.; Springer Science & Business Media: Dordrecht, The Netherlands, 2008; Volume 1, pp. 51–78. [Google Scholar] [CrossRef]
- Chi, Y.; Bhonsle, J.B.; Canteenwala, T.; Huang, J.-P.; Shiea, J.; Chen, B.-J.; Chiang, L.Y. Novel watersoluble hexakis(4-sulfobutylfullerenes as potent free radical scavengers. Chem. Lett. 1998, 27, 465–466. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Lu, Z.; Gao, X. Syntheses, structures and antioxidant activities of fullerenols: Knowledge learned at the atomistic level. J. Clust. Sci. 2015, 26, 375–388. [Google Scholar] [CrossRef]
- Mirkov, S.M.; Djordjevic, A.N.; Andric, N.L.; Andric, S.A.; Kostic, S.A.; Bogdanovic, G.M.; Vojinovic-Miloradov, M.B.; Kvacevic, R.A. Nitric oxide-scavenging activity of polyhydroxylated fullerenol, C60(OH)24. Nitric. Oxide. 2004, 11, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Bensasson, R.V.; Brettreich, M.; Frederiksen, J.; Göttinger, H.; Hirsch, A.; Land, E.J.; Leach, S.; McGarvey, D.J.; Schönberger, H. Reactions of e−aq, CO2•−, HO•, O2•− and O2(1Δg) with a dendro [60]fullerene and C60[C(COOH)2]n (n = 2–6). Free Radic. Biol. Med. 2000, 29, 26–33. [Google Scholar] [CrossRef]
- Andreev, I.M.; Romanova, V.S.; Petrukhina, A.O.; Andreev, S.M. Amino-acid derivatives of fullerene C60 behave as lipophilic ions penetrating through biomembranes. Physic. Solid State 2002, 44, 683–685. [Google Scholar] [CrossRef]
- Schreiner, K.M.; Filley, T.R.; Blanchette, R.A.; Bowen, B.B.; Bolskar, R.D.; Hockaday, W.C.; Masiello, C.A.; Raebigeret, J.W. White-Rot Basidiomycete-Mediated decomposition of C60 Fullerol. Environ. Sci. Technol. 2009, 43, 3162–3168. [Google Scholar] [CrossRef] [PubMed]
- Andrievsky, G.V.; Kosevich, M.V.; Vovk, M.; Shelkovsky, V.S.; Vashchenko, L.A. On the production of an aqueous colloidal solution of fullerenes. J. Chem. Soc. Chem. Commun. 1995, 1281–1282. [Google Scholar] [CrossRef]
- Romanova, V.S.; Tsyryapkin, V.A.; Lyakhovetsky, Y.I.; Parnes, Z.N.; Vol’pin, M.E. Addition of amino acids and dipeptides to fullerene C60 giving rise to monoadducts. Russ. Chem. Bull. 1994, 43, 1090–1091. [Google Scholar] [CrossRef]
- Piotrovsky, L.B.; Eropkin, M.Y.; Eropkina, E.M.; Dumpis, M.A.; Kiselev, O.I. Mechanisms of biological action of fullerenes–dependence on aggregate state. Psychopharmacol. Biol. Narcology 2007, 7, 1548–1554. [Google Scholar]
- Heredia, D.A.; Durantini, A.M.; Durantini, J.E.; Durantini, E.N. Fullerene C60 derivatives as antimicrobial photodynamic agents. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100471. [Google Scholar] [CrossRef]
- Dudka, I.A.; Vasser, S.P.; Ellanskaya, I.A.; Koval, E.E.; Gorbik, L.T.; Bilay, V.I. Methods of Experimental Mycology: Handbook; Bilay, V.I., Ed.; Naukova Dumka: Kiev, Ukraine, 1982. (In Russian) [Google Scholar]
- Bondet, W.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH• Free Radical Method. Lebensm. Wiss U. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Volkov, V.A.; Sazhina, N.N.; Pakhomov, P.M.; Misin, V.M. Content and activity of low-molecular antioxidants in food and medicinal plants. Russ. J. Phys. Chem. B 2010, 4, 676–679. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified Thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Volkov, V.A.; Voronkov, M.V.; Misin, V.M.; Yamskova, O.V.; Romanova, V.S.; Kurilov, D.V.; Gagarina, I.N.; Pavlovskaya, N.E.; Gorkova, I.V.; Lushnikov, A.V. New plant growth stimulants based on water-soluble nanoparticles of N-substituted monoamino-acid derivatives of fullerene C60 and the study of their mechanisms of action. Biophysics 2020, 65, 635–641. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Puttaraju, N.G.; Venkateshaiah, S.U.; Dharmesh, S.M.; Urs, S.M.N.; Somasundaram, R. Antioxidant Activity of Indigenous Edible Mushrooms. J. Agric. Food Chem. 2006, 54, 9764–9772. [Google Scholar] [CrossRef]
- Shafiq, F.; Iqbal, M.; Ali, M.; Ashraf, M.A. Seed pre-treatment with polyhydroxy fullerene nanoparticles confer salt tolerance in wheat through up-regulation of H2O2 neutralizing enzymes and phosphorus uptake. J. Soil Sci. Plant Nutr. 2019, 19, 734–742. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef]
- Krsmanović, N.; Rašeta, M.; Mišković, J.; Bekvalac, K.; Bogavac, M.; Karaman, M.; Isikhuemhen, O.S. Effects of UV stress in promoting antioxidant activities in fungal species Trametes versicolor (L.) Lloyd and Flammulina velutipes (Curtis) Singer. Antioxidants 2023, 12, 302. [Google Scholar] [CrossRef]
- Hall, C. Sources of natural antioxidants: Oilseeds, nuts, cereals, legumes, animal products and microbial sources. In Antioxidants in Food: Practical Applications; Pokorn, J., Yanishlieva, N., Gordon, M., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2001; pp. 159–209. [Google Scholar]
- Kim, M.-Y.; Seguin, P.; Ahn, J.-K.; Kim, J.-J.; Chun, S.-C.; Kim, E.-H.; Seo, S.-H.; Kang, E.-Y.; Kim, S.-L.; Park, Y.-J.; et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M.; Agafonova, S.V. Antioxidant components of Laetiporus sulphureus (Bull.: Fr.) Murr. Fruit bodies. Appl. Biochem. Microbiol. 2011, 47, 419–425. [Google Scholar] [CrossRef]
- Rašeta, M.; Karaman, M.; Jakšić, M.; Šibul, F.; Kebert, M.; Novaković, A.; Popovićet, M. Mineral composition, antioxidant and cytotoxic biopotentials of wild-growing Ganoderma species (Serbia): G. lucidum (Curtis) P. Karst vs. G. applanatum (Pers.) Pat. Int. J. Food Sci. Technol. 2016, 51, 2583–2590. [Google Scholar] [CrossRef]
- Volkov, V.A.; Voronkov, M.V.; Sazhina, N.N.; Kurilov, D.V.; Vokhmyanina, D.V.; Yamskova, O.V.; Martirosyan, Y.T.; Atroshenko, D.L.; Martirosyan, L.Y.; Romanova, V.S. Mechanism of the antioxidant activity and structure–activity relationship of N-monosubstituted amino acid derivatives of fullerene C60. Kinet. Catal. 2021, 62, 395–403. [Google Scholar] [CrossRef]
- Krustic, P.J.; Wasserman, E.; Keizer, P.N.; Morton, J.R.; Preston, K.F. Radical reaction of C60. Science 1991, 254, 1183. [Google Scholar] [CrossRef]
- Foley, S.; Growley, C.; Smaihi, M.; Bonfils, C.; Erlanger, B.F.; Seta, P.; Larroque, C. Cellular localisation of a water-soluble fullerene derivative. Biochem. Biophys. Res. Commun. 2002, 294, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Kotelnikova, R.A.; Kotelnikov, A.I.; Bogdanov, G.N.; Romanova, V.S.; Kuleshova, E.F.; Parnes, Z.N.; Volpin, M.E. Membranotropic properties of the water-soluble amino acid and peptide derivatives of fullerene C60. FEBS Lett. 1996, 389, 111–114. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).