Trends of Nanoencapsulation Strategy for Natural Compounds in the Food Industry
Abstract
1. Introduction
2. Nano Encapsulations System
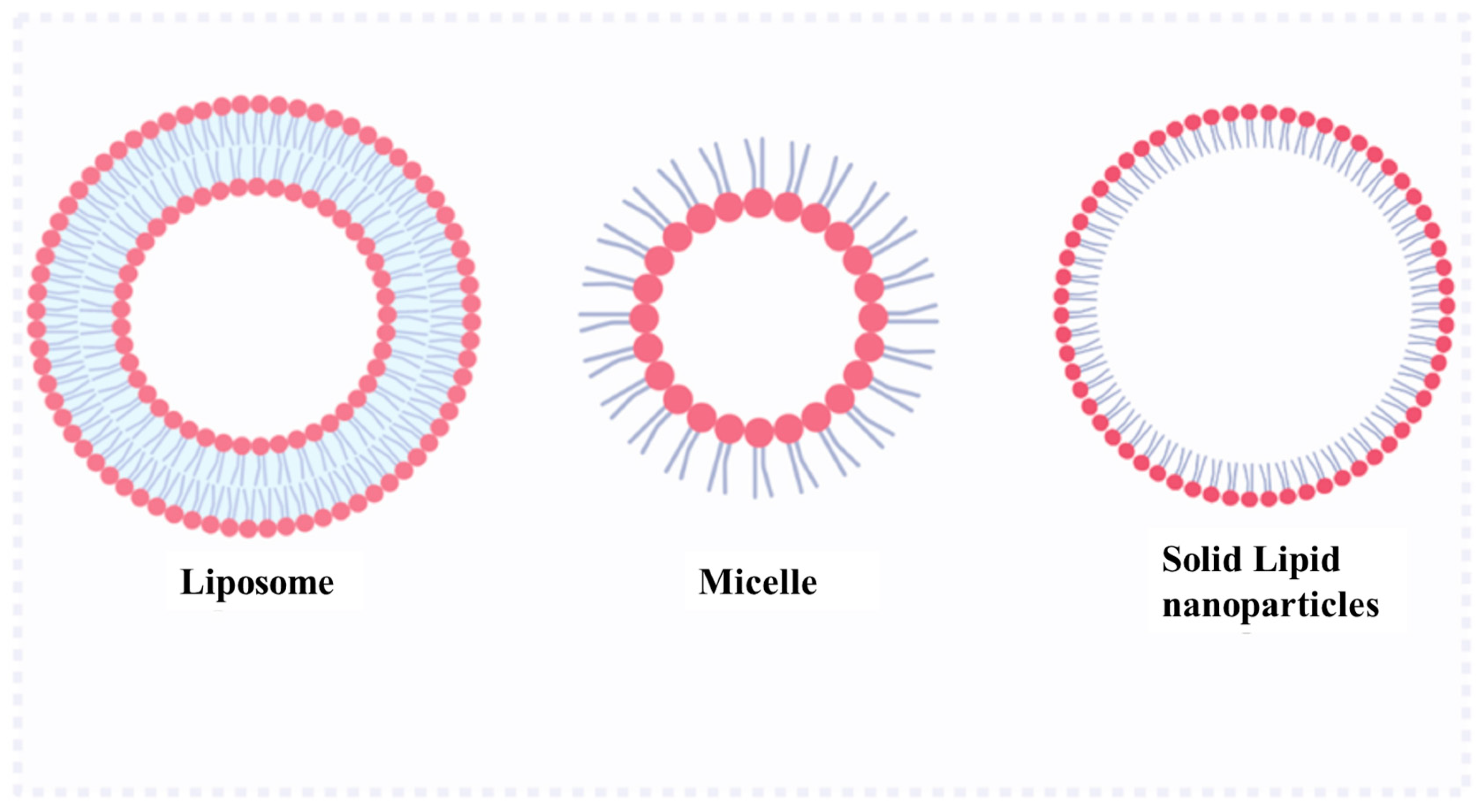
2.1. Lipids Systems
2.1.1. Liposome
2.1.2. Micelle
2.1.3. Solid Lipid Nanoparticles
2.2. Polymer Systems
2.2.1. Alginate
2.2.2. Carrageenan
2.2.3. Chitosan
2.2.4. Cyclodextrin
2.2.5. Pectin
2.2.6. Polyethylene Glycol (PEG)
2.2.7. Polylactic Acid
2.2.8. Protein
2.3. Metallic Systems
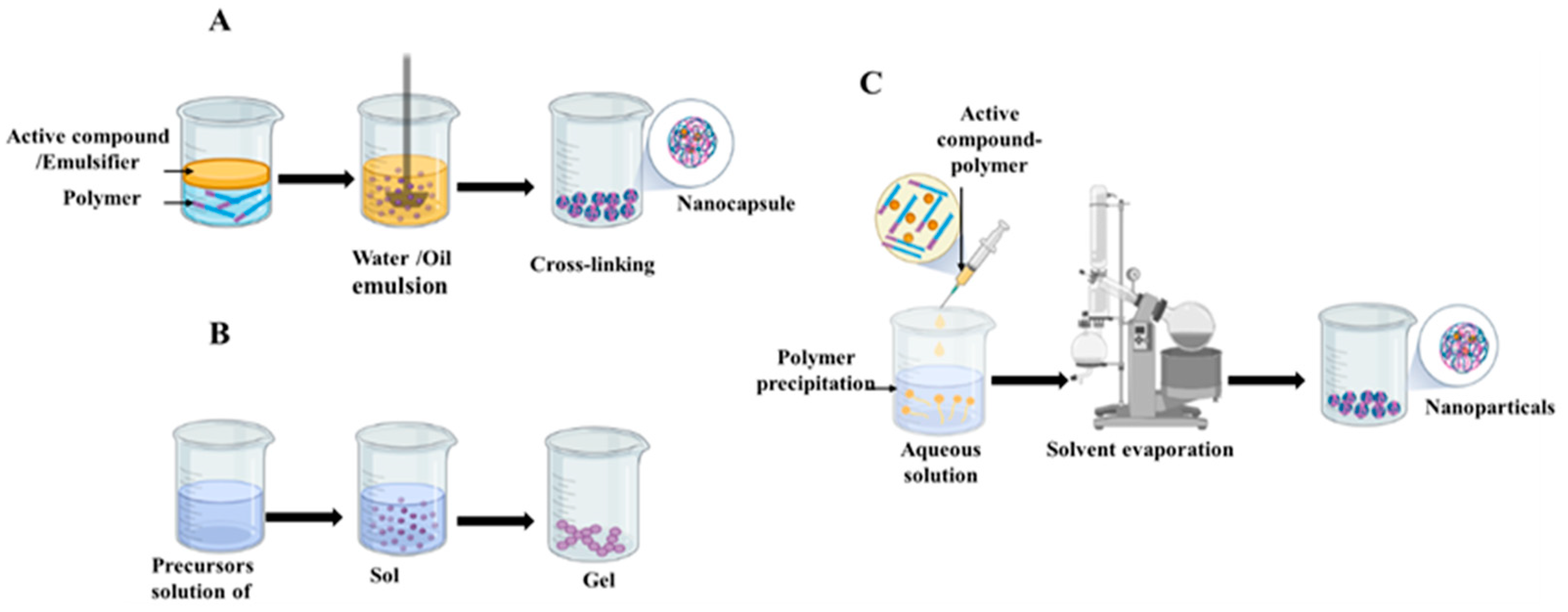
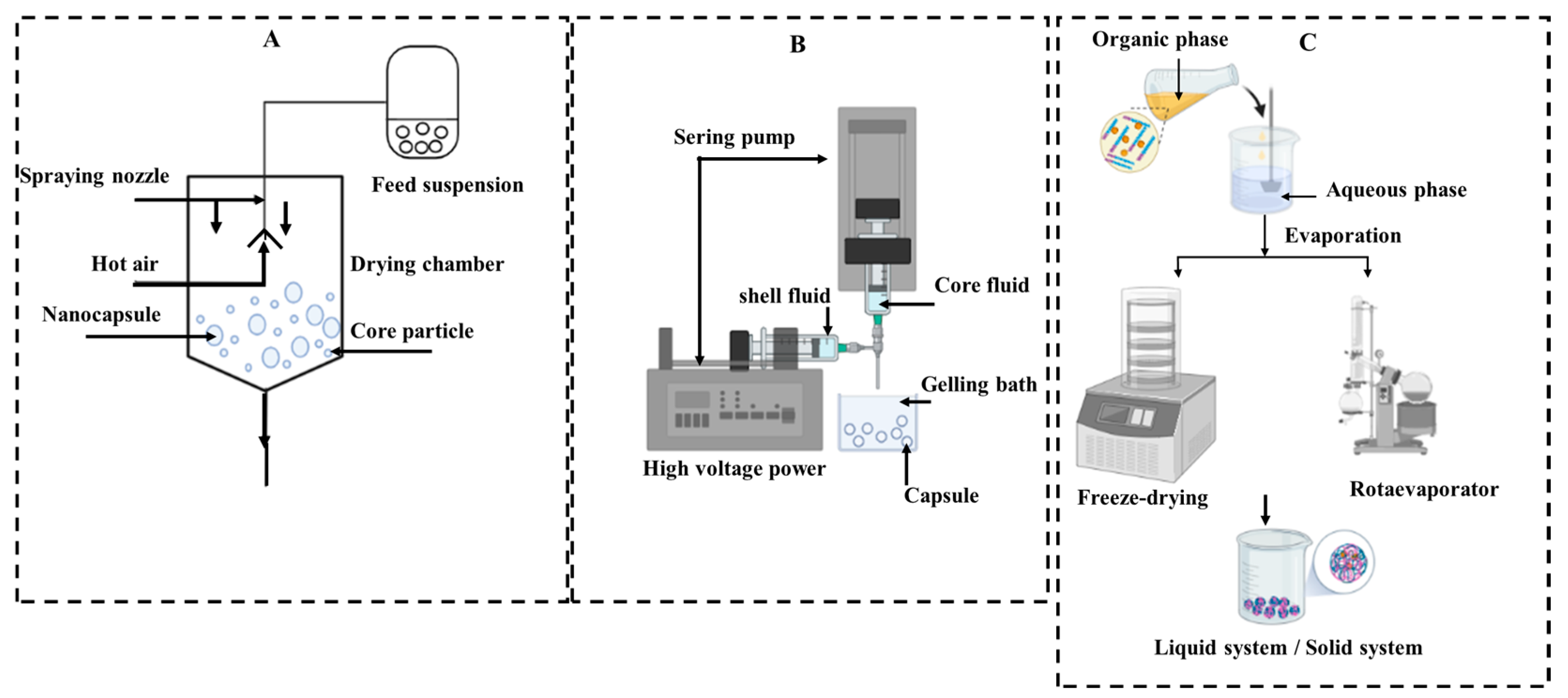
3. Preparation Methods
3.1. Chemical Techniques
3.2. Physicochemical Technique
3.3. Physical-Mechanical Technique
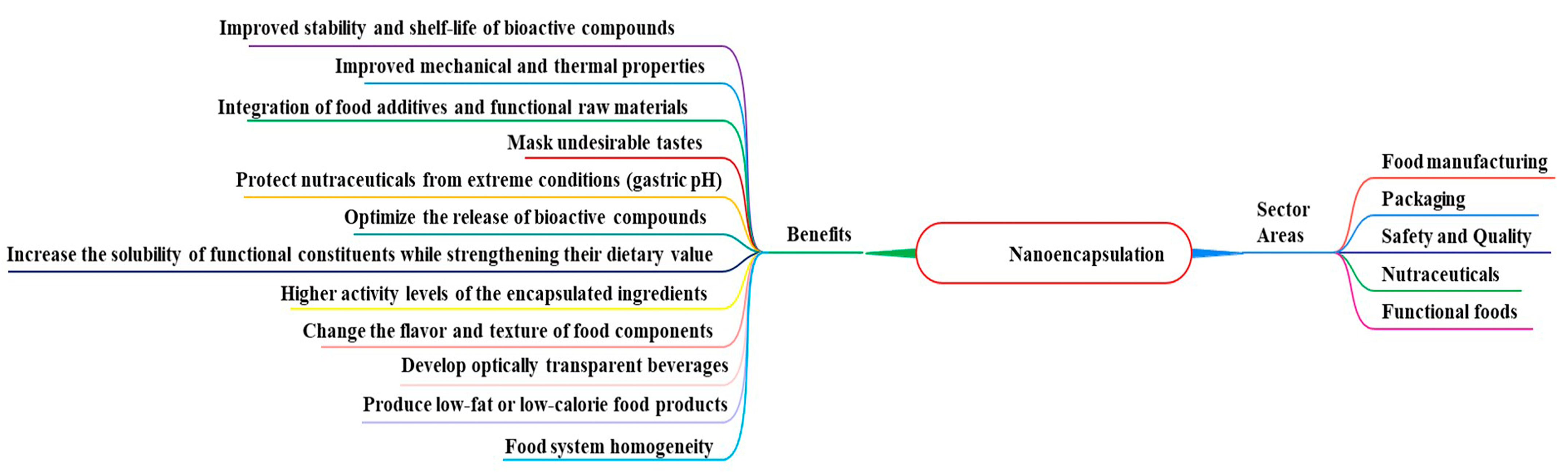
4. Nanoencapsulation Systems Advantages in the Food Industry
5. Case Studies of the Application of Natural Compound Nanoencapsulation in the Food Industry
5.1. Incorporation into Food Matrices
5.1.1. Dairy Products and Beverages
| Agent | Nanoencapsulation Systems | Preparation Technique | Biological Activity | Food Application | Reference |
|---|---|---|---|---|---|
| VitD3 | Liposome | Thin-film dispersion | Improve the usage of spray-dried whey powder as a functional ingredient. | Spray-dried whey powder | [19] |
| Clove Essential Oil (CEO) | Polyethylen glycol | Nanoemulsions | Conservation/ antioxidant potential. | Industrial fresh double cream cheese | [99] |
| Chlorogenic acids | Beta-cyclodextrin | Lyophilization | Polyphenols effect/the profile of volatile aroma compounds. | Caramel cottage cheese | [100] |
| Olive leaf extract | Liposomes | Ethanol injection | Examined considering syneresis, antioxidant activity, pH, acidity, color, and sensorial properties. | Yogurt | [101] |
| Phenolic extract of jaboticaba | Lipid | Nanoemulsions | Evaluated the total phenolic content/cow milk antioxidant activity. | Cow milk | [103] |
| skipjack tuna eyeballs oil (STEO-NL) | Liposomes | Ethanol injection | Fortification | Cow milk | [104] |
| VitD | Lipid | / | Evaluate the effect of 1500 IU nanoencapsulated vitamin D used for fortifying. | Milk and yogurt | [105] |
| VitD | Precirol (Solid lipid) | Homogenization ultrasound | Fortifying dairy Products. | Milk/yogurt | [106] |
| VitD | Lipid | Homogenization with ultrasound | Fortified effect/anti- oxidant balance in adults. | Yogurt | [107] |
| Curcumin | Polyvinylpyrrolidone (PVP) | Solvent | Evaluated the fight against microbial contamination. | Orange juice | [108] |
| Bioactive Compounds Extracted from Egyptian Prickly Pears Peel Fruit | Sodium alginate and chitosan | Solvent evaporation | Protect and improve their stability and bioavailability and evaluate their activity comparing to synthetic ones. | Guava juice | [109] |
| Wheat germ oil (WGO) | Casein micelles | pH changes and ultra-sonication | Fortification of dairy Product. | Labneh | [110] |
| Fish oil | Gum Arabic | Freeze drying | Protecting against environmental conditions. | Fermented milk | [111] |
| Phenolics extract of grape and apple pomace | Chitosan and soy protein | Nanoemulsions | Enhancement of the native antioxidant activity of commercial apple and pineapple juices. | Apple and pineapple juices | [112] |
5.1.2. Meat and Meat Products
| Agent | Nanoencapsulation System | Preparation Technique | Biological Activity | Food Application | Reference | |
|---|---|---|---|---|---|---|
| Cumin essential oil, | Chitosan | Ionic gelation | Growth performance/ immune responses. | Broiler chickens | [115] | |
| Melastoma malabathricum L. Fruit extract (Anthocyanin) | Chitosan | Ionic gelation | Blood lipid profile. | Feed additive | Broiler chicken | [117] |
| Curcumin | Solid lipid nanoparticles | Solvent -evaporation | Growth performance, immune response/ antioxidant activity. | Broilers chickens | [118] | |
| Garlic essential oil | Chitosan | Ionic gelation | Antibacterial//antioxidant activities /growth performance. | Broiler chickens | [119] | |
| Garlic extract | Metal nanoparticles (Calcium) | / | Antioxidant status, lipids profile, immune response. | Broiler chickens | [120] | |
| Mint (Mentha piperita), thyme (Thymus vulgaris), and cinnamon (Cinnamomum Verum) essential oils | Chitosan | Ionic gelation | Growth performance, immune, and intestinal bacteria. | Broiler chickens | [122] | |
| Phaleria macrocarpa fruits extract | Chitosan | Ionic gelation | Antioxidant and antimicrobial activities, and growth performance. | Chickens | [123] | |
| Peppermint (Mentha piperita) Extract | Alginate | Emulsion | Growth performance, blood parameters/immune response. | Broiler chickens | [132] | |
| Silymarin Seeds (Silybum marianum) | Alginate | Emulsion | Antioxidant/hepatoprotective activities. | Broiler chickens | [133] | |
| Flaxseed oil: omega-3 fatty acids | Protein-sodium alginate | Nanoemulsions | Targeted delivery/bioavailability/growth performance/blood lipid profile. | Enrich broiler meat | [134] | |
| Red onion (Alyssum homolocarpum) and seed gum (Lepidium sativum ) extract | Biopolymeric | Nanoemulsion | Antioxidant and antimicrobial activities. | Conservation | Beef fillet | [135] |
| Pomegranate (Punica granatum L.) peel extract | Alginate nanospheres | Nanoemulsion | Antimicrobial activity. | Chicken meat | [136] | |
| clove essential oil (Eugenia Caryophyllata) | Lipid nanoparticles | Nanoemulsion | Antibacterial and antioxidant activities. | Chicken fillets | [124] | |
| Lemon Essential Oil | Chitosan | Nanoemulsion | Antioxidant activity. | Fish burger | [125] | |
| Allium sativum L. essential oil (garlic) | Liposomes | / | Antimicrobial activity. | Beef meat-based hamburger | [126] | |
| Trachyspermum Ammi essential oil | Alginate | Nanoemulsion | Antibacterial activity (against Listeria monocyte genes). | Turkey fillets | [127] | |
| Satureja khuzestanica essential oil | Liposomes | / | Antibacterial and antioxidant activities. | Lamb meat | [128] | |
| Fish oil: omega-3 fatty acids | Liposome | Solvent evaporation | Meat quality and nutritional value. | Enrich pork meat | [129] | |
| Raw and processed chicken breast lipid | β-Cyclodextrin | Nanoemulsion | Lipid protection. | Chicken | [130] | |
| Rosmarinus officinalis essential oil | Chitosan-benzoic/acid nanogel | Solvent evaporation | Antibacterial activity. | Beef cutlet | [137] | |
| Star anise essential oil, Polylysine, and Nisin | Protein isolate | Nanoemulsion | Antimicrobial activity. | Yao meat | [138] | |
| Thyme essential oil (Thymus vulgaris L.) | Chitosan | Nanoemulsion and ionic gelation | Antimicrobial and antioxidant activities. | Beef burgers | [139] | |
| Jujube gum andnettle oil | / | Nanoemulsion | Antimicrobial and antioxidant activities. | Beluga sturgeon fillets Seyed | [140] | |
| Eugenol | Gelatin-chitosan | Solvent evaporation | Antibacterial and antioxidant activities. | Chilled pork | [141] | |
| Laurus nobilis leaf extract | Liposome | Nanoemulsion | Oxidative, microbial, bacterial and sensory properties. | Minced beef | [142] |
5.2. Food Packaging
| Agent | Nanoencapsulation System | Preparation Technique | Biological Activity | Reference |
|---|---|---|---|---|
| Pomegranate peel | Alginate | Emulsification | Antimicrobial | [136] |
| Tea tree oil | Beta-cyclodextrin (β-CD) | Co-precipitation | Antibacterial activity | [144] |
| Chrysanthemum essential oil | Chitosan | Electrospinning | Antibacterial | [146] |
| Essential oil (Ocimum basilicum Ocimum gratissimum) | Polylactic acid | Solution blow spinning | Antifungal activity-antiocratoxigenic activity | [147] |
| Thymol | Polylactic acid Poly (ε-caprolactone) | Electrospinning | Release behavior, antibacterial activity, antioxidant activity | [148] |
| Thymol | Chitosan | Emulsification | Antifungal | [149] |
| α-tocopherol | Solid lipid nanoparticles | High homogenization technique | Antioxidant | [150] |
| Anthocyanins | Pectin, Sodium alginate, Cellulose nanocrystals | Hydrogel film | Antioxidant activity | [151] |
| Garlic essential oil | Liposomal, chitosan | Thin-layer hydration-sonication | Antioxidant activity, Microbial and chemical changes | [152] |
| Oregano essential oil (Carvacrol) | β-cyclodextrin | Kneading | Controlled EO release, antioxidant activity | [153] |
| Gallic acid | Hydroxypropyl methylcellulose | Electrospinning | Antioxidant activity | [154] |
| Phycocyanin | Polymers (PLLA: PVA) | Electrospinning/electrospraying | Antioxidant activity | [155] |
| Cinnamodendron dinisii essential oil | Chitosan-zein | Precipitation/casting method | antioxidant activity and antimicrobial activity | [156] |
| Anthocyanins | Chitosan/Alginat/Protein | Electrospinning | Antioxidant/antibacterial | [157] |
| Clove essential oil | Protein (ZEIN-sodium caseinate) | Modified antisolvent precipitation | Antimicrobial, antibacterial activity | [158] |
| Eugenol | Poly (lactic acid)/nanoparticles (MgO and ZnO/Gelatin | Electrospinning | Antioxidant activity, Antibacterial activity | [159] |
| Mentha spicata L. essential oil | Protein (Sodium caseinate)/MgO nanoparticles | Electrospinning | Antimicrobial activity | [160] |
| Cuminaldehyde | Hydroxypropyl-β-cyclodextrin | Electrospinning | Antibacterial | [161] |
| Coconut oil and solid hydrogenated palm oil (cinnamaldehyde, eugenol, and thymol) | Solid lipid nanoparticles | Nanoemulsion | Antifungal (Rhizopus stolonifer, Alternaria spp., and Aspergillus niger) | [162] |
| Cinnamon and oregano essential oils | Chitosan/polyvinyl alcohol/b-cyclodextrin | Electrospinning | Antifungal activity | [163] |
5.3. Dietary Supplements/Nutraceuticals and Functional Foods
| Agent | Nanoencapsulation Systems | Preparation Technique | Biological Activity | Reference |
|---|---|---|---|---|
| Curcumin | Saponin micelles nanoparticles | pH-driven loading method | The curcumin nanoparticles greatly increased its in vivo bioavailability. | [168] |
| Corn oil/sodium hydroxide | Nanoemulsions | The developed curcumin nanoemulsions gave bio accessibilities that were similar to those of the best commercial formulation tested. | [169] | |
| Nano-micelle soft gel capsules | Industrial product (Exir-Nano-Sina, IRC:1228225765) | A 12-week supplementation with 80 mg/day nano-micelle curcumin improves mean malondialdehyde, total antioxidant capacity, and adiponectin in patients with metabolic syndrome. | [170] | |
| Keto-Curcumin | Lipid systems | Nanoemulsion | Preserving the antioxidant activity and improving their stability. | [180] |
| Curcumin and Vit. D3 | Nanoliposomes | Continuous supercritical CO2 | Improving the stability and antioxidant activity. | [181] |
| Quercetin and Curcumin | Micelles/casein | Vigorous shaking | Encapsulation systems revealed high entrapment efficiency and bio-protection. The cytotoxicity of co-encapsulated bioactives was higher than that of free forms. | [182] |
| Vitamin D3 and Curcumin | Lipid-core nanocapsules | Interfacial deposition of preformed polymer technique | Regulating inflammation and purine metabolism in a model of arthritis. | [183] |
| Anthocyanins | Nanocomplexes of chitosan hydrochloride/carboxymethyl chitosan | Electrostatic interaction | Encapsulated form improved the stability. | [184] |
| Chitosan nanoparticles | Ionotropic gelation | Attenuates hyperlipidemic aberrations in male Wistar rats by inhibiting lipid peroxidation, increasing antioxidant enzyme activity and suppressing development of lipogenesis. | [185] | |
| Quercetin | Cellulose nanofibers | Nano-formulations | Nano-formulation exhibited excellent dietary performance and good antioxidant capacity that was superior to nonencapsulated and displayed a sustained release in an in vitro experiment. | [173] |
| Soluble soybean polysaccharide/chitosan | pH-driven encapsulation procedure | Encapsulated quercetin exhibits better biological activity and enhanced solubility in an aqueous solution. | [186] | |
| Chitosan/lecithin | Electrostatic deposition | The storage stability and antioxidant activity were improved. | [187] | |
| Catechin | Nanocyclodextrin/metal/organic | Solvothermal assisted ultrasound | Improving the storage stability and exhibiting superior bioavailability. | [172] |
| Starch nanoparticles | Ultrasonication treatment and homogenization | Bioactive properties were retained at a higher level upon in vitro digestion. | [188] | |
| Epigallocatechin gallate | Double shell material chitosan recombinant soybean seed H-2 ferritin | Electrostatic interactions | Promote stabilization and absorption. | [189] |
| Carotenoids | TritonX100/Tween 80 | Nanoemulsions | Retention of antioxidant efficiency. | [190] |
| β-carotene | Pure pullulan/whey protein isolate | Coaxial electrospinning | Provided greater protection against oxidative degradation under different storage temperatures. Improved stability at low humidity environments | [167] |
| Long or medium chain triglycerides | Excipient nanoemulsions | Nanoemulsions have considerable potential for improving nutraceutical bioavailability from dietary supplements. | [191] | |
| Vit.C and β-Carotene | Liposomes (lecithin, cholesterol/phosphate-buffer solution | Ethanol injection | Improving the storage stability and bioavailability. | [192] |
| Hydroxytyrosol | Polyethyleneglycol/polyethyleneimine nanogels | Multi-step procedure (activation of PEG; PEI functionalization; formation of nanogel network) | Nanogels improved therapeutic effects against hepatic steatosis (significant decrease in the intracellular triglyceride levels, restoring cell viability). | [171] |
| Picrorhiza kurroa extract | Pluronic-F-68 copolymer-based biodegradable PLA nanoparticles | Nanoprecipitation | Provides nutraceutical with increased suitability for better hepato-protection by enhancing intestinal absorption and bioavailability. | [193] |
| Phenolic extracts | Liposomes | Nanoemulsions | Protection of phenolic compounds under stomach conditions and increase their bioaccessibility. | [194] |
| Bioactive compounds of Tinospora cordifolia leaf extract | Whey protein isolate | Electrospray | Increase of 28.12% in vitro antidiabetic activity. | [166] |
| Saffron extract | Tragacanth/zein nanofibers | Electrospinning | Enhancing thermal stability. | [164] |
| Saffron | Nanoliposomes | Heating method | Increase solubility. | [165] |
| Fucoxanthin | Casein/chitosan | Homogenization, and injection into the electrospray system | The nanomaterial improved water solubility and bioavailability. | [195] |
| Crocin, Safranal/Picrocrocin | Pectin/whey protein/water–oil | Nanoemulsions and nanodroplets | High storage stability and controlled release. | [196] |
| Fish oil | Caseinate/glycated caseinate | Nanoemulsions | Improved stability during in vitro digestion, increased oxidative stability, and reduced the rate of lipolysis. | [197] |
| Fish oil and Garlic essential oil | Persian gum/chitosan | Electrostatic layer-by-layer deposition | Improve physicochemical characteristics and thermal stability (excellent thermal stability of >250 °C). | [174] |
| Omega-3 fatty acids | Solid lipid nanoparticle | Emulsion | Enhancing the growth inhibitory effects of ω-3 PUFAs in human HT-29 CRC cells after encapsulation. | [198] |
| Lipid | Nanoemulsion | Nanoparticles caused greater and more sustained formation of NO and enhanced their anti-aggregatory effects. | [199] | |
| Probiotic strains | Starch/sodium alginate | Electrospinning | The viability rate of lactobacilli and bifidobacteria strains in the acidic environment and simulated gastrointestinal condition enhanced significantly. | [200] |
| Vit. D3 | Ovalbumin-pectin nano complexes | Antisolvent precipitation | Enhanced storage stability and sustained release in simulated gastrointestinal digestion. | [175] |
| Lipid | Nanoemulsion | Improvement of bioaccessibility, bioavailability, and stability. | [176] | |
| Lipid | Homogenization/sonication | Improved bioavailability. | [201] | |
| Oil (ethylene glycol, kolliphor-RH-40 in-water emulsion | Sonication | Increased gut bioavailability, and improved stability. | [202] | |
| Lipid | Hot high-pressure homogenization | Improved the oral bioavailability. | [203] | |
| Nano-niosomes (Span 60 and Tween 80/isopropyl alcohol) | Thin layer hydration and sonication | Increasing effects on encapsulation efficiency and antioxidant capacity. | [204] | |
| Vit. C | Modified cellulose nanocrystal/chitosan nanocapsules | Ionic gelation | Improving the stability, antimicrobial activity, and vitamin release. | [177] |
| Nanoliposomes | Thin-film evaporation | Control the stability. | [205] | |
| Vit. B2 | Alginate/chitosan | Ionotropic polyelectrolyte/pre-gelation | Increase vitamin stability and release. | [206] |
| Vit. B9 (folic acid) | Casein nanoparticles | Coacervation/dried using spray-drying | Preventing its release in an acid environment and promoting its oral bioavailability through in vitro and in vivo studies. | [207] |
| Liposome | Proliposome/suspension | Prolonged release and solubility of folic acid. | [208] | |
| Vit. A | Lipid nanoparticle | Organic solvent-free sonication | Biocompatibility of the formulations showed no toxicity in fibroblasts and assured oral bioaccessibility. | [179] |
6. Nanoencapsulation Application in Food Safety Sensors
7. Challenges and Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loh, H.C.; Seah, Y.K.; Looi, I. The COVID-19 pandemic and diet change. Prog. Microbes Mol. Biol. 2021, 4, 1. [Google Scholar] [CrossRef]
- De Abreu Figueiredo, J.; de Paula Silva, C.R.; Oliveira, M.F.S.; Norcino, L.B.; Campelo, P.H.; Botrel, D.A.; Borges, S.V. Microencapsulation by spray chilling in the food industry: Opportunities, challenges, and innovations. Trends Food Sci. Technol. 2022, 120, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Rajoka, M.S.R.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present, and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Mudgal, D.; Anand, R.; Jindal, S.; Mishra, V. Recent Development in Nanoencapsulation and Delivery of NaturalBioactives through Chitosan Scaffolds for Various Biological Applications. Int. J. Biol. Macromol. 2022, 220, 537–572. [Google Scholar] [CrossRef] [PubMed]
- Guía-García, J.L.; Charles-Rodríguez, A.V.; Reyes-Valdés, M.H.; Ramírez-Godina, F.; Robledo-Olivo, A.; García-Osuna, H.T.; Cerqueira, M.A.; Flores-López, M.L. Micro and Nanoencapsulation of Bioactive Compounds for Agri-Food Applications: A Review. Ind. Crops Prod. 2022, 186, 115198. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef]
- Assadpour, E.; Mahdi Jafari, S. A Systematic Review on Nanoencapsulation of Food Bioactive Ingredients and Nutraceuticals by Various Nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef]
- Hosseini, H.; Jafari, S.M. Introducing Nano/Microencapsulated Bioactive Ingredients for Extending the Shelf-Life of Food Products. Adv. Colloid Interface Sci. 2020, 282, 102210. [Google Scholar] [CrossRef]
- Norkaew, O.; Thitisut, P.; Mahatheeranont, S.; Pawin, B.; Sookwong, P.; Yodpitak, S.; Lungkaphin, A. Effect of Wall Materials on Some Physicochemical Properties and Release Characteristics of Encapsulated Black Rice Anthocyanin Microcapsules. Food Chem. 2019, 294, 493–502. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S. Green Biopolymers from By-Products as Wall Materials for Spray Drying Microencapsulation of Phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Maqsoudlou, A.; Assadpour, E.; Mohebodini, H.; Jafari, S.M. The influence of nanodelivery systems on the antioxidant activity of natural bioactive compounds. Crit. Rev. Food Sci. Nutr. 2022, 62, 3208–3231. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Micro and nanoencapsulation of natural colors: A holistic view. Appl. Biochem. Biotechnol. 2021, 193, 3787–3811. [Google Scholar] [CrossRef] [PubMed]
- Momin, J.K.; Joshi, B.H. Nanotechnology in Foods. In Nanotechnologies in Food and Agriculture; Rai, M., Ribeiro, C., Mattoso, L., Duran, N., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 3–24. ISBN 978-3-319-14023-0. [Google Scholar]
- Sahoo, M.; Vishwakarma, S.; Panigrahi, C.; Kumar, J. Nanotechnology: Current Applications and Future Scope in Food. Food Front. 2021, 2, 3–22. [Google Scholar] [CrossRef]
- Mohammad, Z.H.; Ahmad, F.; Ibrahim, S.A.; Zaidi, S. Application of Nanotechnology in Different Aspects of the Food Industry. Discov. Food 2022, 2, 12. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.; Macena, M.; Guiné, R.P.F. Application of Nanotechnologies along the Food Supply Chain. Open Agric. 2021, 6, 749–760. [Google Scholar] [CrossRef]
- Sharma, A.; Nagarajan, J.; Gopalakrishnan, K.; Bodana, V.; Singh, A.; Prabhakar, P.K.; Suhag, R.; Kumar, R. Nanotechnology Applications and Implications in Food Industry. In Nanotechnology Applications for Food Safety and Quality Monitoring; Elsevier: Amsterdam, The Netherlands, 2023; pp. 171–182. ISBN 978-0-323-85791-8. [Google Scholar]
- Soni, M.; Maurya, A.; Das, S.; Prasad, J.; Yadav, A.; Singh, V.K.; Singh, B.K.; Dubey, N.K.; Dwivedy, A.K. Nanoencapsulation Strategies for Improving Nutritional Functionality, Safety and Delivery of Plant-Based Foods: Recent Updates and Future Opportunities. Plant Nano Biol. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Valorisation of Micro/Nanoencapsulated Bioactive Compounds from Plant Sources for Food Applications Towards Sustainability. Foods 2022, 12, 32. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Morya, S.; Dendegh, T.A.; Okpala, C.O.R.; Korzeniowska, M. Nanoencapsulation of Food Bioactive Constituents and Its Associated Processes: A Revisit. Bioresour. Technol. Rep. 2022, 19, 101088. [Google Scholar] [CrossRef]
- Lim, D.Y.; Lee, J.-S.; Lee, H.G. Nano-Encapsulation of a Combination of Clove Oil and Thymol and Their Application in Fresh-Cut Apples and Raw Minced Beef. Food Control 2023, 148, 109683. [Google Scholar] [CrossRef]
- Choudhary, M.; Kaur, A.; Kaur, P. Recent Development in Nanoencapsulation of β-Sitosterol and γ-Oryzanol and Food Fortification. In Handbook of Nanoencapsulation; CRC Press: Boca Raton, FL, USA, 2023; pp. 65–81. ISBN 978-1-00-325918-3. [Google Scholar]
- Oprea, I.; Fărcaș, A.C.; Leopold, L.F.; Diaconeasa, Z.; Coman, C.; Socaci, S.A. Nano-Encapsulation of Citrus Essential Oils: Methods and Applications of Interest for the Food Sector. Polymers 2022, 14, 4505. [Google Scholar] [CrossRef] [PubMed]
- Vasisht, N. Nanoencapsulation in the Food Industry. In Microencapsulation in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 209–213. ISBN 978-0-12-821683-5. [Google Scholar]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, V.; Sadeghi, M.; Hadi, A. Physical and Chemical Properties of Nano-Liposome, Application in Nano Medicine. J. Appl. Comput. Appl. Mech. 2021, 52, 751–767. [Google Scholar] [CrossRef]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-Processing Techniques for the Improvement of Liposome Stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef] [PubMed]
- Sriwidodo; Umar, A.K.; Wathoni, N.; Zothantluanga, J.H.; Das, S.; Luckanagul, J.A. Liposome-Polymer Complex for Drug Delivery System and Vaccine Stabilization. Heliyon 2022, 8, e08934. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Cano, A.; Andreani, T.; Martins-Gomes, C.; Silva, A.M.; Szalata, M.; Słomski, R.; Souto, E.B. Lipid-Drug Conjugates and Nanoparticles for the Cutaneous Delivery of Cannabidiol. IJMS 2022, 23, 6165. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.R.; Margalit, D.; Ryskin, M.; Dor, M.; Shuali, U.; Nir, S.; Polubesova, T.; Ben-Ari, J.; Kertsnus-Banchik, J.; Undabeytia, T. Modified Compositions of Micelle–Clay and Liposome–Clay Composites for Optimal Removal from Water of Bacteria and Hydrophobic Neutral Chemicals. Appl. Sci. 2022, 12, 3044. [Google Scholar] [CrossRef]
- Blanco-Llamero, C.; Fonseca, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Señoráns, F.J.; Souto, E.B. Nutraceuticals and Food-Grade Lipid Nanoparticles: From Natural Sources to a Circular Bioeconomy Approach. Foods 2022, 11, 2318. [Google Scholar] [CrossRef]
- Madkhali, O.A. Perspectives and Prospective on Solid Lipid Nanoparticles as Drug Delivery Systems. Molecules 2022, 27, 1543. [Google Scholar] [CrossRef]
- Tan, C.; McClements, D.J. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef]
- Priyanka, K.R.; Rajaram, R.; Sivakumar, S.R. A Critical Review on Pharmacological Properties of Marine Macroalgae. Biomass Conv. Bioref. 2022, 1–25. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef] [PubMed]
- Lomartire, S.; Gonçalves, A.M.M. Novel Technologies for Seaweed Polysaccharides Extraction and Their Use in Food with Therapeutically Applications—A Review. Foods 2022, 11, 2654. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, M.M.; Vanniarachchy, M.P.G.; Wijesekara, I. Seaweed Derived Alginate, Agar, and Carrageenan Based Edible Coatings and Films for the Food Industry: A Review. Food Meas. 2022, 16, 1195–1227. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.; Xue, C. Recent Advances in Carrageenan-Based Delivery Systems for Bioactive Ingredients: A Review. Trends Food Sci. Technol. 2021, 112, 348–361. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Souto, S.; Flórez-Fernández, N.; Torres, M.D.; Bandín, I.; Domínguez, H. Antiviral Activity of Carrageenans and Processing Implications. Mar. Drugs 2021, 19, 437. [Google Scholar] [CrossRef]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Rehman, A.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S. A Comprehensive Review on the Nanocomposites Loaded with Chitosan Nanoparticles for Food Packaging. Crit. Rev. Food Sci. Nutr. 2022, 62, 1383–1416. [Google Scholar] [CrossRef]
- Khajavian, M.; Vatanpour, V.; Castro-Muñoz, R.; Boczkaj, G. Chitin and Derivative Chitosan-Based Structures—Preparation Strategies Aided by Deep Eutectic Solvents: A Review. Carbohydr. Polym. 2022, 275, 118702. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Sabaghi, M.; Tavasoli, S.; Hoseyni, S.Z.; Mozafari, M.R.; Degraeve, P.; Katouzian, I. A Critical Review on Approaches to Regulate the Release Rate of Bioactive Compounds from Biopolymeric Matrices. Food Chem. 2022, 382, 132411. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z. Cyclodextrin Polymers: Structure, Synthesis, and Use as Drug Carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef] [PubMed]
- Rohasmizah, H.; Azizah, M. Pectin-Based Edible Coatings and Nanoemulsion for the Preservation of Fruits and Vegetables: A Review. Appl. Food Res. 2022, 2, 100221. [Google Scholar] [CrossRef]
- Taouzinet, L.; Fatmi, S.; Lahiani-Skiba, M.; Skiba, M.; Iguer-Ouada, M. Encapsulation Nanotechnology in Sperm Cryopreservation: Systems Preparation Methods and Antioxidants Enhanced Delivery. Cryolettres 2021, 42, 1–12. [Google Scholar]
- Mundel, R.; Thakur, T.; Chatterjee, M. Emerging Uses of PLA–PEG Copolymer in Cancer Drug Delivery. 3 Biotech 2022, 12, 41. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical Review on Polylactic Acid: Properties, Structure, Processing, Biocomposites, and Nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Zuhri, M.Y.M.; Aisyah, H.A.; Asyraf, M.R.M.; Hassan, S.A.; Zainudin, E.S.; Sapuan, S.M.; Sharma, S.; Bangar, S.P.; Jumaidin, R. Natural Fiber-Reinforced Polylactic Acid, Polylactic Acid Blends and Their Composites for Advanced Applications. Polymers 2022, 14, 202. [Google Scholar] [CrossRef]
- Numata, K. How to Define and Study Structural Proteins as Biopolymer Materials. Polym. J. 2020, 52, 1043–1056. [Google Scholar] [CrossRef]
- Tang, C.-H. Assembly of Food Proteins for Nano-Encapsulation and Delivery of Nutraceuticals (a Mini-Review). Food Hydrocoll. 2021, 117, 106710. [Google Scholar] [CrossRef]
- Susanti, D.; Haris, M.S.; Taher, M.; Khotib, J. Natural Products-Based Metallic Nanoparticles as Antimicrobial Agents. Front. Pharmacol. 2022, 13, 895616. [Google Scholar] [CrossRef] [PubMed]
- Ameta, S.K.; Rai, A.K.; Hiran, D.; Ameta, R.; Ameta, S.C. Use of Nanomaterials in Food Science. In Biogenic Nano-Particles and their Use in Agro-Ecosystems; Ghorbanpour, M., Bhargava, P., Varma, A., Choudhary, D.K., Eds.; Springer: Singapore, 2020; pp. 457–488. ISBN 9789811529849. [Google Scholar]
- Tirado-Kulieva, V.A.; Sánchez-Chero, M.; Palacios Jimenez, D.P.; Sánchez-Chero, J.; Ygnacio Santa Cruz, A.G.; Minchán Velayarce, H.H.; Pozo Suclupe, L.A.; Carbajal Garcia, L.O. A Critical Review on The Integration of Metal Nanoparticles in Biopolymers: An Alternative for Active and Sustainable Food Packaging. Curr. Res. Nutr. Food Sci. 2022, 10, 1–18. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M. Nanoencapsulation of Phase Change Materials (PCMs) and Their Applications in Various Fields for Energy Storage and Management. Adv. Colloid Interface Sci. 2020, 283, 102226. [Google Scholar] [CrossRef]
- Håkansson, A. Emulsion Formation by Homogenization: Current Understanding and Future Perspectives. Annu. Rev. Food Sci. Technol. 2019, 10, 239–258. [Google Scholar] [CrossRef]
- El-hoshoudy, A.N.M.B. Emulsion Polymerization Mechanism. In Recent Research in Polymerization; Cankaya, N., Ed.; InTech: London, UK, 2018; ISBN 978-953-51-3746-7. [Google Scholar]
- Parimala, B.; Dinesha, B.L.; Vijayakumar, S.H.; Manjunath, B. A Review on Application of Nano-Technology in Food Industry: Nano-encapsulation, Nano-Sensors and Nano-Emulsions. Pharma Innov. J. 2021, 10, 333–337. [Google Scholar]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 1–21. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y.; Lu, L.; Shi, C.; Huang, Y.; Mao, Z.; Duan, C.; Ren, X.; Guo, Y.; Huang, C. Hydrodynamic Cavitation: A Feasible Approach to Intensify the Emulsion Cross-Linking Process for Chitosan Nanoparticle Synthesis. Ultrason. Sonochem. 2021, 74, 105551. [Google Scholar] [CrossRef]
- Azagury, A.; Fonseca, V.C.; Cho, D.Y.; Perez-Rogers, J.; Baker, C.M.; Steranka, E.; Goldenshtein, V.; Calvao, D.; Darling, E.M.; Mathiowitz, E. Single Step Double-Walled Nanoencapsulation (SSDN). J. Control Release 2018, 280, 11–19. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Bahmid, N.A.; Taha, A.; Abdel-Moneim, A.-M.E.; Shehata, A.M.; Tan, C.; Kharazmi, M.S.; Li, Y.; Assadpour, E.; Castro-Muñoz, R. Bioactive-Loaded Nanodelivery Systems for the Feed and Drugs of Livestock; Purposes, Techniques and Applications. Adv. Colloid Interface Sci. 2022, 308, 102772. [Google Scholar] [CrossRef]
- Tahir, A.; Shabir Ahmad, R.; Imran, M.; Ahmad, M.H.; Kamran Khan, M.; Muhammad, N.; Nisa, M.U.; Tahir Nadeem, M.; Yasmin, A.; Tahir, H.S. Recent Approaches for Utilization of Food Components as Nano-Encapsulation: A Review. Int. J. Food Prop. 2021, 24, 1074–1096. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.-S.; Kim, S.; Ha, E.-S.; Kim, M.-S.; Hwang, S.-J. Pharmaceutical Applications of Supercritical Fluid Extraction of Emulsions for Micro-/Nanoparticle Formation. Pharmaceutics 2021, 13, 1928. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, E.A.; Giménez, G.; Lombardo, M.V.; Zelcer, A.; Soler-Illia, G.J.A.A. Nanoencapsulation of Isotropic and Anisotropic Particles through a Green Chemistry Aerosol Method: A Scalable Approach for Ad-Hoc Surface Tuning. J. Sol-Gel Sci. Technol. 2022, 102, 208–218. [Google Scholar] [CrossRef]
- Han, S.Y.; Lee, H.; Nguyen, D.T.; Yun, G.; Kim, S.; Park, J.H.; Choi, I.S. Single-Cell Nanoencapsulation of Saccharomyces Cerevisiae by Cytocompatible Layer-by-Layer Assembly of Eggshell Membrane Hydrolysate and Tannic Acid. Adv. NanoBio. Res. 2021, 1, 2000037. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Maudhuit, A.; Gaiani, C.; Desobry, S. Encapsulation of Bioactive Compounds Using Competitive Emerging Techniques: Electrospraying, Nano Spray Drying, and Electrostatic Spray Drying. J. Food Eng. 2023, 339, 111260. [Google Scholar] [CrossRef]
- Vázquez-León, L.A.; Aparicio-Saguilán, A.; Martínez-Medinilla, R.M.; Utrilla-Coello, R.G.; Toruco-Uco, J.G.; Carpintero-Tepole, V.; Páramo-Calderón, D.E. Physicochemical and morphological characterization of black bean (Phaseolus vulgaris L.) starch and potential application in nano-encapsulation by spray drying. J. Food Meas. Charact. 2022, 16, 547–560. [Google Scholar] [CrossRef]
- Khairnar, S.V.; Pagare, P.; Thakre, A.; Nambiar, A.R.; Junnuthula, V.; Abraham, M.C.; Kolimi, P.; Nyavanandi, D.; Dyawanapelly, S. Review on the Scale-Up Methods for the Preparation of Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 1886. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polysaccharides as Carriers of Polyphenols: Comparison of Freeze-Drying and Spray-Drying as Encapsulation Techniques. Molecules 2022, 27, 5069. [Google Scholar] [CrossRef]
- Greque De Morais, M.; Pires Alvarenga, A.G.; Vaz Da Silva, B.; Vieira Costa, J.A. Nanoencapsulation of Spirulina Biomass by Electrospraying for Development of Functional Foods a Review. Biotechnol. Res. Innov 2021, 5, e2021009. [Google Scholar] [CrossRef]
- Ibrahim, A.; Moodley, D.; Uche, C.; Maboza, E.; Olivier, A.; Petrik, L. Antimicrobial and Cytotoxic Activity of Electrosprayed Chitosan Nanoparticles against Endodontic Pathogens and Balb/c 3T3 Fibroblast Cells. Sci. Rep. 2021, 11, 24487. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, W.; Jiang, P.; Hong, T. Coaxial Electrohydrodynamic Atomization for the Production of Drug-Loaded Micro/Nanoparticles. Micromachines 2019, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, J.; Itel, F.; Wuertz-Kozak, K.; Fortunato, G.; Rossi, R.M. PH-Responsive Electrospun Nanofibers and Their Applications. Polym. Rev. 2022, 62, 351–399. [Google Scholar] [CrossRef]
- Yoda, M.; Garden, J.-L.; Bourgeois, O.; Haque, A.; Kumar, A.; Deyhle, H.; Hieber, S.; Müller, B.; Cano-Sarabia, M.; Maspoch, D. Nano(Evenescent-Wave)-Particle Image Velocimetry. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; p. 1485. ISBN 978-90-481-9750-7. [Google Scholar]
- Gupta, V.; Biswas, D.; Roy, S. A Comprehensive Review of Biodegradable Polymer-Based Films and Coatings and Their Food Packaging Applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef]
- Alhnan, M.A.; Kidia, E.; Basit, A.W. Spray-drying enteric polymers from aqueous solutions: A novel, economic, and environmentally friendly approach to produce pH-responsive microparticles. Eur. J. Pharm. Biopharm. 2011, 79, 432–439. [Google Scholar] [CrossRef]
- Luo, Y.; Ning, T.; Pei, Y.; Feng, X.; Zhang, S.; Lu, B.; Wang, L. High-performance and tailored honeycombed Ag nanowire networks fabricated by a novel electrospray assisted etching process. Appl. Surf. Sci. 2022, 571, 151081. [Google Scholar] [CrossRef]
- Bensid, A.; El Abed, N.; Houicher, A.; Regenstein, J.M.; Özogul, F. Antioxidant and antimicrobial preservatives: Properties, mechanism of action and applications in food—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2985–3001. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.M.; Elahi, F.; Chelliah, R.; Lee, B.H.; Oh, D.H. New insights on the use of polyphenols as natural preservatives and their emerging safety concerns. Front. Sustain. Food Syst. 2020, 4, 525810. [Google Scholar] [CrossRef]
- Ahmed, T.M.K. Side Effects of Preservatives on Human Life. Sci. Res. J. Pharm. 2022, 2, 5. [Google Scholar]
- Al-Maqtari, Q.A.; Rehman, A.; Mahdi, A.A.; Al-Ansi, W.; Wei, M.; Yanyu, Z.; Phyo, H.M.; Galeboe, O.; Yao, W. Application of essential oils as preservatives in food systems: Challenges and future prospectives—A review. Phytochem. Rev. 2021, 21, 1209–1246. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of natural antimicrobials in food preservation: Recent views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- Padovano, M.; Aromatario, M.; D’Errico, S.; Concato, M.; Manetti, F.; David, M.C.; Scopetti, M.; Frati, P.; Fineschi, V. Sodium Nitrite Intoxication and Death: Summarizing Evidence to Facilitate Diagnosis. Int. J. Environ. Res. Public Health 2022, 19, 13996. [Google Scholar] [CrossRef] [PubMed]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in cured meats, health risk issues, alternatives to nitrites: A review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.K.; Akhila, S.; Rao, Y.S.; Devi, B.R. Alternative to artificial preservatives. Syst. Rev. Pharm. 2019, 10, 99–102. [Google Scholar]
- Gupta, M.K.; Basavaraj, G.V. Sulphites in food & drinks in asthmatic adults & children: What we need to know. Indian J. Allergy Asthma Immunol. 2021, 35, 43. [Google Scholar] [CrossRef]
- Mitra, P.; Chatterjee, S.; Paul, N.; Ghosh, S.; Das, M. An Overview of Endocrine Disrupting Chemical Paraben and Search for An Alternative–A Review. In Proceedings of the Zoological Society; Springer: New Delhi, India, 2021; Volume 74, pp. 479–493. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Dwivedy, A.K.; Singh, V.K.; Das, S.; Singh, A.; Dubey, N.K. Essential oils and their bioactive compounds as green preservatives against fungal and mycotoxin contamination of food commodities with special reference to their nanoencapsulation. Environ. Sci. Pollut. Res. 2019, 26, 25414–25431. [Google Scholar] [CrossRef]
- Weaver, C.M. Role of Dairy Beverages in the Diet. Physiol. Behav. 2010, 100, 63–66. [Google Scholar] [CrossRef]
- Van de Langerijt, T.M.; James, A.O.M.; Shane, V.C. Structural, Binding and Functional Properties of Milk Protein-Polyphenol Systems: A Review. Molecules 2023, 28, 2288. [Google Scholar] [CrossRef]
- Silva, S.; Veiga, M.; Costa, E.; Oliveira, A.; Madureira, A.; Pintado, M. Nanoencapsulation of Polyphenols towards Dairy Beverage Incorporation. Beverages 2018, 4, 61. [Google Scholar] [CrossRef]
- Bedoya-Serna, C.M.; Dacanal, G.C.; Fernandes, A.M.; Pinho, S.C. Antifungal Activity of Nanoemulsions Encapsulating Oregano (Origanum Vulgare) Essential Oil: In Vitro Study and Application in Minas Padrão Cheese. Braz. J. Microbiol. 2018, 49, 929–935. [Google Scholar] [CrossRef]
- Budryn, G.; Zaczyńska, D.; Oracz, J. Effect of Addition of Green Coffee Extract and Nanoencapsulated Chlorogenic Acids on Aroma of Different Food Products. LWT 2016, 73, 197–204. [Google Scholar] [CrossRef]
- Tavakoli, H.; Hosseini, O.; Jafari, S.M.; Katouzian, I. Evaluation of Physicochemical and Antioxidant Properties of Yogurt Enriched by Olive Leaf Phenolics within Nanoliposomes. J. Agric. Food Chem. 2018, 66, 9231–9240. [Google Scholar] [CrossRef] [PubMed]
- Delshadi, R.; Bahrami, A.; Tafti, A.G.; Barba, F.J.; Williams, L.L. Micro and Nano-Encapsulation of Vegetable and Essential Oils to Develop Functional Food Products with Improved Nutritional Profiles. Trends Food Sci. Technol. 2020, 104, 72–83. [Google Scholar] [CrossRef]
- Di Maio, G.; Pittia, P.; Mazzarino, L.; Maraschin, M.; Kuhnen, S. Cow Milk Enriched with Nanoencapsulated Phenolic Extract of Jaboticaba (Plinia Peruviana). J. Food Sci. Technol. 2019, 56, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Pudtikajorn, K.; Sae-leaw, T.; Benjakul, S. Characterization of Fortified Pasteurized Cow Milk with Nanoliposome Loaded with Skipjack Tuna Eyeball Oil. Int. J. Food Sci. Technol. 2021, 56, 5893–5903. [Google Scholar] [CrossRef]
- Ghayour-Mobarhan, M.; Sharifan, P.; Hassanzadeh, E.; Mohammadi Bajgiran, M.; Dabbagh, V.R.; Aminifar, E.; Ghazizadeh, H.; Saffar Soflaei, S.; Darroudi, S.; Tanbakouchi, D. Effects of Vitamin D3 Fortified Low-Fat Dairy Products on Bone Density Measures in Adults with Abdominal Obesity: A Randomized Clinical Trial. ABJS 2022, 10, 601–610. [Google Scholar] [CrossRef]
- Sharifan, P.; Bagherniya, M.; Bajgiran, M.M.; Safarian, M.; Vatanparast, H.; Eslami, S.; Tayefi, M.; Khadem-Rezaiyan, M.; Baygan, A.; Khoshakhlagh, M. The Efficacy of Dairy Products Fortified with Nano-Encapsulated Vitamin D3 on Physical and Mental Aspects of the Health in Obese Subjects; the Protocol of the SUVINA Trial. Transl. Metab. Syndr. Res. 2021, 4, 1–9. [Google Scholar] [CrossRef]
- Taghizadeh, N.; Sharifan, P.; Ekhteraee Toosi, M.S.; Najar Sedgh Doust, F.; Darroudi, S.; Afshari, A.; Rezaie, M.; Safarian, M.; Vatanparast, H.; Eslami, S. The Effects of Consuming a Low-Fat Yogurt Fortified with Nano Encapsulated Vitamin D on Serum pro-Oxidant-Antioxidant Balance (PAB) in Adults with Metabolic Syndrome; a Randomized Control Trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102332. [Google Scholar] [CrossRef]
- Dutra, T.V.; de Menezes, J.L.; Mizuta, A.G.; De Oliveira, A.; Moreira, T.F.M.; Barros, L.; Mandim, F.; Pereira, C.; Gonçalves, O.H.; Leimann, F.V. Use of Nanoencapsulated Curcumin against Vegetative Cells and Spores of Alicyclobacillus spp. in Industrialized Orange Juice. Int. J. Food Microbiol. 2021, 360, 109442. [Google Scholar] [CrossRef]
- Mahmoud, K.F.; Ali, H.S.; Amin, A.A. Nanoencapsulation of Bioactive Compounds Extracted from Egyptian Prickly Pears Peel Fruit: Antioxidant and Their Application in Guava Juice. Asian J. Sci. Res. 2018, 11, 574–586. [Google Scholar] [CrossRef]
- Nour Solim, T.; Farrag Far, A.; Abdel-Hady, H.; EL-Hossien, M. Preparation and Properties Nano-Encapsulated Wheat Germ Oil and Its Use in the Manufacture of Functional Labneh Cheese. Pak. J. Biol. Sci. 2019, 22, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, F.V.; Pourahmad, R.; Mortazavi, A.; Davoodi, D.; Azizinezhad, R. Use of Fish Oil Nanoencapsulated with Gum Arabic Carrier in Low Fat Probiotic Fermented Milk. Food Sci. Anim. Resour. 2019, 39, 309–323. [Google Scholar] [CrossRef]
- Gaber Ahmed, G.H.; Fernández-González, A.; Díaz García, M.E. Nano-Encapsulation of Grape and Apple Pomace Phenolic Extract in Chitosan and Soy Protein via Nanoemulsification. Food Hydrocoll. 2020, 108, 105806. [Google Scholar] [CrossRef]
- Pereira, P.M.d.C.C.; Vicente, A.F.d.R.B. Meat Nutritional Composition and Nutritive Role in the Human Diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Saavedra, A.; García-Betanzos, C.; Zambrano-Zaragoza, M.; Quintanar-Guerrero, D.; Mendoza-Elvira, S.; Velasco-Bejarano, B. Recent Developments and Applications of Nanosystems in the Preservation of Meat and Meat Products. Foods 2022, 11, 2150. [Google Scholar] [CrossRef] [PubMed]
- Amiri, N.; Afsharmanesh, M.; Salarmoini, M.; Meimandipour, A.; Hosseini, S.A.; Ebrahimnejad, H. Effects of Nanoencapsulated Cumin Essential Oil as an Alternative to the Antibiotic Growth Promoter in Broiler Diets. J. Appl. Poult. Res. 2020, 29, 875–885. [Google Scholar] [CrossRef]
- Yaseen, A.; Gaafar, K.; Abou-elkhaire, R. Influence of Nano Encapsulated Essential Oils on Broiler Performance: An Overview. J. Curr. Vet. Res. 2022, 4, 173–179. [Google Scholar] [CrossRef]
- Dani, M.; Rusman, R.; Zuprizal, Z. The Influence of Nano-Encapsulation of Melastoma malabathricum L. Fruit Extract to Lipid Profile of Broiler Chicken. BuletinPeternak 2019, 43, 4. [Google Scholar] [CrossRef]
- Badran, A. Effect of dietary curcumin and curcumin nanoparticles supplementation on growth performance, immune response and antioxidant of broilers chickens. EPSJ 2020, 40, 325–343. [Google Scholar] [CrossRef]
- Amiri, N.; Afsharmanesh, M.; Salarmoini, M.; Meimandipour, A.; Hosseini, S.A.; Ebrahimnejad, H. Nanoencapsulation (in Vitro and in Vivo) as an Efficient Technology to Boost the Potential of Garlic Essential Oil as Alternatives for Antibiotics in Broiler Nutrition. Animal 2021, 15, 100022. [Google Scholar] [CrossRef] [PubMed]
- El-Gogary, M.R.; El-Khateeb, A.Y.; Megahed, A.M. Effect of physiological and chemical nano garlic supplementation on broiler chickens. Plant Arch. 2019, 19, 695–705. [Google Scholar]
- Ahmadi, M.; Ahmadian, A.; Seidavi, A.R. Effect of Different Levels of Nano-Selenium on Performance, Blood Parameters, Immunity and Carcass Characteristics of Broiler Chickens. PSJ 2018, 6, 99–108. [Google Scholar] [CrossRef]
- Nouri, A. Chitosan Nano-Encapsulation Improves the Effects of Mint, Thyme, and Cinnamon Essential Oils in Broiler Chickens. Br. Poult. Sci. 2019, 60, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Zuprizal, Z.; Ningsih, N.; Zulfian, T.A. The Effect of Nano-Encapsulation Phaleria Macrocarpa Fruits Extract in Drinking Water on the Digestive Tract and Carcass Characteristic of Broiler Chickens. BuletinPeternak 2020, 44, 1. [Google Scholar] [CrossRef]
- Ahmadabadi, L.R.; Hosseini, S.E.; Seyedein Ardebili, S.M.; Mousavi Khaneghah, A. Application of Clove Essential Oil-Loaded Nanoemulsions in Coating of Chicken Fillets. Food Meas. 2022, 16, 819–828. [Google Scholar] [CrossRef]
- Hasani, S.; Ojagh, S.M.; Ghorbani, M.; Hasani, M. Nano-Encapsulation of Lemon Essential Oil Approach to Reducing the Oxidation Process in Fish Burger during Refrigerated Storage. J. Food Biosci. Technol. 2020, 10, 35–46. [Google Scholar]
- Homayounpour, P.; Alizadeh Sani, M.; Shariatifar, N. Application of nano-encapsulated Allium sativum L. essential oil to increase the shelf life of hamburger at refrigerated temperature with analysis of microbial and physical properties. J. Food Process. Preserv. 2021, 45, e15907. [Google Scholar] [CrossRef]
- Kazemeini, H.; Azizian, A.; Adib, H. Inhibition of Listeria Monocytogenes Growth in Turkey Fillets by Alginate Edible Coating with Trachyspermum Ammi Essential Oil Nano-Emulsion. Int. J. Food Microbiol. 2021, 344, 109104. [Google Scholar] [CrossRef]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of Chitosan Coatings Incorporating with Free or Nano-Encapsulated Satureja Plant Essential Oil on Quality Characteristics of Lamb Meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- Ojha, K.S.; Perussello, C.A.; García, C.Á.; Kerry, J.P.; Pando, D.; Tiwari, B.K. Ultrasonic-Assisted Incorporation of Nano-Encapsulated Omega-3 Fatty Acids to Enhance the Fatty Acid Profile of Pork Meat. Meat Sci. 2017, 132, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hădărugă, N.G.; Chirilă, C.A.; Szakal, R.N.; Gălan, I.M.; Simandi, M.D.; Bujancă, G.S.; David, I.; Riviş, A.; Stanciu, S.M.; Hădărugă, D.I. FTIR–PCA Approach on Raw and Thermally Processed Chicken Lipids Stabilized by Nano-Encapsulation in β-Cyclodextrin. Foods 2022, 11, 3632. [Google Scholar] [CrossRef] [PubMed]
- Elbarbary, A.M.; El-Sawy, N.M.; Hegazy, E.-S.A. Antioxidative Properties of Irradiated Chitosan/Vitamin C Complex and Their Use as Food Additive for Lipid Storage. J. Appl. Polym. Sci. 2015, 132, 24. [Google Scholar] [CrossRef]
- Yarmohammadi Barbarestani, S.; Samadi, F.; Hassani, S.; Asadi, G. Effects of Encapsulated Nano- and Microparticles of Peppermint (Mentha piperita) Alcoholic Extract on the Growth Performance, Blood Parameters and Immune Function of Broilers under Heat Stress Condition. Iran. J. Appl. Anim. Sci. 2017, 7, 669–677. [Google Scholar]
- Yousefdoost, S.; Samadi, F.; Jafari, S.M.; Ramezanpour, S.S.; Hassani, S.; Ganji, F. Application of Nanoencapsulated Silymarin to Improve Its Antioxidant and Hepatoprotective Activities against Carbon Tetrachloride-Induced Oxidative Stress in Broiler Chickens. Livest. Sci. 2019, 228, 177–186. [Google Scholar] [CrossRef]
- Abbasi, F.; Samadi, F.; Jafari, S.M.; Ramezanpour, S.; Shams-Shargh, M. Production of Omega-3 Fatty Acid-Enriched Broiler Chicken Meat by the Application of Nanoencapsultsed Flaxseed Oil Prepared via Ultrasonication. J. Funct. Foods 2019, 57, 373–381. [Google Scholar] [CrossRef]
- Sarvinehbaghi, M.B.; Ahmadi, M.; Shiran, M.; Azizkhani, M. Antioxidant and Antimicrobial Activity of Red Onion (Allium cepa L.) Extract Nanoencapsulated in Native Seed Gums Coating and Its Effect on Shelf-Life Extension of Beef Fillet. Food Meas. 2021, 15, 4771–4780. [Google Scholar] [CrossRef]
- Rahnemoon, P.; Sarabi-Jamab, M.; Bostan, A.; Mansouri, E. Nano-Encapsulation of Pomegranate (Punica granatum L.) peel extract and evaluation of its antimicrobial properties on coated chicken meat. Food Biosci. 2021, 43, 101331. [Google Scholar] [CrossRef]
- Hadian, M.; Rajaei, A.; Mohsenifar, A.; Tabatabaei, M. Encapsulation of Rosmarinus Officinalis Essential Oils in Chitosan-Benzoic Acid Nanogel with Enhanced Antibacterial Activity in Beef Cutlet against Salmonella Typhimurium during Refrigerated Storage. LWT 2017, 84, 394–401. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Shi, J.; Huang, X.; Zou, X.; Zhang, D.; Zhai, X.; Yang, Z.; Li, Z.; Li, Y. Preparation and Comparison of Two Functional Nanoparticle-Based Bilayers Reinforced with a κ-Carrageenan–Anthocyanin Complex. Int. J. Biol. Macromol. 2020, 165, 758–766. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Azizi, M.H. Nanoencapsulation Approach to Improve Antimicrobial and Antioxidant Activity of Thyme Essential Oil in Beef Burgers During Refrigerated Storage. Food Bioprocess Technol. 2016, 9, 1187–1201. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Mohammadnabi, S. Effect of Novel Bioactive Edible Coatings Based on Jujube Gum and Nettle Oil-Loaded Nanoemulsions on the Shelf-Life of Beluga Sturgeon Fillets. Int. J. Biol. Macromol. 2017, 95, 769–777. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Ding, W. Eugenol Nanocapsules Embedded with Gelatin-Chitosan for Chilled Pork Preservation. Int. J. Biol. Macromol. 2020, 158, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Tometri, S.S.; Ahmady, M.; Ariaii, P.; Soltani, M.S. Extraction and Encapsulation of Laurus Nobilis Leaf Extract with Nano-Liposome and Its Effect on Oxidative, Microbial, Bacterial and Sensory Properties of Minced Beef. Food Meas. 2020, 14, 3333–3344. [Google Scholar] [CrossRef]
- Brandelli, A.; Brum, L.F.W.; Dos Santos, J.H.Z. Nanostructured Bioactive Compounds for Ecological Food Packaging. Environ. Chem. Lett. 2017, 15, 193–204. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Lin, L. Plasma-Treated Poly(Ethylene Oxide) Nanofibers Containing Tea Tree Oil/Beta-Cyclodextrin Inclusion Complex for Antibacterial Packaging. Carbohydr. Polym. 2018, 179, 360–369. [Google Scholar] [CrossRef]
- Karimifar, P.; Saei-Dehkordi, S.S.; Izadi, Z. Antibacterial, Antioxidative and Sensory Properties of Ziziphora Clinopodioides–Rosmarinus Officinalis Essential Oil Nanoencapsulated Using Sodium Alginate in Raw Lamb Burger Patties. Food Biosci. 2022, 47, 101698. [Google Scholar] [CrossRef]
- Lin, L.; Mao, X.; Sun, Y.; Rajivgandhi, G.; Cui, H. Antibacterial Properties of Nanofibers Containing Chrysanthemum Essential Oil and Their Application as Beef Packaging. Int. J. Food Microbiol. 2019, 292, 21–30. [Google Scholar] [CrossRef]
- Magalhães Brandão, R.; Roberto Batista, L.; Elvis de Oliveira, J.; Bispo Barbosa, R.; Lee Nelson, D.; Graças Cardoso, M. In Vitro and in Vivo Efficacy of Poly(Lactic Acid) Nanofiber Packaging Containing Essential Oils from Ocimum basilicum L. and Ocimum gratissimum L. Against Aspergillus Carbonarius Aspergillus Niger Table Grapes. Food Chem. 2023, 400, 134087. [Google Scholar] [CrossRef]
- Zeng, J.; Ji, Q.; Liu, X.; Yuan, M.; Yuan, M.; Qin, Y. Electrospun Polylactic Acid/Poly (ε-Caprolactone) Fibrous Encapsulated Thymol/MIL-68(Al) as a Food Packaging Material. J. Mater. Res. Technol. 2022, 18, 5032–5044. [Google Scholar] [CrossRef]
- Álvarez-Hernández, M.H.; Martínez-Hernández, G.B.; Castillejo, N.; Martínez, J.A.; Artés-Hernández, F. Development of an Antifungal Active Packaging Containing Thymol and an Ethylene Scavenger. Valid. Dur. Storage Cherry Tomatoes. Food Packag. Shelf Life 2021, 29, 100734. [Google Scholar] [CrossRef]
- de Carvalho, S.M.; Noronha, C.M.; da Rosa, C.G.; Sganzerla, W.G.; Bellettini, I.C.; Nunes, M.R.; Bertoldi, F.C.; Manique Barreto, P.L. PVA Antioxidant Nanocomposite Films Functionalized with Alpha-Tocopherol Loaded Solid Lipid Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123793. [Google Scholar] [CrossRef]
- Lei, Y.; Yao, Q.; Jin, Z.; Wang, Y.-C. Intelligent Films Based on Pectin, Sodium Alginate, Cellulose Nanocrystals, and Anthocyanins for Monitoring Food Freshness. Food Chem. 2023, 404, 134528. [Google Scholar] [CrossRef] [PubMed]
- Kamkar, A.; Molaee-Aghaee, E.; Khanjari, A.; Akhondzadeh-Basti, A.; Noudoost, B.; Shariatifar, N.; Alizadeh Sani, M.; Soleimani, M. Nanocomposite Active Packaging Based on Chitosan Biopolymer Loaded with Nano-Liposomal Essential Oil: Its Characterizations and Effects on Microbial, and Chemical Properties of Refrigerated Chicken Breast Fillet. Int. J. Food Microbiol. 2021, 342, 109071. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, A.; Navarro-Martínez, A.; Martínez-Hernández, G.B. Active Paper Sheets Including Nanoencapsulated Essential Oils: A Green Packaging Technique to Control Ethylene Production and Maintain Quality in Fresh Horticultural Products—A Case Study on Flat Peaches. Foods 2020, 9, 1904. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Fabrication of Gallic Acid Loaded Hydroxypropyl Methylcellulose Nanofibers by Electrospinning Technique as Active Packaging Material. Carbohydr. Polym. 2019, 208, 241–250. [Google Scholar] [CrossRef]
- Schmatz, D.A.; Costa, J.A.V.; Morais, d.M.G. A Novel Nanocomposite for Food Packaging Developed by Electrospinning and Electrospraying. Food Packag. Shelf Life 2019, 20, 100314. [Google Scholar] [CrossRef]
- Xavier, L.O.; Sganzerla, W.G.; Rosa, G.B.; da Rosa, C.G.; Agostinetto, L.; Veeck, A.P.d.L.; Bretanha, L.C.; Micke, G.A.; Dalla Costa, M.; Bertoldi, F.C.; et al. Chitosan Packaging Functionalized with Cinnamodendron Dinisii Essential Oil Loaded Zein: A Proposal for Meat Conservation. Int. J. Biol. Macromol. 2021, 169, 183–193. [Google Scholar] [CrossRef]
- Li, L.; Xia, L.; Xiao, F.; Xiao, Y.; Liu, L.; Jiang, S.; Wang, H. Colorimetric Active Carboxymethyl Chitosan/Oxidized Sodium Alginate-Oxalis Triangularis Ssp. Papilionacea Anthocyanins Film@gelatin/Zein-Linalool Membrane for Milk Freshness Monitoring and Preservation. Food Chem. 2023, 405, 134994. [Google Scholar] [CrossRef]
- Hua, L.; Deng, J.; Wang, Z.; Wang, Y.; Chen, B.; Ma, Y.; Li, X.; Xu, B. Improving the Functionality of Chitosan-Based Packaging Films by Crosslinking with Nanoencapsulated Clove Essential Oil. Int. J. Biol. Macromol. 2021, 192, 627–634. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Fabrication of Eugenol Loaded Gelatin Nanofibers by Electrospinning Technique as Active Packaging Material. LWT 2021, 139, 110800. [Google Scholar] [CrossRef]
- Eghbalian, M.; Shavisi, N.; Shahbazi, Y.; Dabirian, F. Active Packaging Based on Sodium Caseinate-Gelatin Nanofiber Mats Encapsulated with Mentha spicata L. Essential Oil and MgO Nanoparticles: Preparation, Properties, and Food Application. Food Packag. Shelf Life 2021, 29, 100737. [Google Scholar] [CrossRef]
- Sharif, N.; Golmakani, M.-T.; Hajjari, M.M.; Aghaee, E.; Ghasemi, J.B. Antibacterial Cuminaldehyde/Hydroxypropyl-β-Cyclodextrin Inclusion Complex Electrospun Fibers Mat: Fabrication and Characterization. Food Packag. Shelf Life 2021, 29, 100738. [Google Scholar] [CrossRef]
- McDaniel, A.; Tonyali, B.; Yucel, U.; Trinetta, V. Formulation and Development of Lipid Nanoparticle Antifungal Packaging Films to Control Postharvest Disease. J. Agric. Food Res. 2019, 1, 100013. [Google Scholar] [CrossRef]
- Munhuweyi, K.; Caleb, O.J.; van Reenen, A.J.; Opara, U.L. Physical and Antifungal Properties of β-Cyclodextrin Microcapsules and Nanofibre Films Containing Cinnamon and Oregano Essential Oils. LWT 2018, 87, 413–422. [Google Scholar] [CrossRef]
- Dehcheshmeh, M.A.; Fathi, M. Production of Core-Shell Nanofibers from Zein and Tragacanth for Encapsulation of Saffron Extract. Int. J. Biol. Macromol. 2019, 122, 272–279. [Google Scholar] [CrossRef]
- Hadavi, R.; Jafari, S.M.; Katouzian, I. Nanoliposomal Encapsulation of Saffron Bioactive Compounds; Characterization and Optimization. Int. J. Biol. Macromol. 2020, 164, 4046–4053. [Google Scholar] [CrossRef]
- Jain, A.; Dasgupta, N.; Ranjan, S.; Singh, V.; Singh, H.; Purohit, S.D.; Mishra, N.C.; Yadav, N.P.; Haque, S.; Mishra, B.N. Whey Protein Based Electrosprayed Nanospheres for Encapsulation and Controlled Release of Bioactive Compounds from Tinospora Cordifolia Extract. Innov. Food Sci. Emerg. Technol. 2021, 69, 102671. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M.; Biliaderis, C.G. Encapsulation of β-Carotene into Food-Grade Nanofibers via Coaxial Electrospinning of Hydrocolloids: Enhancement of Oxidative Stability and Photoprotection. Food Hydrocoll. 2022, 133, 107949. [Google Scholar] [CrossRef]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Improving Curcumin Solubility and Bioavailability by Encapsulation in Saponin-Coated Curcumin Nanoparticles Prepared Using a Simple PH-Driven Loading Method. Food Funct. 2018, 9, 1829–1839. [Google Scholar] [CrossRef]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of Delivery System Type on Curcumin Bioaccessibility: Comparison of Curcumin-Loaded Nanoemulsions with Commercial Curcumin Supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef]
- Bateni, Z.; Behrouz, V.; Rahimi, H.R.; Hedayati, M.; Afsharian, S.; Sohrab, G. Effects of Nano-Curcumin Supplementation on Oxidative Stress, Systemic Inflammation, Adiponectin, and NF-ΚB in Patients with Metabolic Syndrome: A Randomized, Double-Blind Clinical Trial. J. Herb. Med. 2022, 31, 100531. [Google Scholar] [CrossRef]
- Mauri, E.; Gori, M.; Giannitelli, S.M.; Zancla, A.; Mozetic, P.; Abbruzzese, F.; Merendino, N.; Gigli, G.; Rossi, F.; Trombetta, M.; et al. Nano-Encapsulation of Hydroxytyrosol into Formulated Nanogels Improves Therapeutic Effects against Hepatic Steatosis: An in Vitro Study. Mater. Sci. Eng. 2021, 124, 112080. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, F.; Du, M.; Xie, C.; Xie, X.; Zhang, H.; Meng, X.; Li, A.; Deng, T. Encapsulation of Catechin into Nano-Cyclodextrin-Metal-Organic Frameworks: Preparation, Characterization, and Evaluation of Storage Stability and Bioavailability. Food Chem. 2022, 394, 133553. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Yu, Y.; Chen, W.; Liu, Y.; Yu, H. Nanoformulations of Quercetin and Cellulose Nanofibers as Healthcare Supplements with Sustained Antioxidant Activity. Carbohydr. Polym. 2019, 207, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, S.; Ojagh, S.M.; Quek, S.Y.; Pourashouri, P.; Salaün, F. Nano-Encapsulation of Fish Oil and Garlic Essential Oil by a Novel Composition of Wall Material: Persian Gum-Chitosan. LWT 2019, 116, 108494. [Google Scholar] [CrossRef]
- Xiang, C.; Gao, J.; Ye, H.; Ren, G.; Ma, X.; Xie, H.; Fang, S.; Lei, Q.; Fang, W. Development of Ovalbumin-Pectin Nanocomplexes for Vitamin D3 Encapsulation: Enhanced Storage Stability and Sustained Release in Simulated Gastrointestinal Digestion. Food Hydrocoll. 2020, 106, 105926. [Google Scholar] [CrossRef]
- Zhang, X.; Song, R.; Liu, X.; Xu, Y.; Wei, R. Fabrication of Vitamin D3 Nanoemulsions Stabilized by Tween 80 and Span 80 as a Composite Surface-Active Surfactant: Characterization and Stability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128873. [Google Scholar] [CrossRef]
- Baek, J.; Ramasamy, M.; Willis, N.C.; Kim, D.S.; Anderson, W.A.; Tam, K.C. Encapsulation and Controlled Release of Vitamin C in Modified Cellulose Nanocrystal/Chitosan Nanocapsules. Curr. Res. Food Sci. 2021, 4, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, S.; Muthukumar, S.P.; Anandharamakrishnan, C. The Influence of Droplet Size on the Stability, in Vivo Digestion, and Oral Bioavailability of Vitamin E Emulsions. Food Funct. 2016, 7, 2294–2302. [Google Scholar] [CrossRef]
- Resende, D.; Costa Lima, S.A.; Reis, S. Nanoencapsulation Approaches for Oral Delivery of Vitamin A. Colloids Surf. B: Biointerfaces 2020, 193, 111121. [Google Scholar] [CrossRef]
- Sharmila, D.J.S.; Lakshmanan, A. Molecular Dynamics Study of Plant Bioactive Nutraceutical Keto-Curcumin Encapsulated in Medium Chain Triglyceride Oil-in-Water Nanoemulsion That Are Stabilized by Globular Whey Proteins. J. Mol. Liq. 2022, 362, 119753. [Google Scholar] [CrossRef]
- Chaves, M.A.; Baldino, L.; Pinho, S.C.; Reverchon, E. Co-Encapsulation of Curcumin and Vitamin D3 in Mixed Phospholipid Nanoliposomes Using a Continuous Supercritical CO2 Assisted Process. J. Taiwan Inst. Chem. Eng. 2022, 132, 104120. [Google Scholar] [CrossRef]
- Ghayour, N.; Hosseini, S.M.H.; Eskandari, M.H.; Esteghlal, S.; Nekoei, A.-R.; Hashemi Gahruie, H.; Tatar, M.; Naghibalhossaini, F. Nanoencapsulation of Quercetin and Curcumin in Casein-Based Delivery Systems. Food Hydrocoll. 2019, 87, 394–403. [Google Scholar] [CrossRef]
- da Silva, J.L.G.; Passos, D.F.; Bernardes, V.M.; Cabral, F.L.; Schimites, P.G.; Manzoni, A.G.; de Oliveira, E.G.; de Bona da Silva, C.; Beck, R.C.R.; Jantsch, M.H. Co-Nanoencapsulation of Vitamin D3 and Curcumin Regulates Inflammation and Purine Metabolism in a Model of Arthritis. Inflammation 2019, 42, 1595–1610. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Yue, P.; Chi, J.; Liang, J.; Gao, X. Formation and Stability of Anthocyanins-Loaded Nanocomplexes Prepared with Chitosan Hydrochloride and Carboxymethyl Chitosan. Food Hydrocoll. 2018, 74, 23–31. [Google Scholar] [CrossRef]
- Sreerekha, P.R.; Dara, P.K.; Vijayan, D.K.; Chatterjee, N.S.; Raghavankutty, M.; Mathew, S.; Ravishankar, C.N.; Anandan, R. Dietary Supplementation of Encapsulated Anthocyanin Loaded-Chitosan Nanoparticles Attenuates Hyperlipidemic Aberrations in Male Wistar Rats. Carbohydr. Polym. Technol. Appl. 2021, 2, 100051. [Google Scholar] [CrossRef]
- Moon, H.; Lertpatipanpong, P.; Hong, Y.; Kim, C.-T.; Baek, S.J. Nano-Encapsulated Quercetin by Soluble Soybean Polysaccharide/Chitosan Enhances Anti-Cancer, Anti-Inflammation, and Anti-Oxidant Activities. J. Funct. Foods 2021, 87, 104756. [Google Scholar] [CrossRef]
- Hao, J.; Guo, B.; Yu, S.; Zhang, W.; Zhang, D.; Wang, J.; Wang, Y. Encapsulation of the Flavonoid Quercetin with Chitosan-Coated Nano-Liposomes. LWT Food Sci. Technol. 2017, 85, 37–44. [Google Scholar] [CrossRef]
- Ahmad, M.; Mudgil, P.; Gani, A.; Hamed, F.; Masoodi, F.A.; Maqsood, S. Nano-Encapsulation of Catechin in Starch Nanoparticles: Characterization, Release Behavior and Bioactivity Retention during Simulated in-Vitro Digestion. Food Chem. 2019, 270, 95–104. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Gao, Y.; Yang, Z.; Zhao, S.; Wang, Y.; Blanchard, C.; Zhou, Z. Nano-Encapsulation of Epigallocatechin Gallate in the Ferritin-Chitosan Double Shells: Simulated Digestion and Absorption Evaluation. Food Res. Int. 2018, 108, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chaari, M.; Theochari, I.; Papadimitriou, V.; Xenakis, A.; Ammar, E. Encapsulation of Carotenoids Extracted from Halophilic Archaea in Oil-in-Water (O/W) Micro- and Nano-Emulsions. Colloids Surf. B Biointerfaces 2018, 161, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; McClements, D.J. Improvement of β-Carotene Bioaccessibility from Dietary Supplements Using Excipient Nanoemulsions. J. Agric. Food Chem. 2016, 64, 4639–4647. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, P.; Zou, Y.-X.; Luo, Z.-G.; Tamer, T.M. Co-Encapsulation of Vitamin C and β-Carotene in Liposomes: Storage Stability, Antioxidant Activity, and in Vitro Gastrointestinal Digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef]
- Jia, D.; Barwal, I.; Thakur, S.; Yadav, S.C. Methodology to Nanoencapsulate Hepatoprotective Components from Picrorhiza Kurroa as Food Supplement. Food Biosci. 2015, 9, 28–35. [Google Scholar] [CrossRef]
- Machado, A.R.; Pinheiro, A.C.; Vicente, A.A.; Souza-Soares, L.A.; Cerqueira, M.A. Liposomes Loaded with Phenolic Extracts of Spirulina LEB-18: Physicochemical Characterization and Behavior under Simulated Gastrointestinal Conditions. Food Res. Int. 2019, 120, 656–667. [Google Scholar] [CrossRef]
- Koo, S.Y.; Mok, I.-K.; Pan, C.-H.; Kim, S.M. Preparation of Fucoxanthin-Loaded Nanoparticles Composed of Casein and Chitosan with Improved Fucoxanthin Bioavailability. J. Agric. Food Chem. 2016, 64, 9428–9435. [Google Scholar] [CrossRef] [PubMed]
- Faridi Esfanjani, A.; Jafari, S.M.; Assadpour, E. Preparation of a Multiple Emulsion Based on Pectin-Whey Protein Complex for Encapsulation of Saffron Extract Nanodroplets. Food Chem. 2017, 221, 1962–1969. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Salt, L.J.; Ridout, M.J.; Ding, Y.; Wilde, P.J. Fish Oil Emulsions Stabilized with Caseinate Glycated by Dextran: Physicochemical Stability and Gastrointestinal Fate. J. Agric. Food Chem. 2019, 67, 452–462. [Google Scholar] [CrossRef]
- Serini, S.; Cassano, R.; Corsetto, P.; Rizzo, A.; Calviello, G.; Trombino, S. Omega-3 PUFA Loaded in Resveratrol-Based Solid Lipid Nanoparticles: Physicochemical Properties and Antineoplastic Activities in Human Colorectal Cancer Cells In Vitro. Int. J. Mol. Sci. 2018, 19, 586. [Google Scholar] [CrossRef]
- Remila, L.; Belcastro, E.; Guenday-Tuereli, N.; Park, S.; Houngue, U.; Vandamme, T.; Tuereli, E.; Kerth, P.; Auger, C.; Schini-Kerth, V. Nanoencapsulation of the Omega-3 EPA:DHA 6:1 Formulation Enhances and Sustains NO-Mediated Endothelium-Dependent Relaxations in Coronary Artery Rings and NO Formation in Endothelial Cells. J. Funct. Foods 2021, 87, 104851. [Google Scholar] [CrossRef]
- Atraki, R.; Azizkhani, M. Survival of Probiotic Bacteria Nanoencapsulated within Biopolymers in a Simulated Gastrointestinal Model. Innov. Food Sci. Emerg. Technol. 2021, 72, 102750. [Google Scholar] [CrossRef]
- Hosny, K.M.; Bahmdan, R.H.; Alhakamy, N.A.; Alfaleh, M.A.; Ahmed, O.A.; Elkomy, M.H. Physically Optimized Nano-Lipid Carriers Augment Raloxifene and Vitamin D Oral Bioavailability in Healthy Humans for Management of Osteoporosis. J. Pharm. Sci. 2020, 109, 2145–2155. [Google Scholar] [CrossRef] [PubMed]
- Jan, Y.; Al-Keridis, L.A.; Malik, M.; Haq, A.; Ahmad, S.; Kaur, J.; Adnan, M.; Alshammari, N.; Ashraf, S.A.; Panda, B.P. Preparation, Modelling, Characterization and Release Profile of Vitamin D3 Nanoemulsion. LWT 2022, 169, 113980. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Development of Nanostructured Lipid Carriers for the Encapsulation and Controlled Release of Vitamin D3. Food Chem. 2017, 225, 213–219. [Google Scholar] [CrossRef]
- Talebi, V.; Ghanbarzadeh, B.; Hamishehkar, H.; Pezeshki, A.; Ostadrahimi, A. Effects of Different Stabilizers on Colloidal Properties and Encapsulation Efficiency of Vitamin D3 Loaded Nano-Niosomes. J. Drug Deliv. Sci. Technol. 2021, 61, 101284. [Google Scholar] [CrossRef]
- Hassane Hamadou, A.; Huang, W.-C.; Xue, C.; Mao, X. Formulation of Vitamin C Encapsulation in Marine Phospholipids Nanoliposomes: Characterization and Stability Evaluation during Long Term Storage. LWT 2020, 127, 109439. [Google Scholar] [CrossRef]
- Azevedo, M.A.; Bourbon, A.I.; Vicente, A.A.; Cerqueira, M.A. Alginate/Chitosan Nanoparticles for Encapsulation and Controlled Release of Vitamin B2. Int. J. Biol. Macromol. 2014, 71, 141–146. [Google Scholar] [CrossRef]
- Penalva, R.; Esparza, I.; Agüeros, M.; Gonzalez-Navarro, C.J.; Gonzalez-Ferrero, C.; Irache, J.M. Casein Nanoparticles as Carriers for the Oral Delivery of Folic Acid. Food Hydrocoll. 2015, 44, 399–406. [Google Scholar] [CrossRef]
- Batinić, P.M.; Đorđević, V.B.; Stevanović, S.I.; Balanč, B.D.; Marković, S.B.; Luković, N.D.; Mijin, D.Ž.; Bugarski, B.M. Formulation and Characterization of Novel Liposomes Containing Histidine for Encapsulation of a Poorly Soluble Vitamin. J. Drug Deliv. Sci. Technol. 2020, 59, 101920. [Google Scholar] [CrossRef]
- Fu, X.; Chen, J. A review of hyperspectral imaging for chicken meat safety and quality evaluation: Application, hardware, and software. Compr. Rev. Food Sci. Food Saf. 2019, 18, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Kristi, N.; Ye, Z. Atomic force microscopy in food preservation research: New insights to overcome spoilage issues. Food Res. Int. 2021, 140, 110043. [Google Scholar] [CrossRef] [PubMed]
- Chalklen, T.; Jing, Q.; Kar-Narayan, S. Biosensors based on mechanical and electrical detection techniques. Sensors 2020, 20, 5605. [Google Scholar] [CrossRef] [PubMed]
- Pavase, T.R.; Lin, H.; Hussain, S.; Li, Z.; Ahmed, I.; Lv, L.; Sun, L.; Shah, S.B.H.; Kalhoro, M.T. Recent advances of conjugated polymer (CP) nanocomposite-based chemical sensors and their applications in food spoilage detection: A comprehensive review. Sens. Actuators B Chem. 2018, 273, 1113–1138. [Google Scholar] [CrossRef]
- Weston, M.; Geng, S.; Chandrawati, R. Food sensors: Challenges and opportunities. Adv. Mater. Technol. 2021, 6, 2001242. [Google Scholar] [CrossRef]
- Mathew, S.; Radhakrishnan, E.K. (Eds.) Nano-innovations in Food Packaging: Functions and Applications; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Dev, A.; Karmakar, S. Nanosensors and nanobiosensors in food and agriculture. Environ. Chem. Lett. 2018, 16, 161–182. [Google Scholar] [CrossRef]
- Thakur, M.; Wang, B.; Verma, M.L. Development and applications of nanobiosensors for sustainable agricultural and food industries: Recent developments, challenges and perspectives. Environ. Technol. Innov. 2022, 26, 102371. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S.; Husen, A. Metal-based nanoparticles, sensors, and their multifaceted application in food packaging. J. Nanobiotechnol. 2021, 19, 256. [Google Scholar] [CrossRef]
- Borah, N.; Gogoi, D.; Ghosh, N.N.; Tamuly, C. GA-AuNP@ Tollens’ complex as a highly sensitive plasmonic nanosensor for detection of formaldehyde and benzaldehyde in preserved food products. Food Chem. 2023, 399, 133975. [Google Scholar] [CrossRef]
- Mazur, F.; Tran, H.; Kuchel, R.P.; Chandrawati, R. Rapid Detection of Listeriolysin O Toxin Based on a Nanoscale Liposome–Gold Nanoparticle Platform. ACS Appl. Nano Mater. 2020, 3, 7270–7280. [Google Scholar] [CrossRef]
- Weston, M.; Mazur, F.; Chandrawati, R. Monitoring of Food Spoilage Using Polydiacetylene-and Liposome-Based Sensors. Smart Sens. Environ. Med. Appl. 2020, 81–102. [Google Scholar] [CrossRef]
- Soni, V.; Raizada, P.; Singh, P.; Cuong, H.N.; Rangabha, S.; Saini, A.; Saini, R.V.; Le, Q.V.; Nadda, A.K.; Le, T.-T.; et al. Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: A review. Environ. Res. 2021, 202, 111622. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.R.; Teli, S.B.; Ghosh, J.; Prasad, N.R.; Shaikh, V.S.; Nazeruddin, G.M.; Abdullah, G.A.; Imran, P.; Shaikh, Y.I. A review on bio-inspired synthesis of silver nanoparticles: Their antimicrobial efficacy and toxicity. Eng. Sci. 2021, 16, 90–128. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trace Elem. Res. 2023, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Wypij, M.; Trzcińska-Wencel, J.; Golinska, P.; Avila-Quezada, G.D.; Avinash, P.I.; Rai, M. The strategic applications of natural polymer nanocomposites in food packaging and agriculture: Chances, challenges, and consumers’ perception. Front. Chem. 2023, 10, 1633. [Google Scholar] [CrossRef]
- Niu, L.; Li, Z.; Fan, W.; Zhong, X.; Peng, M.; Liu, Z. Nano-strategies for enhancing the bioavailability of tea polyphenols: Preparation, applications, and challenges. Foods 2022, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on nanoparticles and nanostructured materials: Bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; Abdalla, S.M.; Bechelany, M. Review on natural, incidental, bioinspired, and engineered nanomaterials: History, definitions, classifications, synthesis, properties, market, toxicities, risks, and regulations. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Siddiqui, A.J.; Abd Elmoneim, O.E.; Khan, M.I.; Patel, M.; Alreshidi, M.; Moin, A.; Singh, R.; Snoussi, M.; Adnan, M. Innovations in nanoscience for the sustainable development of food and agriculture with implications on health and environment. Sci. Total Environ. 2021, 768, 144990. [Google Scholar] [CrossRef]
- Domingues, C.; Santos, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Jarak, I.; Veiga, F.; Barbosa, I.; Dourado, M.; Figueiras, A. Where is nano today and where is it headed? A review of nanomedicine and the dilemma of nanotoxicology. ACS Nano 2022, 16, 9994–10041. [Google Scholar] [CrossRef]
- Trivedi, R.; Shende, P. Nanotech-based Food: An Initiative for Alternative Pharmaceuticals. Curr. Pharm. Biotechnol. 2022, 23, 1739–1749. [Google Scholar] [CrossRef] [PubMed]


| Synthetic Preservative | Side Effects | Reference |
|---|---|---|
| Nitrates and nitrites | Methemoglobin, loss of consciousness and death, especially in infants. | [89] |
| Carcinogenic. | [90] | |
| Alzheimer’s, Parkinson’s, and type 2 diabetes fatalities. | [91] | |
| Headache, sweating, redness of skin, nausea and weakness. | ||
| Formaldehyde | Potent irritants (skin, eye and lung). | [91] |
| Sperm DNA damage | ||
| Sulfites | Severe allergic reactions and asthma. | [92] |
| Parabens | Neurological damage (in rats), potent irritants, and allergens. | [93] |
| Pregnant women’s exposure of certain toxic chemicals may have an adverse effect on embryonic brain development. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taouzinet, L.; Djaoudene, O.; Fatmi, S.; Bouiche, C.; Amrane-Abider, M.; Bougherra, H.; Rezgui, F.; Madani, K. Trends of Nanoencapsulation Strategy for Natural Compounds in the Food Industry. Processes 2023, 11, 1459. https://doi.org/10.3390/pr11051459
Taouzinet L, Djaoudene O, Fatmi S, Bouiche C, Amrane-Abider M, Bougherra H, Rezgui F, Madani K. Trends of Nanoencapsulation Strategy for Natural Compounds in the Food Industry. Processes. 2023; 11(5):1459. https://doi.org/10.3390/pr11051459
Chicago/Turabian StyleTaouzinet, Lamia, Ouarda Djaoudene, Sofiane Fatmi, Cilia Bouiche, Meriem Amrane-Abider, Hind Bougherra, Farouk Rezgui, and Khodir Madani. 2023. "Trends of Nanoencapsulation Strategy for Natural Compounds in the Food Industry" Processes 11, no. 5: 1459. https://doi.org/10.3390/pr11051459
APA StyleTaouzinet, L., Djaoudene, O., Fatmi, S., Bouiche, C., Amrane-Abider, M., Bougherra, H., Rezgui, F., & Madani, K. (2023). Trends of Nanoencapsulation Strategy for Natural Compounds in the Food Industry. Processes, 11(5), 1459. https://doi.org/10.3390/pr11051459








