Ketones in Low-Temperature Oxidation Products of Crude Oil
Abstract
:1. Introduction
2. Experimental Section
2.1. Samples and Regents
2.2. Oxidation Process
2.3. Bulk Property and Chemical Composition Analysis
2.4. Derivatization and Separation of Ketones
2.5. FT-ICR MS Analysis and Data Processing
3. Results and Discussion
3.1. Properties of Crude Oil
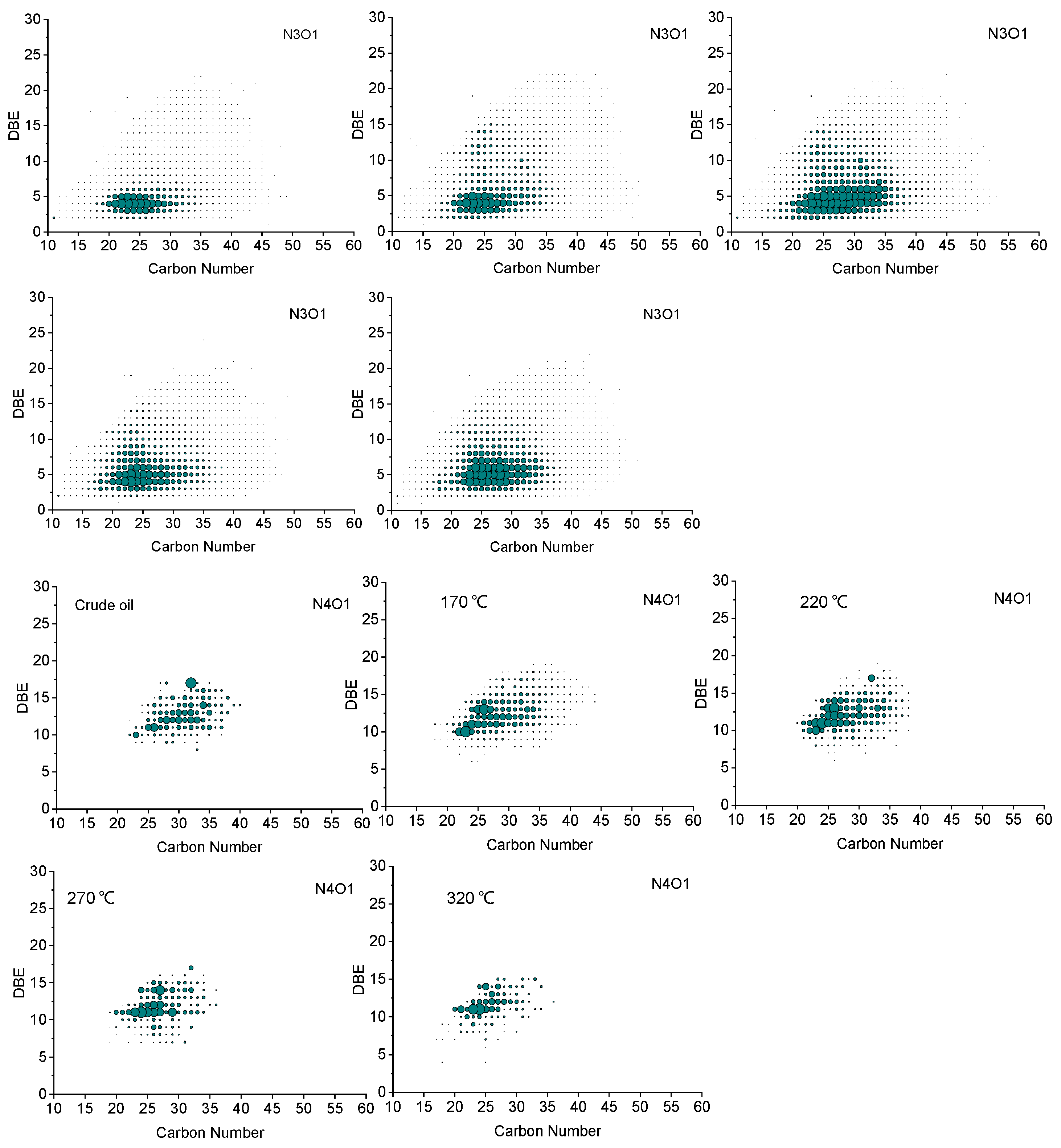
3.2. Molecular Composition of Ketones in the Oxidation Products

4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Wang, Z.; Hu, Z.; Xu, B.; Li, Y.; Pu, W.; Zhao, J. A review of in situ upgrading technology for heavy crude oil. Pet 2021, 7, 117–122. [Google Scholar] [CrossRef]
- Horne, J.; Bousaid, I.; Dore, T.L.; Smith, L.B. Initiation of an in-situ combustion project in a thin oil column underlain by water. J. Pet. Technol. 1982, 34, 2233–2243. [Google Scholar] [CrossRef]
- Ali, S.F. A current appraisal of in-situ combustion field tests. J. Pet. Technol. 1972, 24, 477–486. [Google Scholar] [CrossRef]
- Howell, J.C.; Peterson, M.E. The Fry In Situ Combustion Project Performance And Economic Status. In Proceedings of the SPE Annual Technical Conference and Exhibition, Las Vegas, NV, USA, 23–26 September 1979. [Google Scholar] [CrossRef]
- Turta, A. Chapter 18—In Situ Combustion. In Enhanced Oil Recovery Field Case Studies; Sheng, J.J., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2013; pp. 447–541. [Google Scholar]
- Hascakir, B.; Ross, C.M.; Castanier, L.M.; Kovscek, A.R. Fuel Formation and Conversion During In-Situ Combustion of Crude Oil. Spe J. 2013, 18, 1217–1228. [Google Scholar] [CrossRef]
- Li, Y.-B.; Chen, Y.; Pu, W.-F.; Gao, H.; Bai, B. Experimental investigation into the oxidative characteristics of Tahe heavy crude oil. Fuel 2017, 209, 194–202. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.-F.; Su, L.; Shang, C.; Song, Y.; Li, W.; He, H.-Z.; Liu, Y.-G.; Liu, Z.-Z. Properties, combustion behavior, and kinetic triplets of coke produced by low-temperature oxidation and pyrolysis: Implications for heavy oil in-situ combustion. Pet. Sci. 2021, 18, 1483–1491. [Google Scholar] [CrossRef]
- Huo, J.; Zhao, S.; Pan, J.; Pu, W.; Varfolomeev, M.A.; Emelianov, D.A. Evolution of mass losses and evolved gases of crude oil and its SARA components during low-temperature oxidation by isothermal TG-FTIR analyses. J. Therm. Anal. Calorim. 2021, 147, 4099–4112. [Google Scholar] [CrossRef]
- Kok, M.V.; Varfolomeev, M.A.; Nurgaliev, D.K. Low-temperature oxidation reactions of crude oils using TGA-DSC techniques. J. Therm. Anal. Calorim. 2020, 141, 775–781. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.F.; Varfolomeev, M.A.; Yuan, C.D.; Zhang, J.Z.; Han, X.Q.; Yang, Y.; Peng, X.Q.; Wu, J.X. Comprehensive investigations into low temperature oxidation of heavy crude oil. J. Pet. Sci. Eng. 2018, 171, 835–842. [Google Scholar] [CrossRef]
- Niu, B.; Ren, S.; Liu, Y.; Wang, D.; Tang, L.; Chen, B. Low-Temperature Oxidation of Oil Components in an Air Injection Process for Improved Oil Recovery. Energy Fuels 2011, 25, 4299–4304. [Google Scholar] [CrossRef]
- Li, Y.-B.; Pu, W.-F.; Sun, L.; Jin, F.-Y.; Zhao, J.-Y.; Zhao, J.-Z.; Huang, T. Effect of formation factors on light crude oil oxidation via TG-FTIR. J. Therm. Anal. Calorim. 2014, 118, 1685–1695. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.; Varfolomeev, M.A.; Yuan, C.; Rodionov, A.A. Integrative Investigation of Low-Temperature Oxidation Characteristics and Mechanisms of Heavy Crude Oil. Ind. Eng. Chem. Res. 2019, 58, 14595–14602. [Google Scholar] [CrossRef]
- Greaves, M.; Ren, S.; Rathbone, R. Air injection technique (LTO Process) for IOR from light oil reservoirs: Oxidation. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 19–22 April 1998. [Google Scholar]
- Petrov, L.V.; Solyanikov, V.M. Acid-catalyzed formation of free radicals in the reaction of hydroperoxides with ketones. Russ. Chem. Bull. 1996, 45, 340–345. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, J.; Fang, Q.; Wei, Y.; Song, G.; Xu, C.; Hsu, C.S.; Shi, Q. Evolution of Acidic Compounds in Crude Oil during In Situ Combustion. Energy Fuels 2017, 31, 5926–5932. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.L.; Hou, J.J.; Zhou, C.G. A mechanism study on the viscosity evolution of heavy oil upon peroxide oxidation and pyrolysis. Fuel 2018, 214, 123–126. [Google Scholar] [CrossRef]
- Smith, D.F.; Schaub, T.M.; Rahimi, P.; Teclemariam, A.; Rodgers, R.P.; Marshall, A.G. Self-association of organic acids in petroleum and Canadian bitumen characterized by low- and high-resolution mass spectrometry. Energy Fuels 2007, 21, 1309–1316. [Google Scholar] [CrossRef]
- Fu, J.; Klein, G.C.; Smith, D.F.; Kim, S.; Rodgers, R.P.; Hendrickson, C.L.; Marshall, A.G. Comprehensive compositional analysis of hydrotreated and untreated nitrogen-concentrated fractions syncrude oil by electron ionization, field desorption ionization, and electrospray ionization ultrahigh-resolution FT-ICR mass spectrometry. Energy Fuels 2006, 20, 1235–1241. [Google Scholar] [CrossRef]
- Purcell, J.M.; Juyal, P.; Kim, D.G.; Rodgers, R.P.; Hendrickson, C.L.; Marshall, A.G. Sulfur speciation in petroleum: Atmospheric pressure photoionization or chemical derivatization and electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2007, 21, 2869–2874. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Y.; Chung, K.H.; Zhao, S.; Xu, C. Molecular Characterization of Fossil and Alternative Fuels Using Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry: Recent Advances and Perspectives. Energy Fuels 2021, 35, 18019–18055. [Google Scholar] [CrossRef]
- Ruddy, B.M.; Huettel, M.; Kostka, J.E.; Lobodin, V.V.; Bythell, B.J.; McKenna, A.M.; Aeppli, C.; Reddy, C.M.; Nelson, R.K.; Marshall, A.G.; et al. Targeted petroleomics: Analytical investigation of macondo well oil oxidation products from pensacola beach. Energy Fuels 2014, 28, 4043–4050. [Google Scholar] [CrossRef]
- Long, H.; Shi, Q.; Pan, N.; Zhang, Y.; Cui, D.; Chung, K.H.; Zhao, S.; Xu, C. Characterization of Middle-Temperature Gasification Coal Tar. Part 2: Neutral Fraction by Extrography Followed by Gas Chromatography–Mass Spectrometry and Electrospray Ionization Coupled with Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2012, 26, 3424–3431. [Google Scholar] [CrossRef]
- Neto, C.C.; Maçaira, A.; Pinto, R.; Nakayama, H.; Cardoso, J. New analytical approaches to organic geochemistry: Solid phase functional group extraction for bitumens and functional group markers for kerogens. Phys. Chem. Earth 1980, 12, 249–263. [Google Scholar] [CrossRef]
- Strel’nikova, E.B.; Stakhina, L.D.; Petrenko, T.V. Preconcentration of petroleum organic acids and ketones by two-stage chromatography using a modified adsorbent. J. Anal. Chem. 2009, 64, 8–13. [Google Scholar] [CrossRef]
- Harvey, T.G.; Matheson, T.W.; Pratt, K.C. Chemical class separation of organics in shale oil by thin-layer chromatography. Anal. Chem. 1984, 56, 1277–1281. [Google Scholar] [CrossRef]
- Alhassan, A.; Andersson, J.T. Ketones in fossil materials—A mass spectrometric analysis of a crude oil and a coal tar. Energy Fuels 2013, 27, 5770–5778. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.; Xu, C.; Zhang, W.; Zhu, G.; Li, Z.; Ji, H.; Shi, Q. Molecular Characterization of Ketones in a Petroleum Source Rock. Energy Fuels 2018, 32, 11136–11142. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Liang, Y.; Shi, Q. Molecular characterization of carbonyl compounds in atmospheric fine particulate matters (PM2.5) in Beijing by derivatization with Girard’s reagent T combined with positive-ion ESI Orbitrap MS. Atmos. Res. 2022, 273. [Google Scholar] [CrossRef]
- Chen, X.; Xu, C.; Zhang, W.; Ma, C.; Liu, X.; Zhao, S.; Shi, Q. Separation and Molecular Characterization of Ketones in a Low-Temperature Coal Tar. Energy Fuels 2018, 32, 4662–4670. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, Y.; Bi, S.; Liang, Y.; Shi, Q. Molecular characterization of organic aerosol in winter from Beijing using UHPLC-Orbitrap MS. Sci. Total Environ. 2022, 812. [Google Scholar] [CrossRef]
- Li, Y.; Liao, G.; Wang, Z.; Su, R.; Ma, S.; Zhang, H.; Wang, L.; Wang, X.; Pan, J.; Shi, Q. Molecular composition of low-temperature oxidation products in a simulated crude oil In-situ combustion. Fuel 2022, 316. [Google Scholar] [CrossRef]
- Shi, Q.; Pan, N.; Long, H.; Cui, D.; Guo, X.; Long, Y.; Chung, K.H.; Zhao, S.; Xu, C.; Hsu, C.S. Characterization of Middle-Temperature Gasification Coal Tar. Part 3: Molecular Composition of Acidic Compounds. Energy Fuels 2013, 27, 108–117. [Google Scholar] [CrossRef]
- Hsu, C.S.; Qian, K.; Chen, Y.C. An innovative approach to data analysis in hydrocarbon characterization by on-line liquid chromatography-mass spectrometry. Anal. Chim. Acta 1992, 264, 79–89. [Google Scholar] [CrossRef]
- Zhang, S.; Huo, J.; Sun, X.; Yang, F.; Wang, P.; Wu, J.; Zhang, Y.; Shi, Q. Molecular Composition Reveals Unique Rheological Property of Karamay Heavy Crude Oil. Energy Fuels 2021, 35, 473–478. [Google Scholar] [CrossRef]
- Chen, X. Analysis on Separation and Molecular Composition of Ketone Compounds from Low-Temperature Tar; China University of Petroleum: Beijing, China, 2017. [Google Scholar]
- Khansari, Z.; Kapadia, P.; Mahinpey, N.; Gates, I.D. A new reaction model for low temperature oxidation of heavy oil: Experiments and numerical modeling. Energy 2014, 64, 419–428. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, S. Influence of nitrogen compounds on oxidation property of saturated hydrocarbon. J. Pet. Univ. Nat. Sci. Ed. 2001, 25, 33–35. [Google Scholar]



| Sample | C, wt% | H, wt% | O, wt% | N, wt% | O/C | H/C |
|---|---|---|---|---|---|---|
| Crude oil | 85.91 | 12.38 | 1.42 | 0.28 | 0.012 | 1.73 |
| 170 °C | 85.72 | 12.58 | 2.03 | 0.28 | 0.018 | 1.76 |
| 220 °C | 86.42 | 12.18 | 1.87 | 0.28 | 0.016 | 1.69 |
| 270 °C | 86.22 | 11.81 | 2.10 | 0.24 | 0.018 | 1.64 |
| 320 °C | 86.68 | 11.45 | 2.12 | 0.21 | 0.018 | 1.58 |
| Sample | Saturates (wt%) | Aromatics (wt%) | Resins (wt%) | Asphaltenes (wt%) | Yield (wt%) |
|---|---|---|---|---|---|
| Crude oil | 53.21 | 22.20 | 12.81 | 0.55 | 88.78 |
| 170 °C | 53.02 | 21.69 | 13.82 | 3.46 | 91.98 |
| 220 °C | 45.68 | 17.26 | 15.87 | 13.08 | 91.90 |
| 270 °C | 43.42 | 17.95 | 18.64 | 11.93 | 91.95 |
| 320 °C | 40.18 | 18.07 | 20.61 | 18.94 | 97.80 |
| Oxidation Temperature | ΔN3O1 (%) | ΔN4O1 (%) |
|---|---|---|
| 170 °C | 9.22 | 93.65 |
| 220 °C | 28.69 | −1.59 |
| 270 °C | 24.03 | −55.77 |
| 320 °C | 44.94 | −63.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Li, Y.; Su, R.; Wu, J.; Xie, L.; Tang, J.; Wang, X.; Pan, J.; Wang, Y.; Shi, Q.; et al. Ketones in Low-Temperature Oxidation Products of Crude Oil. Processes 2023, 11, 1664. https://doi.org/10.3390/pr11061664
Ma S, Li Y, Su R, Wu J, Xie L, Tang J, Wang X, Pan J, Wang Y, Shi Q, et al. Ketones in Low-Temperature Oxidation Products of Crude Oil. Processes. 2023; 11(6):1664. https://doi.org/10.3390/pr11061664
Chicago/Turabian StyleMa, Shuai, Yunyun Li, Rigu Su, Jianxun Wu, Lingyuan Xie, Junshi Tang, Xusheng Wang, Jingjun Pan, Yuanfeng Wang, Quan Shi, and et al. 2023. "Ketones in Low-Temperature Oxidation Products of Crude Oil" Processes 11, no. 6: 1664. https://doi.org/10.3390/pr11061664
APA StyleMa, S., Li, Y., Su, R., Wu, J., Xie, L., Tang, J., Wang, X., Pan, J., Wang, Y., Shi, Q., Liao, G., & Xu, C. (2023). Ketones in Low-Temperature Oxidation Products of Crude Oil. Processes, 11(6), 1664. https://doi.org/10.3390/pr11061664






