Synergistic Ball Milling–Enzymatic Pretreatment of Brewer’s Spent Grains to Improve Volatile Fatty Acid Production through Thermophilic Anaerobic Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. BSG Pretreatment
2.3. Characterization of the Raw and Pretreated BSG Samples
2.3.1. Composition and Proximate and Ultimate Analysis
2.3.2. Surface Morphology Analysis
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR) and X-ray Powder Diffraction (XRD)
2.4. Thermophilic AD for VFA Production
2.5. Statistical Analysis
3. Results and Discussion
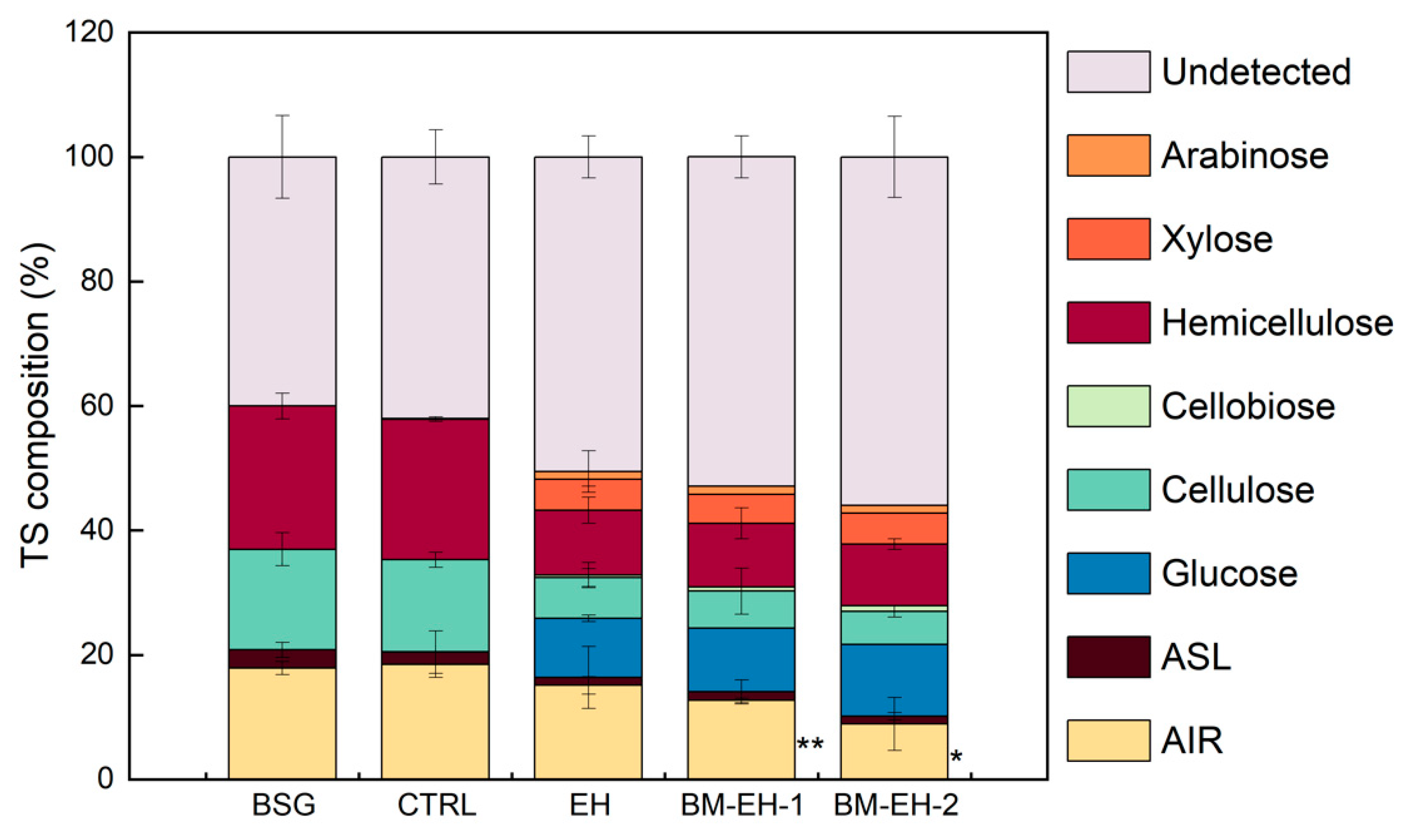
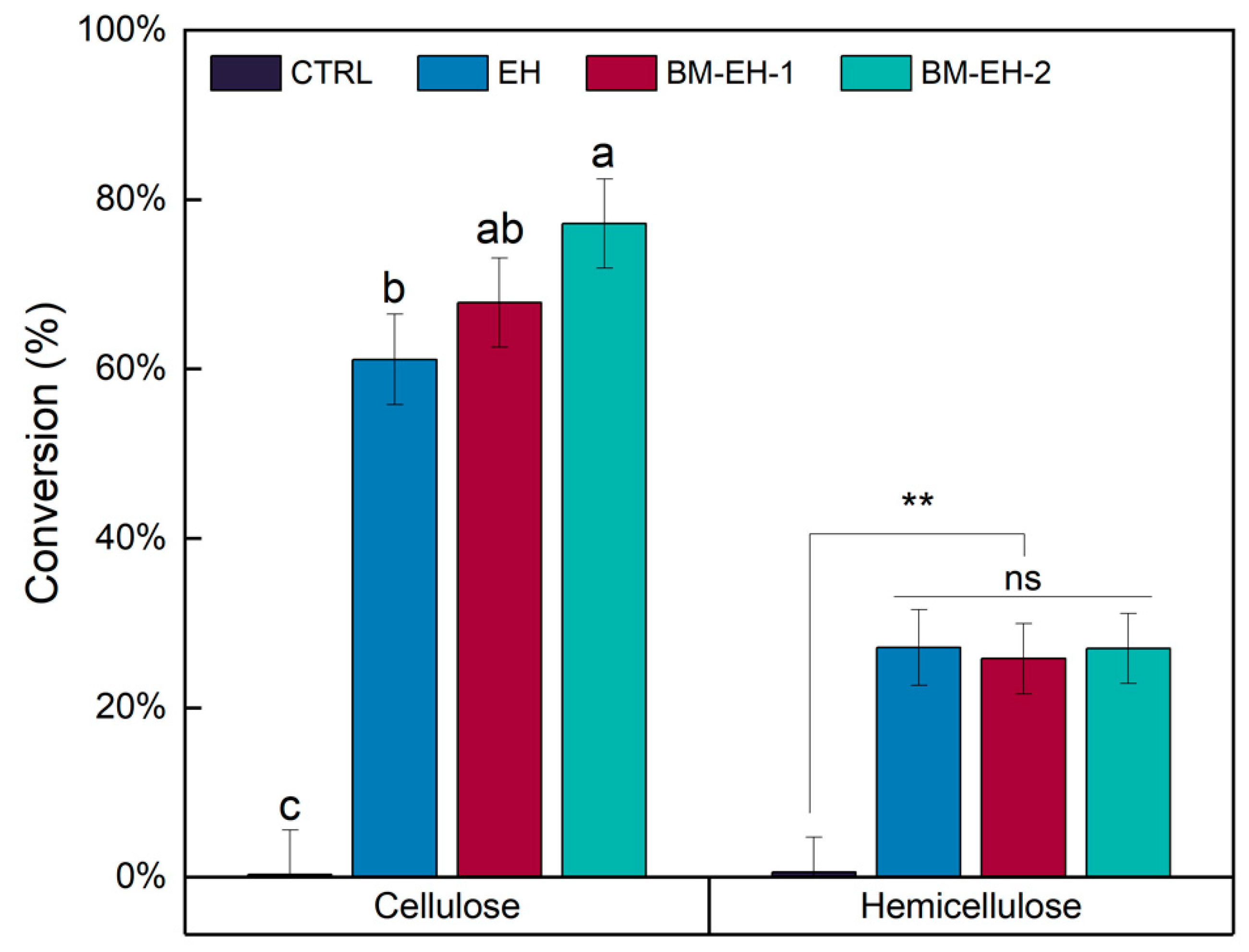
3.1. Effects of the BM-EH Pretreatment on BSG to Liberate Monosaccharides
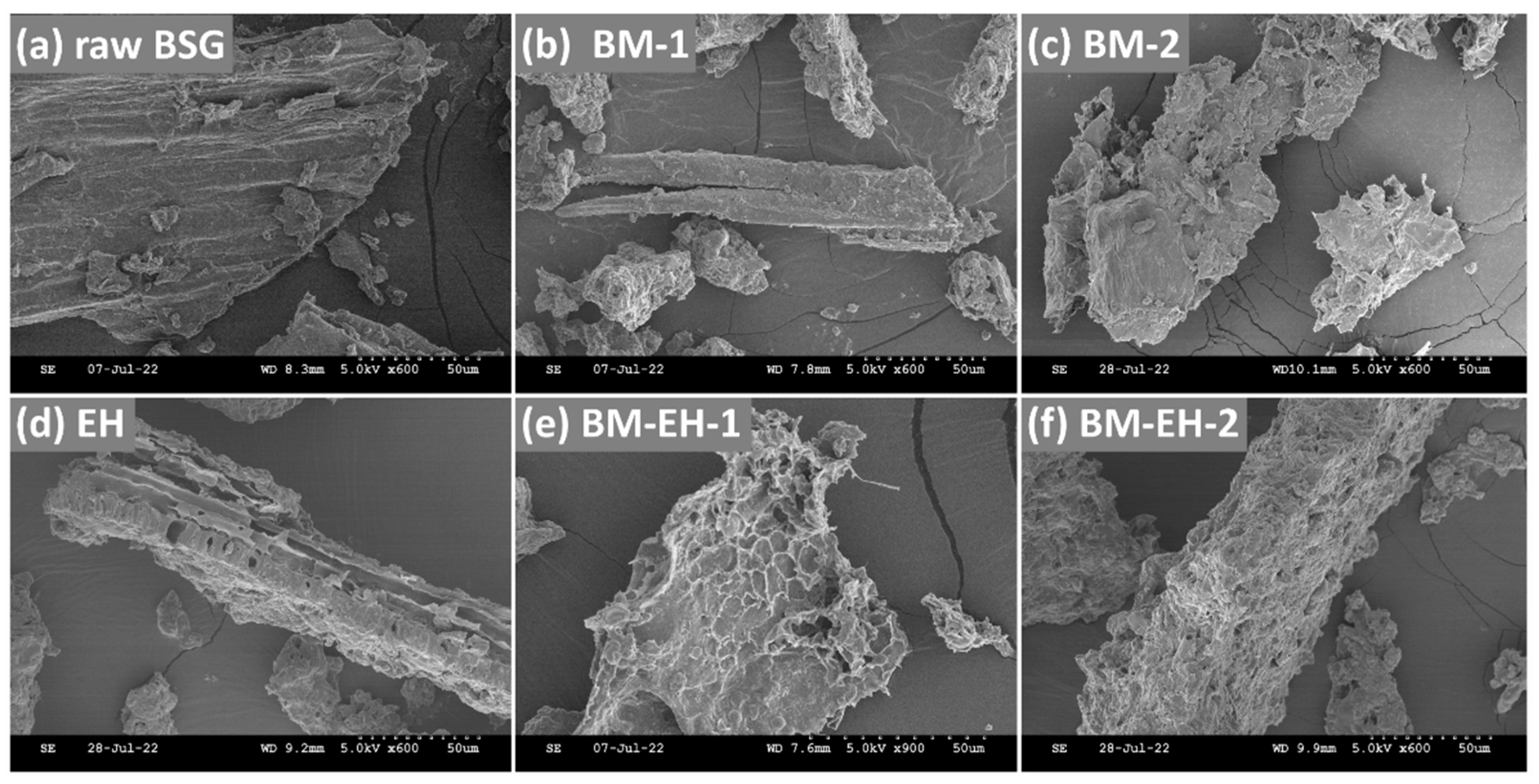
3.2. Characterization of Materials
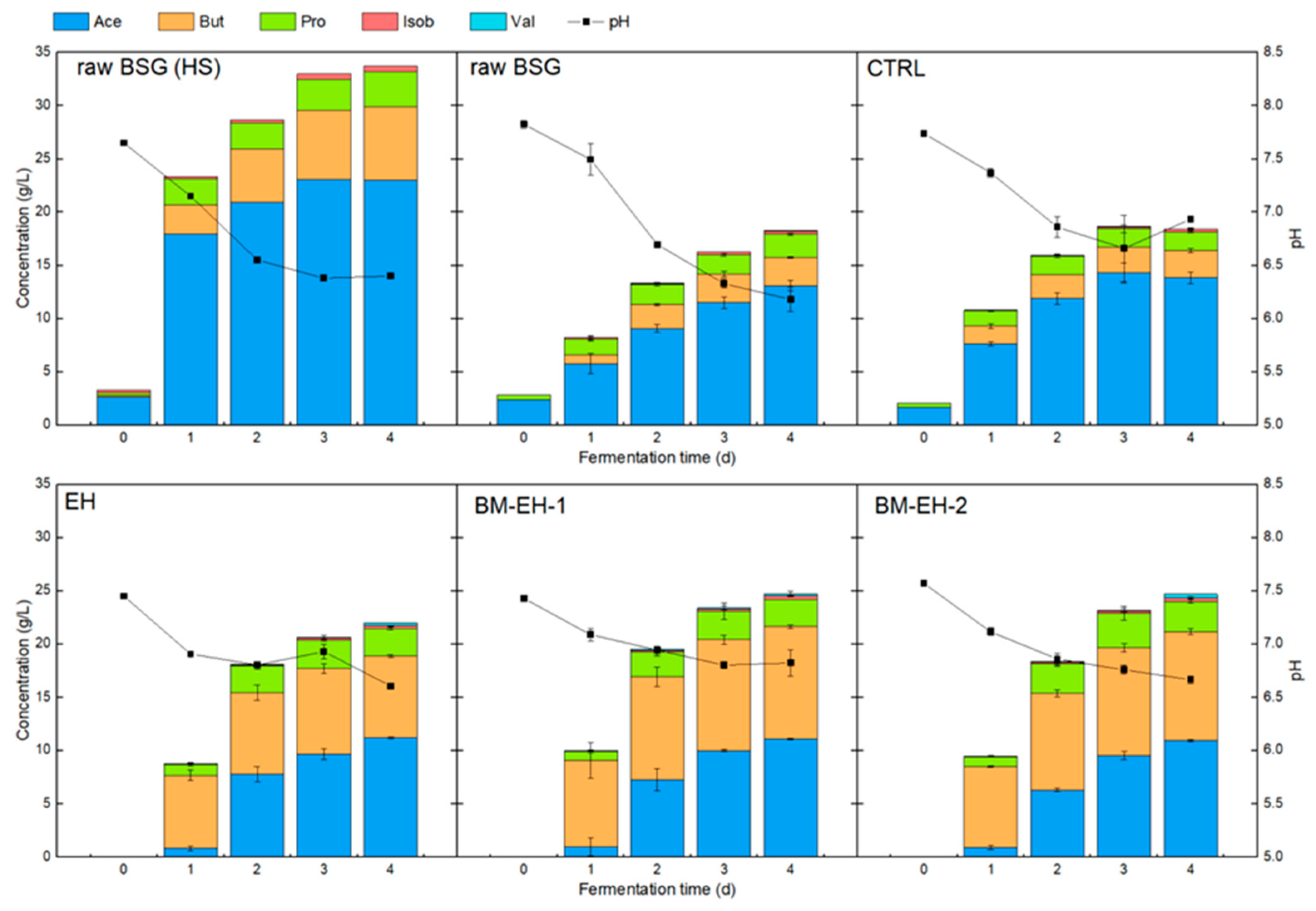
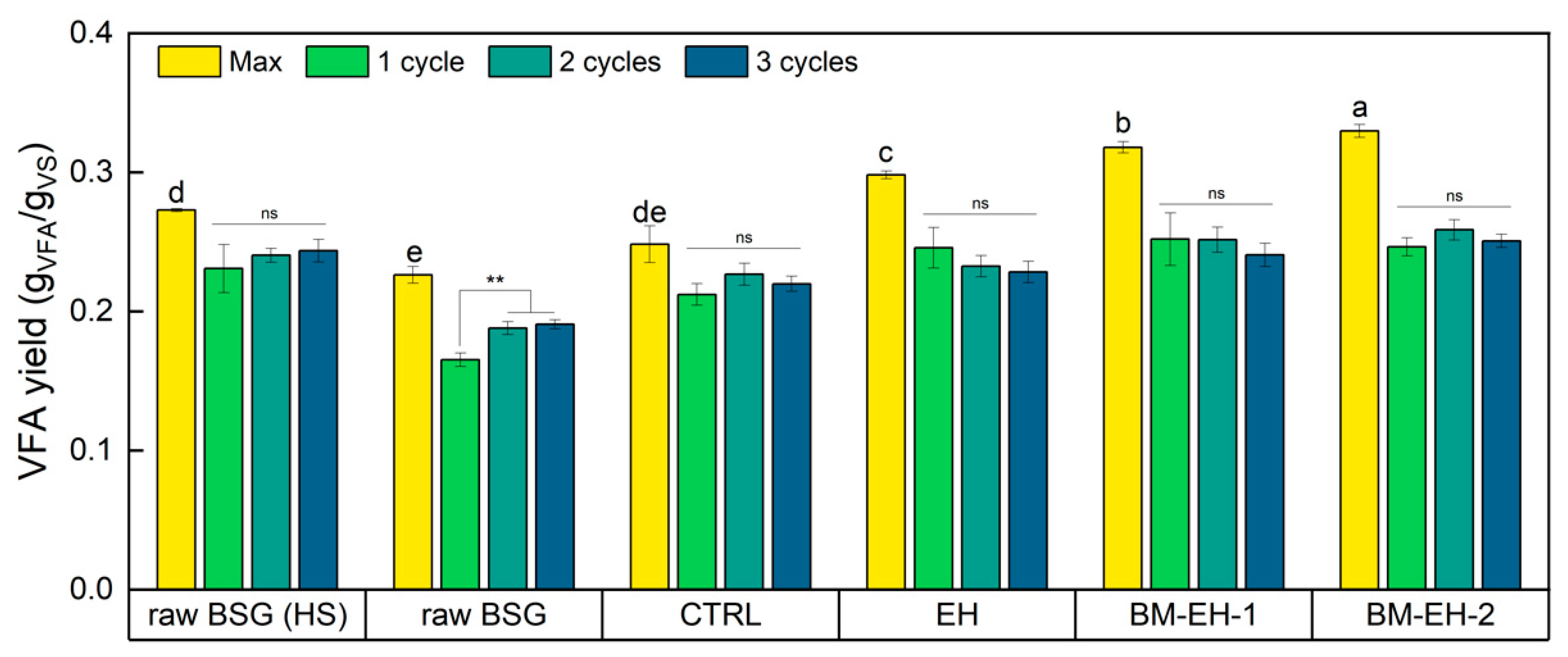
3.3. VFA Fermentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Petit, G.; Korbel, E.; Jury, V.; Aider, M.; Rousselière, S.; Audebrand, L.K.; Turgeon, S.L.; Mikhaylin, S. Environmental Evaluation of New Brewer’s Spent Grain Preservation Pathways for Further Valorization in Human Nutrition. ACS Sustain. Chem. Eng. 2020, 8, 17335–17344. [Google Scholar] [CrossRef]
- Mitri, S.; Salameh, S.-J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of Brewers’ spent grains: Pretreatments and Fermentation, a Review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Llamas, M.; Magdalena, J.A.; González-Fernández, C.; Tomás-Pejó, E.J.B. Volatile fatty acids as novel building blocks for oil-based chemistry via oleaginous yeast fermentation. Biotechnol. Bioeng. 2020, 117, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Marang, L.; Jiang, Y.; van Loosdrecht, M.C.; Kleerebezem, R.J.B.T. Butyrate as preferred substrate for polyhydroxybutyrate production. Bioresour. Technol. 2013, 142, 232–239. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sarvari-Horvath, I.; Taherzadeh, M.J. Factors influencing volatile fatty acids production from food wastes via anaerobic digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Z.; Stiverson, J.A.; Yu, Z.; Li, Y. Reactor performance and microbial community dynamics during solid-state anaerobic digestion of corn stover at mesophilic and thermophilic conditions. Bioresour. Technol. 2013, 136, 574–581. [Google Scholar] [CrossRef]
- Vanwonterghem, I.; Jensen, P.D.; Rabaey, K.; Tyson, G.W. Temperature and solids retention time control microbial population dynamics and volatile fatty acid production in replicated anaerobic digesters. Sci. Rep. 2015, 5, 8496. [Google Scholar] [CrossRef]
- Guarda, E.C.; Oliveira, A.C.; Antunes, S.; Freitas, F.; Castro, P.M.L.; Duque, A.F.; Reis, M.A.M. A Two-Stage Process for Conversion of Brewer’s Spent Grain into Volatile Fatty Acids through Acidogenic Fermentation. Appl. Sci. 2021, 11, 3222. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L.J.B.T. Influence of initial uncontrolled pH on acidogenic fermentation of brewery spent grains to biohydrogen and volatile fatty acids production: Optimization and scale-up. Bioresour. Technol. 2021, 319, 124233. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Guarda, E.C.; Freitas, E.B.; Galinha, C.F.; Duque, A.F.; Reis, M.A.J.N.B. Valorization of raw brewers’ spent grain through the production of volatile fatty acids. New Biotechnol. 2020, 57, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M.J.M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [PubMed]
- Zeko-Pivač, A.; Tišma, M.; Žnidaršič-Plazl, P.; Kulisic, B.; Sakellaris, G.; Hao, J.; Planinić, M. The potential of brewer’s spent grain in the circular bioeconomy: State of the art and future perspectives. Biotechnology 2022, 10, 870744. [Google Scholar]
- Larsson, S.; Pålsson, B.I.; Parian, M.; Jonsén, P.J.M.E. A novel approach for modelling of physical interactions between slurry, grinding media and mill structure in wet stirred media mills. Miner. Eng. 2020, 148, 106180. [Google Scholar] [CrossRef]
- Sakthivel, S.; Venkatesh, R.P. Solid state synthesis of nano-mineral particles. Int. J. Min. Sci. Technol. 2012, 22, 651–655. [Google Scholar] [CrossRef]
- Martin-Sampedro, R.; Filpponen, I.; Hoeger, I.C.; Zhu, J.Y.; Laine, J.; Rojas, O.J. Rapid and Complete Enzyme Hydrolysis of Lignocellulosic Nanofibrils. ACS Macro Lett. 2012, 1, 1321–1325. [Google Scholar] [CrossRef]
- Zhong, Y.; Frost, H.; Bustamante, M.; Li, S.; Liu, Y.S.; Liao, W.J.R.; Reviews, S.E. A mechano-biocatalytic one-pot approach to release sugars from lignocellulosic materials. Renew. Sustain. Energy Rev. 2020, 121, 109675. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- American Society for Testing and Materials. Standard Test Methods for Analysis of Wood Fuels; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Olszewski, M.; Arauzo, P.; Wądrzyk, M.; Kruse, A.J.J.O.A.; Pyrolysis, A. Py-GC-MS of hydrochars produced from brewer’s spent grains. J. Anal. Appl. Pyrolysis 2019, 140, 255–263. [Google Scholar] [CrossRef]
- Zeraatkar Dehnavi, G. Fractionation of the Main Components of Barley Spent Grains from a Microbrewery; University of Borås/School of Engineering: Borås, Sweden, 2009. [Google Scholar]
- Sitotaw, Y.W.; Habtu, N.G.; Gebreyohannes, A.Y.; Nunes, S.P.; Van Gerven, T. Biorefinery. Ball milling as an important pretreatment technique in lignocellulose biorefineries: A review. Biomass Convers. Biorefinery 2021, 1–24. [Google Scholar] [CrossRef]
- Santos, D.M.D.; Bukzem, A.d.L.; Ascheri, D.P.R.; Signini, R.; Aquino, G.L.B.D. Microwave-assisted carboxymethylation of cellulose extracted from brewer’s spent grain. Carbohydr. Polym. 2015, 131, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.H.F.; Voorwald, H.C.J.; Cioffi, M.O.H.; Mullinari, D.R.; Da Luz, S.M.; Da Silva, M.L.C.P.J.B. Sugarcane bagasse pulping and bleaching: Thermal and chemical characterization. BioResources 2011, 6, 2471–2482. [Google Scholar]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. A comparative analysis of pretreatment strategies on the properties and hydrolysis of brewers’ spent grain. Bioresour. Technol. 2018, 248, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Gregor, T.; Wimmer, R. Utilising brewer’s spent grain as a source of cellulose nanofibres following separation of protein-based biomass. BioResources 2017, 12, 107–116. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, J.H.; Kim, T.H.; Choi, W.I. Impact of planetary ball mills on corn stover characteristics and enzymatic digestibility depending on grinding ball properties. Bioresour. Technol. 2017, 241, 1094–1100. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Fujimoto, S.; Hirata, S.; Hassan, M.A. Ball milling pretreatment of oil palm biomass for enhancing enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2014, 173, 1778–1789. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Loh, K.C. Review and perspectives of enhanced volatile fatty acids production from acidogenic fermentation of lignocellulosic biomass wastes. Bioresour. Bioprocess. 2021, 8, 68. [Google Scholar] [CrossRef]
- Temudo, M.F.; Mato, T.; Kleerebezem, R.; van Loosdrecht, M.C.M. Xylose anaerobic conversion by open-mixed cultures. Appl. Microbiol. Biotechnol. 2009, 82, 231–239. [Google Scholar] [CrossRef]
- Qiao, W.; Takayanagi, K.; Niu, Q.; Shofie, M.; Li, Y.Y. Long-term stability of thermophilic co-digestion submerged anaerobic membrane reactor encountering high organic loading rate, persistent propionate and detectable hydrogen in biogas. Bioresour. Technol. 2013, 149, 92–102. [Google Scholar] [CrossRef]
- Wang, K.; Chang, Z.; Ma, Y.; Lei, C.; Wang, J.; Zhu, T.; Liu, H.; Zuo, Y.; Li, X. Study on solvent extraction of propionic acid from simulated discharged water in vitamin B12 production by anaerobic fermentation. Bioresour. Technol. 2009, 100, 2878–2882. [Google Scholar] [CrossRef]
- Li, Y.F.; Shi, J.; Nelson, M.C.; Chen, P.H.; Graf, J.; Li, Y.; Yu, Z. Impact of different ratios of feedstock to liquid anaerobic digestion effluent on the performance and microbiome of solid-state anaerobic digesters digesting corn stover. Bioresour. Technol. 2016, 200, 744–752. [Google Scholar] [CrossRef] [PubMed]

| Component | BSG | CTRL | EH | BM-EH-1 | BM-EH-2 | SLG |
|---|---|---|---|---|---|---|
| Volatile Matter, % 2 | 80.72 ± 1.18 | 83.60 ± 1.68 | 83.12 ± 0.81 | 81.84 ± 0.93 | 81.36 ± 0.70 | 57.89 ± 0.58 |
| Fixed Carbon, % 2 | 16.09 ± 0.19 | 8.99 ± 0.34 | 10.44 ± 0.16 | 11.42 ± 0.47 | 11.65 ± 0.13 | 1.65 ± 0.28 |
| Ash, % 2 | 3.18 ± 0.33 | 7.41 ± 0.49 | 6.44 ± 0.51 | 6.74 ± 0.09 | 6.99 ± 0.28 | 40.47 ± 0.48 |
| Hydrogen, % | 7.00 ± 0.10 | 6.64 ± 0.04 | 6.62 ± 0.17 | 6.63 ± 0.08 | 6.64 ± 0.06 | 5.30 ± 0.16 |
| Carbon, % | 47.35 ± 0.46 | 45.11 ± 0.25 | 44.61 ± 0.48 | 44.15 ± 0.13 | 43.95 ± 0.05 | 28.71 ± 0.07 |
| Nitrogen, % | 4.14 ± 0.10 | 3.82 ± 0.06 | 3.88 ± 0.07 | 3.78 ± 0.02 | 3.80 ± 0.04 | 4.03 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Ullah, A.; Gao, X.; Shi, J. Synergistic Ball Milling–Enzymatic Pretreatment of Brewer’s Spent Grains to Improve Volatile Fatty Acid Production through Thermophilic Anaerobic Fermentation. Processes 2023, 11, 1648. https://doi.org/10.3390/pr11061648
Liu C, Ullah A, Gao X, Shi J. Synergistic Ball Milling–Enzymatic Pretreatment of Brewer’s Spent Grains to Improve Volatile Fatty Acid Production through Thermophilic Anaerobic Fermentation. Processes. 2023; 11(6):1648. https://doi.org/10.3390/pr11061648
Chicago/Turabian StyleLiu, Can, Ahamed Ullah, Xin Gao, and Jian Shi. 2023. "Synergistic Ball Milling–Enzymatic Pretreatment of Brewer’s Spent Grains to Improve Volatile Fatty Acid Production through Thermophilic Anaerobic Fermentation" Processes 11, no. 6: 1648. https://doi.org/10.3390/pr11061648
APA StyleLiu, C., Ullah, A., Gao, X., & Shi, J. (2023). Synergistic Ball Milling–Enzymatic Pretreatment of Brewer’s Spent Grains to Improve Volatile Fatty Acid Production through Thermophilic Anaerobic Fermentation. Processes, 11(6), 1648. https://doi.org/10.3390/pr11061648









