Progress on the Microwave-Assisted Recycling of Spent Lithium Battery Graphite
Abstract
1. Introduction
2. Microwave-Assisted Regeneration of Spent Graphite by Inducing Flash Evaporation
3. Microwave-Assisted Preparation of Graphite Intercalation Compounds
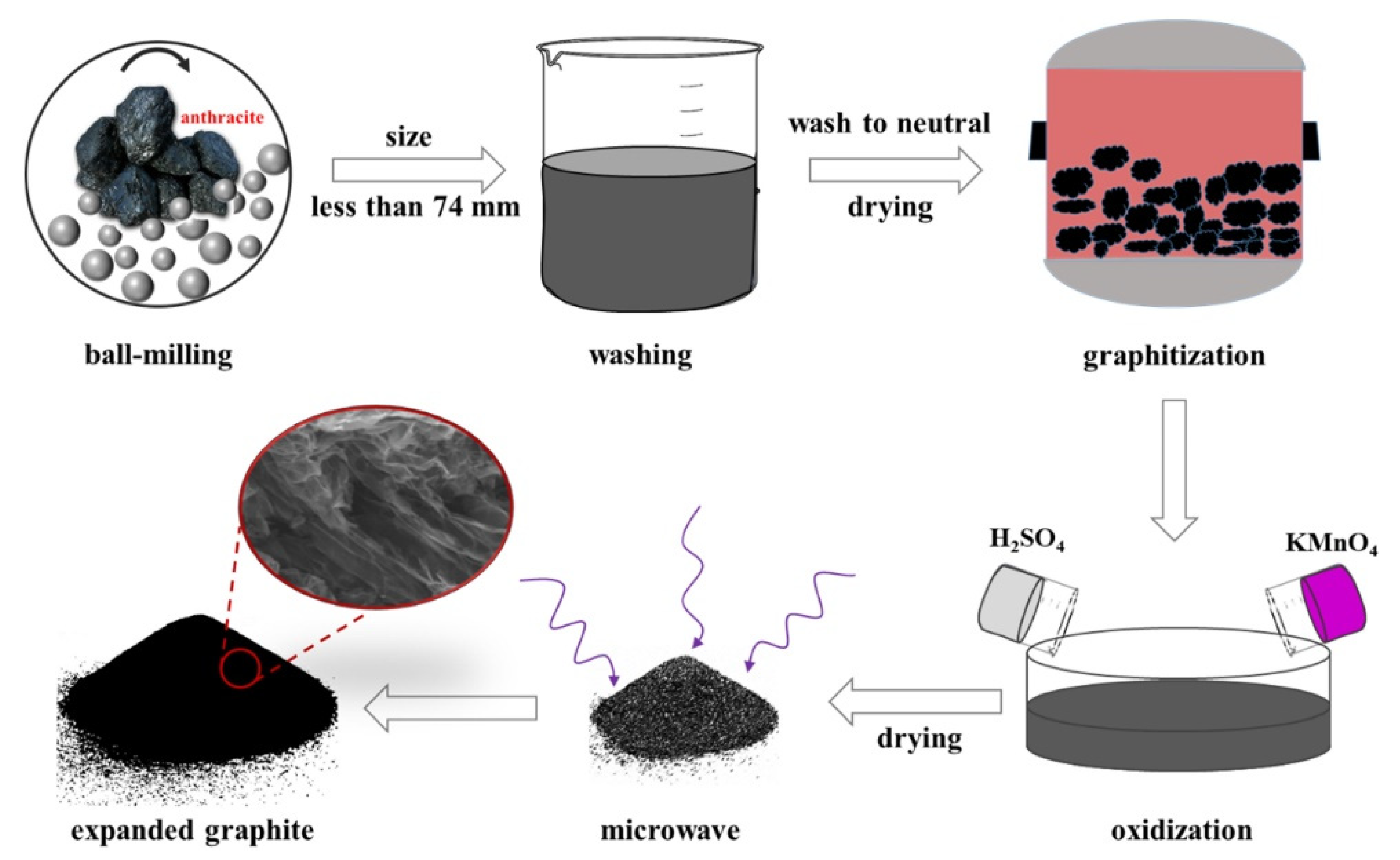
4. Microwave-Assisted Preparation of Expanded Graphite
5. Microwave-Assisted Preparation of Graphene and Graphene-Derivative Functional Material
5.1. Graphene
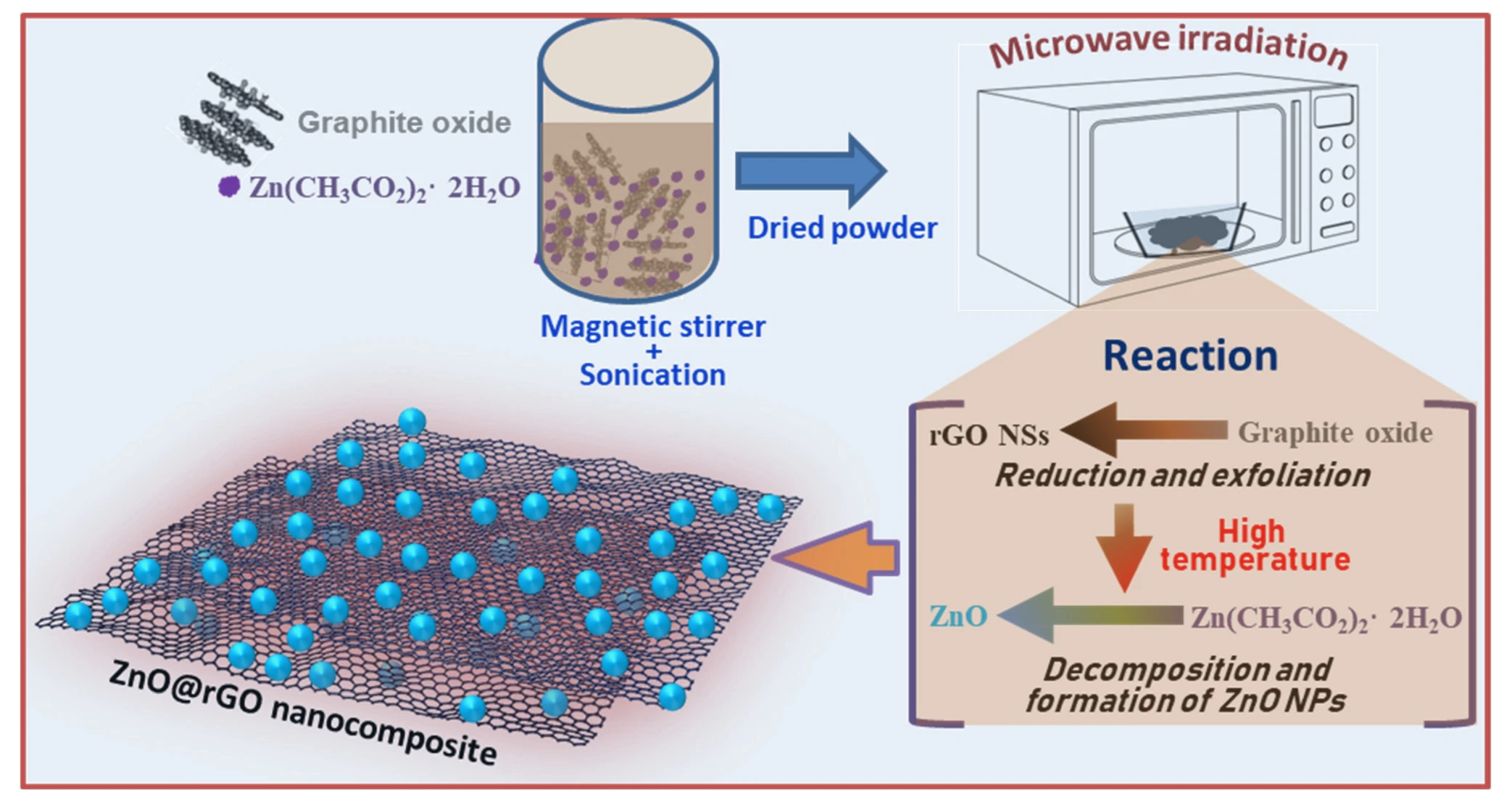
5.2. Graphene-Derivative Functional Material
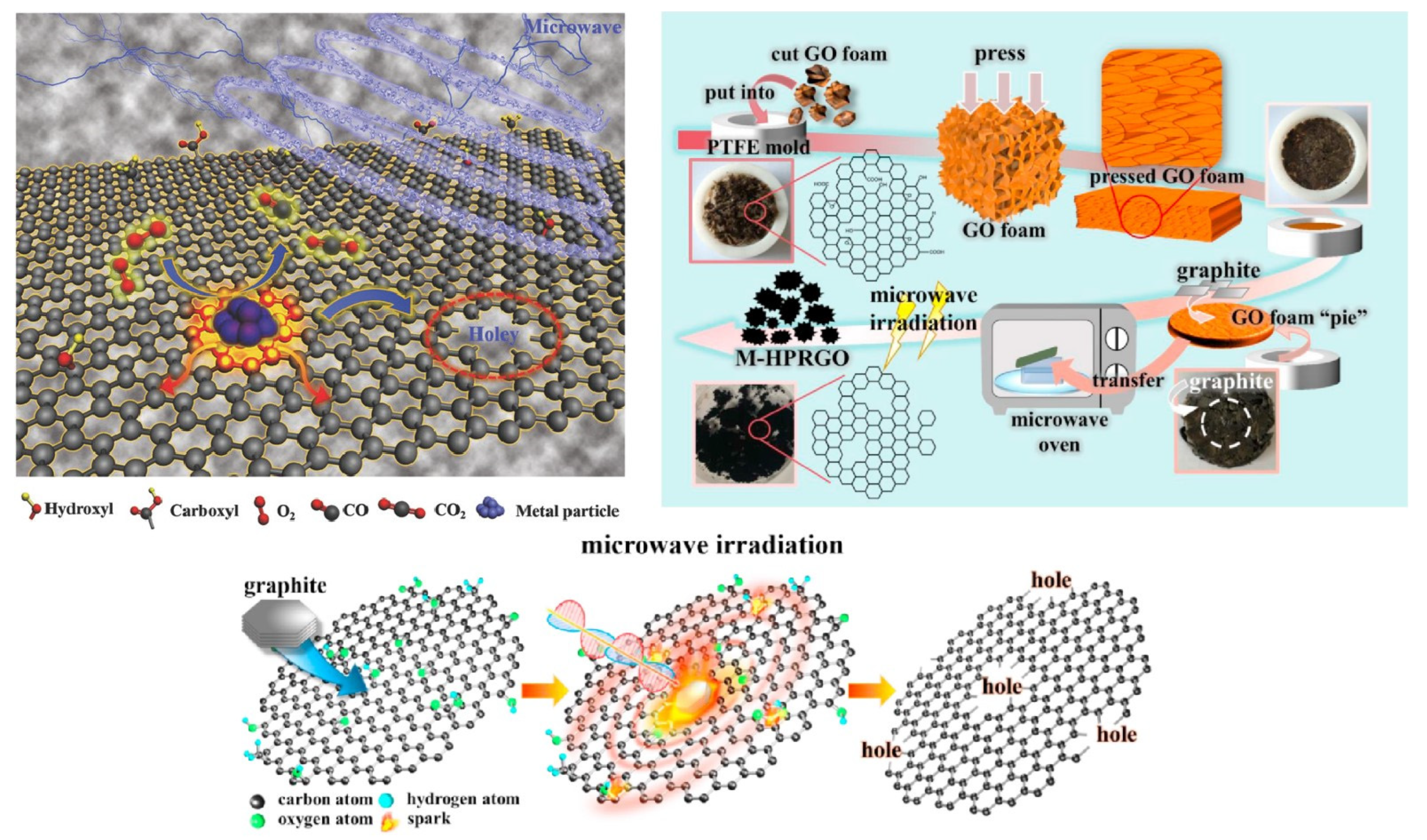
6. Microwave-Assisted Preparation of Porous Graphene
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Production and Sales of the Automobile Industry in 2022. Available online: http://www.caam.org.cn/chn/4/cate_32/con_5236639.html (accessed on 5 April 2023).
- Wang, S.; Ren, P.; Takyi-Aninakwa, P.; Jin, S.; Fernandez, C. A Critical Review of Improved Deep Convolutional Neural Network for Multi-Timescale State Prediction of Lithium-Ion Batteries. Energies 2022, 15, 5053. [Google Scholar] [CrossRef]
- Wang, S.; Takyi-Aninakwa, P.; Jin, S.; Yu, C.; Fernandez, C.; Stroe, D.-I. An Improved Feedforward-Long Short-Term Memory Modeling Method for the Whole-Life-Cycle State of Charge Prediction of Lithium-Ion Batteries Considering Current-Voltage-Temperature Variation. Energy 2022, 254, 124224. [Google Scholar] [CrossRef]
- Wang, S.; Jin, S.; Bai, D.; Fan, Y.; Shi, H.; Fernandez, C. A Critical Review of Improved Deep Learning Methods for the Remaining Useful Life Prediction of Lithium-Ion Batteries. Energy Rep. 2021, 7, 5562–5574. [Google Scholar] [CrossRef]
- Wu, C.; Hu, J.; Ye, L.; Su, Z.; Fang, X.; Zhu, X.; Zhuang, L.; Ai, X.; Yang, H.; Qian, J. Direct Regeneration of Spent Li-Ion Battery Cathodes via Chemical Relithiation Reaction. Acs Sustain. Chem. Eng. 2021, 9, 16384–16393. [Google Scholar] [CrossRef]
- Yang, Y.; Song, S.; Lei, S.; Sun, W.; Hou, H.; Jiang, F.; Ji, X.; Zhao, W.; Hu, Y. A Process for Combination of Recycling Lithium and Regenerating Graphite from Spent Lithium-Ion Battery. Waste Manag. 2019, 85, 529–537. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, C.; Yu, Z.; Ma, W.; Jin, Q.; Du, R.; Qian, B.; Jin, X.; Wu, H.; Zhang, Q.; et al. Slidable and Highly Ionic Conductive Polymer Binder for High-Performance Si Anodes in Lithium-Ion Batteries. Adv. Sci. 2023, 10, 2205590. [Google Scholar] [CrossRef]
- Yu, J.; Lin, M.; Tan, Q.; Li, J. High-Value Utilization of Graphite Electrodes in Spent Lithium-Ion Batteries: From 3D Waste Graphite to 2D Graphene Oxide. J. Hazard. Mater. 2021, 401, 123715. [Google Scholar] [CrossRef]
- Cheng, Q.; Marchetti, B.; Chen, X.; Xu, S.; Zhou, X.-D. Separation, Purification, Regeneration and Utilization of Graphite Recovered from Spent Lithium-Ion Batteries—A Review. J. Environ. Chem. Eng. 2022, 10, 107312. [Google Scholar] [CrossRef]
- Li, Y.; Lv, W.; Zhao, H.; Xie, Y.; Ruan, D.; Sun, Z. Regeneration of Anode Materials from Complex Graphite Residue in Spent Lithium-Ion Battery Recycling Process. Green Chem. 2022, 24, 9315–9328. [Google Scholar] [CrossRef]
- Ma, Z.; Zhuang, Y.; Deng, Y.; Song, X.; Zuo, X.; Xiao, X.; Nan, J. From Spent Graphite to Amorphous Sp2 + sp3 Carbon-Coated Sp2 Graphite for High-Performance Lithium Ion Batteries. J. Power Sources 2018, 376, 91–99. [Google Scholar] [CrossRef]
- Arshad, F.; Li, L.; Amin, K.; Fan, E.; Manurkar, N.; Ahmad, A.; Yang, J.; Wu, F.; Chen, R. A Comprehensive Review of the Advancement in Recycling the Anode and Electrolyte from Spent Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2020, 8, 13527–13554. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Hu, X.; Geng, Y.; Yang, L.; Shao, P.; Luo, X. Critical Strategies for Recycling Process of Graphite from Spent Lithium-Ion Batteries: A Review. Sci. Total Environ. 2022, 816, 151621. [Google Scholar] [CrossRef]
- Sugiyama, K.; Suzuki, K.; Kuwasima, S.; Aoki, Y.; Yajima, T. Decomposition of Poly(Amide-Imide) Film Enameled on Solid Copper Wire Using Atmospheric Pressure Non-Equilibrium Plasma. J. Environ. Sci. 2009, 21, S166–S169. [Google Scholar] [CrossRef]
- Chung, J.Y.; Kodama, S.; Sekiguchi, H. Preparation of a Pd/Al2O3 Catalyst with Microwave-Induced Plasma Jet Irradiation under Atmospheric Pressure. Nanomaterials 2019, 9, 1734. [Google Scholar] [CrossRef]
- Fahimi, A.; Alessandri, I.; Cornelio, A.; Frontera, P.; Malara, A.; Mousa, E.; Ye, G.; Valentim, B.; Bontempi, E. A Microwave-Enhanced Method Able to Substitute Traditional Pyrometallurgy for the Future of Metals Supply from Spent Lithium-Ion Batteries. Resour. Conserv. Recycl. 2023, 194, 106989. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, C.; Huang, H.; Guo, W.; Zhao, C.; Ren, W.; Xie, Y.; Qiu, J. Energy Accumulation Enabling Fast Synthesis of Intercalated Graphite and Operando Decoupling for Lithium Storage. Adv. Funct. Mater. 2021, 31, 2009801. [Google Scholar] [CrossRef]
- Yi, C.; Yang, Y.; Zhang, T.; Wu, X.; Sun, W.; Yi, L. A Green and Facile Approach for Regeneration of Graphite from Spent Lithium Ion Battery. J. Clean. Prod. 2020, 277, 123585. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Z.; Xia, J.; Li, F.; He, W.; Li, G.; Huang, J. Preparing Graphene from Anode Graphite of Spent Lithium-Ion Batteries. Front. Environ. Sci. Eng. 2017, 11, 6. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, L.; Liu, J.; Luo, Y.; Chen, Y. A New Approach to Regenerate High-Performance Graphite from Spent Lithium-Ion Batteries. Carbon 2022, 189, 293–304. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. An Urgent Call to Spent LIB Recycling: Whys and Wherefores for Graphite Recovery. Adv. Energy Mater. 2020, 10, 2002238. [Google Scholar] [CrossRef]
- Yu, H.; Dai, H.; Zhu, Y.; Hu, H.; Zhao, R.; Wu, B.; Chen, D. Mechanistic Insights into the Lattice Reconfiguration of the Anode Graphite Recycled from Spent High-Power Lithium-Ion Batteries. J. Power Sources 2021, 481, 229159. [Google Scholar] [CrossRef]
- Niu, B.; Xiao, J.; Xu, Z. Advances and Challenges in Anode Graphite Recycling from Spent Lithium-Ion Batteries. J. Hazard. Mater. 2022, 439, 129678. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Wang, R.; Poyraz, S.; Cook, J.; Bozack, M.J.; Das, S.; Zhang, X.; Hu, L. Ultrafast Microwave Nano-Manufacturing of Fullerene-Like Metal Chalcogenides. Sci Rep 2016, 6, 22503. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, H.; Ru, Y.; Zhang, X.; Qiao, J. Conductive Graphene–Melamine Sponge Prepared via Microwave Irradiation. Acs Appl. Mater. Interfaces 2018, 10, 24776–24783. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G.; Bahri, M. Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics. ChemistryOpen 2021, 10, 1202–1226. [Google Scholar] [CrossRef]
- Yuwen, C.; Liu, B.; Zhang, H.; Tian, S.; Zhang, L.; Guo, S.; Zhou, B. Efficient Recovery and Regeneration of Waste Graphite through Microwave Stripping from Spent Batteries Anode for High-Performance Lithium-Ion Batteries. J. Cleaner Prod. 2022, 333, 130197. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, J.; Ma, R.; Chen, Y.; Wang, C. Regeneration of Graphite Anode from Spent Lithium-Ion Batteries via Microwave Calcination. J. Electroanal. Chem. 2022, 908, 116087. [Google Scholar] [CrossRef]
- Hou, D.; Guo, Z.; Wang, Y.; Hou, X.; Yi, S.; Zhang, Z.; Hao, S.; Chen, D. Microwave-Assisted Reconstruction of Spent Graphite and Its Enhanced Energy-Storage Performance as LIB Anodes. Surf. Interfaces 2021, 24, 101098. [Google Scholar] [CrossRef]
- An, Y.; Fei, H.; Zeng, G.; Ci, L.; Xi, B.; Xiong, S.; Feng, J. Commercial Expanded Graphite as a Low–Cost, Long-Cycling Life Anode for Potassium–Ion Batteries with Conventional Carbonate Electrolyte. J. Power Sources 2018, 378, 66–72. [Google Scholar] [CrossRef]
- Zhang, W.-J. A Review of the Electrochemical Performance of Alloy Anodes for Lithium-Ion Batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Yang, J.; Fan, E.; Lin, J.; Arshad, F.; Zhang, X.; Wang, H.; Wu, F.; Chen, R.; Li, L. Recovery and Reuse of Anode Graphite from Spent Lithium-Ion Batteries via Citric Acid Leaching. Acs Appl. Energy Mater. 2021, 4, 6261–6268. [Google Scholar] [CrossRef]
- Krawczyk, P.; Gurzęda, B.; Bachar, A.; Buchwald, T. Formation of a N2O5–Graphite Intercalation Compound by Ozone Treatment of Natural Graphite. Green Chem. 2020, 22, 5463–5469. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Sundararajan, A.; Omprakash, P.; Bhat Panemangalore, D. Review—Energy Storage through Graphite Intercalation Compounds. J. Electrochem. Soc. 2021, 168, 040541. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Li, M. Preparation of Expandable Graphite with Ultrasound Irradiation. Mater. Lett. 2007, 61, 5070–5073. [Google Scholar] [CrossRef]
- Chen, Y.P.; Li, S.Y.; Luo, R.Y.; Lv, X.M.; Wang, X.J. Optimization of Initial Redox Potential in the Preparation of Expandable Graphite by Chemical Oxidation. New Carbon Mater. 2013, 28, 435–441. [Google Scholar] [CrossRef]
- He, J.; Yuan, M.; Ren, H.; Song, T.; Zhang, Y. The Electrochemical Preparation and Characterization of Sulfur-Free Expanded Graphite. J. Chem. Sci. 2023, 135, 17. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Zhang, L. Green and Simple Production of Graphite Intercalation Compound Used Sodium Bicarbonate as Intercalation Agent. BMC Chem. 2022, 16, 13. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, L.; Tang, Z.; Xu, Z.; Xie, C.; Guo, L.; Li, W. High-Performance Expanded Graphite from Flake Graphite by Microwave-Assisted Chemical Intercalation Process. J. Ind. Eng. Chem. 2023, 122, 562–572. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Han, F.; Zhang, F.; Zhou, D.; Xu, S.; Liu, H.; Li, X.; Liu, J. Improving the Cycle Stability of FeCl3-Graphite Intercalation Compounds by Polar Fe2O3 Trapping in Lithium-Ion Batteries. Nano Res. 2019, 12, 1836–1844. [Google Scholar] [CrossRef]
- Frąc, M.; Pichór, W.; Szołdra, P. Cement Composites with Expanded Graphite as Resistance Heating Elements. J. Compos. Mater. 2020, 54, 3821–3831. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H.; Kim, W.Y.; Lee, H.S.; Kim, S.Y.; Khil, M.-S. Volume Control of Expanded Graphite Based on Inductively Coupled Plasma and Enhanced Thermal Conductivity of Epoxy Composite by Formation of the Filler Network. Carbon 2017, 119, 40–46. [Google Scholar] [CrossRef]
- Chung, D. Exfoliation of Graphite. J. Mater. Sci. 1987, 22, 4190–4198. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, C.; Zhang, W.; Pan, W.; Wang, Q.; Li, L. Expanded Graphite-Based Materials for Supercapacitors: A Review. Molecules 2022, 27, 716. [Google Scholar] [CrossRef]
- Chriaa, I.; Karkri, M.; Trigui, A.; Jedidi, I.; Abdelmouleh, M.; Boudaya, C. The Performances of Expanded Graphite on the Phase Change Materials Composites for Thermal Energy Storage. Polymer 2021, 212, 123128. [Google Scholar] [CrossRef]
- Hoang, N.B.; Nguyen, T.T.; Nguyen, T.S.; Bui, T.P.Q.; Bach, L.G. The Application of Expanded Graphite Fabricated by Microwave Method to Eliminate Organic Dyes in Aqueous Solution. Cogent Eng. 2019, 6, 1584939. [Google Scholar] [CrossRef]
- Sykam, N.; Jayram, N.D.; Rao, G.M. Highly Efficient Removal of Toxic Organic Dyes, Chemical Solvents and Oils by Mesoporous Exfoliated Graphite: Synthesis and Mechanism. J. Water Process Eng. 2018, 25, 128–137. [Google Scholar] [CrossRef]
- Tryba, B.; Morawski, A.W.; Inagaki, M. Preparation of Exfoliated Graphite by Microwave Irradiation. Carbon 2005, 43, 2417–2419. [Google Scholar] [CrossRef]
- Falcao, E.H.L.; Blair, R.G.; Mack, J.J.; Viculis, L.M.; Kwon, C.-W.; Bendikov, M.; Kaner, R.B.; Dunn, B.S.; Wudl, F. Microwave Exfoliation of a Graphite Intercalation Compound. Carbon 2007, 45, 1367–1369. [Google Scholar] [CrossRef]
- Hua, H.L.; Wang, Y.; Wang, Y.J.; Ruan, S.J.; Zeng, C.; Zhang, T.; Zhu, M.C.; Zhang, Y.C.; Li, D.X. Preparation of Expanded Graphite Using Recycling Graphite Rods by Microwave Irradiation. Adv. Mat. Res. 2012, 610–613, 2356–2360. [Google Scholar]
- Wei, T.; Fan, Z.; Luo, G.; Zheng, C.; Xie, D. A Rapid and Efficient Method to Prepare Exfoliated Graphite by Microwave Irradiation. Carbon 2009, 47, 337–339. [Google Scholar] [CrossRef]
- Deng, R.; Chu, F.; Yu, H.; Kwofie, F.; Qian, M.; Zhou, Y.; Wu, F. Electrochemical Performance of Expanded Graphite Prepared from Anthracite via a Microwave Method. Fuel Process. Technol. 2022, 227, 107100. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Zhang, X.-W.; Zhang, W.-J.; Wei, X.-X.; Liang, P. Microwave-Assisted Fabrication of Slight-Expanded Graphite under Normal Temperature. Mater. Sci. Technol. 2019, 36, 251–254. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Yu, K.; Geng, Y.; Hu, X.; Yi, G.; Zhang, J.; Luo, X. Regeneration and Reuse of Anode Graphite from Spent Lithium-Ion Batteries with Low Greenhouse Gas (GHG) Emissions. Chin. Chem. Lett. 2023, 108274. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Huang, M.; Deng, B.; Dong, F.; Zhang, L.; Zhang, Z.; Chen, P. Substrate Engineering for CVD Growth of Single Crystal Graphene. Small Methods 2021, 5, 2001213. [Google Scholar] [CrossRef]
- Hadi, A.; Zahirifar, J.; Karimi-Sabet, J.; Dastbaz, A. Graphene Nanosheets Preparation Using Magnetic Nanoparticle Assisted Liquid Phase Exfoliation of Graphite: The Coupled Effect of Ultrasound and Wedging Nanoparticles. Ultrason. Sonochem. 2018, 44, 204–214. [Google Scholar] [CrossRef]
- Cooil, S.P.; Song, F.; Williams, G.T.; Roberts, O.R.; Langstaff, D.P.; Jørgensen, B.; Høydalsvik, K.; Breiby, D.W.; Wahlström, E.; Evans, D.A.; et al. Iron-Mediated Growth of Epitaxial Graphene on SiC and Diamond. Carbon 2012, 50, 5099–5105. [Google Scholar] [CrossRef]
- Dong, L.; Yang, J.; Chhowalla, M.; Loh, K.P. Synthesis and Reduction of Large Sized Graphene Oxide Sheets. Chem. Soc. Rev. 2017, 46, 7306–7316. [Google Scholar] [CrossRef]
- Qi, B.; He, L.; Bo, X.; Yang, H.; Guo, L. Electrochemical Preparation of Free-Standing Few-Layer Graphene through Oxidation–Reduction Cycling. Chem. Eng. J. 2011, 171, 340–344. [Google Scholar] [CrossRef]
- Tran-Van, A.-F.; Wegner, H.A. Strategies in Organic Synthesis for Condensed Arenes, Coronene, and Graphene. In Polyarenes I; Siegel, J.S., Wu, Y.-T., Eds.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2013; Volume 349, pp. 121–157. ISBN 978-3-662-43378-2. [Google Scholar]
- Chen, X.; Zhu, Y.; Peng, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Direct Exfoliation of the Anode Graphite of Used Li-Ion Batteries into Few-Layer Graphene Sheets: A Green and High Yield Route to High-Quality Graphene Preparation. J. Mater. Chem. A 2017, 5, 5880–5885. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, N.; He, J.; Chen, R.; Li, X. Lithiation-Aided Conversion of End-of-Life Lithium-Ion Battery Anodes to High-Quality Graphene and Graphene Oxide. Nano Lett. 2019, 19, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Chen, Y.N.; Wang, Z.J. Effect of Microwave Irradiation on Reduction of Graphene Oxide Films. RSC Adv. 2015, 5, 92940–92946. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Shin, H.S.; Chhowalla, M. High-Quality Graphene via Microwave Reduction of Solution-Exfoliated Graphene Oxide. Science 2016, 353, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, J.; Jia, P.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y. A Sustainable Strategy for Spent Li-Ion Battery Regeneration: Microwave-Hydrothermal Relithiation Complemented with Anode-Revived Graphene to Construct a LiFePO4/MWrGO Cathode Material. Sustain. Energy Fuels 2022, 6, 2207–2222. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, Q.; Wang, J. A Simple and Fast Microwave Assisted Approach for the Reduction of Graphene Oxide. Ceram. Int. 2016, 42, 3007–3013. [Google Scholar] [CrossRef]
- Sreedhar, D.; Devireddy, S.; Veeredhi, V.R. Synthesis and Study of Reduced Graphene Oxide Layers under Microwave Irradiation. Mater. Today Proc. 2018, 5, 3403–3410. [Google Scholar] [CrossRef]
- Song, S.; Yang, H.; Su, C.; Jiang, Z.; Lu, Z. Ultrasonic-Microwave Assisted Synthesis of Stable Reduced Graphene Oxide Modified Melamine Foam with Superhydrophobicity and High Oil Adsorption Capacities. Chem. Eng. J. 2016, 306, 504–511. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, K.; Li, L.; Zhang, T. Synthesis of Nitrogen-Doped Graphene with Microwave. Int. J. Electrochem. Sci. 2013, 8, 9384–9389. [Google Scholar]
- Fei, H.; Dong, J.; Wan, C.; Zhao, Z.; Xu, X.; Lin, Z.; Wang, Y.; Liu, H.; Zang, K.; Luo, J.; et al. Microwave-Assisted Rapid Synthesis of Graphene-Supported Single Atomic Metals. Adv. Mater. 2018, 30, 1802146. [Google Scholar] [CrossRef]
- Khdair, A.I.; Ibrahim, A. Effect of Graphene Addition on the Physicomechanical and Tribological Properties of Cu Nanocomposites. Int. J. Miner. Metall. Mater. 2022, 29, 161–167. [Google Scholar] [CrossRef]
- Hu, Z.; Dai, R.; Wang, D.; Wang, X.; Chen, F.; Fan, X.; Chen, C.; Liao, Y.; Nian, Q. Preparation of Graphene/Copper Nanocomposites by Ball Milling Followed by Pressureless Vacuum Sintering. New Carbon Mater. 2021, 36, 420–428. [Google Scholar] [CrossRef]
- Jabbarzare, S.; Bakhsheshi-Rad, H.R.; Nourbakhsh, A.A.; Ahmadi, T.; Berto, F. Effect of Graphene Oxide on the Corrosion, Mechanical and Biological Properties of Mg-Based Nanocomposite. Int. J. Miner. Metall. Mater. 2022, 29, 305–319. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Li, J.J.; She, X.L.; Xia, L.H. MnO2/Graphene Nanocomposite for Use in High Performance Lithium-Ion Batteries. Adv. Mat. Res. 2013, 709, 157–160. [Google Scholar]
- Kim, H.W.; Na, H.G.; Kwon, Y.J.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Wu, P.; Kim, S.S. Microwave-Assisted Synthesis of Graphene–SnO2 Nanocomposites and Their Applications in Gas Sensors. ACS Appl. Mater. Interfaces 2017, 9, 31667–31682. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, C. Preparation and Lithium Storage Performance of a Carbon-Coated Si/Graphene Nanocomposite. Carbon 2015, 81, 851. [Google Scholar] [CrossRef]
- Liang, L.; Huang, C.; Wang, C.; Sun, X.; Yang, M.; Wang, S.; Cheng, Y.; Ning, Y.; Li, J.; Yin, W.; et al. Ultratough Conductive Graphene/Alumina Nanocomposites. Compos. Part A Appl. Sci. Manuf. 2022, 156, 106871. [Google Scholar] [CrossRef]
- Kumar, R.; Youssry, S.M.; Abdel-Galeil, M.M.; Matsuda, A. One-Pot Synthesis of Reduced Graphene Oxide Nanosheets Anchored ZnO Nanoparticles via Microwave Approach for Electrochemical Performance as Supercapacitor Electrode. J. Mater. Sci. Mater. Electron. 2020, 31, 15456–15465. [Google Scholar] [CrossRef]
- Zhang, M.; Du, H.; Wei, Z.; Zhang, X.; Wang, R. Ultrafast Microwave Synthesis of Nickel-Cobalt Sulfide/Graphene Hybrid Electrodes for High-Performance Asymmetrical Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 8262–8274. [Google Scholar] [CrossRef]
- Hou, D.; Bostwick, J.E.; Shallenberger, J.R.; Zofchak, E.S.; Colby, R.H.; Liu, Q.; Hickey, R.J. Simultaneous Reduction and Polymerization of Graphene Oxide/Styrene Mixtures to Create Polymer Nanocomposites with Tunable Dielectric Constants. ACS Appl. Nano Mater. 2020, 3, 962–968. [Google Scholar] [CrossRef]
- Aldosari, M.; Othman, A.; Alsharaeh, E. Synthesis and Characterization of the in Situ Bulk Polymerization of PMMA Containing Graphene Sheets Using Microwave Irradiation. Molecules 2013, 18, 3152–3167. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Z.-S.; Ren, W.; Cheng, H.-M.; Bao, X. Graphene: A Promising 2D Material for Electrochemical Energy Storage. Sci. Bull. 2017, 62, 724–740. [Google Scholar] [CrossRef]
- Tao, Y.; Sui, Z.-Y.; Han, B.-H. Advanced Porous Graphene Materials: From in-Plane Pore Generation to Energy Storage Applications. J. Mater. Chem. A 2020, 8, 6125–6143. [Google Scholar] [CrossRef]
- Chen, C.-M.; Zhang, Q.; Huang, C.-H.; Zhao, X.-C.; Zhang, B.-S.; Kong, Q.-Q.; Wang, M.-Z.; Yang, Y.-G.; Cai, R.; Sheng Su, D. Macroporous ‘Bubble’ Graphene Film via Template-Directed Ordered-Assembly for High Rate Supercapacitors. Chem. Commun. 2012, 48, 7149. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-Dimensional Holey-Graphene/Niobia Composite Architectures for Ultrahigh-Rate Energy Storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shi, Y.; Shi, W.; Lu, G.; Huang, X.; Yan, Q.; Zhang, Q.; Zhang, H. Preparation of Novel 3D Graphene Networks for Supercapacitor Applications. Small 2011, 7, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yu, G.; An, K.; Wang, W.; Wang, B.; Jiang, Z.; Sun, C.; Mao, Y.; Zhao, X.; Song, Z. Microwave-Induced High-Energy Sites and Targeted Energy Transition Promising for Efficient Energy Deployment. Front. Energy 2022, 16, 931–942. [Google Scholar] [CrossRef]
- Wan, J.; Huang, L.; Wu, J.; Xiong, L.; Hu, Z.; Yu, H.; Li, T.; Zhou, J. Microwave Combustion for Rapidly Synthesizing Pore-Size-Controllable Porous Graphene. Adv. Funct. Mater. 2018, 28, 1800382. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J. Superfast Microwave Synthesis of Hierarchically Porous RGO by Graphite Ignited Reduction Propagation. Carbon 2021, 178, 734–742. [Google Scholar] [CrossRef]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. The Chemistry and Promising Applications of Graphene and Porous Graphene Materials. Adv. Funct. Mater. 2020, 30, 1909035. [Google Scholar] [CrossRef]
- Wang, D.; Dai, R.; Zhang, X.; Liu, L.; Zhuang, H.; Lu, Y.; Wang, Y.; Liao, Y.; Nian, Q. Scalable and Controlled Creation of Nanoholes in Graphene by Microwave-Assisted Chemical Etching for Improved Electrochemical Properties. Carbon 2020, 161, 880–891. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Q.; Yang, N. Recent Advances of Porous Graphene: Synthesis, Functionalization, and Electrochemical Applications. Small 2019, 15, 1903780. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Chen, W.; Jia, K.; Li, S.; Jia, P.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y.; Chen, S. Progress on the Microwave-Assisted Recycling of Spent Lithium Battery Graphite. Processes 2023, 11, 1451. https://doi.org/10.3390/pr11051451
Sun J, Chen W, Jia K, Li S, Jia P, Wang W, Song Z, Zhao X, Mao Y, Chen S. Progress on the Microwave-Assisted Recycling of Spent Lithium Battery Graphite. Processes. 2023; 11(5):1451. https://doi.org/10.3390/pr11051451
Chicago/Turabian StyleSun, Jing, Wenxin Chen, Kexin Jia, Su Li, Pingshan Jia, Wenlong Wang, Zhanlong Song, Xiqiang Zhao, Yanpeng Mao, and Shouyan Chen. 2023. "Progress on the Microwave-Assisted Recycling of Spent Lithium Battery Graphite" Processes 11, no. 5: 1451. https://doi.org/10.3390/pr11051451
APA StyleSun, J., Chen, W., Jia, K., Li, S., Jia, P., Wang, W., Song, Z., Zhao, X., Mao, Y., & Chen, S. (2023). Progress on the Microwave-Assisted Recycling of Spent Lithium Battery Graphite. Processes, 11(5), 1451. https://doi.org/10.3390/pr11051451