Properties of FAPbI3-Based Alloy Perovskite Thin Films and Their Application in Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Solution Preparation and Device Fabrication
2.2. Thin Film and Device Characterizations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saliba, M.; Correa-Baena, J.P.; Grätzel, M.; Hagfeldt, A.; Abate, A. Perovskite Solar Cells: From the Atomic Level to Film Quality and Device Performance. Angew. Chem.—Int. Ed. 2018, 57, 2554–2569. [Google Scholar] [CrossRef]
- Boix, P.; Nonomura, K.; Mathews, N.; Mhaisalkar, S.G. 2014 undefined. Current progress and future perspectives for organic/inorganic perovskite solar cells. Mater. Today 2014, 17, 16–23. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Li, J.; Li, H.; Tian, C.; Su, J.; Tong, G.; Ono, L.K.; Wang, C.; Lin, Z.; Chai, N.; et al. Lead halide-templated crystallization of methylamine-free perovskite for efficient photovoltaic modules. Science 2021, 372, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Yi, C.; Luo, J.; Décoppet, J.D.; Zhang, F.; Zakeeruddin, S.M.; Li, X.; Hagfeldt, A.; Grätzel, M. Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%. Nat. Energy 2016, 1, 16142. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, S.N.; Chu, Y.X.; Jeng, M.J.; Chang, L.B. The Characteristics of Perovskite Solar Cells Fabricated Using DMF and DMSO/GBL Solvents. J. Electron. Mater. 2020, 49, 6823–6828. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Nasti, G.; Abate, A.; Nasti, G.; Abate, A. Tin Halide Perovskite (ASnX3) Solar Cells: A Comprehensive Guide toward the Highest Power Conversion Efficiency. Adv. Energy Mater. 2020, 10, 1902467. [Google Scholar] [CrossRef]
- Tang, S.; Huang, S.; Wilson, G.; Ho-Baillie, A. Progress and opportunities for Cs incorporated perovskite photovoltaics. Trends Chem. 2020, 2, 638–653. [Google Scholar] [CrossRef]
- Park, B.; Seok, S.I. Intrinsic instability of inorganic–organic hybrid halide perovskite materials. Adv. Mater. 2019, 31, 1805337. [Google Scholar] [CrossRef]
- Yu, D.; Hu, Y.; Shi, J.; Tang, H.; Zhang, W.; Meng, Q.; Han, H.; Ning, Z.; Tian, H. Stability improvement under high efficiency—Next stage development of perovskite solar cells. Sci. China Chem. 2019, 62, 684–707. [Google Scholar] [CrossRef]
- Tan, S.; Yavuz, I.; Weber, M.H.; Huang, T.; Chen, C.H.; Wang, R.; Wang, H.C.; Ko, J.H.; Nuryyeva, S.; Xue, J.; et al. Shallow indine defects accelerate the degradation of a-phase formamidinium perovskite. Joule 2020, 4, 2426–2442. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, Z.; Fu, C.; Xiao, Q.; Zhang, S.; Zhang, Y.; Song, Y. FAPbI3 Perovskite Solar Cells: From Film Morphology Regulation to Device Optimization. Sol. RRL 2022, 6, 2200120. [Google Scholar] [CrossRef]
- Haris, M.P.U.; Kazim, S.; Ahmad, S. Low-temperature-processed perovskite solar cells fabricated from presynehesized CsFAPbI3 powder. ACS Appl. Energy Mater. 2021, 4, 2600–2606. [Google Scholar]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S., Il. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef]
- Ono, L.K.; Juarez-Perez, E.J.; Qi, Y. Progress on Perovskite Materials and Solar Cells with Mixed Cations and Halide Anions. ACS Appl. Mater. Interfaces 2017, 9, 30197–30246. [Google Scholar] [CrossRef] [PubMed]
- Schelhas, L.T.; Li, Z.; Christians, J.A.; Goyal, A.; Kairys, P.; Harvey, S.P.; Kim, D.H.; Stone, K.H.; Luther, J.M.; Zhu, K.; et al. Insights into operational stability and processing of halide perovskite active layers. Energy Environ. Sci. 2019, 12, 1341–1348. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, D.-H.; Kim, H.-S.; Seo, S.-W.; Min Cho, S.; Park, N.-G.; Lee, J.; Kim, D.; Kim, H.; Seo, S.; et al. Formamidinium and Cesium Hybridization for Photo- and Moisture-Stable Perovskite Solar Cell. Adv. Energy Mater. 2015, 5, 1501310. [Google Scholar] [CrossRef]
- Yi, C.; Luo, J.; Meloni, S.; Boziki, A.; Ashari-Astani, N.; Grätzel, C.; Zakeeruddin, S.M.; Röthlisberger, U.; Grätzel, M. Entropic stabilization of mixed A-cation ABX3 metal halide perovskites for high performance perovskite solar cells. Energy Environ. Sci. 2016, 9, 656–662. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Q.; Chmiel, F.; Sakai, N.; Herz, L.M.; Snaith, H.J. Efficient ambient-air-stable solar cells with 2D–3D heterostructured butylammonium-caesium-formamidinium lead halide perovskites. Nat. Energy 2017, 2, 17135. [Google Scholar] [CrossRef]
- Xia, X.; Wu, W.; Li, H.; Zheng, B.; Xue, Y.; Xu, J.; Zhang, D.; Gao, C.; Liu, X. Spray reaction prepared FA1−xCsxPbI3 solid solution as a light harvester for perovskite solar cells with improved humidity stability. RSC Adv. 2016, 6, 14792–14798. [Google Scholar] [CrossRef]
- Manzoor, S.; Häusele, J.; Bush, K.A.; Palmstrom, A.F.; Carpenter, J.; Yu, Z.J.; Bent, S.F.; Mcgehee, M.D.; Holman, Z.C. Optical modeling of wide-bandgap perovskite and perovskite/silicon tandem solar cells using complex refractive indices for arbitrary-bandgap perovskite absorbers. Opt. Express 2018, 26, 27441–27460. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-W.; Song, J.-Y.; Wu, H.-T.; Leu, C.-C.; Shih, C.-C. Properties of halide perovskite photodetectors with little rubidium incorporation. Nanomaterials 2022, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, C.; Grice, C.R.; Shrestha, N.; Chen, J.; Zhao, D.; Liao, W.; Cimaroli, A.J.; Roland, P.J.; Yan, Y. Improving the performance of formamidinium and cesium lead triiodide perovskite solar cells using lead thiocyanate additives. ChemSusChem 2016, 9, 3288–3297. [Google Scholar] [CrossRef]
- Yang, J.; Lim, E.L.; Tan, L.; Wei, Z. Ink Engineering in blade-coating large-area perovskite solar cells. Adv. Energy Mater. 2022, 12, 2200975. [Google Scholar] [CrossRef]
- Xu, H.; Wu, Y.; Cui, J.; Ni, C.; Xu, F.; Cai, J.; Hong, F.; Fang, Z.; Wang, W.; Zhu, J.; et al. Formation and evolution of the unexpected PbI2 phase at the interface during the growth of evaporated perovskite films. Phys. Chem. Chem. Phys. 2016, 18, 18607–18613. [Google Scholar] [CrossRef]
- Zhou, N.; Shen, Y.; Zhang, Y.; Xu, Z.; Zheng, G.; Li, L.; Chen, Q.; Zhou, H. CsI Pre-Intercalation in the Inorganic Framework for Efficient and Stable FA1−x CsxPbI3(Cl) Perovskite Solar Cells. Small 2017, 13, 1700484. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef]
- Ruan, S.; McMeekin, D.P.; Fan, R.; Webster NA, S.; Ebendorff-Heidepriem, H.; Cheng, Y.-B.; Lu, J.; Ruan, Y.; McNeill, R. Raman spectroscopy of formamidinium-based lead halide perovskite single crystals. J. Phys. Chem. C 2020, 124, 2265–2272. [Google Scholar] [CrossRef]
- Basumatary, P.; Agarwal, P. Photocurrent transient measurements in MAPbI3 thin films. J. Mater. Sci. Mater. Electron. 2020, 31, 10047–10054. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Cai, B.; Shan, Q.; Song, J.; Li, J.; Zhang, F.; Chen, J.; Fang, T.; Ji, Q.; Xu, X.; et al. Stable, Efficient Red Perovskite Light-Emitting Diodes by (α,δ)-CsPbI3 Phase Engineering. Adv. Funct. Mater. 2018, 28, 1804285. [Google Scholar] [CrossRef]
- Chang, S.H.; Huang, W.C.; Chen, C.C.; Chen, S.H.; Wu, C.G. Effects of anti-sovlent (iobobenzene) volume on the formation of CH3NH3PbI3 thin films and their application in photovoltaic cells. Appl. Surf. Sci. 2018, 445, 24–29. [Google Scholar] [CrossRef]
- Huang, L.; Wang, J.; Zhu, Y. Efficient p−n Heterojunction Perovskite Solar Cell without a Redundant Electron Transport Layer and Interface Engineering. J. Phys. Chem. Lett. 2021, 12, 2266–2272. [Google Scholar] [CrossRef]
- Zhao, X.; Vasenko, A.S.; Prezhdo, O.V.; Long, R. Anion Doping Delays Nonradiative Electron−Hole Recombination in Cs-Based All-Inorganic Perovskites: Time Domain ab Initio Analysis. J. Phys. Chem. Lett. 2022, 13, 11375–11382. [Google Scholar] [CrossRef]
- Murugadoss, G.; Arunachalam, P.; Panda, S.K.; Kumar, M.R.; Rajabathar, J.R.; Al-Lohedan, H.; Wasmiah, M.D. Crystal stabilization of α-FAPbI3 perovskite by rapid annealing method in industrial scale. J. Mater. Res.Technol. 2021, 12, 1924–1930. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, D.; Yang, R.; Yang, B.; Yang, Z.; Ren, X.; Zhang, J.; Niu, J.; Feng, J.; Liu, S. Superior stability for perovskite solar cells with 20% efficiency using vacuum co-evaporation. Nanoscale 2017, 9, 12316–12323. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, X.; Liu, B.; Munir, R.; Zhu, X.; Yang, D.; Li, J.; Liu, Y.; Smilgies, D.-M.; Li, R.; et al. Stable high efficiency two-dimensional perovskite solar cells via cesium doping. Energy Environ. Sci. 2017, 10, 2095–2102. [Google Scholar] [CrossRef]
- Deepa, M.; Salado, M.; Calio, L.; Kazim, S.; Shivaprasad, S.M.; Ahmad, S. Cesium power: Low Cs+ levels impart stability to perovskite solar cells. Phys. Chem. Chem. Phys. 2017, 19, 4069–4077. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, K.F.; Chiu, Y.C.; Tsai, C.L.; Cheng, H.M.; Yeh, S.C.; Wu, W.T.; Chen, W.N.; Chen, C.T.; Chen, S.H.; et al. Improving the efficiency of CH3NH3PbI3 based photovoltaics by tuning the work function of the PEDOT:PSS hole transport layer. Sol. Energy 2015, 122, 892–899. [Google Scholar] [CrossRef]
- Yu, H.; Lu, H.; Xie, F.; Zhou, S.; Zhao, N. Native defect-induced hysteresis behavior in organolead iodide perovskite solar cells. Adv. Funct. Mater. 2016, 26, 1411–1419. [Google Scholar] [CrossRef]
- Yun, J.S.; Kim, J.; Young, T.; Patterson, P.J.; Kim, D.; Seidel, J.; Lim, S.; Green, M.A.; Huang, S.; Ho-Baillie, A. Humidity-induced degradation via grain boundaries of HC(NH2)2PbI3 planar perovskite solar cells. Adv. Funct. Mater. 2018, 28, 1705363. [Google Scholar] [CrossRef]
- Chaing, S.E.; Wu, J.R.; Cheng, H.M.; Hsu, C.L.; Shen, J.L.; Yuan, C.T.; Chang, S.H. Origins of the s-shape characteristic in J-V curve of inverted-type perovskite solar cells. Nanotechnology 2020, 31, 115403. [Google Scholar] [CrossRef]
- Chandel, A.; Ke, Q.B.; Chiang, S.E.; Cheng, H.M.; Chang, S.H. Effects of drying time on the formation merged and soft MAPbI3 grains and their photovoltaic responses. Nanoscale Adv. 2023, 5, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zou, X.; Cheng, J.; Bai, X.; Che, D.; Yao, Y.; Chang, C.; Yu, X.; Zhou, Z.; Wang, J.; et al. Effects of PbI2 passivation to grain boundary of perovskite film with cation and anion co-mixing on performance of photovoltaic devices. Mater. Lett. 2020, 273, 127979. [Google Scholar] [CrossRef]
- Tress, W.; Baena JP, C.; Saliba, M.; Abate, A.; Graetzel, M. Inverted current-voltage hysteresis in mixed perovskite solar cells: Polarization, energy barriers, and defect recombination. Adv. Energy Mater. 2016, 6, 1600396. [Google Scholar] [CrossRef]
- Ke, Q.B.; Wu, J.K.; Lin, C.C.; Chang, S.H. Understanding the PEDOT:PSS, PTAA and P3CT-X hole-transport-layer-based inverted perovskite solar cells. Polymers 2022, 14, 823. [Google Scholar] [CrossRef]
- Chang, S.H.; Chen, W.N.; Chen, C.C.; Yeh, S.C.; Cheng, H.M.; Tseng, Z.L.; Chen, L.C.; Chiu, K.Y.; Wu, W.T.; Chen, C.T.; et al. Manipulating the molecular structure of PEDOT chains through controlling the viscosity of PEDOT:PSS solutions to improve the photovoltaic performance of CH3NH3PbI3 solar cells. Sol. Energy Mater. Sol. Cells 2017, 161, 7–13. [Google Scholar] [CrossRef]
- Tang, H.; Shang, Y.; Zhou, W.; Peng, Z.; Ning, Z. Energy level tuning of PEDOT:PSS for high performance tin-lead mixed perovskite solar cells. Sol. RRL 2018, 3, 1800256. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Zhang, N.; Yu, Z.; Sun, K.; Tang, B.; Shi, D.; Yao, H.; Ouyang, J.; Gon. Multifunction RbCl dopants fro efficient inverted planar perovskite solar cell with ultra-high fill factor, negligible hysteresis and improved stability. Nano Energy 2018, 52, 567–578. [Google Scholar] [CrossRef]
- Chen, C.-J.; Chandel, A.; Thakur, D.; Wu, J.-R.; Chiang, S.-E.; Zeng, G.-S.; Shen, J.-L.; Chen, S.-H.; Chang, S.H. Ag modified bathocuproine:ZnO nanoparticles electron buffer layer based bifacial inverted-type perovskite solar cells. Org. Electron. 2021, 92, 106110. [Google Scholar] [CrossRef]
- Chang, S.H.; Chiang, C.-H.; Kao, F.-S.; Tien, C.-L.; Wu, C.-G. Unraveling the enhanced electrical conductivity of PEDOT:PSS thin films for ITO-free organic photovoltaics. IEEE Photonics J. 2014, 6, 8400307. [Google Scholar]


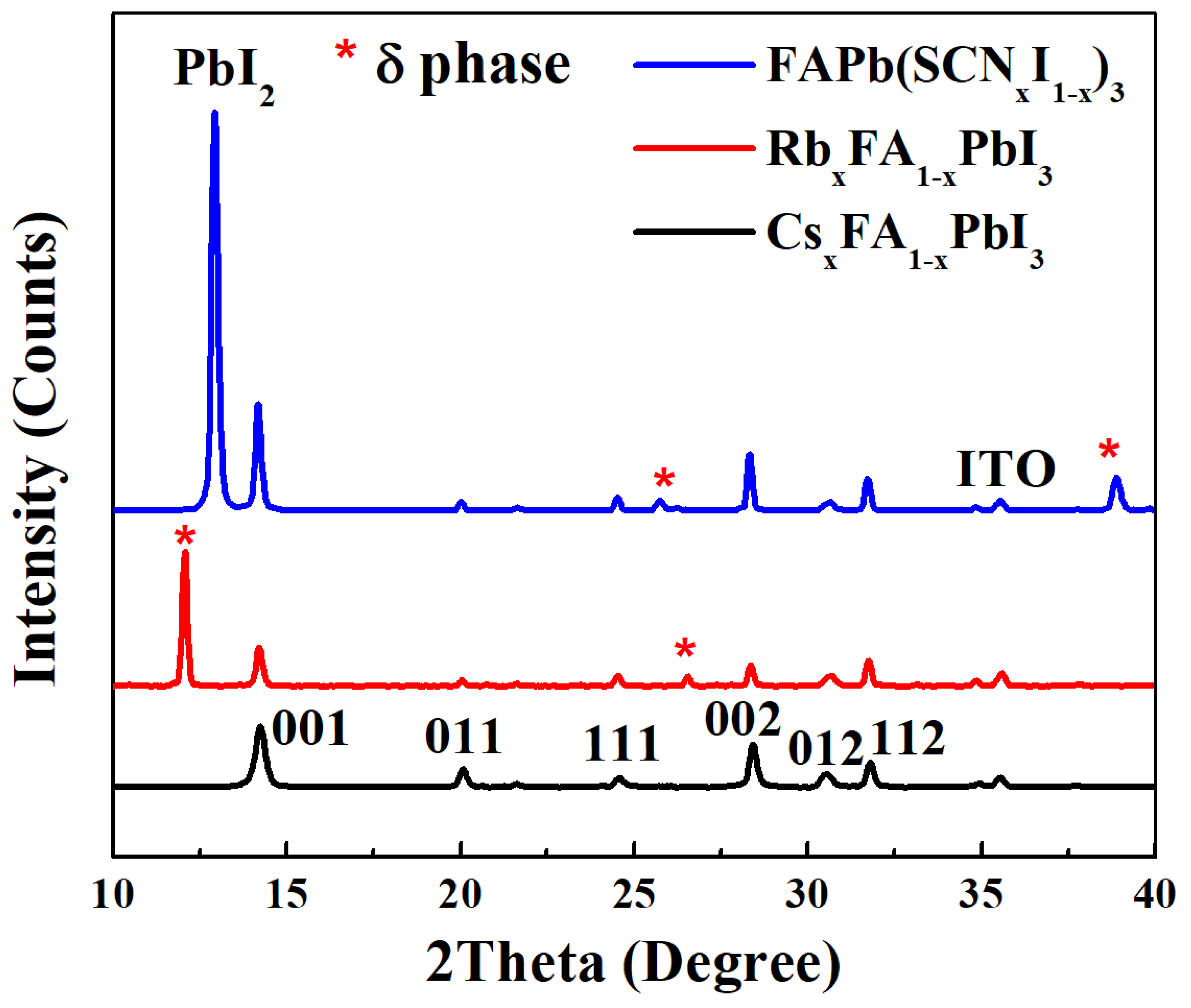
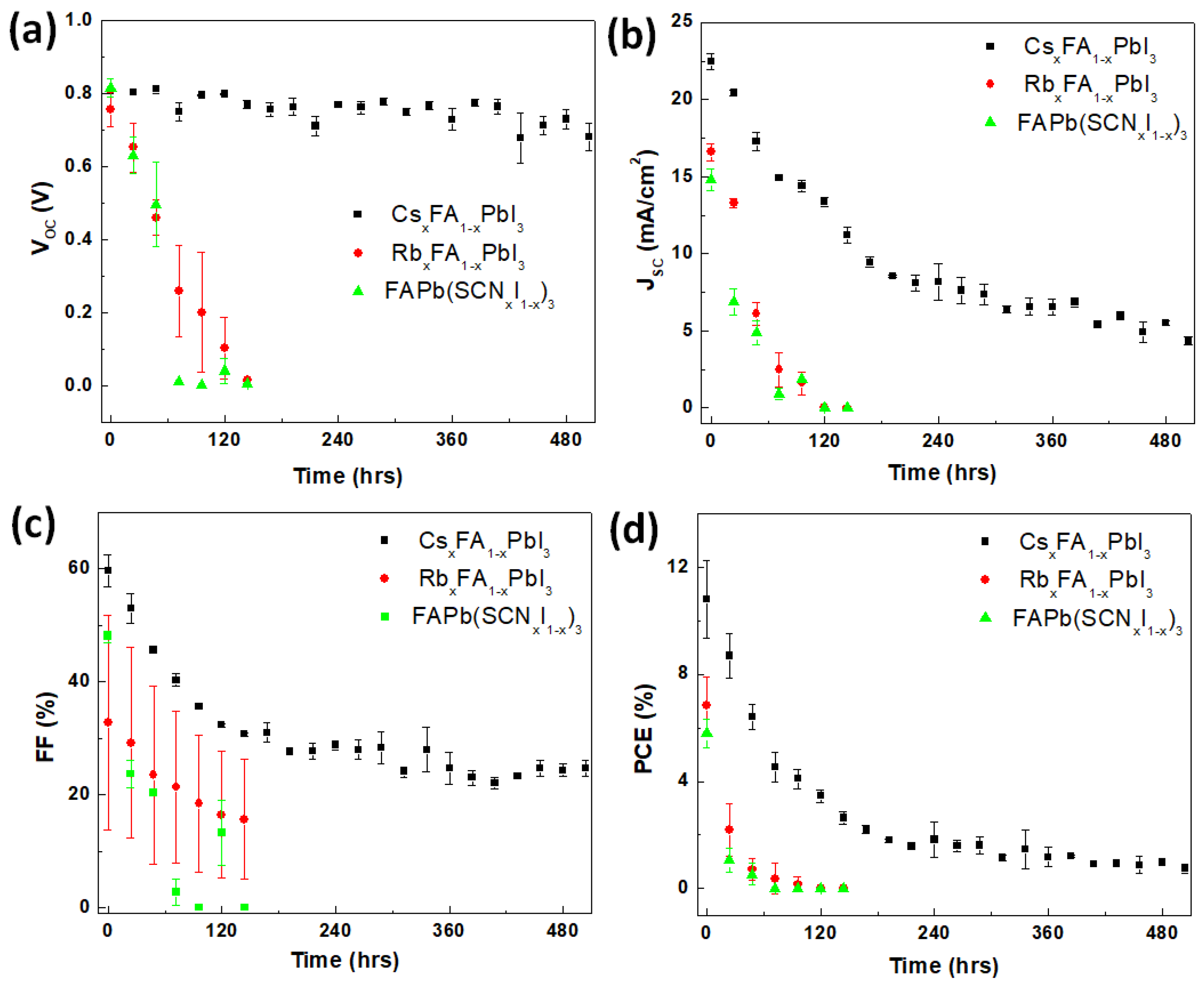
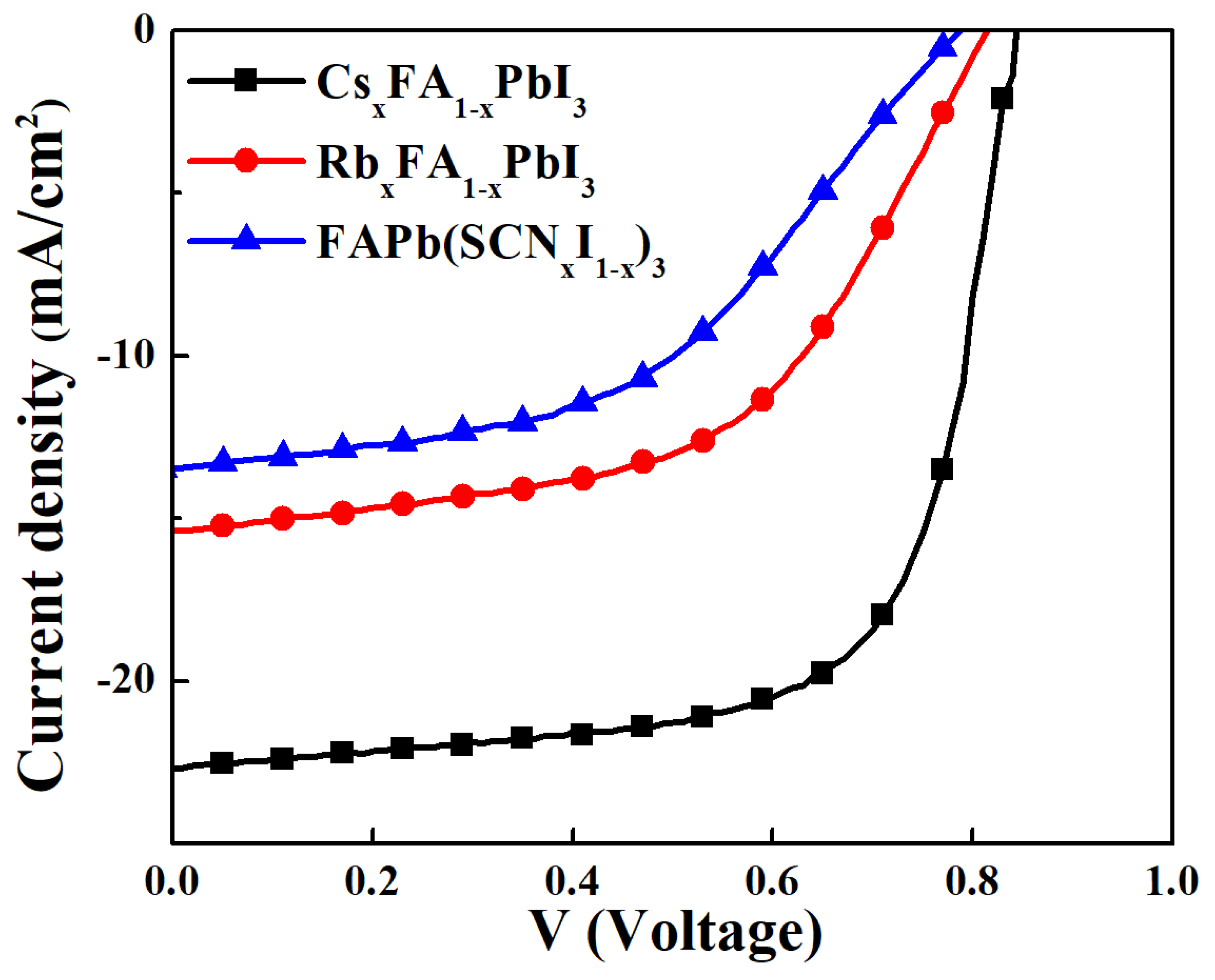

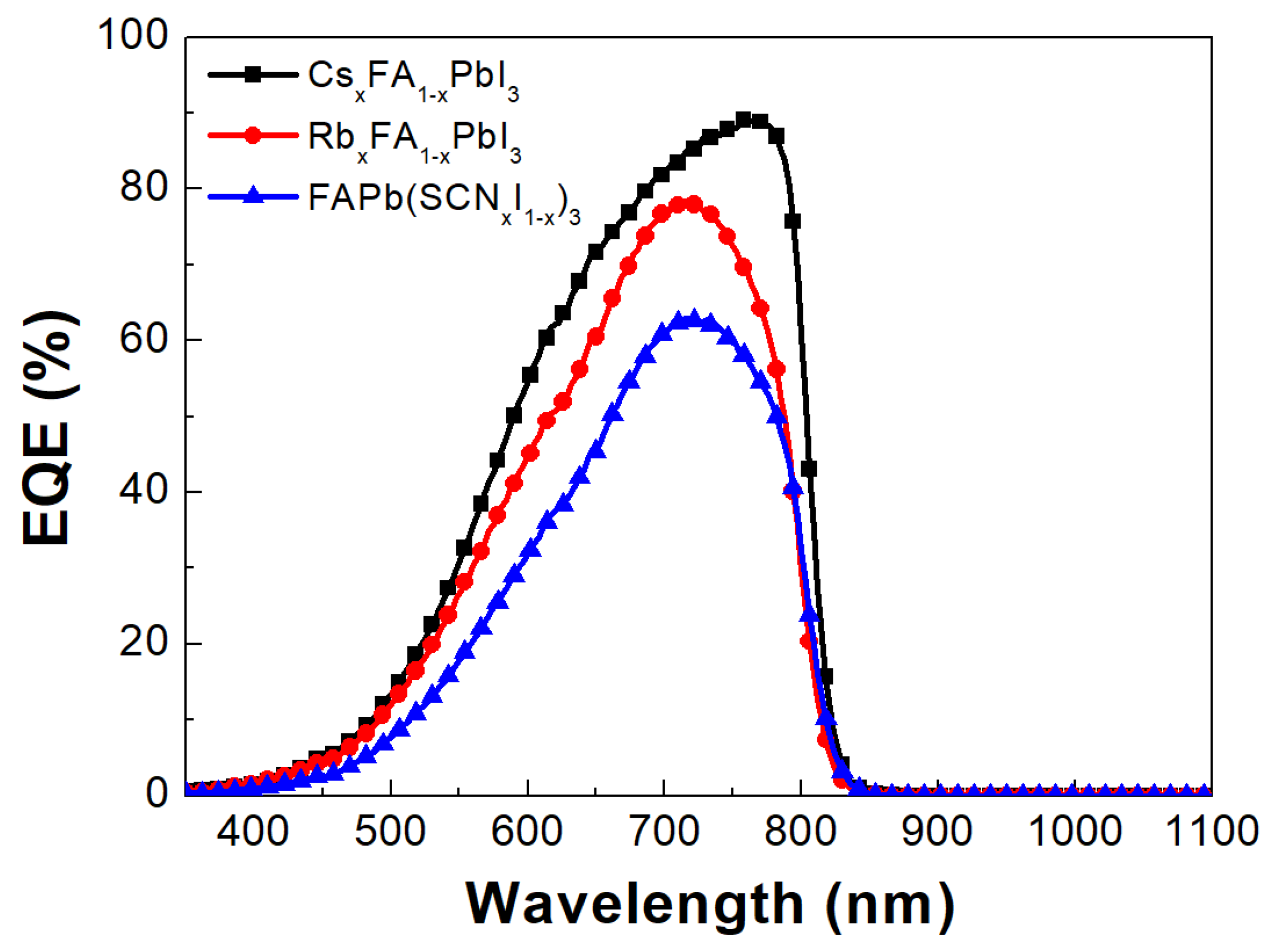
| Perovskite | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| CsxFA1−xPbI3 | 0.83 ± 0.01 | 21.9 ± 0.7 | 66 ± 2 | 11.99 ± 0.90 |
| RbxFA1−xPbI3 | 0.80 ± 0.01 | 13.9 ± 1.4 | 51 ± 3 | 5.67 ± 0.80 |
| FAPb(SCNxI1−x)3 PSCs | 0.75 ± 0.03 | 13.3 ± 0.1 | 46 ± 2 | 4.90 ± 0.10 |
| Perovskite | Integration Intensity (mW/cm2) Under Forward Scan | Integration Intensity (mW/cm2) Under Backward Scan | Integration InteIntensity Difference (mW/cm2) |
|---|---|---|---|
| CsxFA1−xPbI3 | 11.11 | 11.25 | 0.12 |
| RbxFA1−xPbI3 | 9.92 | 10.32 | 0.40 |
| FAPb(SCNxI1−x)3 | 7.58 | 6.25 | −1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-L.; Manjunatha, S.N.; Chang, S.H.; Jeng, M.-J.; Chang, L.-B.; Chang, C.-H.; Sharma, M.; Yuan, C.-T. Properties of FAPbI3-Based Alloy Perovskite Thin Films and Their Application in Solar Cells. Processes 2023, 11, 1450. https://doi.org/10.3390/pr11051450
Tsai C-L, Manjunatha SN, Chang SH, Jeng M-J, Chang L-B, Chang C-H, Sharma M, Yuan C-T. Properties of FAPbI3-Based Alloy Perovskite Thin Films and Their Application in Solar Cells. Processes. 2023; 11(5):1450. https://doi.org/10.3390/pr11051450
Chicago/Turabian StyleTsai, Chia-Lung, S. N. Manjunatha, Sheng Hsiung Chang, Ming-Jer Jeng, Liann-Be Chang, Chun-Huan Chang, Mukta Sharma, and Chi-Tsu Yuan. 2023. "Properties of FAPbI3-Based Alloy Perovskite Thin Films and Their Application in Solar Cells" Processes 11, no. 5: 1450. https://doi.org/10.3390/pr11051450
APA StyleTsai, C.-L., Manjunatha, S. N., Chang, S. H., Jeng, M.-J., Chang, L.-B., Chang, C.-H., Sharma, M., & Yuan, C.-T. (2023). Properties of FAPbI3-Based Alloy Perovskite Thin Films and Their Application in Solar Cells. Processes, 11(5), 1450. https://doi.org/10.3390/pr11051450












