Abstract
It has been estimated and demonstrated that the antioxidant capacity of proteins is increased as a result of digestion in the gastrointestinal tract, which can be contributed by denaturation and digestion. This study aimed to evaluate the effect of denaturation and proteolytic digestion on the antioxidant activity of bovine serum albumin (BSA) and chicken egg white proteins in model systems. Denaturation with an anionic detergent (sodium dodecyl sulfate) and digestion with papain and trypsin increased the antioxidant activity/capacity of the proteins, apparently due to the increased exposure of amino acid residues responsible for the antioxidant activity of proteins (tyrosine, tryptophan, cysteine, histidine, arginine, and cystine in the ABTS● decolorization assay; cysteine, tryptophan, tyrosine, and cystine in the FRAP assay). As the increase in the protein antioxidant activity/capacity was limited in extent, it does not invalidate the use of the antioxidant capacity of proteins to be consumed as a rough measure of their antioxidant capacity after modifications in the gastrointestinal tract.
1. Introduction
There is a permanent interest in the antioxidant properties of food as the source of antioxidant vitamins and other exogenous antioxidants. Databases of the antioxidant capacities of various meals have been constructed to estimate the antioxidant intake [1,2,3,4,5]. One such database was made publicly available on the website of the United States Department of Agriculture but withdrawn since the reported values were misused by manufacturing companies when promoting their products, as well as by consumers misinterpreting them when choosing food and dietary supplements [6,7].
It is obvious, however, that the conditions of the assay of food antioxidant capacity may not fully correspond to the conditions in which antioxidant capacity is manifested in vivo. This statement refers especially to proteins, which are often subjected to denaturation during food preparation and denaturation as well as digestion in the gastrointestinal tract. Most studies have reported an increase in the antioxidant capacity of proteins as a result of simulated gastrointestinal digestion; however, the results are not always concordant. Various models of in vitro digestion are used to evaluate the effect of this process on the total antioxidant capacity (TAC) of food [8]. Release of antioxidant compounds from the food matrix may increase the TAC of plant food [9]. Simulated digestion was found to increase the availability and antioxidant capacity of Maillard reaction products of breakfast cereals [10]. In vitro digestion was found to increase the TAC of cooked mushrooms [11]. However, polyphenols are highly sensitive to mildly alkaline conditions in the small intestine, where most dietary polyphenols are degraded or transformed into other compounds, and, generally, the polyphenol-dependent total antioxidant capacity (TAC) of plant-derived food may decrease after simulated digestion [12,13]. In vitro digestion of the 36 most popular Brazilian foods increased the antioxidant capacity of cereals, legumes, vegetables, tuberous vegetables, chocolates, and fruits but reduced the TAC of beverages (red wine, coffee, and yerba mate), in which the phenolic components were not protected by the matrix against enzymatic action and alteration in pH during digestion [14]. The content of bioavailable phenolics was decreased by simulated gastrointestinal digestion when compared with fresh leaves of Centella asiatica [15]. The cellular antioxidant activity of feijoada, a traditional Brazilian plant-rich meal, was decreased after simulated digestion [16]. On the contrary, simulated gastrointestinal digestion augmented the antioxidant capacity of bovine whey proteins [17]. The antioxidant capacity of amaranth peptides was increased by 20–25% after simulated gastrointestinal digestion [18]. Simulated gastrointestinal digestion augmented the antioxidant capacity of the loach peptide by 5–77% (depending on the assay) [19]. Protease action on salmon byproduct protein from the pectoral fin increased the antioxidant capacity and generated peptides of high antioxidant activity [20]. Simulated digestion was reported to significantly increase the TAC of dairy products, which contributed up to 60% of the daily antioxidant capacity intake. Nevertheless, it was reported that most of the TAC (90–98%) was released from dairy products by microbial fermentation, simulating that taking place in the intestine [21]. For plant-derived food, the fraction of TAC released by fermentation was estimated to range from 80 to 98% [22]. In other words, TAC measured in the food to be consumed contributed only several percent to the TAC, which would be exhibited in the colon. However, this result is difficult to interpret because of the contribution of microbes and products of their metabolism to the TAC measured after food fermentation.
To avoid the ambiguity of results, this study aimed to examine the effect of processes simulating those occurring in the digestive system (denaturation and proteolytic digestion) of bovine serum albumin as a model protein and egg white as a protein-rich food on their antioxidant capacity in model systems to avoid complications arising from the interference of other factors in physiologically relevant situations.
2. Materials and Methods
2.1. Materials
L-Arginine (CAS no. 74-79-3; cat. no. 11009, purity ≥ 99.5%), ferric chloride hexahydrate (CAS no. 10025-77-1; cat. no. 236489, purity ≥ 97%), 6-hydroxy-2,5,7,8-tetramethylochromane-2-carboxylic acid (Trolox) (CAS no. 53188-07-1; cat. no. 238813, purity ≥ 97), L-lysine monohydrochloride (CAS no. 657-27-2; cat. no. L5626, purity ≥ 98%), neocuproine (CAS no. 484-11-7; cat. no. N1501, purity ≥ 98%), papain from papaya latex (CAS no. 9001-73-4; cat. no. P3125, highly purified by chromatography), sodium dodecyl sulfate (SDS) (CAS no.151-21-3; cat. no. L4509, purity ≥ 98.5%), 2,4,6-tri-(2-pyridyl)-s-triazine (TPTZ) (CAS no. 3682-35-7; cat. no. 93285, purity ≥ 99%), cysteine (CAS no. 52-90-4; cat. no. 168149, purity ≥ 97%), as well as trypsin (CAS no. 9002-07-7; cat. no. T1326, purity 99%) were purchased from Merck (Poznań), and L-Cystine (CAS no. 56-89-3; cat. no. 2/03/75, purity 99.9%) was obtained from Biomed (Lublin, Poland).
DL-Dithiothreitol (DTT) (CAS- no. 3483-12-3; cat. no. DTT001.5, purity ≥ 99.5%), L-histidine (CAS no. 71-00-1; cat. no. HIS100.25, purity ≥ 98.5%), phosphate-buffered saline (PBS; cat. no. PBS404.200), sodium phosphate monobasic (CAS no. 10049-21-5; cat. no. SPM306.500, purity 98–103%), and sodium phosphate dibasic (CAS no. 7782-85-6; cat. no. SPD579.1, purity 98–102%) were from LAB EMPIRE (Rzeszów, Poland).
Tryptophan (CAS no. 73-22-3; cat. no. 4858, purity ≥ 98.5%) and tyrosine (CAS no. 60-18-4; cat. no. T207, purity ≥ 99%) were purchased from Roth (Zielona Góra, Poland), and 2,2’-azino-bis(3-ethylobenzthiazoline-6-sulfonic acid) (ABTS) (CAS no. 504-14-6; cat. no. 10102946001, purity > 98%) were from Roche (Warsaw, Poland).
Ethanol (CAS no. 64-17-5; cat. no. 396480111, purity ≥ 99.8%), copper (II) sulfate pentahydrate (CAS no. 7758-99-8; cat. no. 658310422, purity ≥ 98%), and sodium acetate anhydrous (CAS no. 127-09-3; cat. no. BN60/6191, purity ≥ 99%) were from Avantor Performance Materials Poland (Gliwice, Poland). Acetic acid (CAS no. 64-19-7; cat. No. 425687339, purity 80%), hydrochloric acid (CAS no. 7647-01-0; cat. no. 115752837, 35–38%), hydrogen peroxide (CAS no. 7722-84-1; cat. no. 118851934, 30%), sodium nitrite (CAS no. 7632-00-0; cat. no. 792690115, purity ≥ 97.5%), and Tris-HCl (CAS no. 77-86-1; cat. no. 118534707, purity ≥ 99%) were provided by Chempur (Piekary Śląskie, Poland).
Albumin Fraction V (BSA) (CAS no. 9038-46-8; cat. no. A1391,0025, purity ≥ 97%) was bought from AppliChem (Darmstadt, Germany). β-Mercaptoethanol (BME) (CAS no. 60-24-2; cat. no. Z523A, 48.7%) was provided by Promega (Madison, WI, USA), and NaOH (CAS no. 1310-73-2; cat. no. 056992, purity ≥ 98%) was from Warchem (Warsaw, Poland). All other reagents, if not mentioned otherwise, were purchased from Merck (Poznan, Poland) and were of analytical grade. Distilled water was purified using a Milli-Q system (Millipore, Bedford, MA).
Stock solutions of BSA were made in PBS. The chicken egg white was diluted 10 times with PBS.
Transparent flat-bottom 96-well plates (Greiner, Kremsmünster, Austria) were used for the assays. Absorptiometric measurements were conducted in a Spark multimode microplate reader (Tecan Group Ltd., Mannedorf, Switzerland).
2.2. ABTS● Decolorization Assay
The assay was conducted as described previously [23]. Briefly, aliquots of the amino acids or protein solutions containing increasing amounts of the reactants were added to wells of a 96-well plate containing 200 μL of ABTS● solution diluted with PBS so as to provide absorbance of 1.0 at 734 nm in a plate reader. The decrease in absorbance was read after 30 min of incubation at ambient temperature. Antioxidant activity/capacity was calculated using the formula:
and expressed in moles of Trolox equivalents (TEs) per mole of amino acid or gram of BSA or mL of non-diluted egg white [23]. The term “antioxidant activity” is used consequently in this paper with respect to a defined compound and “antioxidant capacity” with respect to a complex material containing antioxidants [24], such as egg white.
Antioxidant activity = (slope of dependence of absorbance change on the amount of tested compound)/(slope of dependence of absorbance change on the amount of Trolox)
2.3. FRAP Assay
The assay was performed according to a modified procedure of Benzie and Strain [25]. In brief, aliquots of the amino acids or protein solutions containing increasing amounts of the reactant were added to wells of a 96-well plate containing 200 μL of the working solution, freshly prepared by mixing ten volumes of 0.3 M acetate buffer, pH 3.6, one volume of 10 mM TPTZ in 40 mM HCl, and one volume of 20 mM FeCl3. After 30 min incubation at ambient temperature, absorbance was measured at 593 nm against a reagent blank. Antioxidant activity was calculated and expressed in TEs as above [23]. Protein-containing samples became slightly turbid after their addition to the working solution, so they were centrifuged before the measurements.
2.4. CUPRAC Assay
A modification of the procedure of Özyürek et al. [26] was used. Briefly, 50 μL of 50 mM Tris-HCl buffer, pH 7.0, were mixed with 50 μL of 10 mM CuSO4, 50 μL of 7.5 mM neocuproin solution in ethanol and 50 μL of PBS containing increasing amounts of amino acids. After 60 min incubation at ambient temperature, absorbance was measured at 450 nm against a reagent blank. Antioxidant activity was calculated and expressed in TEs as above. It was not possible to use the CUPRAC assay to determine the protein antioxidant activity/capacity since significant protein precipitation was observed, apparently due to the high ethanol concentration in the samples.
2.5. Protein Denaturation
BSA (500 μg/mL) in PBS (9 volumes) was added with 1 volume of 5% SDS and compared with BSA solution added with deionized water in the same proportion. Egg white diluted 10× with PBS was treated in the same manner.
2.6. Protein Digestion
BSA solution in PBS (10 mg/mL) and egg white diluted 10× with PBS were added with papain (2 mg/mL) and incubated at 37 °C for 24 h. In parallel, control samples containing no papain and papain alone (2 mg/mL PBS) were incubated. Alternatively, 9 volumes of BSA solution in PBS (5 mg/mL) were added with 1 volume of 0.05% trypsin (or PBS for control preparations) and incubated at 37 °C for 3 h. Then, the protein antioxidant activity/capacity was measured. The antioxidant activity of papain or trypsin was subtracted from that of protein digested with papain or trypsin, respectively.
2.7. Statistics
All measurements were performed at least in triplicate and repeated at least three times on different preparations. As the dependencies of absorbance changes on the concentration of amino acids were linear, the slopes were calculated with the REGLINP function (Excel). For proteins, the linear portions of these dependencies were used for the calculation of antioxidant activity/capacity. The error of antioxidant activity/capacity was calculated from errors of the slopes of dependences of absorbance changes on the amount for amino acids/proteins and for Trolox using the total differential method: error of antioxidant activity = [(error of slopeamino acid or protein)2 + (error of slopeTrolox)2]1/2. Statistical significance of differences was evaluated using the two-tailed Student’s t-test (Excel).
3. Results
3.1. Amino Acids Contributing to the Antioxidant Activity/Capacity of Proteins
The antioxidant activity of a protein molecule and the antioxidant capacity of protein mixtures are conditioned by the reactivity of only some amino acid residues. Six amino acids showed reactivity in the ABTS● decolorization assay: Tyr > Trp > Cys > His > Arg > cystine. Other amino acids, including Met, did not exhibit any detectable reactivity. In the FRAP assay, three amino acids were reactive: Cys > Trp > Tyr; cystine showed very weak antioxidant activity. In the CUPRAC assay, these three amino acids were also reactive, but the sequence of reactivity was different: Cys > Tyr > Trp (Table 1).

Table 1.
Reactivity of amino acids in three assays of antioxidant activity.
Therefore, the antioxidant activity/capacity of proteins depends on the accessibility of these amino acid residues to ABTS● or Fe3+ and Cu2+, respectively. In order to check whether this accessibility can be altered by protein digestion and denaturation, we compared the antioxidant activity of BSA and the antioxidant capacity of egg white subjected to denaturation with SDS and digestion by trypsin and papain.
3.2. Effect of Denaturation on Protein Antioxidant Activity/Capacity
Protein denaturation by SDS caused an increase in the total antioxidant activity of BSA and the total antioxidant capacity of egg white in the ABTS● decolorization assay. The dependence of the extent of ABTS● reduction was not linear, showing saturation for the higher amounts of BSA/egg white, approaching the limit of ABTS● available for reduction. For both BSA and egg white, the increase in antioxidant activity was apparent, especially in the non-linear part of the plot (Figure 1). SDS alone did not react with ABTS● or with the FRAP reagent.
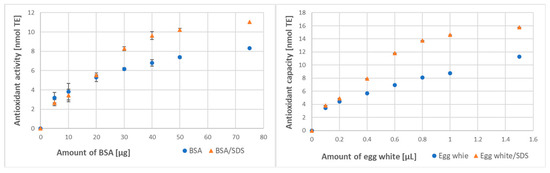
Figure 1.
Effect of denaturation with SDS (0.5% final) on the antioxidant activity of bovine serum albumin (BSA) and antioxidant capacity of egg white assayed by ABTS● decolorization. In some cases, standard deviations were lower than the symbol size (also in other Figures).
The SDS-induced increase in the antioxidant activity of BSA and the antioxidant capacity of egg white was smaller in magnitude but still detectable for BSA and well visible for egg white in the FRAP assay (Figure 2).
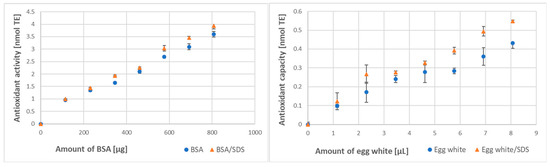
Figure 2.
Effect of denaturation with SDS (0.5% final) on the antioxidant activity of BSA and antioxidant capacity of egg white assayed by the FRAP method.
3.3. Effect of Digestion on the Protein Antioxidant Activity/Capacity
Papain digestion brought about a significant increase in the antioxidant activity of BSA and, especially, in the antioxidant capacity of egg white (Figure 3).
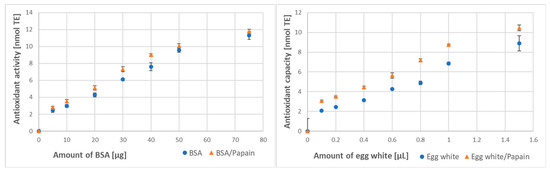
Figure 3.
Effect of digestion with papain on the antioxidant activity of BSA and antioxidant capacity of egg white assayed by ABTS● decolorization.
The increases in the antioxidant activity of BSA and the antioxidant capacity of egg white treated with papain were also detectable in the FRAP assay. This effect was not visible only for the lowest concentration of BSA when subtraction of the antioxidant activity of papain brought the antioxidant activity of papain-treated BSA below the level of the control BSA (Figure 4). This result may be artefactual since self-digestion of papain may be more exhaustive and increase its own antioxidant activity more than in the presence of substrate excess.
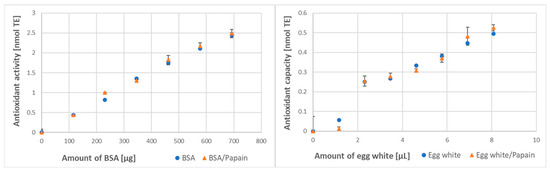
Figure 4.
Effect of papain digestion on the antioxidant activity of BSA and antioxidant capacity of egg white assayed by the FRAP method.
Digestion with trypsin brought a similar effect, significantly increasing the antioxidant activity of BSA assayed by ABTS● decolorization. The augmentation of the antioxidant capacity of egg white proteins treated with trypsin was lower and detectable only for some amounts of egg white (Figure 5).
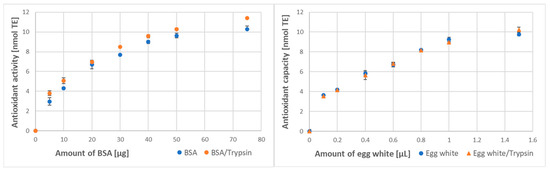
Figure 5.
Effect of digestion with trypsin on the antioxidant activity of BSA and antioxidant capacity of egg white assayed by ABTS● decolorization.
The effect of detergent denaturation and proteolytic digestion on the antioxidant activity of BSA and the antioxidant capacity of chicken egg white is summarized in Table 2. The percent increase in the antioxidant activity/capacity was the highest in the case of SDS-induced denaturation, somewhat smaller for papain digestion, and the smallest for the case of trypsin digestion for both BSA and egg white. In the case of egg white, the increase induced by trypsin digestion did not reach the level of statistical significance.

Table 2.
Effect of detergent denaturation and protease digestion on the antioxidant activity of BSA and antioxidant capacity of egg white.
4. Discussion
One asset of this study is the indication of the contribution of individual amino acid residues to the antioxidant activity of proteins by analysis of the antioxidant activities of individual amino acids. These activities were different when estimated with different assays. The highest number of amino acids (tyrosine, tryptophan, cysteine, histidine, arginine, and cystine) showed reactivity in the ABTS● decolorization assay. Cysteine, tyrosine, and tryptophan were also reactive in two other assays; their activities were the highest in the ABTS decolorization assay and higher in the CUPRAC assay than in the FRAP assay. These differences in reactivity are apparently due to differences in the reaction mechanisms with various reagents and to pH differences (pH 3.6 in the FRAP assay, 7.0 in the CUPRAC assay, and 7.4 in the ABTS● assay). Interestingly, cystine showed some reactivity in the ABTS● decolorization assay and a trace reactivity in the FRAP assay, apparently due to ABTS●- and Fe3+-induced fission of the S-S bond and sulfur oxidation to sulfinic or sulfonic acids [27,28].
The antioxidant activities of reactive amino acids in the ABTS● decolorization and CUPRAC assays were higher than 1 with respect to Trolox. Trolox reacts with ABTS● in two one-electron steps, each Trolox molecule eventually consuming two ABTS● radicals [29,30]. Two consecutive one-electron reactions with ABTS● transform a reducing group in an antioxidant molecule into a stable oxidized form. If one such group in the antioxidant molecule reacts with ABTS● or Fe3+, the stoichiometry with respect to Trolox will be 1. Stoichiometry higher than 1 (with respect to Trolox) indicates more complex reactions and/or further reactivity of amino acid oxidation products with ABTS● or Cu2+. In the case of ABTS●, other reactions of the ABTS● radical, apart from reduction (addition, degradation, etc.), can also contribute to the increased stoichiometry of amino acids in the decolorization assay [30].
Results shown in Table 1 demonstrate that only some amino acid residues have antioxidant activity. Thus, only these amino acid residues determine the antioxidant activity of protein molecules. These residues may be not accessible for the reactions with ABTS● or Fe3+ if buried inside a native protein molecule. It is usually assumed that the thiol groups of cysteine are the main groups responsible for the antioxidant activity of proteins [31,32,33]. However, our results indicate that tyrosine and tryptophan residues (and, to a smaller extent, histidine, arginine, and cystine in the ABTS● decolorization assay) also contribute to the antioxidant activity of proteins. We employed 30 min incubation times in our assays (which are more relevant than shorter assay times for protein interactions in vivo). The relative contribution of various amino acid residues to protein antioxidant activity/capacity can be different for shorter assay times.
This study demonstrates the effects of defined treatments relevant to phenomena occurring during food digestion on the antioxidant activity of a model protein (BSA) and the antioxidant capacity of a protein-rich food (egg white).
The antioxidant activity of BSA estimated by our ABTS assay was about 170–180 μmol/g (Table 2). Taking into account the amount of reactive amino acids in BSA (172 μmol Arg, 193 μmol His, 29 μmol Trp, 127 μmol Tyr [34], 15 μmol Cys, and 250 μmol cystine [35] per g BSA) and considering that only about 60% of the Cys residues are in the reduced state in BSA [36], and assuming antioxidant activities of amino acids shown in Table 1, the total antioxidant activity of BSA should be about 847 μmol/g. Thus, only a fraction of amino acid residues is available for ABTS●, and there is great room for an increase in the reactivity of BSA with ABTS●, as observed after denaturation with SDS and papain digestion. The total antioxidant activity of BSA calculated in the same way for the FRAP assay (about 66 μmol/g) is also higher than that determined experimentally. In this assay, the difference between the maximal and determined antioxidant activity is smaller; it may be due to the low pH of the assay, which causes BSA denaturation. A transition from the normal (N) form of the protein to the partly open, fast migrating (F) form occurs at a pH lower than 4.5 [37]. This transition can be expected to increase the accessibility of more reactive amino acid residues in the protein to Fe3+. The denaturation of many proteins in the FRAP assay may account for lower effects of the procedures applied in this assay in comparison with the ABTS● decolorization assay.
Protein denaturation can occur in the stomach due to low pH but also in the intestine due to the detergent action of bile acids [38]. SDS is a model anionic detergent binding not specifically to proteins and is used for the denaturation of proteins prior to polyacrylamide gel electrophoresis to enable their separation according to apparent molecular weight. Generally, proteins bind up to 1.4 g SDS/g; the binding is independent of ionic strength and primarily hydrophobic in nature [39]. The amounts of SDS used in this study corresponded to 1.0 g/g BSA and 0.51 g/g egg white protein, assuming the egg white protein content of 10.5% and density of 0.93 g/mL [40,41]. The onset of protein denaturation coincides with the critical micelle concentration (cmc) of SDS, which is in the range of several mM, depending on the concentration of SDS-binding proteins [42]. Below cmc, the detergent does not significantly modify the native protein conformation of BSA [43].
The binding of SDS in amounts close to saturating ones unfolds protein molecules due to electrostatic repulsion between negatively charged SDS molecules, eventually to rod-like structures exposing residues that may be not accessible in the native protein structure. Tyr and Trp residues, which contribute significantly to the antioxidant activity of proteins, are often buried inside the hydrophobic interior of protein globules, and unfolding of the molecules may make them accessible for ABTS● or Fe3+. Detergent-induced dissociation of protein complexes may expose reactive amino acid residues. However, extensive detergent binding can also limit the access of the reagents, decreasing the yield of the reaction, so the net effect of detergents on the antioxidant capacity of proteins is not easy to predict. Under the experimental conditions applied, SDS increased the antioxidant activity of BSA and the antioxidant capacity of egg white, apparently due to the unfolding of BSA and egg white proteins.
Similarly, the digestion of proteins may increase the accessibility of reactive amino acid residues if a protein molecule is fragmented. The increase in the antioxidant activity/capacity was more pronounced after the papain treatment than after the trypsin treatment. This is apparently due to the higher substrate specificity of trypsin than papain, enabling papain to hydrolyze more peptide bonds in protein molecules [43]. However, the fragments produced by proteolysis also adopt a conformation most favorable energetically, burying hydrophobic residues inside; therefore, the digestion-induced increase in antioxidant activity of proteins may be limited, as observed in the present study. Denaturation by low pH in the stomach or by heating may also decrease protein antioxidant capacity by the aggregate formation and decrease the protein solubility [44] and availability of redox-active amino acid residues. In agreement with this expectation, an increase in the egg white antioxidant capacity after digestion with papain and papain+pancreatin, but not by boiling, was found by other authors [45].
5. Conclusions
This study points out that several amino acids are reactive in antioxidant activity assays: Tyr > Trp > Cys > His > Arg > cystine in the ABTS● decolorization assay and Cys > Trp > Tyr >> cystine in the FRAP assay; reactions of these amino acids determine the antioxidant activities of proteins. The results of this study demonstrate that both detergent-induced denaturation and proteolytic digestion increased the antioxidant activity of BSA and the antioxidant capacity of egg white, but the effects were limited in extent, being contained in the range of 5–67%.
Author Contributions
Conceptualization, I.S.-B. and G.B.; methodology K.K., G.B. and I.S.-B.; investigation, K.K. and I.S.-B.; validation, K.K., G.B. and I.S.-B.; writing—original draft preparation, K.K., G.B. and I.S.-B.; writing—review and editing, K.K., G.B. and I.S.-B.; supervision, I.S.-B.; project administration, I.S.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data available on reasonable request from the corresponding author.
Acknowledgments
The authors are indebted to Edyta Bieszczad-Bedrejczuk, (Laboratory of Analytical Biochemistry, University of Rzeszów, Poland) for excellent technical help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, X.; Gu, L.; Holden, J.; Haytowitz, D.B.; Gebhardt, S.E.; Beecher, G.; Prior, R.L. Development of a database for total antioxidant capacity in foods: A preliminary study. J. Food Composit. Anal. 2004, 17, 407–422. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Pellegrini, N.; Vitaglione, P.; Granato, D.; Fogliano, V. Twenty-five years of total antioxidant capacity measurement of foods and biological fluids: Merits and limitations. J. Sci. Food Agric. 2020, 100, 5064–5078. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Composit. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Song, W.O.; Fernandez, M.L.; Bruno, R.S.; Koo, S.I.; Chun, O.K. Development and validation of an algorithm to establish a total antioxidant capacity database of the US diet. Int. J. Food Sci. Nutr. 2010, 61, 600–623. [Google Scholar] [CrossRef] [PubMed]
- Pompella, A.; Sies, H.; Wacker, R.; Brouns, F.; Grune, T.; Biesalski, H.K.; Frank, J. The use of total antioxidant capacity as surrogate marker for food quality and its effect on health is to be discouraged. Nutrition 2014, 30, 791–793. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X. Diet Antioxidant Capacity: Relationships to Oxidative Stress and Health. Am. J. Biomed. Sci. 2013, 5, 126–139. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Alvarez, J.A.; Fernández-López, J. In vitro digestion models suitable for foods: Opportunities for new fields of application and challenges. Food Res. Int. 2018, 107, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; Delgado-Andrade, C. Effect of digestive process on Maillard reaction indexes and antioxidant properties of breakfast cereals. Food Res. Int. 2009, 42, 394–400. [Google Scholar] [CrossRef]
- Ng, Z.X.; Rosman, N.F. In vitro digestion and domestic cooking improved the total antioxidant activity and carbohydrate-digestive enzymes inhibitory potential of selected edible mushrooms. J. Food Sci. Technol. 2019, 56, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols-A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Reginio, F.C., Jr.; Thuengtung, S.; Ogawa, Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4684–4705. [Google Scholar] [CrossRef]
- Koehnlein, E.A.; Koehnlein, É.M.; Corrêa, R.C.G.; Nishida, V.S.; Correa, V.G.; Bracht, A.; Peralta, R.M. Analysis of a whole diet in terms of phenolic content and antioxidant capacity: Effects of a simulated gastrointestinal digestion. Int. J. Food Sci. Nutr. 2016, 67, 614–623. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Change of phenolics, carotenoids, and antioxidant capacity following simulated gastrointestinal digestion and dialysis of selected edible green leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Faller, A.L.; Fialho, E.; Liu, R.H. Cellular antioxidant activity of feijoada whole meal coupled with an in vitro digestion. J. Agric. Food Chem. 2012, 60, 4826–4832. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Sariçay, Y.; Arranz, E.; Kelly, P.M.; Buckin, V.; Giblin, L. Comparison of antioxidant activities of bovine whey proteins before and after simulated gastrointestinal digestion. J. Dairy Sci. 2019, 102, 54–67. [Google Scholar] [CrossRef]
- Delgado, M.C.O.; Galleano, M.; Añón, M.C.; Tironi, V.A. Amaranth peptides from simulated gastrointestinal digestion: Antioxidant activity against reactive species. Plant Foods Hum. Nutr. 2015, 70, 27–34. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chem. 2010, 120, 810–816. [Google Scholar] [CrossRef]
- Ahn, C.B.; Kim, J.G.; Je, J.Y. Purification and antioxidant properties of octapeptide from salmon byproduct protein hydrolysate by gastrointestinal digestion. Food Chem. 2014, 147, 78–83. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Valverde-Moya, Á.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Effect of Cooking Methods on the Antioxidant Capacity of Foods of Animal Origin Submitted to In Vitro Digestion-Fermentation. Antioxidants 2021, 10, 445. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Valverde-Moya, Á.J.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Effect of Cooking Methods on the Antioxidant Capacity of Plant Foods Submitted to In Vitro Digestion-Fermentation. Antioxidants 2020, 9, 1312. [Google Scholar] [CrossRef] [PubMed]
- Kut, K.; Cieniek, B.; Stefaniuk, I.; Bartosz, G.; Sadowska-Bartosz, I. A Modification of the ABTS• Decolorization Method and an Insight into Its Mechanism. Processes 2022, 10, 1288. [Google Scholar] [CrossRef]
- Bartosz, G. Total antioxidant capacity. Adv. Clin. Chem. 2003, 37, 219–292. [Google Scholar] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Çelik, S.E.; Baki, S.; Leyla, Y.; Karaman, S.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Meth. 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Finley, J.W.; Wheeler, E.L.; Walker, H.G., Jr.; Finlayson, A.J. Effect of cystine oxidation on lysinoalanine formation in proteins. J. Agric. Food Chem. 1982, 30, 818–820. [Google Scholar] [CrossRef]
- Karimi, M.; Crossett, B.; Cordwell, S.J.; Pattison, D.I.; Davies, M.J. Characterization of disulfide (cystine) oxidation by HOCl in a model peptide: Evidence for oxygen addition, disulfide bond cleavage and adduct formation with thiols. Free Radic. Biol. Med. 2020, 154, 62–74. [Google Scholar] [CrossRef]
- Yi, H.; Cheng, Y.; Zhang, Y.; Xie, Q.; Yang, X. Potentiometric and UV-Vis spectrophotometric titrations for evaluation of the antioxidant capacity of chicoric acid. RSC Adv. 2020, 10, 11876–11882. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Atanasiu, R.L.; Stea, D.; Mateescu, M.A.; Vergely, C.; Dalloz, F.; Briot, F.; Maupoil, V.; Nadeau, R.; Rochette, L. Direct evidence of caeruloplasmin antioxidant properties. Mol. Cell. Biochem. 1998, 189, 127–135. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Maiani, G.; Azzini, E.; Ferro-Luzzi, A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic. Biol. Med. 1995, 18, 29–36. [Google Scholar] [CrossRef]
- Cui, J.; Chong, B.; Rutherfurd, S.M.; Wilkinson, B.; Singh, H.; Moughan, P.J. Gross and true ileal digestible amino acid contents of several animal body proteins and their hydrolysates. Meat Sci. 2013, 94, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Siriwardana, K.; Wang, A.; Gadogbe, M.; Collier, W.E.; Fitzkee, N.C.; Zhang, D. Studying the effects of cysteine residues on protein interactions with silver nanoparticles. J. Phys. Chem. C 2015, 119, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.B.; Saroff, H.A. Decrease in Sulfhydryl Titer of Serum Albumin1. J. Am. Chem. Soc. 1958, 80, 2129–2131. [Google Scholar] [CrossRef]
- Michnik, A.; Michalik, K.; Drzazga, Z. Stability of bovine serum albumin at different pH. J. Therm. Anal. Calorim. 2005, 80, 399–406. [Google Scholar] [CrossRef]
- Robic, S.; Linscott, K.B.; Aseem, M.; Humphreys, E.A.; McCartha, S.R. Bile acids as modulators of enzyme activity and stability. Protein J. 2011, 30, 539–545. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Tanford, C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc. Natl. Acad. Sci. USA 1970, 66, 1002–1007. [Google Scholar] [CrossRef]
- Mann, K. The chicken egg white proteome. Proteomics 2007, 7, 3558–3568. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Y.Q. An insight on egg white: From most common functional food to biomaterial application. J. Biomed. Materials Res. B Appl. Biomater. 2021, 109, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.M.; Andersen, K.K.; Westh, P.; Otzen, D.E. Unfolding of beta-sheet proteins in SDS. Biophys. J. 2007, 92, 674–685. [Google Scholar] [CrossRef]
- van der Hoorn, R.A. Plant proteases: From phenotypes to molecular mechanisms. Annu. Rev. Plant Biol. 2008, 59, 191–223. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.F.; Zanette, D.; Fischer, H.; Itri, R. A systematic study of bovine serum albumin (BSA) and sodium dodecyl sulfate (SDS) interactions by surface tension and small angle X-ray scattering. J. Coll. Interface Sci. 2003, 262, 400–408. [Google Scholar] [CrossRef]
- Rao, P.S.; Nolasco, E.; Handa, A.; Naldrett, M.J.; Alvarez, S.; Majumder, K. Effect of pH and Heat Treatment on the Antioxidant Activity of Egg White Protein-Derived Peptides after Simulated In-Vitro Gastrointestinal Digestion. Antioxidants 2020, 9, 1114. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).