Analysis of Hydrochemical Characteristics and Causes of Drinking Water Sources in South China: A Case Study in Zhanjiang City
Abstract
1. Introduction
2. Materials and Methods
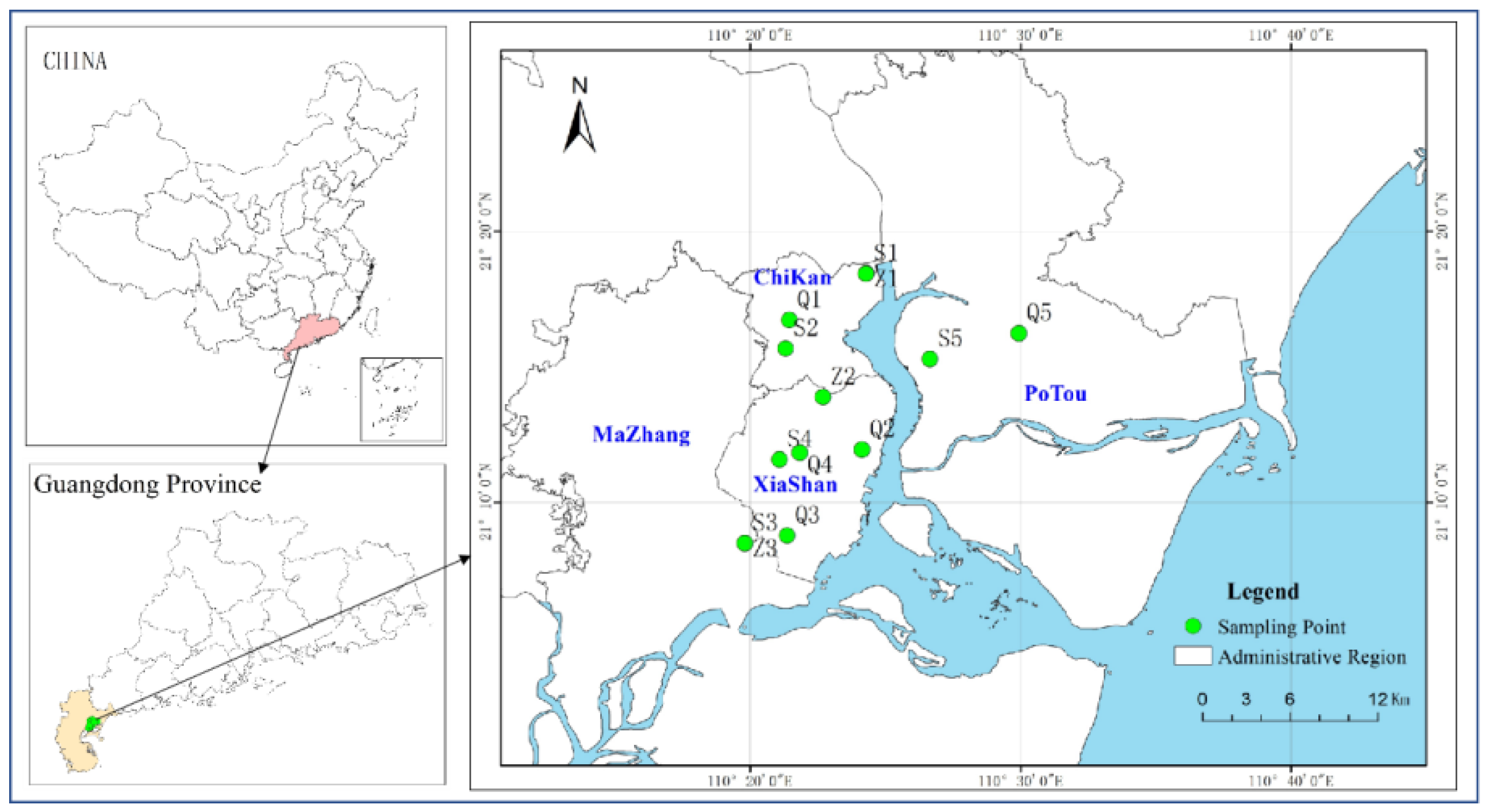
2.1. Information of Study Area
2.2. Sample Collection and Measurement
3. Results and Discussion
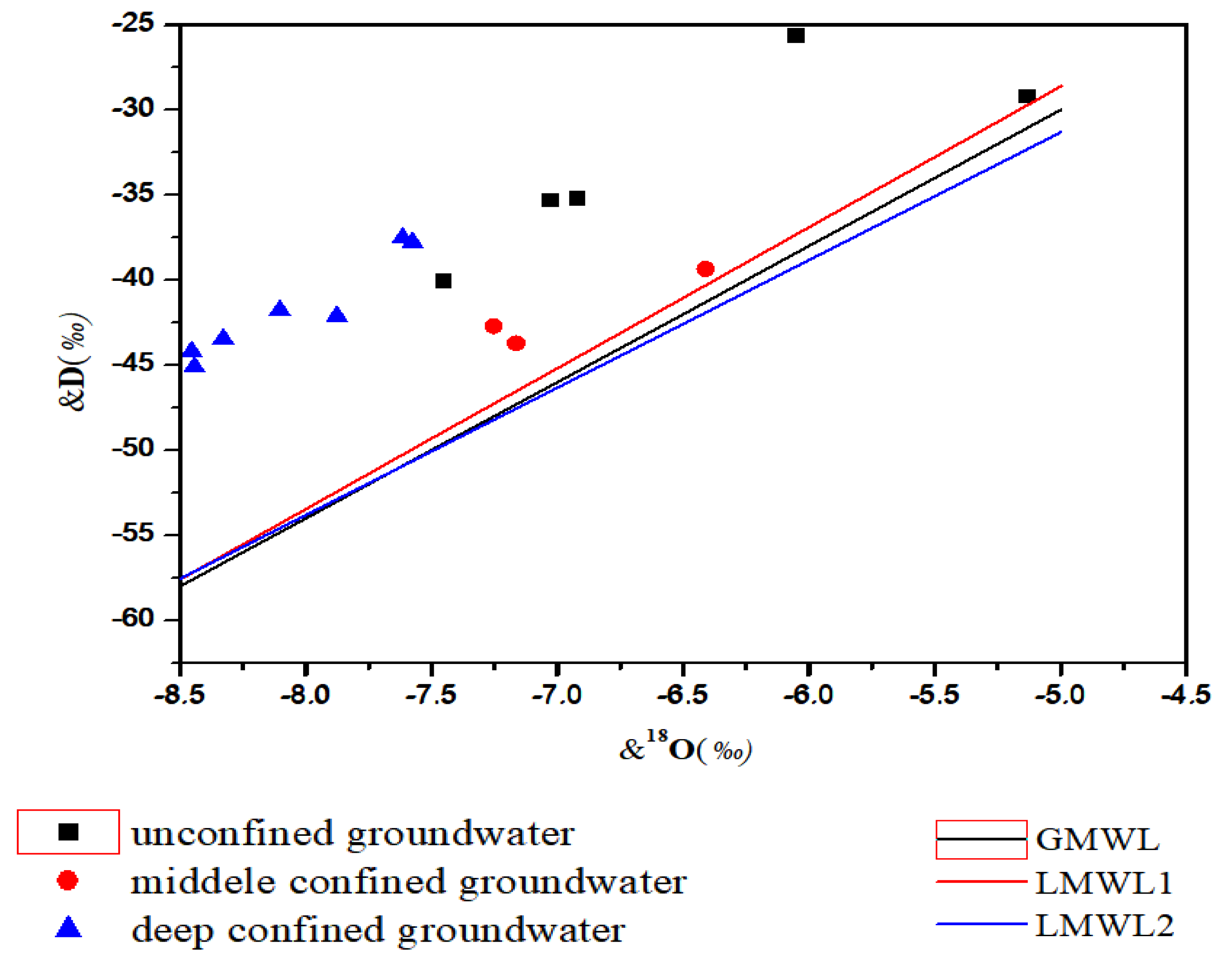
3.1. Characteristic of Isotopes
3.2. Hydrochemical Characteristics
3.3. Hydrochemical Pattern of Groundwater
3.4. Correlation Relationship
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Tang, Z.; Gao, M.; Hou, G. Evolutionary process of saline-water intrusion in Holocene and Late Pleistocene groundwater in southern Laizhou Bay. Sci. Total. Environ. 2017, 607–608, 586–599. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Du, S. The Investigation and Assessment on Groundwater Organic Pollution. Org. Pollut. Monit. Risk Treat 2013, 4, 87–110. [Google Scholar] [CrossRef]
- Wang, S.Y.; Sun, C.J.; Chen, W.; Zhou, S.J.; Zhang, X. Analysis of Water Chemistry Characteristics and Hydraulic Relationships of Different Water Bodies in Typical Mountain-oasis Systems in the Northwest Inland Area. Environ. Sci. 2022, 44, 1416–1428. (In Chinese) [Google Scholar]
- Soltanian, M.; Dargahi, A.; Asadi, F.; Ivani, A.; Setareh, P. Variation of PhysicoChemical Quality of Groundwater Watershed in Gharehsou during 2003–2012. J. Maz. Univ. Med. Sci. 2015, 24, 275–287. [Google Scholar]
- Hu, M.; Wang, Y.; Du, P.; Shui, Y.; Cai, A.; Lv, C.; Bao, Y.; Li, Y.; Li, S.; Zhang, P. Tracing the sources of nitrate in the rivers and lakes of the southern areas of the Tibetan Plateau using dual nitrate isotopes. Sci. Total Environ. 2019, 658, 132–140. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Z.; Bing, W. An Analysis of the Hydro-chemical Characteristics and Causes of Drinking Water Source of Concentrated Deep Groundwater in Fuyang City. China Rural. Water Hydropower 2020, 3, 78–82. (In Chinese) [Google Scholar]
- Al-Barakah, F.N.; Al-jassas, A.M.; Aly, A.A. Water quality assessment and hydrochemical characterization of Zamzam groundwater, Saudi Arabia. Appl. Water Sci. 2017, 7, 3985–3996. [Google Scholar] [CrossRef]
- Zhao, W.; Liang, H.; Gong, S.J. Hydrochemical Characteristics and Oxygen Isotope Tracing Study of Shallow Groundwater Seawater—Intrusion in Tianjin. J. Cap. Norm. Univ. (Nat. Sci. Ed.) 2019, 40, 66–69. [Google Scholar]
- Wang, Y.; Guo, Y.; Zhou, Y.; Li, S.; Wang, Q. Quantifications of spatial and temporal variations in groundwater discharge into a river using hydrochemical and isotopic tracers. Arid. Land Geigraphy 2020, 43, 290–298. (In Chinese) [Google Scholar]
- Zhang, W.; Yang, X.; Huang, L.; Wang, L.; Zheng, N.; Xie, Y. Characteristics and means of comprehensive management of typical groundwater overexploitation areas in southern China—Taking Zhanjiang City of Guangdong Province as an example. China Water Resour. 2022, 7, 86–88. (In Chinese) [Google Scholar]
- Dargahi, A.; Azizi, A.; Karami, A.; Amirian, F.; Mohammadi, M.; Almasi, A. Evaluating the chemical and microbial quality of drinking water in harsin city. Int. J. Pharm. Technol. 2016, 8, 16709–16719. [Google Scholar]
- Sheikhy, N.T.; Sefie, A.; Aris, A.Z. The long-term impacts of anthropogenic and natural processes on groundwater deterioration in a multilayered aquifer. Sci. Total Environ. 2018, 630, 931–942. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, F.; Jin, Z. Spatial characteristics and controlling factors of chemical weathering of loess in the dry season in the middle Loess Plateau, China. Hydrol. Process. 2016, 30, 4855–4869. [Google Scholar] [CrossRef]
- Su, F.; Wu, J.; Wang, D.; Zhao, H.; Wang, Y.; He, X. Moisture movement, soil salt migration, and nitrogen transformation under different irrigation conditions: Field experimental research. Chemosphere 2022, 300, 134569. [Google Scholar] [CrossRef]
- Alcalá, F.J.; Custodio, E. Using the Cl/Br ratio as a tracer to identify the origin of salinity in aquifers in Spain and Portugal. J. Hydrol. 2008, 359, 189–207. [Google Scholar] [CrossRef]
- Aminiyan, M.M.; Aminiyan, F.M. Comprehensive integrated index-based geochemistry and hydrochemical analyses of groundwater resources for multiple consumptions under coastal conditions. Environ. Sci. Pollut. Res. Int. 2020, 27, 21386–21406. [Google Scholar] [CrossRef]
- Santoni, S.; Huneau, F.; Garel, E.; Aquilina, L.; Vergnaud-Ayraud, V.; Labasque, T.; Celle-Jeanton, H. Strontium isotopes as tracers of water-rocks interactions, mixing processes and residence time indicator of groundwater within the granite-carbonate coastal-aquifer of Bonifacio (Corsica, France). Sci. Total Environ. 2016, 573, 233–246. [Google Scholar] [CrossRef]
- Zabala, M.E.; Manzano, M.; Vives, L. The origin of groundwater composition in the Pampeano Aquifer underlying the Del Azul Creek basin, Argentina. Sci. Total Environ. 2015, 518–519, 168–188. [Google Scholar] [CrossRef]
- Mark, Y.S.; Eric, K. The groundwater recharge regime of some slightly metamorphosed neoproterozoic sedimentary rocks: An application of natural environmental tracers. Hydrol. Process. 2014, 28, 3104–3117. [Google Scholar]
- Tamborski, J.J.; Cochran, J.K.; Bokuniewicz, H.J. Application Of 224ra And 222rn For Evaluating Seawater Residence Times In A Tidal Subterranean Estuary. Mar. Chem. 2017, 189, 32–45. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, M.; Wu, X.; Pan, M. The relationship between δ18O characteristics of the precipitation (heavy rainfall or rainstorm) and its water vapor sources in Guilin, China. Carsologica Sin. 2017, 36, 139–161. (In Chinese) [Google Scholar]
- Yang, N.; Wang, G. Moisture sources and climate evolution during the last 30 kyr in northeastern Tibetan Plateau: Insights from groundwater isotopes (2H, 18O, 3H and 14C) and water vapour trajectories modeling. Quat. Sci. Rev. 2020, 242, 106426. [Google Scholar] [CrossRef]
- Chowdhury, A.H.; Scanlon, B.R.; Reedy, R.C.; Young, S. Fingerprinting groundwater salinity sources in the Gulf Coast Aquifer System, USA. Hydrogeol. J. 2017, 26, 197–213. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, F.; Liu, H.; Deng, G. Application of hydrochemical and isotopic analysis to research a typical karst groundwater system: A case study at xianrendong, Xichang City. Sci. Technol. Eng. 2019, 19, 76–83. (In Chinese) [Google Scholar]
- Jebreen, H.; Wohnlich, S.; Banning, A.; Wisotzky, F.; Niedermayr, A.; Ghanem, M. Recharge, geochemical processes and water quality in karst aquifers: Central West Bank, Palestine. Environ. Earth Sci. 2018, 77, 261. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, J.; Evaristo, J.; Li, Z. Spatiotemporal variations in the hydrochemical characteristics and controlling factors of streamflow and groundwater in the Wei River of China. Environ. Pollut. 2019, 254, 113006. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, B.; Mengoni, A.; Dong, J.; Peng, X. Distribution of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans in sediments from the Xiangjiang River, China. Environ. Monit. Assess. 2012, 184, 7083–7092. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Zhu, H.; Chen, Z.; Su, C.; He, Z.; Chen, X.; Qiu, J.; Wang, T. Spatial Distribution and Source Apportionment of Soil Heavy Metals in Pearl River Delta, China. Sustainability 2021, 13, 9651. [Google Scholar] [CrossRef]
- Yan, J.; Chen, J.; Zhang, W. Study on the groundwater quality and its influencing factor in Songyuan City, Northeast China, using integrated hydrogeochemical method. Sci. Total Environ. 2021, 773, 144958. [Google Scholar] [CrossRef]
- Ako, A.A.; Shimada, J.; Hosono, T.; Kagabu, M.; Richard, A.; Nkeng, G.E.; Tongwa, A.F.; Ono, M.; Eyong, G.E.T.; Tandia, B.K.; et al. Flow dynamics and age of groundwater within a humid equatorial active volcano (Mount Cameroon) deduced by δD, δ18O, 3H and chlorofluorocarbons (CFCs). J. Hydrol. 2013, 502, 156–176. [Google Scholar] [CrossRef]
- IAEA/WMO. Global Network of Isotopes in Precipitation. The GNIP Database. 2022. Available online: https://nucleusiaeaorg/wiser (accessed on 31 October 2022).
- Jin, J.; Wang, Z.; Zhao, Y.; Ding, H.; Zhang, J. Delineation of Hydrochemical Characteristics and Tracing Nitrate Contamination of Groundwater Based on Hydrochemical Methods and Isotope Techniques in the Northern Huangqihai Basin, China. Water 2022, 14, 3168. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rammohan, V.; Sahayam, J.D.; Jeevanandam, M. Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environ. Model. Assess. 2009, 159, 341–351. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Zhang, Y.; Sun, Z.; Sun, T.; Fan, H.; Wu, B.; Li, M.; Qian, L. Hydrochemical evaluation of groundwater quality and human health risk assessment of nitrate in the largest peninsula of China based on high-density sampling: A case study of Weifang. J. Clean. Prod. 2021, 322, 129164. [Google Scholar] [CrossRef]
- GB/T 14848–2017; Standards for Groundwater Quality. General Administration of Quality Supervision: Beijing, China, 2017.
- Kenniche, S.; Bekkoussa, B.; M’Nassri, S.; Teffahi, M.; Taupin, J.-D.; Patris, N.; Zaagane, M.; Majdoub, R. Hydrochemical characterization, physicochemical and bacteriological quality of groundwater in Sidi Kada Mountains, northwest of Algeria. Arab. J. Geosci. 2022, 15, 1–18. [Google Scholar] [CrossRef]
- Jones, S.; Rosen, M. Controls on the chemical composition of groundwater from alluvial aquifers in the Wanaka and Wakatipu basins, Central Otago, New Zealand. Hydrogeol. J. 1998, 6, 264–281. [Google Scholar] [CrossRef]
- Böhlke, J.-K. Groundwater recharge and agricultural contamination. Hydrogeol. J. 2002, 10, 153–179. [Google Scholar] [CrossRef]
- Busico, G.; Kazakis, N.; Colombani, N.; Mastrocicco, M.; Voudouris, K.; Tedesco, D. A modifed SINTACS method for groundwater vulnerability and pollution risk assessment in highly anthropized regions based on NO3− and SO42− concentrations. Sci. Total Environ. 2017, 609, 1512–1523. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Mohammadi, M.; Karami, A.; Tabandeh, L.; Dargahi, A.; Amirian, F. Residue Analysis of Pesticides, Herbicides, and Fungicides in Various Water Sources Using Gas Chromatography-Mass Detection. Pol. J. Environ. Stud. 2017, 26, 2189–2195. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Yeh, T.J.; Zhen, P.; Wang, L.; Shi, L. Using multivariate statistical techniques and geochemical modelling to identify factors controlling the evolution of groundwater chemistry in a typical transitional area between Taihang Mountains and North China Plain. Hydrol. Process. 2020, 34, 1888–1905. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.; Zhan, H.; Chen, X.; Liu, M.; Wang, M. Identifcation of hydrogeochemical processes and transport paths of a multiaquifer system in closed mining regions. J. Hydrol. 2020, 589, 125344. [Google Scholar] [CrossRef]
- Ren, X.; Li, P.; He, X.; Su, F.; Elumalai, V. Hydrogeochemical processes afecting groundwater chemistry in the central part of the Guanzhong Basin, China. Arch. Environ. Contam. Toxicol. 2021, 80, 74–91. [Google Scholar] [CrossRef]
- Hosono, T.; Masaki, Y. Post-seismic hydrochemical changes in regional groundwater flow systems in response to the 2016 Mw 7.0 Kumamoto earthquake. J. Hydrol. 2020, 580, 124340. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, C.L.; Ma, Y.H.; Liu, W.J. Indicators of Groundwater Evolution Processes Based on Hydrochemistry and Environmental Isotopes: A Case Study of the Dongyuan Drinking Water Source Area in Ji’nan City. Environ. Sci. 2019, 40, 2667–2674. [Google Scholar]
- Zhao, Y.; Han, J.; Zhang, B.; Gong, J. Impact of transferred water on the hydrochemistry and water quality of surface water and groundwater in Baiyangdian Lake, North China. Geosci. Front. 2021, 12, 101086. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Thakur, T.; Rishi, M.S.; Naik, P.K.; Sharma, P. Elucidating hydrochemical properties of groundwater for drinking and agriculture in parts of Punjab, India. Environ. Earth Sci. 2016, 75, 1–15. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Wang, L.; Shi, L.; Song, X.; Yeh, T.C.J.; Zhen, P. Coupling hydrochemistry and stable isotopes to identify the major factors afecting groundwater geochemical evolution in the Heilongdong Spring Basin, North China. J. Geochem. Explor. 2019, 205, 106352. [Google Scholar] [CrossRef]
- Kim, K.-H.; Yun, S.-T.; Choi, B.-Y.; Chae, G.-T.; Joo, Y.; Kim, K.; Kim, H.-S. Hydrochemical and multivariate statistical interpretations of spatial controls of nitrate concentrations in a shallow alluvial aquifer around oxbow lakes (Osong area, central Korea). J. Contam. Hydrol. 2009, 107, 114–127. [Google Scholar] [CrossRef]
- Su, C.; Wang, Y.; Pan, Y. Hydrogeochemical and isotopic evidences of the groundwater regime in Datong Basin, Northern China. Environ. Earth Sci. 2013, 70, 877–885. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Wang, D.; Ren, X.; Wei, M. Statistical and multivariate statistical techniques to trace the sources and afecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Hum. Ecol. Risk Assess. 2020, 26, 1603–1621. [Google Scholar] [CrossRef]



| Sample ID | pH | Ca2− (mg/L) | Mg2− (mg/L) | K+ (mg/L) | Na+ (mg/L) | Cl− (mg/L) | SO42− (mg/L) | HCO3− (mg/L) | NO3− (mg/L) | NO2− (mg/L) | TDS (mg/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unconfined aquifer | Q1 | 4.15 | 24.96 | 9.28 | 15.88 | 43.96 | 44.10 | 44.87 | 2.55 | 25.00 | 0.05 | 175.60 |
| Q2 | 7.02 | 115.19 | 10.28 | 27.48 | 62.44 | 29.98 | 3.49 | 180.97 | 9.00 | 7.42 | 169.41 | |
| Q3 | 5.91 | 84.94 | 9.29 | 24.65 | 63.28 | 38.23 | 40.52 | 6.24 | 50.00 | 0.01 | 322.90 | |
| Q4 | 7.21 | 128.72 | 18.43 | 72.48 | 19.85 | 50.25 | 70.93 | 281.17 | 35.00 | 0.02 | 239.80 | |
| Q5 | 4.62 | 54.39 | 14.18 | 8.30 | 59.25 | 55.54 | 89.54 | 2.99 | 8.00 | ND | 216.32 | |
| Middle confined aquifer | Z1 | 6.39 | 29.31 | 4.23 | 22.75 | 11.76 | 2.66 | 19.72 | 61.74 | ND | ND | 92.10 |
| Z2 | 6.19 | 24.32 | 8.84 | 9.47 | 11.20 | 10.43 | 10.72 | 52.35 | 2.00 | ND | 109.23 | |
| Z3 | 5.57 | 17.81 | 12.92 | 11.11 | 28.62 | 28.53 | 30.98 | 10.42 | ND | ND | 122.12 | |
| Deep confined aquifer | S1 | 8.11 | 15.59 | 1.01 | 6.18 | 227.90 | 5.19 | 7.15 | 251.52 | ND | ND | 332.40 |
| S2 | 6.61 | 14.24 | 5.31 | 18.65 | 18.52 | 16.84 | 8.34 | 92.65 | 0.60 | ND | 136.02 | |
| S3 | 6.32 | 21.69 | 11.29 | 12.63 | 23.93 | 17.38 | 14.30 | 138.29 | ND | 0.02 | 212.18 | |
| S4 | 6.65 | 22.65 | 11.25 | 14.71 | 17.84 | 4.43 | 5.96 | 95.59 | 0.60 | ND | 142.58 | |
| S5 | 6.43 | 15.91 | 5.34 | 20.17 | 13.72 | 24.15 | 12.98 | 50.72 | ND | ND | 199.88 | |
| S6 | 6.42 | 24.13 | 3.87 | 16.30 | 29.24 | 15.07 | 1.19 | 91.18 | 9.00 | 0.01 | 226.08 | |
| S7 | 6.51 | 23.85 | 3.24 | 17.42 | 33.78 | 3.49 | 3.59 | 73.11 | ND | ND | 167.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Zou, Q.; Chen, Z.; Cao, Y.; Wang, S.; Zhu, F.; Liu, X. Analysis of Hydrochemical Characteristics and Causes of Drinking Water Sources in South China: A Case Study in Zhanjiang City. Processes 2023, 11, 1196. https://doi.org/10.3390/pr11041196
Wei H, Zou Q, Chen Z, Cao Y, Wang S, Zhu F, Liu X. Analysis of Hydrochemical Characteristics and Causes of Drinking Water Sources in South China: A Case Study in Zhanjiang City. Processes. 2023; 11(4):1196. https://doi.org/10.3390/pr11041196
Chicago/Turabian StyleWei, Hang, Qi Zou, Zhiliang Chen, Yingjie Cao, Shuang Wang, Fen Zhu, and Xulong Liu. 2023. "Analysis of Hydrochemical Characteristics and Causes of Drinking Water Sources in South China: A Case Study in Zhanjiang City" Processes 11, no. 4: 1196. https://doi.org/10.3390/pr11041196
APA StyleWei, H., Zou, Q., Chen, Z., Cao, Y., Wang, S., Zhu, F., & Liu, X. (2023). Analysis of Hydrochemical Characteristics and Causes of Drinking Water Sources in South China: A Case Study in Zhanjiang City. Processes, 11(4), 1196. https://doi.org/10.3390/pr11041196









